Published online Jan 18, 2026. doi: 10.5312/wjo.v17.i1.112262
Revised: September 4, 2025
Accepted: November 14, 2025
Published online: January 18, 2026
Processing time: 170 Days and 20.4 Hours
Tuberculous osteitis is a chronic, granulomatous bone infection that frequently results in impaired bone healing following surgery. Despite surgical intervention and prolonged anti-tuberculous therapy, complete bone regeneration often re
To investigate the efficacy and safety of pamidronate in promoting bone regene
A controlled randomized basic study of rabbit femoral tuberculosis induced by Mycobacterium tuberculosis strain H37Rv included surgical removal of infected tissue and implantation of osteoinductive bone grafts with the following animal allocation to one of three groups: (1) Bisphosphonates alone; (2) Bisphosphonates combined with anti-tuberculous therapy; and (3) Anti-tuberculous therapy alone. The control group consisted of animals that received no surgical or medical treatment. Clinical evaluations, biochemical markers, micro-computed tomography imaging, and histomorphometry analyses were conducted at 3 months and 6 months postoperatively.
Pamidronate treatment significantly reduced early implant resorption, increased osteoblastic activity, improved trabecular bone regeneration, and maintained graft integrity compared to the anti-tuberculous therapy-only group. Histologically, pamidronate led to enhanced vascular remodeling and increased bone matrix formation. Crucially, bisphosphonate therapy demonstrated safety, compatibility with anti-tuberculous medications, and did not exacerbate tuberculous inflammation. Furthermore, micro-computed tomography analysis revealed a significant increase in trabecular thickness and density in pamidronate-treated groups, underscoring the anabolic effects of bisphosphonates. Morphometric evaluation confirmed a marked reduction in osteoclast number and activity at graft interfaces. These combined radiological, histological, and biochemical data collectively demonstrate the efficacy of pamidronate as an adjunctive agent in enhancing bone repair outcomes following surgical intervention for tuberculous osteitis.
A single intravenous dose of pamidronate significantly enhances bone regeneration and prevents implant resorp
Core Tip: A single intravenous dose of pamidronate significantly enhances bone regeneration after surgical treatment of experimental tuberculous osteitis. Pamidronate effectively inhibits early implant resorption, increases osteoblastic activity, and promotes prolonged osteogenesis, showing compatibility and safety when combined with antituberculosis therapy. This finding suggests that bisphosphonates may be a promising adjunctive therapeutic strategy for enhancing clinical outcomes in patients undergoing skeletal tuberculosis surgery.
- Citation: Petukhova VV, Mushkin AY, Maletin AS, Dogonadze MZ, Zabolotnykh NV, Dyakova ME, Esmedlyaeva DS, Vinogradova TI, Kostik MM. Novel use of bisphosphonates to improve surgical outcomes in experimental bone tuberculosis. World J Orthop 2026; 17(1): 112262
- URL: https://www.wjgnet.com/2218-5836/full/v17/i1/112262.htm
- DOI: https://dx.doi.org/10.5312/wjo.v17.i1.112262
Tuberculous (TB) osteitis is a chronic, granulomatous bone infection caused by the Mycobacterium tuberculosis (M. tuberculosis) complex, leading to progressive osteolysis and subsequent skeletal destruction. Despite surgical debridement and long-term anti- TB therapy (ATT), complete bone regeneration remains a challenge due to persistent inflammation and impaired osteogenesis, often resulting in orthopedic sequelae such as deformities, instability, and chronic pain[1-3]. The current surgical osteoregenerative strategies include bone grafts and biologically active substitutes. However, these materials are often subject to early resorption in inflammatory environments, which limits their long-term structural integration[4,5]. Alternative strategies for enhancing bone regeneration include implant materials with antimicrobial properties or the combination of osteoconductive materials with bone morphogenetic proteins (BMPs) or autologous bone marrow[6,7]. Unfortunately, these approaches tend to be costly and involve additional procedures, such as har
In contrast, bisphosphonates offer a more cost-effective and efficient solution for improving bone healing, making them an attractive alternative for enhancing post-surgical outcomes, especially in inflammatory conditions such as TB osteitis. Bisphosphonates are agents that could modulate bone turnover, and they have emerged as promising candidates for preserving graft integrity by inhibiting osteoclastic activity and prolonging the osteoconductive phase of healing[8]. Recent studies have also suggested that bisphosphonates may possess anti-inflammatory and angiogenic properties, potentially aiding in the integration of bone implants even in the presence of infection or immune dysregulation[9,10]. Despite the widespread use of bisphosphonates in osteometabolic and oncologic bone diseases, their application in skeletal infections, particularly in bone TB, has been limited. While bisphosphonates have been used to manage osteoporosis and to stabilize metal implants in orthopedic surgeries for patients with skeletal TB, the intravenous administration of bisphosphonates specifically to improve implant integration post-surgery remains unexplored[11]. Studies have indicated the use of bisphosphonates in combination with other treatments for TB, such as soaking allografts in bisphosphonate solutions to enhance their integration in joint prosthesis surgeries[12]. Moreover, bisphosphonates are effective in treating chronic refractory osteomyelitis, where surgical treatment was challenging due to the extent of the infection[13]. However, the targeted use of intravenous bisphosphonates in postoperative settings to enhance bone re
The purpose of this experimental study was to evaluate the efficacy and safety of bisphosphonates, specifically pamidronate, as adjunctive therapy in the surgical treatment of TB osteitis, focusing on effects on implant preservation, bone regeneration, and compatibility with ATT.
The study was approved by the Independent Ethical Committee of the Saint Petersburg Research Institute of Phthisiopulmonology, Ministry of Health, Russian Federation (Approval No. 74). All animal handling procedures were strictly followed the principles of the European Commission[15], as well as Russian national standards for laboratory animal care[16,17].
The experimental study was conducted on 21 male rabbits (“Soviet chinchilla” breed), with a body weight of 3456 ± 321 g, provided by the certified animal breeding facility (Rappolovo Laboratory Animal Nursery, National Research Center “Kurchatov Institute”, Russia). Animals were housed individually under standardized, controlled conditions: A temperature of 23 °C - 25 °C, a humidity of 50%-70%, a 12-hour light/dark cycle, and ad libitum access to water and food. They were maintained in accordance with local ethical guidelines and regulations.
TB osteitis was induced in the medial condyle of the right femur, according to the previously described and validated method[18]. Under general anesthesia (Zoletil®, Virbac, France, 25 mg/kg intramuscular injection; Xyla®, Interchemie werken De Adelaar BV, Netherlands, 2% solution, 1.0-1.5 mL intramuscular injection), the right hind limb was prepared, and a surgical incision (5 cm length) was made on the anteromedial surface of the distal femur. A small bone tunnel (0.5-0.8 cm deep, diameter 0.8 mm) was created using a trocar. A suspension containing 1 × 106 CFU of M. tuberculosis strain H37Rv (drug-sensitive strain tuberculosis #1/47, Institute of Hygiene and Epidemiology, Prague, 1976) was embedded in a sterile collagen sponge (2 mm × 2 mm) and placed inside the bone tunnel. The site of injury was sealed with a bone post from the trocar, and the wound was sutured layer by layer.
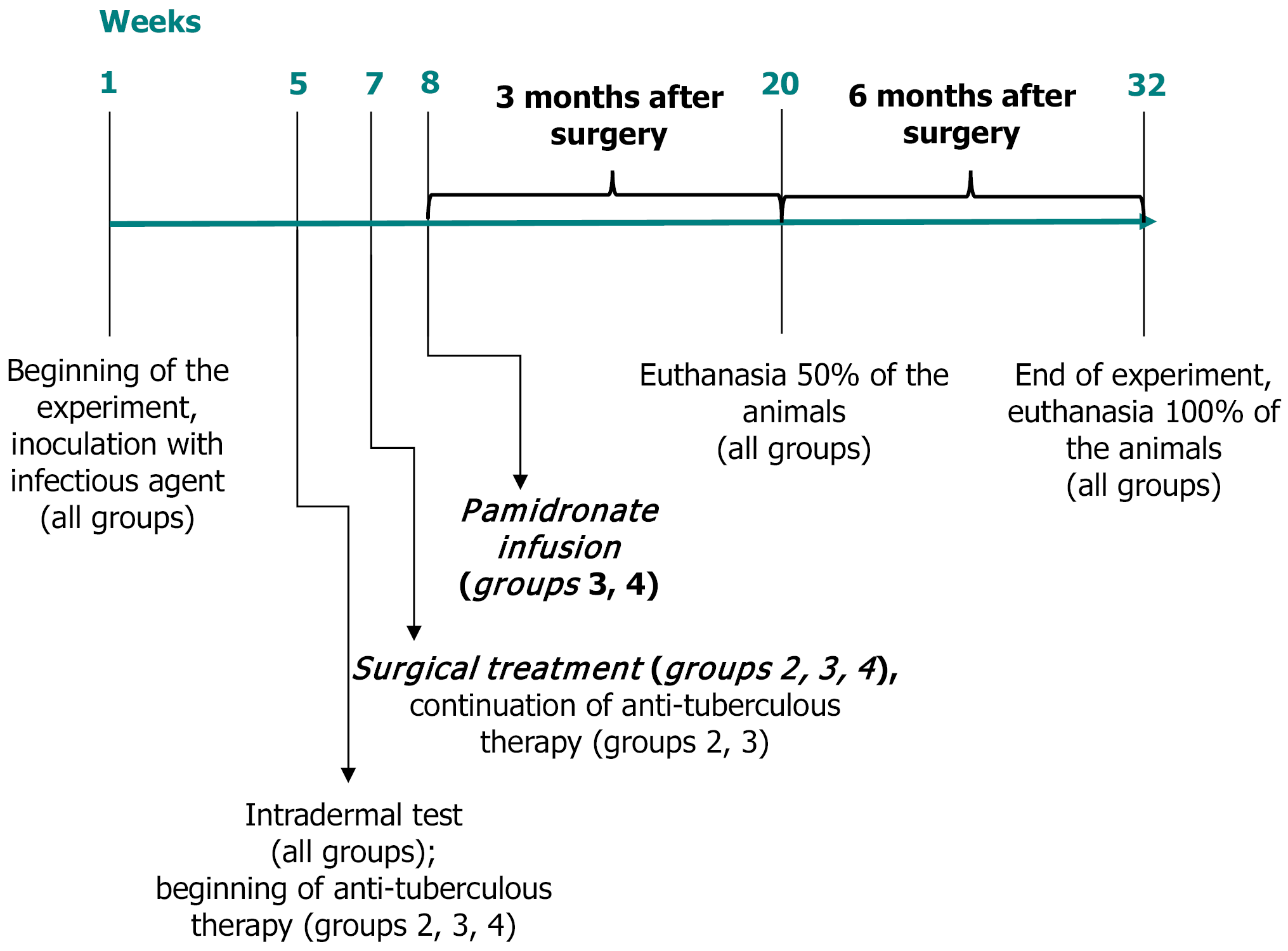
Four weeks post-infection, tuberculin skin tests (Diaskintest®, Generium, Russia) confirmed successful infection in all animals. Twenty-one rabbits were then randomly allocated into four groups.
Control group (n = 3): No further treatment (infection only, no surgery, no medications).
ATT-only group (n = 4): Surgical debridement, Osteoset®2DBM pellets (Wright Medical Technology, Inc., TN, United States) implantation, followed by ATT regimen (isoniazid 10 mg/kg, ethambutol 20 mg/kg, pyrazinamide 20 mg/kg orally, 5 days/week).
ATT + pamidronate group (n = 7): Surgical treatment as in group 2, followed by combined ATT and single intravenous pamidronate infusion (Pamidronate Medac, Germany, 1 mg/kg IV at 10 days post-surgery).
Pamidronate-only group (n = 7): Surgical treatment equal to group 2, ATT discontinued post-surgery, single pamidronate infusion (Pamidronate Medac, Germany, 1 mg/kg IV at 10 days post-surgery).
Six weeks post-infection, rabbits underwent surgical debridement of the lesion under the same anesthesia described above. Necrotic tissue and sequestrations were thoroughly removed through the same incision. Bone defects were filled with osteoinductive Osteoset®2DBM pellets (Wright Medical Technology, Inc., TN, United States), which contain BMPs (BMP-2, BMP-4), insulin-like growth factor-1, and transforming growth factor-beta1. The wound was closed in layers. Postoperative antibiotic prophylaxis was provided with Cefazolin (KRKA, d.d., Slovenija; 10 mg/kg intramuscular injection, 5 days).
Pamidronate was administered intravenously as a single infusion at a dose of 1 mg/kg body weight, diluted in 20 mL of sterile saline, delivered slowly over 60 minutes under careful veterinary monitoring. The infusion was performed 10 days following surgical intervention, ensuring optimal conditions for osteogenesis and implant stabilization. Animals were continuously monitored during and after the infusion to detect any adverse reactions, such as changes in vital signs, signs of distress, or injection site reactions. The dose and administration protocol were based on previous pharmacokinetic studies and aimed at achieving effective osteoclast inhibition while maintaining safety and compatibility. Throughout the study period, no significant adverse events or side effects related to pamidronate administration were observed.
Animal health status, activity, food/water intake, body weight, and surgical wound healing were monitored regularly. Blood samples were collected at baseline, 6 weeks post-infection, and at 10 days, 3 months, and 6 months post-surgery. Serum biomarkers related to bone remodeling (total alkaline phosphatase, albumin, osteocalcin, beta-crosslaps, receptor activator of nuclear factor kappa-B ligand, and sclerostin) were analyzed using commercial enzyme-linked immuno
Micro-computed tomography (SkyScan 1172, Bruker microCT, Belgium) scans were performed at euthanasia (3 months and 6 months). Scanning parameters were as follows: Pixel size, 13-27 μm; voltage, 100 kV; current, 100 μA; and rotation step, 0.4°. Images were reconstructed (NRecon, Micro Photonics Inc., Poland) and analyzed (RadiAnt DICOM Viewer, Medixant, Poland; CTAn, CTAN Team, Germany). Qualitative (bone trabeculation, implant integrity) and semi-quantitative scoring (0-2 scale: Absence, mild, severe) were performed to evaluate osteoregeneration and osteolysis.
Following euthanasia, the distal epiphysis of the femur was dissected, fixed in neutral buffered formalin (10%), decalcified, embedded in paraffin, and sectioned (3-5 μm thick). Sections were stained with hematoxylin-eosin and Ziehl-Neelsen. Digital slide scanning (Pannoramic, 3DHISTECH Ltd., Hungary) enabled comprehensive morphometric analysis using Orbit Image Analysis software (Actelion Pharmaceuticals Ltd., Switzerland). Histopathological parameters assessed included the area of specific inflammation, fibrosis, necrosis, implant integrity, osteoblastic and osteoclastic activities, bone trabecular thickness, and vascularity.
Rabbits were withdrawn from the experiment in accordance with the principles outlined by the European Commission[9]. Euthanasia was performed through intravenous administration into the marginal ear vein of a 10% lidocaine solution, at a dose five times higher than the maximum allowable therapeutic level, ensuring rapid, painless, and stress-free cessation of vital functions. Animal welfare procedures were strictly adhered to in compliance with international ethical standards. Following euthanasia, the animal remains and biological waste were safely disposed of by certified personnel using methods compliant with institutional biosafety protocols, ensuring both ethical and environmental safety. The detailed study flow chart is in Figure 1.
Statistical analysis was performed by a biomedical statistician using Statistica version 12.0 (Dell Inc., TX, United States). Data distribution was tested using the Shapiro-Wilk test. Non-parametric tests (Kruskal-Wallis and Mann-Whitney U tests) were applied, and results are presented as median (interquartile ranges). A P-value ≤ 0.05 was considered statistically significant.
All animals, except one rabbit from the untreated control group, maintained a satisfactory health status, exhibited good physical activity, and had a stable intake of food and water throughout the study. One rabbit from the untreated control group developed generalized tuberculosis infection by 4.5 months post-infection, manifesting poor physical condition and reduced limb function.
A postoperative complication (subcutaneous abscess) occurred in one rabbit (ATT + pamidronate group), successfully treated with local antiseptics without further intervention. Pamidronate infusion was well tolerated, with no observed adverse effects or interference with ongoing ATT.
All groups showed a steady body weight gain throughout the study period (4.8%-15.3%). Notably, pamidronate-treated groups exhibited slightly better weight gain dynamics, although differences were not statistically significant (P > 0.05).
Skin tests (Diaskintest®, Generium, Russia) confirmed successful M. tuberculosis infection in all animals (4 weeks after infection). Polymerase chain reaction confirmed the presence of M. tuberculosis DNA in all rabbits, validating the infection model.
Alkaline phosphatase levels significantly decreased in the pamidronate groups (ATT + pamidronate and pamidronate-only; P = 0.07), reflecting reduced bone turnover. Serum sclerostin showed statistically significant intergroup differences at 3 months post-surgery (P = 0.03), with the highest values observed in the control and ATT + pamidronate groups. Levels of osteocalcin, receptor activator of nuclear factor kappa-B ligand, and albumin demonstrated no statistically significant intergroup differences (Table 1).
| Initial level | All animals | Group 1 | Group 2 | Group 3 | Group 4 |
| Weight, g | 3456.2 ± 321.6 | 3446.7 ± 272.9 | 3008.5 ± 274.8 | 3572.0 ± 248.3 | 3600.3 ± 216.9 |
| 3518 (3356-3626) | 3546 (3138-3656) | 2983 (2802-3215) | 3618 (3390-3736) | 3526 (3490-3626) | |
| ALP, U/L | 95.9 ± 30.7 | 89.3 ± 24.0 | 78.0 ± 45.8 | 100.4 ± 25.7 | 104.4 ± 30.1 |
| 94.0 (72.0-120.0) | 99 (62-107) | 64 (45-111) | 94 (91-109) | 120 (72-134) | |
| Osteocalcin, ng/mL | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 |
| 0.1 (0.1-0.2) | 0.1 (0.1-0.1) | 0.1 (0.1-0.1) | 0.1 (0.1-0.1) | 0.2 (0.1-0.2) | |
| RANKL, pg/mL | 61.5 ± 16.2 | 81.6 ± 0.0 | 48.7 ± 0.0 | 53.7 ± 11.6 | 67.4 ± 18.3 |
| 61.9 (48.7-66.6) | 81.6 (81.6-81.6) | 48.7 (48.7-48.7) | 52.5 (43.9-63.5) | 64 (56.2-78.6) | |
| Albumin, g/L | 47.6 ± 3.3 | 49.0 ± 4.6 | 47.7 ± 4.9 | 47.4 ± 3.5 | 47.0 ± 2.3 |
| 47 (45-50) | 50 (44-53) | 50 (42-51) | 46 (45-51) | 47 (45-50) | |
| Sclerostin, ng/mL | 105.2 ± 79.8 | 162.0 ± 0.0 | 71.5 ± 67.2 | 104.0 ± 147.1 | 118.0 ± 0.0 |
| 118.5 (24-162) | 162 (162-162) | 71.5 (24-119) | 104 (0-208) | 118 (118-118) | |
| 1 month after infection | |||||
| Weight, g | 3608.8 ± 325.1 | 3481.3 ± 185.5 | 3212.0 ± 113.3 | 3779.4 ± 315.9 | 3719.4 ± 270.9 |
| 3554 (3350-3756) | 3466 (3304-3674) | 3224 (3119-3305) | 3756 (3468-3924) | 3606 (3520-3994) | |
| ALP, U/L | 65.4 ± 25.5 | 58.7 ± 18.2 | 56.3 ± 27.7 | 60.7 ± 22.3 | 78.1 ± 29.7 |
| 66 (46-79) | 53 (44-79) | 65 (37-75.5) | 66 (45-76) | 81 (46-94) | |
| Osteocalcin, ng/mL | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 |
| 0.2 (0.1-0.2) | 0.2 (0.2-0.2) | 0.2 (0.1-0.2) | 0.2 (0.1-0.2) | 0.2 (0.1-0.2) | |
| RANKL, pg/mL | 58.2 ± 14.1 | 52.9 ± 9.6 | 67.9 ± 20.9 | 60.9 ± 10.4 | 52.2 ± 13.4 |
| 60.2 (49.6-64.7) | 56 (42.1-60.6) | 63.8 (55-80.8) | 62.5 (49.6-68.9) | 53.2 (42.2-62.1) | |
| Albumin, g/L | 45.1 ± 2.8 | 47.3 ± 1.2 | 43.0 ± 3.7 | 46.3 ± 2.8 | 44.1 ± 1.5 |
| 45 (43-48) | 48 (46-48) | 42.5 (40.5-45.5) | 47 (44-48) | 44 (43-45) | |
| Sclerostin, ng/mL | 178.2 ± 102.2 | 155.1 ± 82.4 | 159.7 ± 39.3 | 231.0 ± 130.8 | 146.1 ± 99.9 |
| 141.4 (115-247.9) | 127.3 (90.2-247.9) | 165.2 (118-196) | 191.9 (119.6-353.3) | 133.4 (99.5-227.2) | |
| Before surgery | |||||
| Weight, g | 3659.9 ± 377.6 | 3821.3 ± 356.1 | 3153.0 ± 201.7 | 3872.9 ± 309.9 | 3667.4 ± 281.5 |
| 3580 (3426-4018) | 3682 (3556-4226) | 3143 (2996-3310) | 3914 (3602-4022) | 3514 (3490-4058) | |
| ALP, U/L | 55.9 ± 18.5 | 50.0 ± 24.1 | 40.5 ± 20.9 | 52.1 ± 13.2 | 71.0 ± 10.0 |
| 57 (47-75) | 48 (27-75) | 40 (23-58) | 50 (44-60) | 76 (58-80) | |
| Osteocalcin, ng/mL | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 0.1 (0.1-0.2) | 0.1 (0.1-0.1) | 0.1 (0.1-0.2) | 0.1 (0.1-0.2) | 0.1 (0.1-0.2) | |
| RANKL, pg/mL | 62.9 ± 11.5 | 66.3 ± 18.1 | 68.6 ± 5.1 | 63.3 ± 7.0 | 57.9 ± 14.5 |
| 65.7 (54.4-69.8) | 60.2 (51.9-86.7) | 69.7 (65.1-72.1) | 66.4 (54.4-68.6) | 57.7 (48.5-71) | |
| Albumin, g/L | 44.7 ± 2.9 | 43.7 ± 3.2 | 47.0 ± 1.0 | 45.8 ± 3.2 | 43.3 ± 2.4 |
| 45 (42-47) | 45 (40-46) | 47 (46-48) | 46.5 (42-49) | 43 (41-45) | |
| Sclerostin, ng/mL | 204.0 ± 104.2 | 229.6 ± 107.9 | 145.1 ± 56.2 | 244.7 ± 149.0 | 185.8 ± 59.9 |
| 165 (155.6-231.3) | 177.6 (157.6-353.7) | 137.8 (103.3-186.9) | 181.7 (133.5-370.2) | 164 (158.5-231.3) | |
| 3 months after surgery | |||||
| Weight, g | 3952.9 ± 515.7 | 3786.7 ± 767.7 | 3340.0 ± 307.6 | 4282.9 ± 320.4 | 4044.3 ± 372.7 |
| 3870 (3680-4390) | 3410 (3280-4670) | 3270 (3135-3545) | 4340 (4150-4440) | 3870 (3770-4390) | |
| ALP, U/L | 38.3 ± 12.6 | 41.3 ± 14.0 | 28.0 ± 9.7 | 40.7 ± 14.5 | 40.6 ± 10.9 |
| 37 (28-50) | 40 (28-56) | 25 (22-34) | 39 (28-52) | 37 (30-51) | |
| Osteocalcin, ng/mL | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 0.1 (0.1-0.2) | 0.1 (0.1-0.1) | 0.2 (0.1-0.2) | 0.1 (0.1-0.2) | 0.1 (0.1-0.2) | |
| RANKL, pg/mL | 55.6 ± 13.6 | 52.7 ± 21.4 | 54.8 ± 18.6 | 58.2 ± 10.7 | 54.8 ± 12.8 |
| 56.3 (47.4-63.5) | 48.1 (33.9-75.9) | 50.7 (42.4-67.2) | 60.9 (57.3-63.5) | 56.1 (45-70.5) | |
| Albumin | 44.1 ± 3.6 | 42.3 ± 6.7 | 42.8 ± 3.3 | 44.3 ± 2.4 | 45.4 ± 3.6 |
| 44 (42-46) | 39 (38-50) | 42.5 (40.5-45) | 43 (42-47) | 44 (44-46) | |
| 6 months after surgery | |||||
| Weight, g | 4033.1 ± 637.9 | 3720.0 ± 989.9 | 3400.0 ± 381.8 | 4498.0 ± 289.4 | 3925.0 ± 657.5 |
| 4230 (3670-4420) | 3720 (3020-4420) | 3400 (3130-3670) | 4330 (4310-4760) | 3885 (3470-4380) | |
| ALP, U/L | 23.7 ± 16.8 | 13.0 ± 0.0 | 22.5 ± 13.4 | 19.3 ± 12.2 | 31.5 ± 24.2 |
| 15 (13-35) | 13 (13-13) | 22.5 (13-32) | 18 (10.5-28) | 25.5 (12.5-50.5) | |
| Osteocalcin, ng/mL | 0.2 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.2 | 0.1 ± 0.0 |
| 0.1 (0.1-0.2) | 0.1 (0.1-0.1) | 0.1 (0.1-0.2) | 0.1 (0.1-0.3) | 0.1 (0.1-0.2) | |
| RANKL, pg/mL | 64.6 ± 12.0 | 71.0 ± 0.0 | 55.4 ± 13.1 | 67.7 ± 14.7 | 64.3 ± 11.1 |
| 64.7 (52-71) | 71 (71-71) | 55.4 (46.2-64.7) | 66 (57.2-78.3) | 64.6 (55.8-72.9) | |
| Albumin, g/L | 42.8 ± 2.3 | 41.0 ± 0.0 | 45.0 ± 0.0 | 42.0 ± 1.6 | 43.5 ± 3.0 |
| 42 (42-44) | 41 (41-41) | 45 (45-45) | 42 (41-43) | 42 (42-45) | |
| Sclerostin, ng/mL | 216.8 ± 116.6 | 293.6 ± 0.0 | 175.5 ± 127.1 | 272.4 ± 137.8 | 162.6 ± 99.0 |
| 186.6 (125.7-305.9) | 293.6 (293.6-293.6) | 175.5 (85.6-265.4) | 272.3 (156.9-388) | 132.9 (102.3-223) | |
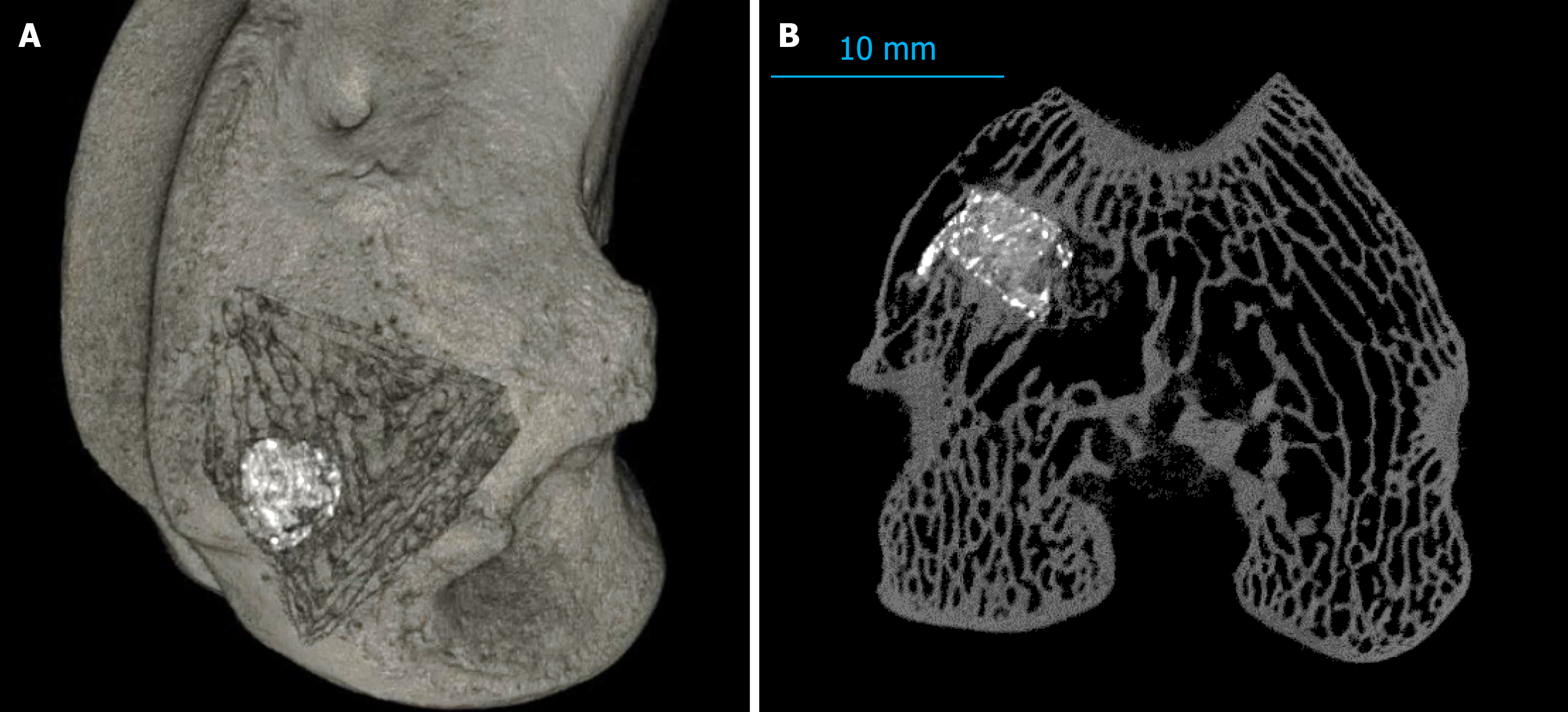
Micro-computed tomography analysis at 3 months and 6 months showed substantial improvements in bone regeneration parameters within the pamidronate-treated groups. These groups demonstrated greater preservation and integration of the implanted osteoinductive material (Osteoset®2DBM pellets), as evidenced by robust trabecular formation and significantly reduced osteolytic lesions compared to the ATT-only and control groups.
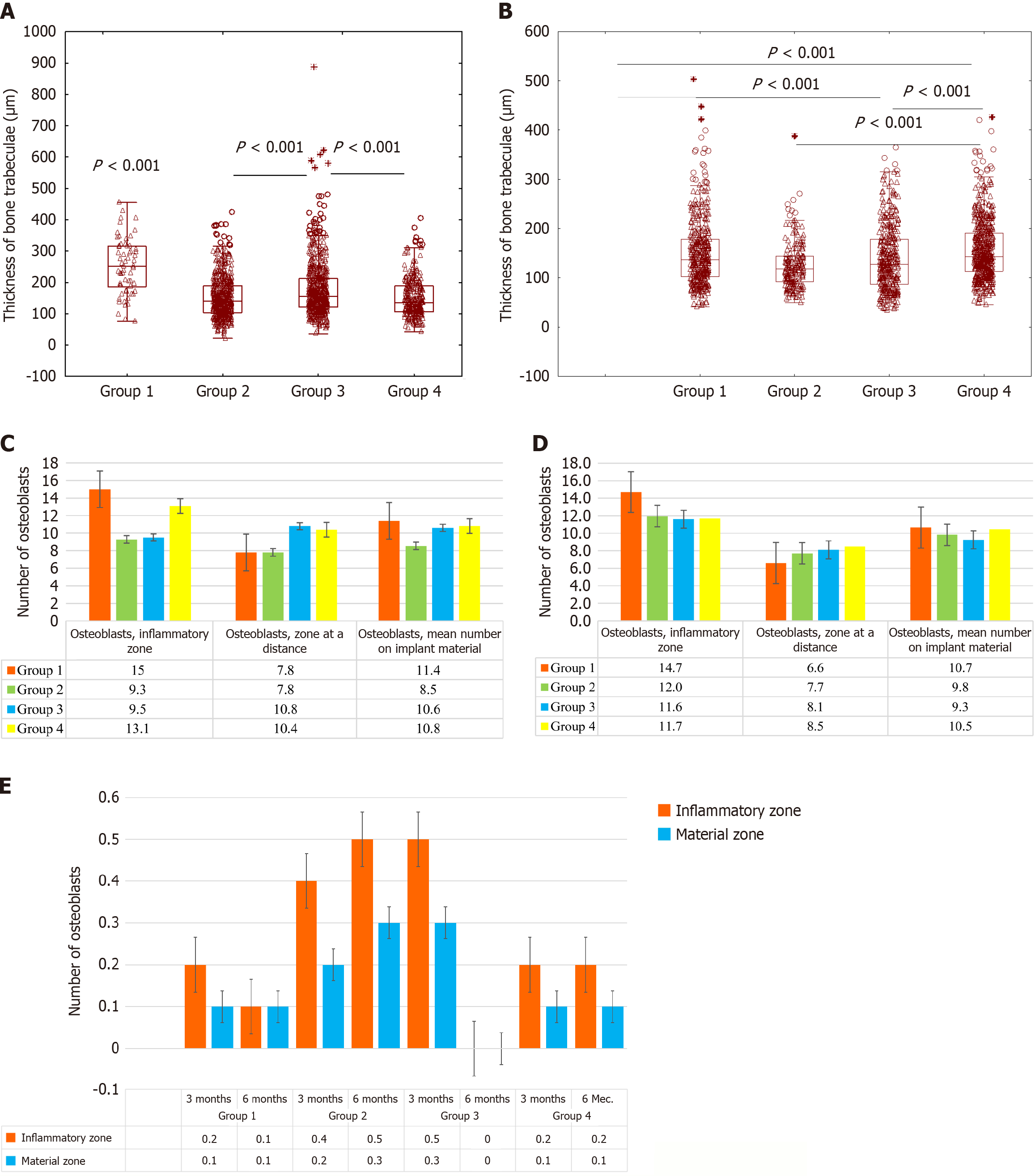
Specifically, at 6 months, the pamidronate-treated groups showed significantly higher scores for bone trabecular integrity and regeneration (median total score, 7.5 vs 4.0 in ATT-only; P < 0.05), with minimal evidence of osteolysis (median score, 0.5 in pamidronate-treated vs 2.0 in ATT-only; P < 0.05; Figure 2).
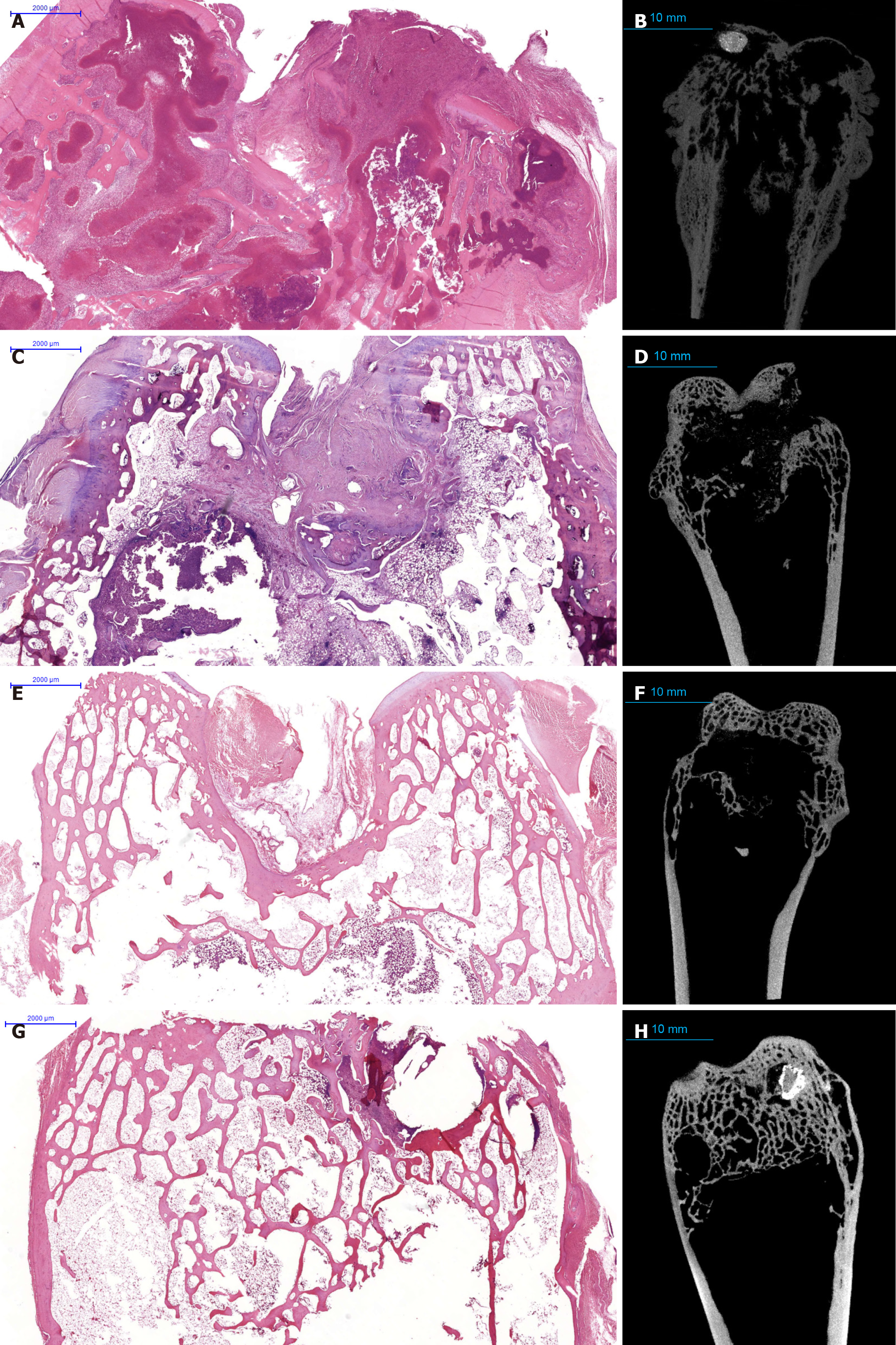
Histological examination of untreated controls revealed severe and extensive specific granulomatous inflammation (40%-50% of the bone area), accompanied by abundant caseous necrosis and numerous acid-fast bacilli (Figure 3A and B).
At 3 months post-surgery, ATT-only animals exhibited incomplete trabecular regeneration and persistent small inflammatory foci. Conversely, the pamidronate-treated groups showed substantially improved histological outcomes, characterized by active osteoblast proliferation, increased vascularity, and organized granulation tissue around the implants (Figures 3C-H and 4).
By 6 months, pamidronate-treated animals demonstrated remarkable improvements in trabecular thickness and overall bone matrix area, significantly greater compared to other groups (P < 0.001). Osteoclast activity markedly reduced following pamidronate administration (0.1 cells per high-power field vs 0.3 cells in ATT-only; P < 0.05). Specific inflammatory changes were minimal or absent in 75% of pamidronate-treated cases, in contrast to persistent residual lesions in ATT-only animals (Figures 3E-H and 4).
An important histological finding in pamidronate-treated animals was the presence of organized fibrous stromal strands extending from the implant, indicative of a favorable remodeling environment. Small fragments of the implant are visualized; histological preparations in this area reveal specific inflammation with small areas of nonspecific lympho-plasmacytic infiltration.
Statistically significant correlations were established between osteoclast numbers and implant density (r = -1.0 at 3 months and r = -1.0 at 6 months; P < 0.05), confirming the direct role of bisphosphonates in preserving implanted materials by reducing osteoclast-mediated resorption.
A strong correlation (r = 1.0 at 3 months; r = 0.9 at 6 months) was also observed between serum sclerostin levels and osteocyte numbers, suggesting that active bone remodeling dynamics are influenced by bisphosphonate therapy.
This experimental, randomized, controlled study demonstrates that pamidronate, a potent bisphosphonate, significantly enhances bone regeneration following surgical treatment of TB osteitis in rabbits. Pamidronate treatment improved the preservation and integration of implanted osteoinductive materials, increased trabecular thickness, and notably suppressed osteoclast activity. These findings highlight the therapeutic potential of bisphosphonates as adjunctive agents for enhancing bone healing and preventing complications after surgical interventions for skeletal TB[2,19-21].
TB osteitis, a form of extrapulmonary TB, poses considerable treatment challenges due to persistent inflammatory osteolysis and poor regenerative responses, even after optimal surgical intervention and extended courses of ATT[22,23]. Prior clinical and experimental data have consistently reported that residual bone defects and incomplete healing are common, leading to significant orthopedic morbidity, deformities, and functional impairment[1,22,24,25]. The search for adjunctive therapies capable of mitigating these complications is therefore of paramount clinical importance.
Despite extensive use in metabolic bone conditions, the application of bisphosphonates in infectious osteitis, particularly bone and joint TB, has been minimally explored[26,27]. Our study addressed this knowledge gap by investigating pamidronate in a validated rabbit model of TB osteitis. We observed significant structural benefits, with enhanced preservation and integration of osteoinductive bone grafts, confirming bisphosphonates’ capacity to protect implants from premature resorption and contribute to bone regeneration[27-29]. One of the key mechanisms underlying the observed improvements in bone regeneration is angiogenesis. Recent studies have shown that bisphosphonates can modulate angiogenic pathways, particularly through the vascular endothelial growth factor (VEGF) signaling pathway. VEGF is a critical mediator of endothelial cell proliferation and new blood vessel formation during tissue repair and regeneration. Bisphosphonates, including pamidronate, have been shown to enhance VEGF-mediated vascular remodeling in bone tissues, promoting improved blood flow to the healing site. The enhanced vascularization in pamidronate-treated animals may contribute to the better integration of the bone grafts and provide an environment conducive to osteogenesis. The increased blood supply ensures the delivery of nutrients, growth factors, and oxygen to the regenerating bone, thus facilitating the repair process and supporting the survival of osteoblasts at the graft site[19,20,30,31].
Additionally, bisphosphonates are known for their anti-inflammatory properties, which could further explain the improved healing observed in our study. Pamidronate has been shown to reduce the release of pro-inflammatory cytokines, such as tumor necrosis factor-α, interleukin-6, and interleukin-1β, which are typically elevated in inflammatory environments. By modulating the immune response, pamidronate may reduce the inflammatory burden at the surgical site, thereby preventing excessive bone resorption that often accompanies chronic inflammation, including TB osteitis. The reduction in inflammatory cytokines not only limits osteoclast activity but also fosters a more favorable environment for osteoblast activity, which is crucial for bone regeneration. Our data, showing a reduction in inflammation and enhanced osteoblast activity in pamidronate-treated groups, support this hypothesis[20,21,32,33].
Moreover, pamidronate may influence osteocyte signaling, an important aspect of bone remodeling. Osteocytes play a critical role in bone homeostasis by coordinating bone formation and resorption through their mechanosensory function and interaction with osteoblasts and osteoclasts. Bisphosphonates, including pamidronate has been shown to affect osteocyte-mediated signaling by altering the release of factors like sclerostin and sclerostin-like molecules. Sclerostin is a potent inhibitor of osteoblastic activity, and its suppression has been associated with increased bone formation. Our study observed an increase in osteoblast numbers and activity in the pamidronate-treated groups, which can be attributed in part to the modulation of osteocyte signaling pathways. Pamidronate may have a dual effect, inhibit osteoclast activity and promote osteoblast function, which ultimately leads to improved bone formation and graft retention.
The significant clinical implication of our study is that even a single administration of pamidronate can substantially enhance bone regeneration outcomes after surgical treatment of TB osteitis. This approach could represent a feasible and effective strategy to reduce postoperative complications, minimize structural deformities, and potentially shorten rehabilitation times for patients with skeletal tuberculosis[34]. However, clinical trials are warranted to confirm these experimental findings in human subjects[21,23].
The underlying pathology determines standard pamidronate dosing regimens. For example, in osteolytic breast cancer bone metastases, pamidronate is typically given at 90 mg intravenous every 3-4 weeks (according to the instructions for use). In situations where there is no ongoing bone destruction (i.e., no progressive osteolysis), there are currently no established protocols for the repeat administration of bisphosphonates. In our study, we elected to administer a single dose of pamidronate in the early postoperative period, primarily for practical reasons. A one-time infusion administered immediately after surgery is easier to integrate into clinical practice, as it enhances patient adherence and is more cost-effective, as it eliminates the need for repeated hospital visits or prolonged therapy. Moreover, when we designed this study, no evidence-based protocols existed for multiple pamidronate doses to enhance bone regeneration in such scenarios. We therefore opted for the most straightforward and safest strategy initially. From a mechanistic standpoint, administering a single dose is supported by the pharmacodynamics of bisphosphonates. Pamidronate binds strongly to the bone matrix and is then slowly released over periods of weeks to even years, providing a long-lasting antiresorptive effect. Therefore, one dose is sufficient to cover the critical early postoperative period, when bone resorption around the implant is at its peak. Additionally, bisphosphonates possess anti-inflammatory properties in addition to their antiresorptive effects. Pamidronate administered postoperatively may have attenuated the local inflammatory response and consequent bone resorption during the initial phase of implant integration (i.e., the first few months after surgery).
Certain limitations must be acknowledged. Firstly, the relatively small number of animals, inherent to ethical and logistical constraints of experimental research, may limit statistical power. Secondly, this study employed only a single bisphosphonate administration; the effects of multiple or prolonged treatments remain to be studied. Lastly, the extrapolation of animal model findings directly to clinical practice requires cautious interpretation. Nevertheless, the significant positive outcomes strongly justify further clinical investigations.
Our experimental data demonstrate that pamidronate significantly promotes bone regeneration and effectively inhibits osteoresorption in TB osteitis following surgical treatment. Given the excellent tolerability and compatibility with ATT, as well as the promising osteoanabolic potential demonstrated in this model, bisphosphonates should be considered as adjunctive therapy for managing patients with skeletal tuberculosis[19-21].
This experimental randomized controlled study demonstrates that a single intravenous dose of pamidronate significantly enhances bone regeneration, inhibits osteoresorption, and improves the integration and stability of bone graft materials following surgical treatment of TB osteitis. Pamidronate therapy was found to be safe and fully compatible with concurrent ATT, effectively mitigating local inflammatory and osteolytic processes. These findings strongly support further clinical evaluation of bisphosphonates as an adjunctive treatment strategy to improve outcomes in patients undergoing surgical intervention for skeletal tuberculosis.
We sincerely thank the staff of the Saint Petersburg Research Institute of Phthisiopulmonology and the Interregional Laboratory Center for their technical support during laboratory and histological analyses, and special thanks to Natalia M. Blum for histological consultations.
| 1. | Alatortsev AV, Kirillova ES, Mushkin AIu, Riasnianskaia TB. [Prediction of orthopedic sequels of operated tuberculous ostitis in children]. Probl Tuberk Bolezn Legk. 2006;58-61. [PubMed] |
| 2. | Gan J, Zhang C, Tang D, Du X. Surgical treatment of spinal tuberculosis: an updated review. Eur J Med Res. 2024;29:588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 3. | Wang W, Yeung KWK. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact Mater. 2017;2:224-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1056] [Cited by in RCA: 1033] [Article Influence: 114.8] [Reference Citation Analysis (0)] |
| 4. | De Pace R, Molinari S, Mazzoni E, Perale G. Bone Regeneration: A Review of Current Treatment Strategies. J Clin Med. 2025;14:1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 5. | Lafzi A, Saravi NSV, Amid R, Kadkhodazadeh M, Shojaei N. Biological reactions of dental pulp stem cells cultured in presence of new xenograft bone substitutes from different sources: An in vitro study. J Indian Soc Periodontol. 2022;26:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Ke Re Mu ALM, Abulikemu M, Liang Z, Abulikemu A, Tuxun A. Anti-Infection Efficacy, Osteogenesis Potential, and Biocompatibility of 3D Printed PLGA/Nano-Hydroxyapatite Porous Scaffolds Grafted with Vancomycin/DOPA/rhBMP-2 in Infected Rabbit Bone Defects. Int J Nanomedicine. 2025;20:6399-6421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Chen Z, Jia M, Liu Y, Zhou H, Wang X, Wu M. Injectable Composite Hydrogel Stents for Bone Defect Management with Enhanced Osteogenesis and Angiogenesis. Int J Nanomedicine. 2025;20:4589-4606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Watson L, V Ramanan A, Oliver E, Segers F, Jones GW, Chew C, Goenka A. Pamidronate-Induced Clinical Remission in Chronic Non-bacterial Osteomyelitis Is Associated with Reduced Vγ9Vδ2 T-Cell Receptor Expression. Eur J Immunol. 2025;55:e202451609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Sun S, Tao J, Sedghizadeh PP, Cherian P, Junka AF, Sodagar E, Xing L, Boeckman RK Jr, Srinivasan V, Yao Z, Boyce BF, Lipe B, Neighbors JD, Russell RGG, McKenna CE, Ebetino FH. Bisphosphonates for delivering drugs to bone. Br J Pharmacol. 2021;178:2008-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Ohlrich EJ, Coates DE, Cullinan MP, Milne TJ, Zafar S, Zhao Y, Duncan WD, Seymour GJ. The bisphosphonate zoledronic acid regulates key angiogenesis-related genes in primary human gingival fibroblasts. Arch Oral Biol. 2016;63:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Jin H, Jin H, Suk KS, Lee BH, Park SY, Kim HS, Moon SH, Park SR, Kim N, Shin JW, Kwon JW. Antiosteoporosis medication in patients with posterior spine fusion: a systematic review and meta-analysis. Spine J. 2025;25:1877-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Shen H, Yang E, Guo M, Yang R, Huang G, Peng Y, Sha W, Wang F, Shen L. Adjunctive Zoledronate + IL-2 administrations enhance anti-tuberculosis Vγ2Vδ2 T-effector populations, and improve treatment outcome of multidrug-resistant tuberculosis(1). Emerg Microbes Infect. 2022;11:1790-1805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 13. | Bosov M, Sproat C, Kwok J, Averely M, Collins L, Patel V. The use of oral bisphosphonates in the management of antibiotic refractory secondary chronic osteomyelitis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2025;140:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Quarta L, Corrado A, Melillo N, Trotta A, Scotto G, d'Onofrio F, Santoro N, Cantatore FP. Combined effect of Neridronate and specific antibiotic therapy in a case of tuberculous spondylodiscitis. Rheumatol Int. 2008;28:495-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Petersen OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SD, Steckler T, Würbel H. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020;18:e3000410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1826] [Cited by in RCA: 3580] [Article Influence: 596.7] [Reference Citation Analysis (0)] |
| 16. | Guide for the Care and Use of Laboratory Animals. Washington (DC): National Academies Press (US); 2011- . [PubMed] |
| 17. | Cho KH, Kim JS, Jeon MS, Lee K, Chung MK, Song CW. Basic Principles of the Validation for Good Laboratory Practice Institutes. Toxicol Res. 2009;25:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Vinogradova TI, Serdobintsev MS, Korzhikova-Vlakh EG, Korzhikov-Vlakh VA, Kaftyrev AS, Blum NM, Semenova NY, Esmedlyaeva DS, Dyakova ME, Nashchekina YA, Dogonadze MZ, Zabolotnykh NV, Yablonsky PK. Comparison of Autografts and Biodegradable 3D-Printed Composite Scaffolds with Osteoconductive Properties for Tissue Regeneration in Bone Tuberculosis. Biomedicines. 2023;11:2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Larrañaga-Vera A, Toti KS, Flatow JS, Haraczy AJ, Warnick E, Rao H, Gao ZG, Sussman SM, Mediero A, Leucht P, Jacobson KA, Cronstein BN. Novel alendronate-CGS21680 conjugate reduces bone resorption and induces new bone formation in post-menopausal osteoporosis and inflammatory osteolysis mouse models. Arthritis Res Ther. 2022;24:265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Hong C, Quach A, Lin L, Olson J, Kwon T, Bezouglaia O, Tran J, Hoang M, Bui K, Kim RH, Tetradis S. Local vs. systemic administration of bisphosphonates in rat cleft bone graft: A comparative study. PLoS One. 2018;13:e0190901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Leerling AT, Cañete AN, Smit F, Hamdy NAT, van de Burgt A, Appelman-Dijkstra NM, Dekkers OM, Winter EM. Pamidronate for pain in adult chronic nonbacterial osteitis: protocol of a randomized, double-blind, placebo-controlled trial. JBMR Plus. 2024;8:ziae114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 22. | Held M, Bruins MF, Castelein S, Laubscher M, Dunn R, Hoppe S. A neglected infection in literature: Childhood musculoskeletal tuberculosis - A bibliometric analysis of the most influential papers. Int J Mycobacteriol. 2017;6:229-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Lansell A, Vasili Y, Suchdev PS, Figueroa J, Kirpalani A. Impact of antibiotic pretreatment on cultures in children with osteomyelitis and septic arthritis: a retrospective review. BMC Pediatr. 2021;21:342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Hogan JI, Hurtado RM, Nelson SB. Mycobacterial Musculoskeletal Infections. Thorac Surg Clin. 2019;29:85-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Huangfu P, Ugarte-Gil C, Golub J, Pearson F, Critchley J. The effects of diabetes on tuberculosis treatment outcomes: an updated systematic review and meta-analysis. Int J Tuberc Lung Dis. 2019;23:783-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 26. | Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society* Clinical Practice Guideline. J Clin Endocrinol Metab. 2019;104:1595-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 542] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 27. | Kirilova IA, Fomichev NG. Issues of reparative regeneration in vertebrology: a historical review of the studies of apprentices of Professor Ya.L. Tsivyan. Russ J Spine Surg. 2020;17:102-112. [DOI] [Full Text] |
| 28. | Kenis VM, Bogdanova SL, Prokopenko TN, Sapogovskiy AV, Kiseleva TI. Bone metabolism biomarkers in walking children with cerebral palsy. Pediatr Traumatol Orthop Reconstr Surg. 2020;7:79-86. [DOI] [Full Text] |
| 29. | Kenis VM, Sapogovskiy AV, Prokopenko TN, Bergaliev AN, Ivanov SV, Kiseleva TI. Bone mineral density in children with cerebral palsy and Spina Bifida treated with ibandronate. Pediatr Traum Orthop Reconstr Surg. 2020;8:129-136. [DOI] [Full Text] |
| 30. | Baas J, Vestermark M, Jensen T, Bechtold J, Soballe K, Jakobsen T. Topical bisphosphonate augments fixation of bone-grafted hydroxyapatite coated implants, BMP-2 causes resorption-based decrease in bone. Bone. 2017;97:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Deliberador FR, Sebastiani AM, Gerber J, Bonetto L, Tórtora G, Giovanini AF, Deliberador TM, Zielak JC, Scariot R. Effect of Local Application of Alendronate and Parathyroid Hormone on Craniofacial Bone Repair - a Preliminary Study. Braz Dent J. 2018;29:435-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Leerling AT, Dekkers OM, Appelman-Dijkstra NM, Winter EM. Clinical and therapeutic diversity in adult chronic nonbacterial osteomyelitis (CNO) of the sternocostoclavicular region: a meta-analysis. Rheumatology (Oxford). 2023;62:512-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 33. | Hedrich CM, Morbach H, Reiser C, Girschick HJ. New Insights into Adult and Paediatric Chronic Non-bacterial Osteomyelitis CNO. Curr Rheumatol Rep. 2020;22:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 34. | Wei Z, Zhang Y, Yang S, Ye J, Hu X, Li T, Chu T. Risk Factors of Bone Nonfusion After Spinal Tuberculosis Debridement Bone Graft Fusion and Internal Fixation. Front Surg. 2022;9:888148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/