Published online Apr 10, 2015. doi: 10.5306/wjco.v6.i2.16
Peer-review started: December 26, 2014
First decision: January 8, 2015
Revised: January 29, 2015
Accepted: February 10, 2015
Article in press: February 12, 2015
Published online: April 10, 2015
Processing time: 105 Days and 1.2 Hours
Ectopic breast tissue is rare and typically presents as an axillary mass. Previous reports have identified ectopic breast tissue in the vulva, but malignancy is exceedingly uncommon. We present a 62 years old with locally advanced breast carcinoma arising in the vulva demonstrates the utilization of sentinel lymph node mapping to identify metastatic lymph nodes previously unable to be identified via traditional surgical exploration. Our case supports the principles of adjuvant therapy for breast cancer to be applied to ectopic breast cancer arising in the vulva. A literature review highlights common key points in similar cases to guide management.
Core tip: Our findings describe the presentation of ectopic breast cancer in the vulva. We demonstrate use of sentinel lymph node technology with identification of the sentinel node, only possible after the use of this technology. We conclude with a review of the literature outlining treatment of this enigmatic disease.
- Citation: Cripe J, Eskander R, Tewari K. Sentinel lymph node mapping of a breast cancer of the vulva: Case report and literature review. World J Clin Oncol 2015; 6(2): 16-21
- URL: https://www.wjgnet.com/2218-4333/full/v6/i2/16.htm
- DOI: https://dx.doi.org/10.5306/wjco.v6.i2.16
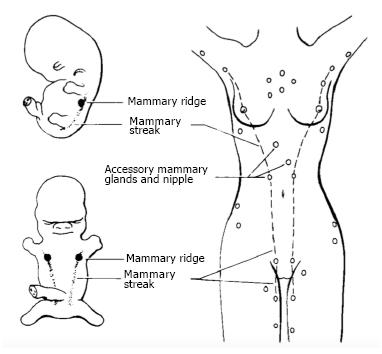
Ectopic breast tissue has been previously reported in various locations along the primitive milk line, from the axilla to the vulva (Figure 1). Axillary ectopic breast tissue is the most frequent location and the vulva being the least common site[1]. Malignant ectopic breast tissue is rare, typically presenting as an axillary mass, with vulvar breast malignancy being exceedingly rare[1]. In 1935, Green et al[2] published the first case report of adenocarcinoma arising from breast tissue in the vulva. Although 22 cases of malignant vulvar breast tissue have been reported since then, there are no clear guidelines regarding surgical or adjuvant treatment. We present a case that outlines the diagnosis and management of primary breast cancer of the vulva, highlighting diagnostic dilemmas, the utility of sentinel node mapping and reinforcing the importance of a multidisciplinary approach in the management of this rare clinical entity.
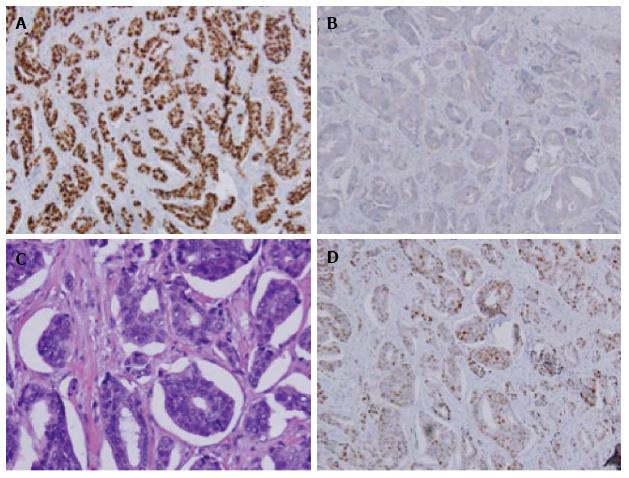
A 62 years old Hispanic multiparous women noted a new 1.3 cm left labial mass for approximately 1 year and presented to her primary gynecologist for evaluation. She underwent a wide local excision that was noteworthy for an invasive ductal carcinoma arising in ectopic breast tissue. Final pathology was confirmed by independent review at two separate institutions. Immunohistochemical staining showed the lesion to be 95% estrogen receptor (ER) positive, 10% progesterone receptor (PR) positive, and human epidermal growth factor 2 (HER2) negative (Figure 2).
The patient underwent an magnetic resonance imaging of the breast that was negative for a breast primary malignancy. Approximately 1 mo after initial presentation in September of 2012, the patient was referred to gynecologic oncology and underwent a partial radical vulvectomy at the prior vulvar scar site. Final pathology was negative for residual disease and the patient, given absence of metastatic disease declined adjuvant therapy. The patient initiated close surveillance and had a Fluoro-deoxyglucose (FDG) Positron emission tomography (PET) scan in January 2013 with findings of suspicious left inguinal-femoral lymphadenopathy, with standard uptake value (SUV) of 8.1.
The patient was counseled to undergo left inguinal-femoral lymphadenectomy (LND). The dissection was completed superficial to the cribiform fascia and final pathology identified 14 lymph nodes ranging from 1.2-2.5 cm that were all negative for tumor. On follow up examination in April 2013, the patient was found to have a 1-2 mm firm, non-tender nodule under her healing scar. In office biopsy confirmed recurrent invasive ductal carcinoma, with identical histology to the previous primary lesion. A repeat wide local excision was performed in June 2013. Pathology from that surgical resection was negative for tumor.
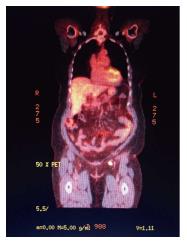
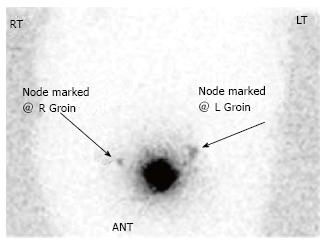
A PET-CT in August 2013 was repeated and was significant for suspicious left inguinal lymph node measuring 1.1 cm × 1.6 cm with SUV of 8.2 (Figure 3). The patient returned to the operating room with preoperative technetium 99 lymphoscintography and lymphazurin blue (injected into the previous left surgical site) lymph node localization (Figure 4). An inguinal incision was created and the Geiger counter was used to identify “hot” areas. Dissection continued until area of maximum radioactivity was encountered. A hot, blue, slightly firm, 1.2 cm left sentinel was identified superficial to the cribiform fascia and excised. Intraoperative frozen section was positive for metastasis and comprehensive LND was performed. Two additional left sentinels (both hot and blue) were positive for ductal carcinoma. A right-sided sentinel node was not identified, but given contralateral positive nodes a comprehensive right LND was performed. Final pathology (Figure 2) confirmed three positive sentinels and 14 negative left and right inguinofemoral nodes.
Metastatic workup was negative and the patient underwent intensity modulated radiation therapy (4500 cGy) with 5900 cGy boosts to the left groin. Chemotherapy included weekly taxol followed by adriamycin and cyclophosphamide. Following adjuvant therapy she started maintenance therapy with an aromatase inhibitor.
She is currently without evidence of disease recurrence 13 mo after sentinel lymph node detection.
Ectopic breast tissue is rare and accounts for 0.2%-0.6% of all breast cancers. Only 4% of these ectopic breast cancers are located in the vulva, making vulvar breast cancer exceedingly rare[3]. Ectopic breast tissue originates in the fetus at the ectodermal mammary streak extending from the axilla to the groin as demonstrated in Figure 1. Most of this structure disappears with small portions persisting in the thorax. This primordial ectoderm penetrates the underlying mesenchyme and gives rise to small solid out buddings that canalize and form the lactiferous ducts and alveoli of the mammary gland[4,5].
There have been 22 reported cases since Greene’s index case report in 1935. The majority of these patients presented with an innocuous solitary lesion of the vulva (Table 1); upon surgical excision, adenocarcinoma or ductal carcinoma arising in normal appearing breast tissue was identified. Extensive preoperative imaging is traditionally used to exclude metastasis of a primary breast malignancy. Two of these reported cases were indeed metastatic from a primary breast lesion[6,7]. Adjuvant chemotherapy and radiation treatment protocols are heterogeneous (Table 2) given the rare frequency of these lesions, and absence of standardized treatment paradigms. Anti-hormonal therapy has been used in 14 (13 Tamoxifen and 1 Aromatase) patients with ER/PR positive specimens with various outcomes. The use of trastuzumab in HER2 positive cases has not been previously reported.
| Ref. | Year | Age (yr) | Location | Size (cm) | Symptoms and duration | Duration (mo) |
| Greene[2] | 1935 | 59 | Right labia majora | 20 × 15 | Mass | 12 |
| Hendrix et al[14] | 1956 | 58 | Right Labia minora | 3 | Ulcerated mass | 84 |
| Guerry et al[6] | 1976 | 62 | Right labia minora | 1.5 | Mass | 4 |
| Guercio et al[15] | 1984 | 49 | Left labia majora | 2 | Mass | Unk |
| Cho et al[16] | 1985 | 70 | Right labia majora | 3 × 4 | Mass | 30 |
| Simon et al[17] | 1988 | 60 | Right labia majora | 2 × 2 | Ulcerated mass | 36 |
| Rose et al[18] | 1990 | 68 | Right labia majora | 3.5 × 3.5 | Mass | 36 |
| Di Bonito et al[19] | 1992 | 46 | Right labia majora | 1.5 | Ulcerated mass | 24 |
| Bailey et al[20] | 1993 | 65 | Right labia majora | 3 × 2 | Ulcerated mass | 36 |
| Levin et al[21] | 1994 | 62 | Left clitorus | 2.5 | Mass | Unk |
| Kennedy et al[7] | 1997 | 71 | Left labia majora | 5 | Ulceration and dysuria | 1 |
| Irvin et al[4] | 1998 | 64 | Left lateral mons | 3 | Indurated mass | 48 |
| Gorisek et al[22] | 2000 | 81 | Left labia majora | 2 × 3 | Ulcerated mass | Unk |
| Piura et al[23] | 2002 | 69 | Left labia majora | 3 | Ulcerated mas | Unk |
| Chung-Park et al[24] | 2002 | 47 | Right labia minora | 2 | Ulcerated mass | 12 |
| Yin et al[25] | 2003 | 84 | Mons | 5 | Swelling | 24 |
| Lopes et al[26] | 2006 | 44 | Left vulva | 2 | Mass | 48 |
| Fracchioli et al[27] | 2006 | 57 | Left vulva | 1 | Mass | Unk |
| North et al[28] | 2006 | 49 | Right labia minora and clitoris | 1.5 | Pain and pressure | Unk |
| Left groin | 2 | |||||
| Naseer et al[29] | 2011 | 57 | Right labia majora | 1.5 | Non painful lesion | Unk |
| McMaster[30] | 2013 | 60 | Left labia majora | 3 | Pedunculated ulcerated mass | 6 |
| Bogani et al[13] | 2013 | 71 | Left labia Majora | 4 | Painful ulcerated mass | Unk |
| Left groin | 3 |
| Ref. | Treatment | Histology | ER | PR | Her2-neu | LN | Status | Follow up (mo) |
| Greene[2] | None | AC + S | NA | NA | NA | NA | DOD | 1 |
| Hendrix et al[14] | Surgery | AC | NA | NA | NA | NA | DOD | 4 |
| Guerry et al[6] | Surgery | DC | NA | NA | NA | NA | DOD | 24 |
| Guercio et al[15] | Surgery + RT | LO | NA | NA | NA | 11/24 | NED | 36 |
| Cho et al[16] | Surgery + tamoxifen | AC | + | + | NA | 2/9 | NED | 24 |
| Simon et al[17] | Surgery + tamoxifen + cyclophosphamide, adriamycin, 5FU | AC | + | + | NA | 3/11 | DOD | 27 |
| Recurrence cisplatin/etoposide then carboplatin/etoposide | ||||||||
| Rose et al[18] | Surgery + RT + tamoxifen | DC | + | - | NA | 1/15 | Unk | Unk |
| Di Bonito et al[19] | Surgery | Unk | NA | 11/13 | NED | 4 | ||
| Bailey et al[20] | Surgery + tamoxifen | DC | + | + | NA | 2/20 | NED | 12 |
| Levin et al[21] | Surgery + tamoxifen | AC | + | - | + | 4/11 | NED | 24 |
| Recurrence restarted tamoxifen/RT | NED | 12 | ||||||
| Kennedy et al[7] | Surgery + adriamycin/cyclophosphamide | Unk | - | - | NA | 9/9 | NED | 15 |
| Irvin et al[4] | Surgery + cytoxan/Mtx/5FU + RT + tamoxifen | AC | + | + | NA | 1/14 | NED | 4 |
| Gorisek et al[22] | Surgery + tamoxifen | AC | + | + | NA | NA | NED | 19 |
| Piura et al[23] | Surgery + adriamycin/cyclophosphamide/paclitaxel + RT + tamoxifen | AC | + | + | NA | 7/15 | NED | 14 |
| Chung-Park et al[24] | Surgery | MU | + | + | - | NA | NED | 36 |
| Yin et al[25] | Surgery | MU | + | + | - | 1/11 | NED | 9 |
| Lopes et al[26] | Surgery + docetaxel/doxorubicin/cyclophosphamide + tamoxifen | MU | + | NA | - | 2/13 | Unk | Unk |
| Fracchioli et al[27] | Surgery + 5FU/adriamycin/cyclophosphamide + tamoxifen | AC | - | NA | NA | 7/7 | Rec | 36 |
| Recurrence-paclitaxel | Unk | Unk | ||||||
| North et al[28] | Surgery + cyclophosphamide/epirubicin/5FU + weekly docetaxel + tamoxifen | DC | + | + | - | 5/7 | Unk | Unk |
| Naseer et al[29] | Surgery + 5FU/epirubicin/cyclophosphamide + weekly docetaxel + RT + aromatase | DC | + | + | - | 3/13 | Unk | Unk |
| McMaster et al[30] | Surgery + RT | DC | + | NA | NA | NA | Unk | Unk |
| Bogani et al[13] | Surgery + epirubicin/cyclophosphamide + tamoxifen | DC | + | + | NA | 1/8 | NED | 24 |
The presence of metastatic tumor in regional lymph nodes remains the most significant prognostic factor for several malignancies, including breast cancer. Sixteen patients underwent inguinal LND with all 16 patients having lymph node involvement. Survival and adjuvant therapy data are outlined in Table 2. Sentinel lymph node mapping is a technique that minimizes morbidity while maintaining diagnostic accuracy by isolating the first or “sentinel” node to drain the affected area burdened with tumor. This is traditionally performed with injection of the tumor with isosulfan blue and a radiolabeled colloid, most often technetium 99. This technique was pioneered by Morton in the treatment of melanoma in the early 1990’s[8]. The assessment of regional lymph nodes in breast cancer paralleled the work in melanoma, in an effort to limit the morbidity of axillary lymph node dissection[9]. Numerous clinical trials have detailed the effectiveness and reduced morbidity associated with sentinel lymph node dissection in breast cancer patients in both the primary surgical setting and following neoadjuvant therapy. Current American Society of Clinical Oncology (ASCO) guidelines recommends sentinel lymph node mapping as standard of care in breast cancer[10].
Sentinel node mapping in vulvar cancer is a more contemporary topic with evolving literature, and has paralleled some advances in penile carcinoma lymphatic mapping. GROINS-V, an observational study, followed 403 patients with primary vulvar tumors less than 4 cm treated with sentinel node mapping. Eight patients had groin recurrence with a false negative rate of 5.9% and a false negative predictive value of 2.9%[11]. Similar results were replicated in GOG protocol 173[12], a phase 3 multi-institutional study of intraoperative lymphatic mapping in patients with invasive squamous cell carcinoma of the vulva. Inclusion criteria included depth of invasion > 1 mm and primary tumor size 2-6 cm. Four hundred and fifty-two patients underwent sentinel node mapping with 418 patients having a sentinel node identified. Eleven (8.3%) patients with negative sentinel lymph nodes had groin recurrence. The false negative rate in primary lesions < 4 cm was 2%, but with lesions > 4 cm the false negative rate was 4%[11]. It is important to note that all patients in GOG protocol 173 underwent comprehensive LND after sentinel LND regardless of its status. GROINS-V also identified a statistically significant decrease in wound breakdown, cellulitis, and lymphedema in patients undergoing sentinel lymph node mapping. Both studies provide evidence to support the incorporation of sentinel LND in the management of vulvar malignancies.
Our case utilized sentinel lymph node mapping at time of recurrence and precisely aided in the identification of the metastatic lymph nodes. This technology was not utilized in the primary surgical management due to uncertainties on how it would perform when the primary lesion was comprised of ectopic breast tissue. However, after failing to identify the PET positive nodes with standard LND, SLD was employed to identify and excise the lymph nodes.
Primary breast cancer originating in the vulva is rare and management strategies stem from individual case reports or case series. Current literature supports the use of sentinel lymph node mapping in vulvar cancer and we anticipate that future cases will utilize this practice. Bogani et al[13] as well as our case have been the only published literature to utilize sentinel lymph node mapping after a previous LND. Both cases identified positive sentinel lymph nodes that were previously unable to have been resected. These findings may support the up-front use of sentinel lymph node localization.
From our review of the literature there are several key concepts in managing this rare malignancy. First, exclusion of a primary breast malignancy needs to be confirmed by pretreatment imaging and physical examination. Next, occurrence of positive nodes is high and can be difficult to locate; primary surgical excision (radical vulvectomy) with sentinel lymph node dissection to help identify the sentinel lymph node should be considered.
Systemic chemotherapy based on adjuvant therapy platforms for breast cancer with docetaxel or paclitaxel, plus doxorubicin, and cyclophosphamide should be considered. Given the biologic parallels between primary breast and vulvar breast cancer, treatment paradigms mimicking primary breast cancer are a rational approach and are advisable. This is supported by the median survival of patients treated with adjuvant therapy for breast cancer had a mean survival of 30 mo in comparison to 12 mo for those receiving a vulvar treatment (surgery followed by radiation). Finally, patients with estrogen and progestin receptor positive specimens may be maintained on tamoxifen or aromatase inhibitors.
A 62 years old Hispanic women presented with a 1.3 cm left labial mass.
Suspected neoplasm originating from the left labia.
Gartners duct cyst, labial myoma, vulvar squamous cell carcinoma, and vulvar melanoma.
Histologic examination identified the ectopic breast tissue and carcinoma present in excised specimen.
Excisional procedure.
Previous reports of vulvar breast cancer present similarly with an isolated labial mass, treated with surgical excision, however many do not receive chemotherapy that parallels breast cancer treatments.
When vulvar breast cancer is encountered, the physician should exclude a primary breast malignancy, perform an excision procedure, and utilize sentinel lymph node mapping as recommended in breast cancer.
Well written case report.
| 1. | Nihon-Yanagi Y, Ueda T, Kameda N, Okazumi S. A case of ectopic breast cancer with a literature review. Surg Oncol. 2011;20:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Greene HJ. Adenocarcinoma of supernumerary breasts of the labia majora in a case of epidermoid carcinoma of the vulva. Am J Obstet Gynecol. 1936;31:660-663. |
| 3. | Visconti G, Eltahir Y, Van Ginkel RJ, Bart J, Werker PM. Approach and management of primary ectopic breast carcinoma in the axilla: where are we? A comprehensive historical literature review. J Plast Reconstr Aesthet Surg. 2011;64:e1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Irvin WP, Cathro HP, Grosh WW, Rice LW, Andersen WA. Primary breast carcinoma of the vulva: a case report and literature review. Gynecol Oncol. 1999;73:155-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Marshall MB, Moynihan JJ, Frost A, Evans SR. Ectopic breast cancer: case report and literature review. Surg Oncol. 1994;3:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Guerry RL, Pratt-Thomas HR. Carcinoma of supernumerary breast of vulva with bilateral mammary cancer. Cancer. 1976;38:2570-2574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Kennedy DA, Hermina MS, Xanos ET, Schink JC, Hafez GR. Infiltrating ductal carcinoma of the vulva. Pathol Res Pract. 1997;193:723-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391-398; discussion 398-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2130] [Cited by in RCA: 2044] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 9. | Edge SB, Niland JC, Bookman MA, Theriault RL, Ottesen R, Lepisto E, Weeks JC. Emergence of sentinel node biopsy in breast cancer as standard-of-care in academic comprehensive cancer centers. J Natl Cancer Inst. 2003;95:1514-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Lyman GH, Temin S, Edge SB, Newman LA, Turner RR, Weaver DL, Benson AB, Bosserman LD, Burstein HJ, Cody H. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2014;32:1365-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1360] [Cited by in RCA: 1305] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 11. | Van der Zee AG, Oonk MH, De Hullu JA, Ansink AC, Vergote I, Verheijen RH, Maggioni A, Gaarenstroom KN, Baldwin PJ, Van Dorst EB. Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J Clin Oncol. 2008;26:884-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 526] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 12. | Levenback C, Coleman RL, Burke TW, Bodurka-Bevers D, Wolf JK, Gershenson DM. Intraoperative lymphatic mapping and sentinel node identification with blue dye in patients with vulvar cancer. Gynecol Oncol. 2001;83:276-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 141] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Bogani G, Uccella S, Cromi A, Casarin J, Donadello N, Ghezzi F. Primary mammary-like ductal carcinoma of the vulva: a case report and analysis of the literature. Am J Dermatopathol. 2013;35:685-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Hendrix RC, Behrman SJ. Adenocarcinoma arising in a supernumerary mammary gland in the vulva. Obstet Gynecol. 1956;8:238-241. [PubMed] |
| 15. | Guercio E, Cesone P, Saracino A, Gatti M, Arisio R, Oberto F. [Adenocarcinoma occurring in an aberrant mammary gland located in the vulva]. Minerva Ginecol. 1984;36:315-319. [PubMed] |
| 16. | Cho D, Buscema J, Rosenshein NB, Woodruff JD. Primary breast cancer of the vulva. Obstet Gynecol. 1985;66:79S-81S. [PubMed] |
| 17. | Simon KE, Dutcher JP, Runowicz CD, Wiernik PH. Adenocarcinoma arising in vulvar breast tissue. Cancer. 1988;62:2234-2238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Rose PG, Roman LD, Reale FR, Tak WK, Hunter RE. Primary adenocarcinoma of the breast arising in the vulva. Obstet Gynecol. 1990;76:537-539. [PubMed] |
| 19. | Di Bonito L, Patriarca S, Falconieri G. Aggressive “breast-like” adenocarcinoma of vulva. Pathol Res Pract. 1992;188:211-214; discussion 214-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Bailey CL, Sankey HZ, Donovan JT, Beith KA, Otis CN, Powell JL. Primary breast cancer of the vulva. Gynecol Oncol. 1993;50:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Levin M, Pakarakas RM, Chang HA, Maiman M, Goldberg SL. Primary breast carcinoma of the vulva: a case report and review of the literature. Gynecol Oncol. 1995;56:448-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Gorisek B, Zegura B, Kavalar R, But I, Krajnc I. Primary breast cancer of the vulva: a case report and review of the literature. Wien Klin Wochenschr. 2000;112:855-858. [PubMed] |
| 23. | Piura B, Gemer O, Rabinovich A, Yanai-Inbar I. Primary breast carcinoma of the vulva: case report and review of literature. Eur J Gynaecol Oncol. 2002;23:21-24. [PubMed] |
| 24. | Chung-Park M, Zheng Liu C, Giampoli EJ, Emery JD, Shalodi A. Mucinous adenocarcinoma of ectopic breast tissue of the vulva. Arch Pathol Lab Med. 2002;126:1216-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Yin C, Chapman J, Tawfik O. Invasive mucinous (colloid) adenocarcinoma of ectopic breast tissue in the vulva: a case report. Breast J. 2003;9:113-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Lopes G, DeCesare T, Ghurani G, Vincek V, Jorda M, Glück S, Silva O. Primary ectopic breast cancer presenting as a vulvar mass. Clin Breast Cancer. 2006;7:278-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Fracchioli S, Puopolo M, De La Longrais IA, Scozzafava M, Bogliatto F, Arisio R, Micheletti L, Katsaros D. Primary “breast-like” cancer of the vulva: a case report and critical review of the literature. Int J Gynecol Cancer. 2006;16 Suppl 1:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | North J, Perez D, Fentiman G, Sykes P, Dempster A, Pearse M. Primary breast cancer of the vulva: case report and literature review. Aust N Z J Obstet Gynaecol. 2007;47:77-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Naseer MA, Mohammed SS, George SM, Das Majumdar SK. Primary ectopic breast cancer mimicking as vulval malignancy. J Obstet Gynaecol. 2011;31:553-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | McMaster J, Dua A, Dowdy SC. Primary breast adenocarcinoma in ectopic breast tissue in the vulva. Case Rep Obstet Gynecol. 2013;2013:721696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
P- Reviewer: AliR A, Park Y, Peng Y S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/