Published online Dec 9, 2025. doi: 10.5409/wjcp.v14.i4.108329
Revised: May 5, 2025
Accepted: July 15, 2025
Published online: December 9, 2025
Processing time: 203 Days and 22.8 Hours
In this case report, we aimed to raise awareness regarding arrhythmogenic cardiomyopathy (ACM) with inflammatory “hot phase” episodes in pediatric patients, which is often misdiagnosed as myocarditis. This condition, caused by aseptic intracellular inflammation, can be misdiagnosed as acute coronary syndrome or myocardial viral infection, with the latter being particularly common in children. Here, we report two pediatric cases of ACM with “hot phase” episo
The first patient (aged 13 years) was hospitalized after experiencing a single episode of syncope, chest pain, and palpitation. Clinical examination revealed elevated troponin levels, complete right bundle branch block, right ventricular dilation, and normal coronary arteries. Cardiac magnetic resonance imaging (MRI) revealed extensive fibrotic changes in the right ventricle, which was consistent with ACM, and a pathogenic variant in DSG2 confirmed the diagnosis. The second patient (aged 4 years) presented with chest pain and elevated troponin levels. Electrocardiography revealed a left bundle branch block, while echocardiography showed reduced left ventricular contractility. Cardiac MRI demonstrated left ventricular dilation and subepicardial fibrosis. The phenotypic features, such as curly-wool hair, hyperkeratosis, and onychodystrophy, suggested a genetic nature of the disease. Two mutations identified in DSP confirmed the diagnosis of Carvajal syndrome with intermittent “hot phase” episodes.
ACM in children can present with nonspecific inflammatory symptoms, which may be misdiagnosed as myocarditis or coronary artery pathology.
Core Tip: Arrhythmogenic cardiomyopathy (ACM) is characterized by intercalated disc remodeling and cardiomyocytes death, which can lead to fibro-fatty replacement and severe heart failure. In some children, ACM presents with a “hot phase” and may be mistaken for acute myoca
- Citation: Nikitina E, Kofeynikova O, Zlotina A, Pervunina T, Vasichkina E, Golovkin A, Kalinina O, Kostareva A. Arrhythmogenic cardiomyopathy in children, on the link between injurious mutations and inflammation: Two case reports and review of the literature. World J Clin Pediatr 2025; 14(4): 108329
- URL: https://www.wjgnet.com/2219-2808/full/v14/i4/108329.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i4.108329
Arrhythmogenic cardiomyopathy (ACM) is an inherited heart disease characterized by malignant ventricular arrhythmias due to myocardial fibro-fatty replacement[1]. The disease phenotype is highly variable, ranging from an early “latent phase” with only slight conduction defects and atrial arrhythmias in structurally normal heart to malignant ventricular arrhythmias and even biventricular heart failure. In most cases, the disease progresses slowly over 10-15 years. However, the intermittent occurrence of “hot phases” that mimics acute myocarditis can be observed in some patients; it represents episodes of active aseptic inflammation response to cellular damage and death, leading to reactive cellular infiltration and life-threatening arrhythmias.
The genetic nature of ACM is diverse and mainly linked to desmosomal structures, which are the tight intercellular contacts between adjacent cells typical of cardiomyocytes and dermal epithelium. Approximately 30%-40% of all ACM cases are caused by mutations in the PKP2 gene encoding the desmosome protein plakophilin-2[2]. Other genes associated with ACM include the desmosomal proteins DSC2, DSG2, DSP, and JUP; area composita component CTNNA3; desmosome-associated intermediate filament DES; intercalated disc-associated kinase ILK; and cardiac sodium channel SCN5A[3]. Desmosomal protein defects result in damage and remodeling of cardiomyocyte intercalated discs, triggering cardiomyocyte death, inflammation, and reactive fibrosis[4-6]. In addition, mutations in genes encoding nuclear lamina proteins, LMNA and TMEM43; components of the circulating calcium system, RYR2 and PLN; growth factor TGFB3; and giant intrasarcomeric protein TTN have been described in association with ACM[3].
Several genotype-phenotype correlations are described for ACM. Thus, mutations in DSP are often associated with left ventricular involvement, heart failure progression, and cardiac transplantation[7], while rare cases linked to TMEM43 are mainly characterized by early and malignant arrhythmias[8]. However, although several clinical case descriptions have been documented, only a few genotype-phenotype correlations have been described for ACM with “hot phases” mimicking acute myocarditis. Here, we report two pediatric cases of “hot phase” ACM episodes, which, along with previously reported cases, underscores the importance of ACM awareness in pediatric patients with signs of myocardial injury and myocarditis.
Case 1: A 13-year-old boy was admitted to the hospital with a single episode of syncope and ventricular tachycardia. During hospitalization, the patient also experienced recurrent chest pain attacks.
Case 2: A 4-year-old boy was hospitalized in the pediatric cardiac department with dyspnea, fatigue, periodic chest pain, and elevated troponin I level.
Case 1: The differential diagnosis initially focused on acute myocarditis and acute coronary syndrome. General inflammatory markers were not elevated; polymerase chain reaction testing for cardiotropic viruses was negative; and typical signs of myocarditis, including early gadolinium enhancement, were absent on magnetic resonance imaging (MRI). However, MRI revealed decreased contractility and extensive fibrotic areas in the right ventricle (RV).
Case 2: The differential diagnosis included myocarditis, dilated cardiomyopathy, and left-dominant ACM. Given the anamnesis data from a previous viral infection, the patient was initially diagnosed with myocarditis and initiated with antiarrhythmic therapy (beta-blocker metoprolol 2 mg/kg/day) and angiotensin-converting enzyme (ACE) for chronic heart failure (CHF). However, over a year of surveillance, the patient demonstrated progressive clinical deterioration, indicating the genetic nature of the disease.
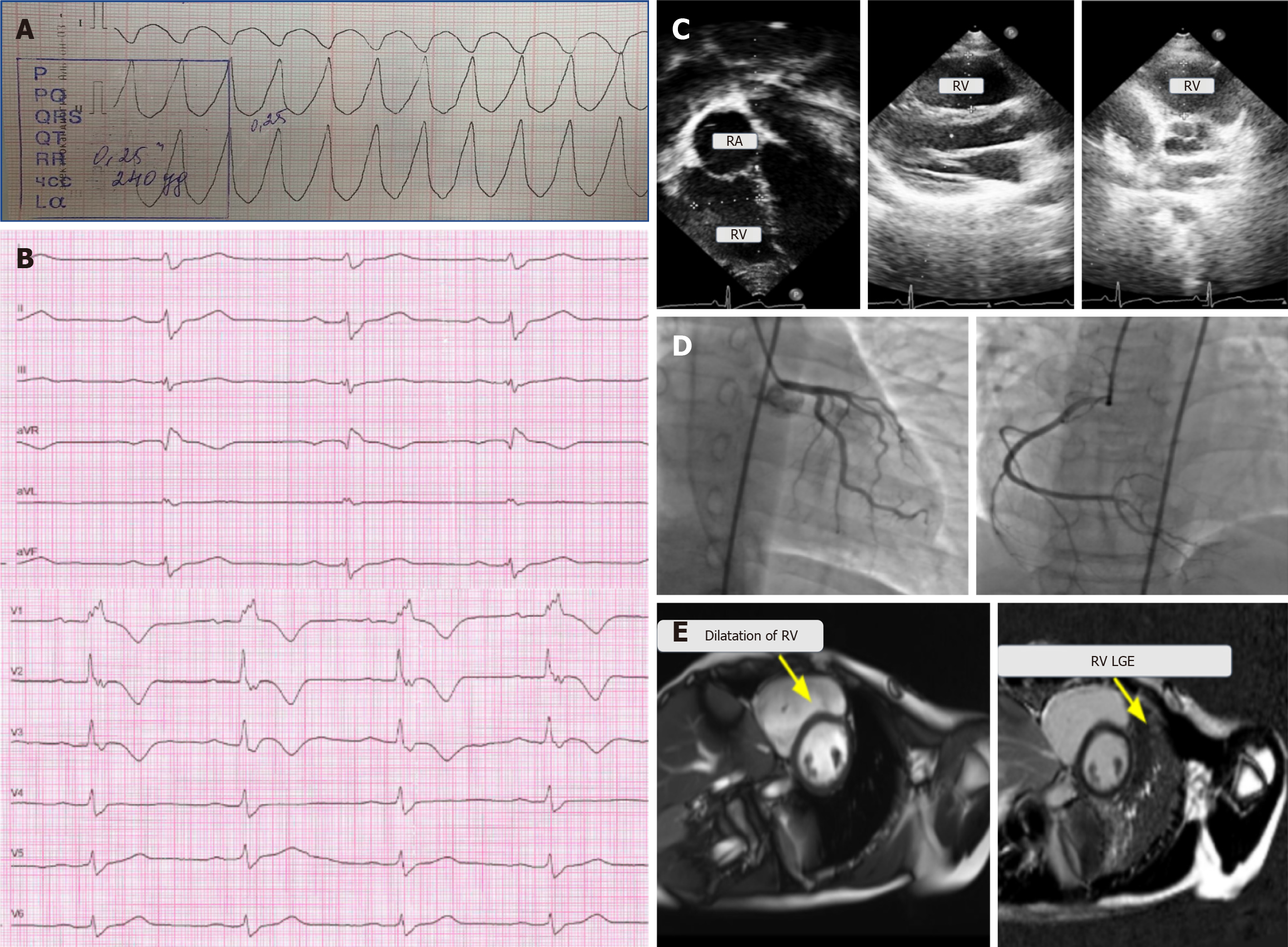
Case 1: About 6 months prior to admission, the patient had experienced a syncopal episode, with heart rate exceeding 250 beats per minute and ventricular tachycardia with wide QRS complexes, which was terminated by electrical cardioversion (Figure 1A). At that time, Holter electrocardiography (ECG) monitoring recorded occasional ventricular extrasystoles, while echocardiography showed no abnormalities. The patient was recommended for propafenone treatment and referred to our center for further examination.
Case 2: The patient had coronavirus infection 2 months prior to hospitalization.
Case 1 and Case 2: No family history of ACM or sudden cardiac death was noted.
Case 1: The physical examination did not reveal notable abnormalities.
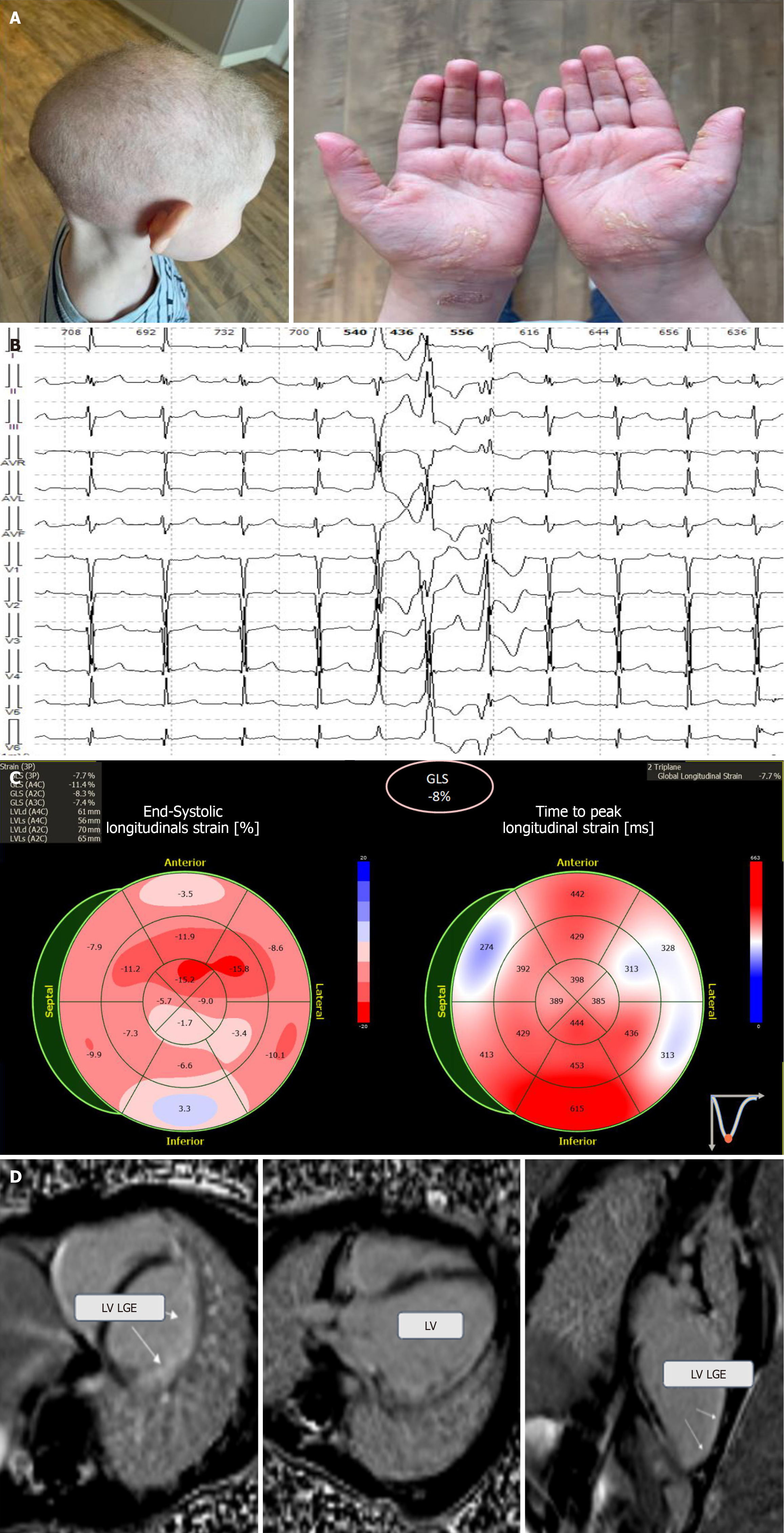
Case 2: Physical examination revealed remarkable phenotypic features such as curly-wool hair, pronounced hyperkeratosis, deep creases on the palms and soles, and onychodystrophy (Figure 2A).
Case 1: The troponin I level increased to 232.744 ng/mL, and NT-proBNP level increased to 16308.5 pg/mL. Routine blood tests revealed no other abnormalities.
Case 2: The troponin I level was elevated to 0.0510 ng/mL, while the NT-proBNP level increased to 786.5 pg/mL. Routine blood tests revealed no other abnormalities.
Case 1: The ECG recording showed a complete right bundle branch block (Figure 1B), and Holter ECG monitoring registered monomorphic ventricular extrasystoles and episodes of monomorphic ventricular tachycardia originating from the RV outlet—a minor criterion of ACM according to the 2010 Task Force Criteria. Echocardiography revealed RV dilation without impaired contractility (Figure 1C). Selective coronary angiography showed no pathological changes (Figure 1D), thereby ruling out any acute coronary events, while MRI demonstrated decreased RV contractility and widespread subepicardial fibrotic changes, in alignment with the major MRI criterion (Figure 1E).
Case 2: ECG revealed left bundle branch block, while the Holter ECG monitoring recorded episodes of unstable mono- and polymorphic ventricular tachycardia and polymorphic ventricular extrasystoles (7682 per day) (Figure 2B). Echocardiography revealed slightly reduced myocardial contractility along with hypokinesis of the posterolateral left ventricle (LV) wall (Figure 2C). Cardiac MRI showed LV dilation and multiple subepicardial fibrotic areas in the anterior, lateral, posterior, and inferior LV walls, as well as interventricular septum fibrosis (Figure 2D).
The presence of two minor and one major MRI criteria according to the 2010 Task Force Criteria allowed us to assume the diagnosis of ACM. Genetic testing revealed a mutation in DSG2 classified as pathogenic (Table 1). Based on the myocardial phenotype, genetic findings, and signs of acute myocardial damage, the patient was diagnosed with ACM “hot phase” episode.
| Patients | Gene | Variants | ACMG classification | CADD |
| Patient 1 | DSG2 | Сhr18:31519867, rs121913006, NM_001943.5: C.146G>A: p.R49H | P (PP5, PP3, PM1, PM5, PM2) | 26 |
| Patient 2 | DSP | Chr6:7559251, NM_004415.4: C.448C>T: p.R150* | P (PVS1, PP5, PM2) | 37 |
| DSP | Chr6:7580877-7580878, NM_004415.4: C.4687_4688del: p.L1563Efs*63 | P (PVS1, PP5, PM2) | No data |
Genetic testing revealed two pathogenic variants in DSP (Table 1), supporting the diagnosis of Carvajal syndrome, which in this case comprised ACM with a prevalent left-dominant form along with skin derivative lesions, such as wooly hair and palmoplantar keratoderma.
Considering several previous episodes of syncope, malignant ventricular arrhythmias, and signs of myocardial damage and fibrosis, a cardioverter-defibrillator (ICD) was implanted along with the prescribed sotalol antiarrhythmic therapy (4 mg/kg/day). Additionally, diuretics, ACE inhibitors, and eplerenone were used to manage CHF symptoms.
Despite the patient’s young age (5 years), the decision of ICD implantation was made based on the recurrent ventricular arrhythmias, decreased contractility, and progressive nature of the disease. Sotalol therapy was initiated (6 mg/kg/day), while the CHF symptoms were managed with diuretics, ACE inhibitors, and eplerenone.
During the 2-year follow-up, the patient did not experience any relapses of the “hot phase”. No ICD discharge was reported, and the CHF symptoms remained stable.
Clinical improvement was observed 6 months after ICD implantation, and no adequate ICD discharges were recorded. During the 3-year follow-up, the patient experienced five additional “hot phase” episodes confirmed by myocardial damage markers and slow progressive worsening of CHF symptoms. The patient is currently on the waiting list for heart transplantation.
The diagnosis of ACM in pediatric patients might be challenging due to its clinical ambiguity and rare occurrence in children. While the typical initial manifestation of ventricular arrhythmias is the most common, patients may also present with chest pain, elevated troponin levels, and inflammatory markers[9]. This condition, referred to as the “hot phase”, may be misdiagnosed as myocarditis or acute coronary syndrome[10]. Only a few publications have described the ACM “hot phase” in pediatric patients, demonstrating that chest pain and myocardial enzyme release at presentation can be a part of the ACM phenotype, especially in children with DSP mutations[10,11]. In addition, ACM in pediatric patients has been reported to present as recurrent myocarditis confirmed by cardiac MRI in the absence of a clear viral infection[12]. Along with our cases, this further underscores the need for ACM diagnostic workup in children with suspected myocarditis.
Since the Padua criteria were proposed in 2020, the definition of ACM—originally focused on the right ventricular form—has expanded to include left-dominant and biventricular forms as well[13], leading to the renaming of the condition from arrhythmogenic right ventricular cardiomyopathy to the broader term ACM. Patients with left-dominant and biventricular subtypes often have similarities to those with dilated cardiomyopathy or myocarditis, leading to the misinterpretation of the diagnosis and even the type of cardiomyopathy[14]. Of note, the last classification of cardiomyopathies by the European Society of Cardiology in 2023 did not specify the left ventricular or biventricular ACM forms, further leading to difficulties in interpreting terms and criteria[15]. However, although the left-dominant or biventricular ACM forms still require precise identification and common acceptance, their existence as distinct clinical forms is beyond doubt. This is further important in light of recent publications focused on genotype-phenotype correlations in ACM[16]; this is especially interesting with regard to left-dominant forms and the “hot phase” phenomenon linked to non-PKP2-associated and DSP-related cases. The cases linked to DSP variants often include LV involvement and “myocarditis-like” episodes of chest pain with laboratory signs of myocardial injury[10]. Consequently, myocardial injury is associated with worse outcomes in DSP-related cases[17]. Similarly, Scheel et al[18] reported several female patients with the left ventricular ACM subtype and genetic variants in DSP and DSG2 to be initially diagnosed with myocarditis. In addition, 10 pediatric cases with DSP causative variants reported by Choi et al[19] emphasized the unfavorable clinical trajectory of such patients with adequate ICD shocks, decrease of contractility, and left ventricular involvement. In line with these correlations, our patients also had “hot phase” manifestations with chest pain, elevated troponin I levels, and LV involvement. In such myocarditis-like cases, the unavailability of precise viral diagnostics due to the risk of cardiac biopsy underscores the importance of advanced visualization methods such as cardiac MRI along with genetic testing and application of the Padua diagnostic criteria in improving diagnostic accuracy[9].
Increasing evidence suggests that inflammation plays a significant role in the pathogenesis of ACM. Moreover, fibrous replacement as a criterion of ACM is considered to be a consequence of inflammation and cardiomyocyte degeneration[20]. In a study of 36 cardiac autopsies from patients with ACM, 39% showed myocardial inflammatory cells[21]. In our cases, we were not able to confirm the tissue signs of inflammation due to the high risk of myocardial perforation during cardiac biopsy in children. However, we could observe the laboratory and functional signs of myocardial damage, namely, increased troponin I level and transient decrease in contractility, which were most likely the consequences of myocardial inflammation. Aseptic inflammation, a molecular process unrelated to infectious pathogens but induced by intracellular damage, is mainly observed in desmosomal forms of ACM. Desmosomal disruption can be a reason for inflammatory cell recruitment through lymphokine secretion and leukocyte attraction, which results in the intensification of myocardium damage[22]. For example, desmocollin-2 overexpression results in necrosis, acute inflammation, and fibrotic remodeling in the heart[23]. In addition, Lubos et al[24] proved that inflammation triggered by cardiomyocyte necrosis is involved in the formation of fibrous scars in the DSG2-mutant mice. These findings demonstrate the importance of adequate cellular junctions (particularly desmosomes) for proper heart muscle organization and function. A major pathway for the realization of inflammation is the NLRP3-dependent inflammasome assembly and its activation[25], which is triggered by NF-κB in response to external Toll-like receptor ligands. This activation leads to the expression of the NLRP3 inflammasome components, including NLRP3, pro-IL-1β, pro-IL-18, and Pycard. Further pharmacologic targeting with an IL-1βR antagonist or inhibition of NLRP3 has been shown to reduce the signs of myocardial inflammation in a mouse model[26]. In addition, Selgrade et al[27] showed that engineered heart tissue containing induced pluripotent stem cells with homozygous mutations in DSP demonstrates transcriptomic molecular signatures of the innate system activation through cytokine release and acquired hypersensitivity to the stimulation of Toll-like receptors. In this model, treatment with colchicine and NF-κB inhibitors improved the force deficit in the DSP-deficient engineered heart tissue. Thus, targeting receptors and pathways involved in the innate immune response along with anti-inflammatory drug discovery might be a prospective approach to ACM treatment.
Genetic defects of desmosomal components could result in the weakening of cell-to-cell junctions and cellular integrity, which is accompanied by cell death, NF-kB pathway activation, and intracellular inflammation[28-30]. The NF-kB signaling pathway can also be activated in response to mechanical stimuli, including changes in the extracellular matrix stiffness and the cell’s internal cytoskeleton[31,32]. Other components of the cellular cytoskeleton, specifically actin microfilaments, may play an additional role in the modulation of intracellular inflammation in cardiomyocytes. Thus, actin filaments modulate the sequestration of NF-κB in cytosol, underlining the importance of the actin polymerization/depolymerization balance in NF-κB activation[32-34].
Cellular inflammation plays a crucial role in ACM pathogenesis, which occurs at least in part via the NF-κB signaling pathway. This pathway can be activated through different mechanisms, such as cytoskeletal or intercalated disc abnormalities and derangements. Genetic mutations associated with these structures result in cell death, inflammation, and fibrosis. Clinically, ACM can present with nonspecific inflammatory signs and symptoms, such as chest pain and elevated troponin I level and may therefore be misdiagnosed as acute coronary syndrome or myocarditis. Recognizing the ACM “hot phase” is essential for timely and accurate diagnosis. This case report presented two clinical cases of the ACM “hot phase” in children, exploring phenotypic disease heterogenicity, underscoring common pathophysiological mechanisms, and focusing on sterile myocardial inflammation. The molecular bases of ACM “hot phase” include cytoskeletal and desmosomal disruption triggering noninfectious intracellular inflammation, which points to the potential benefits of an antiinflammatory pharmacological approach in ACM patients.
| 1. | Corrado D, Basso C, Judge DP. Arrhythmogenic Cardiomyopathy. Circ Res. 2017;121:784-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 324] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 2. | Sheikh F, Ross RS, Chen J. Cell-cell connection to cardiac disease. Trends Cardiovasc Med. 2009;19:182-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Awad MM, Calkins H, Judge DP. Mechanisms of disease: molecular genetics of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Nat Clin Pract Cardiovasc Med. 2008;5:258-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 4. | Nonaka CKV, Sampaio GL, Silva KN, Khouri R, Macedo CT; Chagas Translational Research Consortium; Rogatto SR, Ribeiro Dos Santos R, Souza BSF, Soares MBP. Therapeutic miR-21 Silencing Reduces Cardiac Fibrosis and Modulates Inflammatory Response in Chronic Chagas Disease. Int J Mol Sci. 2021;22:3307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Austin KM, Trembley MA, Chandler SF, Sanders SP, Saffitz JE, Abrams DJ, Pu WT. Molecular mechanisms of arrhythmogenic cardiomyopathy. Nat Rev Cardiol. 2019;16:519-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 197] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 6. | Reichart D, Lindberg EL, Maatz H, Miranda AMA, Viveiros A, Shvetsov N, Gärtner A, Nadelmann ER, Lee M, Kanemaru K, Ruiz-Orera J, Strohmenger V, DeLaughter DM, Patone G, Zhang H, Woehler A, Lippert C, Kim Y, Adami E, Gorham JM, Barnett SN, Brown K, Buchan RJ, Chowdhury RA, Constantinou C, Cranley J, Felkin LE, Fox H, Ghauri A, Gummert J, Kanda M, Li R, Mach L, McDonough B, Samari S, Shahriaran F, Yapp C, Stanasiuk C, Theotokis PI, Theis FJ, van den Bogaerdt A, Wakimoto H, Ware JS, Worth CL, Barton PJR, Lee YA, Teichmann SA, Milting H, Noseda M, Oudit GY, Heinig M, Seidman JG, Hubner N, Seidman CE. Pathogenic variants damage cell composition and single cell transcription in cardiomyopathies. Science. 2022;377:eabo1984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 153] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 7. | Gasperetti A, Carrick RT, Protonotarios A, Murray B, Laredo M, van der Schaaf I, Lekanne RH, Syrris P, Cannie D, Tichnell C, Cappelletto C, Gigli M, Medo K, Saguner AM, Duru F, Gilotra NA, Zimmerman S, Hylind R, Abrams DJ, Lakdawala NK, Cadrin-Tourigny J, Targetti M, Olivotto I, Graziosi M, Cox M, Biagini E, Charron P, Casella M, Tondo C, Yazdani M, Ware JS, Prasad SK, Calò L, Smith ED, Helms AS, Hespe S, Ingles J, Tandri H, Ader F, Peretto G, Peters S, Horton A, Yao J, Dittmann S, Schulze-Bahr E, Qureshi M, Young K, Carruth ED, Haggerty C, Parikh VN, Taylor M, Mestroni L, Wilde A, Sinagra G, Merlo M, Gandjbakhch E, van Tintelen JP, Te Riele ASJM, Elliott PM, Calkins H, James CA. Clinical features and outcomes in carriers of pathogenic desmoplakin variants. Eur Heart J. 2025;46:362-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 39] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 8. | Merner ND, Hodgkinson KA, Haywood AF, Connors S, French VM, Drenckhahn JD, Kupprion C, Ramadanova K, Thierfelder L, McKenna W, Gallagher B, Morris-Larkin L, Bassett AS, Parfrey PS, Young TL. Arrhythmogenic right ventricular cardiomyopathy type 5 is a fully penetrant, lethal arrhythmic disorder caused by a missense mutation in the TMEM43 gene. Am J Hum Genet. 2008;82:809-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 377] [Article Influence: 20.9] [Reference Citation Analysis (1)] |
| 9. | Shaikh T, Nguyen D, Dugal JK, DiCaro MV, Yee B, Houshmand N, Lei K, Namazi A. Arrhythmogenic Right Ventricular Cardiomyopathy: A Comprehensive Review. J Cardiovasc Dev Dis. 2025;12:71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Bariani R, Rigato I, Cipriani A, Bueno Marinas M, Celeghin R, Basso C, Corrado D, Pilichou K, Bauce B. Myocarditis-like Episodes in Patients with Arrhythmogenic Cardiomyopathy: A Systematic Review on the So-Called Hot-Phase of the Disease. Biomolecules. 2022;12:1324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 11. | Bariani R, Cipriani A, Rizzo S, Celeghin R, Bueno Marinas M, Giorgi B, De Gaspari M, Rigato I, Leoni L, Zorzi A, De Lazzari M, Rampazzo A, Iliceto S, Thiene G, Corrado D, Pilichou K, Basso C, Perazzolo Marra M, Bauce B. 'Hot phase' clinical presentation in arrhythmogenic cardiomyopathy. Europace. 2021;23:907-917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 12. | Martins D, Ovaert C, Khraiche D, Boddaert N, Bonnet D, Raimondi F. Myocardial inflammation detected by cardiac MRI in Arrhythmogenic right ventricular cardiomyopathy: A paediatric case series. Int J Cardiol. 2018;271:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Saguner AM, Ganahl S, Kraus A, Baldinger SH, Akdis D, Saguner AR, Wolber T, Haegeli LM, Steffel J, Krasniqi N, Lüscher TF, Tanner FC, Brunckhorst C, Duru F. Electrocardiographic features of disease progression in arrhythmogenic right ventricular cardiomyopathy/dysplasia. BMC Cardiovasc Disord. 2015;15:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Lala IR, Pop-Moldovan A. Inflammation-A Possible Link between Myocarditis and Arrhythmogenic Cardiomyopathy. Diagnostics (Basel). 2024;14:248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Arbelo E, Protonotarios A, Gimeno JR, Arbustini E, Barriales-Villa R, Basso C, Bezzina CR, Biagini E, Blom NA, de Boer RA, De Winter T, Elliott PM, Flather M, Garcia-Pavia P, Haugaa KH, Ingles J, Jurcut RO, Klaassen S, Limongelli G, Loeys B, Mogensen J, Olivotto I, Pantazis A, Sharma S, Van Tintelen JP, Ware JS, Kaski JP; ESC Scientific Document Group. 2023 ESC Guidelines for the management of cardiomyopathies. Eur Heart J. 2023;44:3503-3626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 1490] [Article Influence: 496.7] [Reference Citation Analysis (8)] |
| 16. | Bariani R, Rigato I, Celeghin R, Marinas MB, Cipriani A, Zorzi A, Pergola V, Iliceto S, Basso C, Marra MP, Corrado D, Gregori D, Pilichou K, Bauce B. Phenotypic Expression and Clinical Outcomes in Patients With Arrhythmogenic Cardiomyopathies. J Am Coll Cardiol. 2024;83:797-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Wang W, Murray B, Tichnell C, Gilotra NA, Zimmerman SL, Gasperetti A, Scheel P, Tandri H, Calkins H, James CA. Clinical characteristics and risk stratification of desmoplakin cardiomyopathy. Europace. 2022;24:268-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 18. | Scheel PJ 3rd, Murray B, Tichnell C, James CA, Tandri H, Calkins H, Chelko SP, Gilotra NA. Arrhythmogenic Right Ventricular Cardiomyopathy Presenting as Clinical Myocarditis in Women. Am J Cardiol. 2021;145:128-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 19. | Choi NH, Cherny S, Berul CI, Goodyer WR, Howard TS, Joong A, Liberman L, Silver ES, Villa CR, Lee TM, Zuckerman WA. Desmoplakin Cardiomyopathy in Pediatric Patients: A Distinct, Underrecognized Cohort of Arrhythmogenic Cardiomyopathy. Circ Arrhythm Electrophysiol. 2024;17:e013114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 20. | Asimaki A, Kleber AG, Saffitz JE. Pathogenesis of Arrhythmogenic Cardiomyopathy. Can J Cardiol. 2015;31:1313-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Campuzano O, Alcalde M, Iglesias A, Barahona-Dussault C, Sarquella-Brugada G, Benito B, Arzamendi D, Flores J, Leung TK, Talajic M, Oliva A, Brugada R. Arrhythmogenic right ventricular cardiomyopathy: severe structural alterations are associated with inflammation. J Clin Pathol. 2012;65:1077-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Swirski FK, Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol. 2018;18:733-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 607] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 23. | Brodehl A, Belke DD, Garnett L, Martens K, Abdelfatah N, Rodriguez M, Diao C, Chen YX, Gordon PM, Nygren A, Gerull B. Transgenic mice overexpressing desmocollin-2 (DSC2) develop cardiomyopathy associated with myocardial inflammation and fibrotic remodeling. PLoS One. 2017;12:e0174019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Lubos N, van der Gaag S, Gerçek M, Kant S, Leube RE, Krusche CA. Inflammation shapes pathogenesis of murine arrhythmogenic cardiomyopathy. Basic Res Cardiol. 2020;115:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Cho S, Ying F, Sweeney G. Sterile inflammation and the NLRP3 inflammasome in cardiometabolic disease. Biomed J. 2023;46:100624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Yue R, Zheng Z, Luo Y, Wang X, Lv M, Qin D, Tan Q, Zhang Y, Wang T, Hu H. NLRP3-mediated pyroptosis aggravates pressure overload-induced cardiac hypertrophy, fibrosis, and dysfunction in mice: cardioprotective role of irisin. Cell Death Discov. 2021;7:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 27. | Selgrade DF, Fullenkamp DE, Chychula IA, Li B, Dellefave-Castillo L, Dubash AD, Ohiri J, Monroe TO, Blancard M, Tomar G, Holgren C, Burridge PW, George AL Jr, Demonbreun AR, Puckelwartz MJ, George SA, Efimov IR, Green KJ, McNally EM. Susceptibility to innate immune activation in genetically mediated myocarditis. J Clin Invest. 2024;134:e180254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Zhao G, Qiu Y, Zhang HM, Yang D. Intercalated discs: cellular adhesion and signaling in heart health and diseases. Heart Fail Rev. 2019;24:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 29. | Quarta G, Elliott PM. Diagnostic criteria for arrhythmogenic right ventricular cardiomyopathy. Rev Esp Cardiol (Engl Ed). 2012;65:599-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Chen SN, Gurha P, Lombardi R, Ruggiero A, Willerson JT, Marian AJ. The hippo pathway is activated and is a causal mechanism for adipogenesis in arrhythmogenic cardiomyopathy. Circ Res. 2014;114:454-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 240] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 31. | Lampi MC, Reinhart-King CA. Targeting extracellular matrix stiffness to attenuate disease: From molecular mechanisms to clinical trials. Sci Transl Med. 2018;10:eaao0475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 274] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 32. | Phusuntornsakul P, Jitpukdeebodintra S, Pavasant P, Leethanakul C. Vibration activates the actin/NF-κB axis and upregulates IL-6 and IL-8 expression in human periodontal ligament cells. Cell Biol Int. 2020;44:661-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Alhadidi Q, Shah ZA. Cofilin Mediates LPS-Induced Microglial Cell Activation and Associated Neurotoxicity Through Activation of NF-κB and JAK-STAT Pathway. Mol Neurobiol. 2018;55:1676-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 34. | Pogson M, Holcombe M, Smallwood R, Qwarnstrom E. Introducing spatial information into predictive NF-kappaB modelling--an agent-based approach. PLoS One. 2008;3:e2367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/