Published online Dec 10, 2014. doi: 10.5306/wjco.v5.i5.1068
Revised: June 3, 2014
Accepted: August 27, 2014
Published online: December 10, 2014
Processing time: 268 Days and 15.5 Hours
AIM: To investigate the expression of miR-210 and the role it plays in the cell cycle to regulate radioresistance in oesophageal squamous cell carcinoma (ESCC).
METHODS: MiR-210 expression was evaluated in 37 pairs of ESCC tissues and matched para-tumorous normal oesophageal tissues from surgical patients who had not received neoadjuvant therapy, and in the cells of two novel radioresistant cell lines, TE-1R and Eca-109R, using quantitative reverse transcription-polymerase chain reaction (qRT-PCR). The transient up-regulation of miR-210 expression in TE-1R and Eca-109R cells was studied using liposomes and was confirmed using qRT-PCR. The rate of cell survival after a series of radio-treatment doses was evaluated using the clone formation assay. Flow cytometry was used to detect the changes to the cell cycle patterns due to radiation treatment. RT-PCR and Western blot were used to detect the expression of ataxia telangiectasia mutated (ATM) and DNA dependent protein kinase (DNA-PKcs) after irradiation, and the cell sphere formation assay was used to evaluate the proliferative ability of the cancer stem-like cells.
RESULTS: The level of miR-210 expression was significantly decreased, by 21.3% to 97.2%, with the average being 39.2% ± 16.1%, in the ESCC tissues of most patients (81.1%, 30 of 37 vs patients with high miR-210 expression, P < 0.05). A low level of expression of miR-210 was correlated with a poorly differentiated pathological type (P < 0.01) but was not correlated with the T-stage or lymph node infiltration (both P > 0.05). Early local recurrences (< 18 mo, n = 19) after radiotherapy were significantly related with low miR-210 expression (n = 13, P < 0.05). The level of miR-210 was decreased by approximately 73% (vs TE-1, 0.27 ± 0.10, P < 0.01) in the established radioresistant TE-IR cell line and by 52% (vs Eca-109, 0.48 ± 0.17, P < 0.05) in the corresponding Eca-109R line. Transient transfection with a miR-210 precursor increased the level of miR-210 expression, leading to a significant increase in cell survival after radiotherapy (P < 0.05). Twenty-four hours after radiation, the proportion of pmiR-210 cells in S phase was increased (vs control cells, 30.4% ± 0.4%, and vs untreated TE-1R cells, 23.3% ± 0.7%, P < 0.05 for both). The levels of DNA-PKcs (0.21 ± 0.07) and ATM (0.12 ± 0.03, P < 0.05) proteins were significantly lower in the PmiR-210 cells than in control cells, but no differences were found in the levels of the corresponding mRNAs in the two cell types (P > 0.05 for all). Exogenous miR-210 expression decreased the diameter of pmiR-210 cell spheres (vs control cells, 0.60 ± 0.14, P < 0.05).
CONCLUSION: MiR-210 expression is negatively correlated with the pathological type and the local survival rate after radiotherapy, and high expression of miR-210 may reverse the radioresistance of ESCC stem-like cells.
Core tip: A low level of miR-210 expression, which is common in oesophageal squamous cell carcinoma (ESCC) tissues, was found to be negatively correlated with the tumour pathological type and the prognosis in ESCC patients after radiotherapy, although the sample size was small. A relatively high level of in vitro miR-210 expression reversed the radioresistance of ESCC stem-like cells by decreasing the extent of ataxia telangiectasia mutated/DNA dependent protein kinase-dependent cell cycle arrest, failure of DNA double-strand break repair and stem cell proliferation.
- Citation: Chen X, Guo J, Xi RX, Chang YW, Pan FY, Zhang XZ. MiR-210 expression reverses radioresistance of stem-like cells of oesophageal squamous cell carcinoma. World J Clin Oncol 2014; 5(5): 1068-1077
- URL: https://www.wjgnet.com/2218-4333/full/v5/i5/1068.htm
- DOI: https://dx.doi.org/10.5306/wjco.v5.i5.1068
Oesophageal squamous cell carcinoma (ESCC) has occult symptoms and signs and is difficult to diagnose in the early stages. Radiation therapy is currently one of the main treatments for ESCC, particularly in the case of cervical and upper thoracic lesions. Even with concurrent chemoradiotherapy, the 5-year survival rate is still less than 30%, worse than those of many other squamous cell carcinomas. Local recurrences and the apparently increased radioresistance of recurrent tumours are the main reasons for treatment failure.
The mechanism of tumour resistance to radiotherapy is still unclear. There is a growing body of evidence that microRNAs (miRNAs) involved in the regulation of multiple cellular pathways are associated with radiation resistance. A number of miR-210 target genes have been identified that play roles in the cell cycle[1], DNA repair[2], vascular generation[3] and tumour stem cell survival[4]. MiR-210 was shown to be involved in the radiosensitivity of tumour cells[5,6]. Ataxia telangiectasia mutated (ATM) is a key signalling gene in the early reaction to irradiation, which causes the double-strand break (DSB)-induced DNA damage response[7]. ATM is a Ser/Thr kinase that phosphorylates more than a hundred proteins to orchestrate cell cycle checkpoint activity[8-10].
However, there is no evidence that miR-210 affects the radiosensitivity in ESCC. Thus, the purpose of this study was to evaluate miR-210 expression in oesophageal cancer tissues, to explore the possibility that it participates in regulating cellular radioresistance, and to study its possible role in cell cycle regulation to explore the feasibility of miR-210 as a radiation-sensitive therapeutic target.
This study included 37 male patients with a median age of 54 (range, 42-71) years. All of the patients had been diagnosed with ESCC by biopsy. The para-tumorous normal oesophageal tissues, which comprised the oesophageal mucosa 5 cm from the cancer tissue collection site and close to the resection margin, were normal in appearance. The tissue specimens were collected less than 15 min after resection, fixed for 30 min in liquid nitrogen and stored at -80 °C. All of the selected patients received radiotherapy or concurrent chemoradiation in 2008-2009. All of the patients received radiotherapy no more than 3 mo after surgery and were followed until a local recurrence arose or for at least 35 mo. The median follow-up time was 23.4 (range, 8.7-35.3) mo. This study was approved by the Institutional Review Board of the First Affiliated Hospital of the Medical School of Xi’an Jiaotong University.
The human ESCC cell lines Eca-109 and TE-1 (a gift of the Department of Cardiothoracic Surgery, Second Military Medical University, Shanghai, China) were cultured using high-glucose Dulbecco’s modification of Eagle’s medium (DMEM) that was supplemented with 10% foetal bovine serum (10000 units of penicillin and 10000 μg of streptomycin per mL, all of which were purchased from Gibco Invitrogen, CA, United States). The stem-like radioresistant cell lines were created using fractionated radiation of up to 100 Gy, as previously described[11], and were named TE-1R and Eca-109R. Cell spheres were cultured using DMEM/F12 medium (Gibco Invitrogen) that was supplemented with 2 ng/mL of epidermal growth factor and basic fibroblast growth factor (b-FGF) (all obtained from Pepro Tech Inc., NJ, United States) on 50 g/L agarose-phosphate Buffered Saline (PBS) coated plates. All of the cells were cultivated in a humidified atmosphere containing 50 g/L of CO2 at 37 °C.
Pre-miR-210 (50 pmol; Genetimes Tech Inc., Shanghai, China) and a scrambled control (50 pmol; Genetimes Tech Inc.) were transfected into TE-1R cells growing in six-well dishes (plated at 2 × 105 cells per well 24 h before transfection), which were called PmiR-210 cells and Ctrl cells, respectively. Transfection was conducted using Lipofectamine 2000 (Invitrogen). The transfection efficiency (> 200%) at 24, 36, 48 and 72 h after transfection was determined using quantitative reverse transcription-polymerase chain reaction (qRT-PCR).
The total RNA was extracted from cell lysates according to the manufacturer’s protocol. The concentration of the RNA was determined using Ultraviolet Rays spectrophotometry (NanoDrop® ND-8000 Spectrophotometer, Thermo Fisher Scientific, CA, United States). The quality of RNA was determined by electrophoresis on a 10 g/L agarose gel. All of the RNA samples were confirmed to be non-degraded by visualisation of the distinct 28S and 18S rRNA species. The total RNA was used to synthesise first-strand cDNA. The expression of mature miRNA-210 was profiled using a real-time quantitative PCR assay (all of the kits were obtained from Fermentas, Thermo Scientific, CA, United States) as previously described[11].
Cells (3 × 103 cells per well in a 12-well plate) were irradiated at room temperature with 10 mV photons from a linear accelerator (Electa, WI, United States) at doses of 2, 4, 6 or 8 Gy (a uni-dose of 6 Gy for the cells that were used in the cell-sphere formation and cell-cycle analyses). The controls were handled identically as were the irradiated cells with the exception of the radiation treatment. The cells were allowed to grow for 7 d before analysis of the clones or spheres that formed.
Cells were seeded in 100-mL culture flasks at a density of 5 × 105 cells per flask. After 24 h, the cells were treated with uni-dose irradiation at 6 Gy. The attached and floating cells were harvested at different time points. The cells were then suspended at 1 × 106/mL of propidium iodide solution (3.8 mmol/L of sodium citrate; 0.05 g/L of propidium iodide and 1 g/L of Triton X-100) supplemented with RNaseB and were maintained in the dark at 4 °C. The cells were then analysed using flow cytometry.
The cells were twice washed with phosphate-buffered solution and then directly lysed for 30 min on ice using radio-immunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology). After centrifugation at 12000 ×g for 26 min, the protein concentrations were determined using a bicinchoninic acid protein assay kit (Beyotime Institute of Biotechnology, Jiangsu, China). The proteins were transferred onto nitrocellulose membranes. After blocking with 5% milk in Tris-buffered saline containing 0.5 g/L Tween-20, the membranes were incubated with anti-ATM (rabbit monoclonal antibody, 1:500, Epitomics, CA, United States), anti-DNA-PKcs (rabbit monoclonal antibody, 1:500, Cell Signaling Technology, MA, United States) and anti-glyceraldehyde-3-phosphate dehydrogenase (rabbit monoclonal antibody, 1:1000, Cell Signaling Technology). The immune complexes were detected using horseradish peroxidase-conjugated immunoglobulin G (goat anti-rabbit antibody, Cell Signaling Technology). The labelled antibodies were visualised using an enhanced chemiluminescent substrate (34079, Thermo Fisher Scientific). All of the membranes were exposed to Kodak X-OMAT X-ray film. The details of the method were previously described[11].
The data are expressed as mean and standard deviation or standard error of the mean of the results of two or three independent experiments. Normalisation of the miRNA expression levels was obtained using the comparative DCt method (2-delta-delta computed tomography using the miR-210 expression levels in normal tissues and that of RNAU6B as references). The difference between the times of local recurrence of the groups was tested for significance using the log rank test. The clones were counted using Photoshop cs3 as previously described[12]. After filtration through 100-μm pores and suspension in 100 μL of cold PBS, the cell spheres in three randomised images taken at 100 × were compared using Photoshop cs3. Student’s t-test was used to analyse the significance of the differences between the different treatment groups whenever applicable with the aP-value set at < 0.05 and the bP-value set at < 0.01. The statistical analyses were performed using SPSS version 13 software.
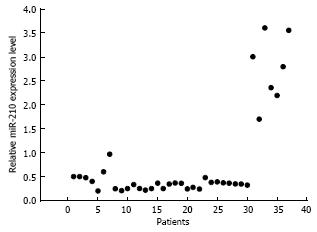
In most of the ESCC patients, the level of miR-210 expression in the ESCC tissue was significantly lower than that in the para-tumorous normal oesophageal tissue from the same patient (81.1%, 30 of 37 patients vs patients with high miR-210 expression, P < 0.05). The expression levels ranged from 21.3% to 97.2%, and the average level was 39.2% ± 16.1% in the patients with low miR-210 expression. Seven patients had a higher level of miR-210 expression in their ESCC tissues than in their normal tissues. The level of high expression ranged from 170.4%-360.8%, and the average level was 193.5% ± 36.2% (Figure 1). A poor pathological type was significantly related to a low level of miR-210 expression in the ESCC tissue (P < 0.01, Table 1). However, there was no significant correlation between the level of miR-210 expression and the T stage (P > 0.05) or lymph-node infiltration (P > 0.05). However, early local recurrences (< 18 mo, n = 19) were significantly correlated with low miR-210 expression (n = 13, P < 0.05). Four patients did not experience local recurrence over the 35-mo observation period.
The level of miR-210 expression was decreased by approximately 73% (vs TE-1, 0.27 ± 0.10, P < 0.01) in radioresistant TE-IR cells and by approximately 52% (vs Eca-109, 0.48 ± 0.17, P < 0.05) in radioresistant Eca-109R cells. Transient transfection of an miR-210 precursor into TE-1R cells led to a high level of miR-210 expression at 72 h (vs TE-1, > 120 times higher, P < 0.001), compared with that of Ctrl cells, which remained high even at 5 d (approximately 19.4 times higher, P < 0.05). PmiR-210 and Ctrl cells were irradiated with a series of Gy doses and a significant difference in cell clone formation at 7 d of culture was observed in the cells that had been irradiated with high dosages (Table 2, Figure 2A). A significant left-downward trend in the pmiR-210 cell-survival curve compared with that of the Ctrl cells was observed (Figure 2B). The radiobiological indices (Dq, N, SF2) of the pmiR-210 cells that were obtained by regression of their survival curve data were lower than those of the Ctrl cells (Table 2, P < 0.05 for all; Table 3), indicating their higher sensitivity to irradiation.
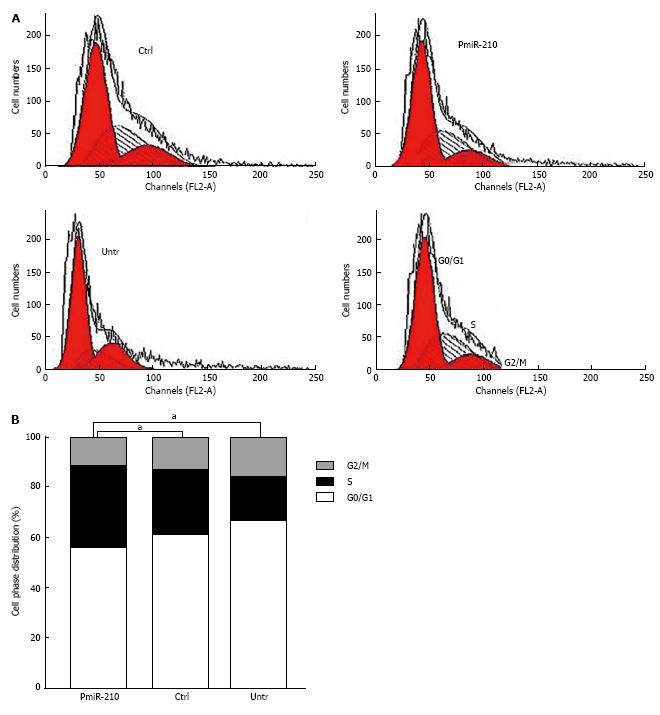
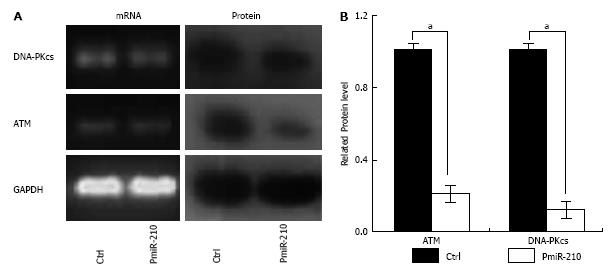
PmiR-210 and Ctrl cells were uni-irradiated with 6 Gy and then the cell cycle distribution was analysed using flow cytometry (Figure 3). There were no significant differences between the cell cycle distributions of the Ctrl and untreated cells in terms of the proportion of cells in G0/G1 phase (64.2% ± 0.5% vs 61.8% ± 0.7%, P > 0.05), in S-phase (23.3% ± 0.8% vs 27.6% ± 0.5%, P > 0.05) or in G2/M phase (13.4% ± 0.4% vs 12.1% ± 0.5%, P > 0.05). However, compared with that of the Ctrl cells, a significantly larger proportion of the pmiR-210-transfected cells were in S phase (30.4% ± 0.4% vs 23.3% ± 0.7%, P < 0.05), although no significant differences in the proportion of cells in the G0/G1 (69.2% ± 0.7% vs 62.5% ± 0.6%, P > 0.05) or G2/M (12.4% ± 0.5% vs 20.2% ± 0.3%, P > 0.05) phases were observed. The levels of DNA-pkcs and ATM mRNAs and proteins were also analysed using RT-PCR and Western blot, respectively, (Figure 4) and no differences in the relevant mRNA levels in the two cell types were found (P > 0.05 for all), but the levels of the proteins were significantly lower in the pmiR-210 cells than in the Ctrl cells, with the DNA-pkcs level at 0.21 ± 0.07 and the ATM expression level at 0.12 ± 0.03 (P < 0.05 for both) in the former cells.
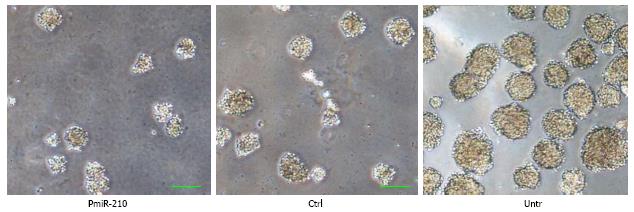
The spheres that had the various cell types had developed in serum-free medium at 7 d after irradiation were compared (Figure 5). The diameters of the cell spheres that pmiR-210 transfected TE-1R cells formed were smaller than those formed by Ctrl cells (0.60 ± 0.14, P < 0.05) or by untreated TE-1R cells (0.25 ± 0.08, P < 0.01).
MiR-210 is highly expressed in glioma[13], melanoma[14,15], renal cell carcinoma[16], pancreatic cancer[17,18], breast cancer[19-21] and lung cancer[22] that are generally associated with a poor disease-free survival rate or poor overall survival rate. The up-regulation of miR-210 expression directly suppressed Bcl-2 adenovirus E1B 19 kDa-interacting protein 3 (BNIP3) expression to maintain the survival of neural progenitor cells[4] and knocking down the expression of miR-210 in combination with radiotherapy was found to have enhanced its anti-tumour effect in human hepatoma xenografts[5]; and moreover, miR-210 expression promoted more efficient DSB repair[7]. Unexpectedly, the significantly lower expression of miR-210 in ESCC tissues was observed in paired tumour/normal tissue sets in our study. In this study, we observed a low level of miR-210 expression in 81.1% of the tumours of male ESCC patients than in the isogenically paired tissues (P < 0.01), which was correlated with the early local recurrence after surgery followed by radiotherapy (P < 0.05). Our results were consistent with those of some of the other ESCC studies[23,24] in which compared to that of normal oesophageal tissue, a low level of miR-210 expression in ESCC was correlated with either poorly differentiated carcinoma or a poor prognosis. Although the sample size (n = 37) is not large, all of our selected patients received radiotherapy after surgery, which most closely resembles the clinical reality. Obviously, the non-randomised patient selection will lead to some bias in our observations and might have caused false-positive outcomes in this study. Therefore, we used two radioresistant cell lines, TE-1R and Eca-109R, to verify the relationship between radioresistance and the level of miR-210 expression.
We found that miR-210 expression was decreased in both of the radioresistant cell lines, by 73% (0.27 ± 0.10, P < 0.01) in TE-IR cells and by 52% (0.48 ± 0.17, P < 0.05) in Eca-109R cells. These results confirmed our result that the down-regulation of miR-210 expression was correlated with bad local tumour control after surgery followed by radiotherapy. We then up-regulated the expression of miR-210 by transiently transfecting the miRNA-210 precursor in the more radioresistant TE-1R cells in which miRNA-210 expression was more down-regulated to analyse the relationship between miR-210 expression and radiosensitivity. The results showed that up-regulated miR-210 expression could reverse the radioresistance of TE-1R cells compared with the effect of transfection with the scrambled sequence (Ctrl cells, Figure 2, Tables 2 and 3). MiR-210 has been previously shown to repress or stimulate cell proliferation, depending on the cellular model. MiR-210 targets proteins that are crucial for cell cycle progression, such as E2F3, FGFRL139, or HOXA1, to inhibit cellular growth. In contrast, miR-210 also targets the Myc-antagonist MNT41 to promote cell cycle progression in some types of cancer cells. Lung adenocarcinoma (A549 or H1975) cells that stably expressed miR-210 did not show any alteration in their proliferation rate but a high level of miR-210 expression after irradiation significantly reduced the apoptosis rate of A549 cells and improved their DSB repair rate[6]. The lack of radiosensitisation in prostate cancer cells treated with an miR-210 inhibitor under anoxic conditions suggested that the extent of inhibition of miR-210 expression may depend on the type of cancer and/or the degree and duration of hypoxia[6]. Our results regarding ESCC were consistent with those of another study, in which miR-210 was found to inhibit cancer cell survival and proliferation by inducing cell death[24].
Consistent with the observed miR-210-mediated sensitivity to radiation, analysis of the cell cycle distribution of the cell populations revealed an increase in S-phase arrest in pmiR-210 cells compared with Ctrl TE-1R and untreated TE-1R cells after irradiation (Figure 3). In contrast, in another study, no significant changes were observed in the cell viability rate or the cell cycle profile when the expression of miR-210 was suppressed[3]. Transfection with an miR-210 inhibitor was shown to decrease the rates of cell viability and accumulation of CAKI-2 cells in the G2 phase of the cell cycle[25]. However, it was previously reported that miR-210 could induce cell-type specific proliferation. In certain transformed cells, inhibiting the expression of the c-Myc antagonist and miR-210 revealed a direct target, MAX-binding protein (MNT), which was demonstrated to be functionally important in controlling the progression of the cell cycle through the reciprocal up-regulation of c-Myc activity. Increasing the level of miR-210 expression in various tumours could, through targeting E2F3 and activin receptor 1b (ACVR1b), activate G1/S-phase cell cycle progression and increase the rate of cellular proliferation[26]. In other types of tumours, depending on the contextual cues, miR-210 could target a different set of mRNAs, such as HOXA3 and HOXA9, and contribute to the reduction of the rate of cell proliferation[27]. The S-phase arresting effect observed under our conditions, which contradicted the results of another study of cell cycle arrest in the G1/G0 and G2/M phases[24], might be explained by the fact that the function of miR-210 has to date been studied within 3 d following transient transfection using a relatively high concentration of an miRNA precursor. The true effect of increased miR-120 expression on the cell cycle may be observed during shorter periods, as was found in arsenic trioxide-treated tumour cells that were arrested at the G2/M phase of the cell cycle at 30 h post-treatment and which overrode the G2/M boundary at 48 h[28].
To understand the potential of increased miR-210 content on the DSB repair function of TE-1R cells, changes in the ATM and DNA-PKcs levels were evaluated in pmiR-210 and Ctrl cells after irradiation. ATM and DNA-PKcs play different roles in the DNA damage response pathway (DDR), but both of them are activated by the occurrence of DSB; they have common targets in the DDR pathway and the absence of either kinase results in faulty DSB repair. The absence of ATM allows timely repair, which nevertheless, is incomplete. In contrast, the absence of DNA-PKcs leads to slower repair, which in turn gives rise to the accumulation of simple and complex chromosomal reorganisations[7]. Consistent with the miR-210-mediated sensitivity to radiation, the levels of ATM and DNA-PKcs proteins were surprisingly significantly lower in PmiR-210 cells that were subjected to 6 Gy compared with those of Ctrl cells (DNA-pkcs, 0.21 ± 0.07 and ATM, 0.12 ± 0.03, P < 0.05 for all), but no differences were found in the corresponding mRNA levels of the two cell types (P > 0.05 for all) (Figure 4) under our conditions. In contrast, it has been shown that miR-210 expression promoted more efficient DSB repair in A549 cells[6]. MiR-210 has been shown to repress mitochondrial metabolism by targeting a number of proteins that are crucial for normal tricarboxylic acid cycle and electron transport chain activity[29]. To date, there is no evidence that miR-210 targets the 3’-UTR of ATM or DNA-PKcs mRNAs. MiR-101 can bind to the 3’-UTR of DNA-PKcs and ATM mRNAs[3]. However, this miRNA targeted not only the 3’-UTR but also the 5’-UTR and coding sequences, which were still present in the expression construct in which only the 3’-UTR of ATM had been substituted, allowing ATM expression to be regulated by miR-181a, miR-326, and miR-345[30]. Thus, there is a potential for miR-210 to target the 5’-UTR or coding sequences of their mRNAs to regulate the expression of the ATM or DNA-PKcs proteins.
A radioresistant cell is considered a type of cancer stem-like cell. Thus, we examined the effect of miR-210 expression on the sphere-formation ability of these stem cells, which is a widely accepted method to determine the proliferative ability of stem cells. The up-regulation of miR-210 content directly suppressed the expression of BNIP3 to maintain the survival of neural progenitor cells under hypoxic conditions[4]. In contrast, it was observed that miR-210 expression inhibited the formation of cell spheres by ESCC radioresistant TE-1R cells (Figure 5), which is first reported here. This result suggested that miR-210 expression reversed the radioresistance of ESCC cancer stem cells.
Taken together, the results of this study demonstrated that a low level of miR-210 expression was common in the tumours of ESCC patients and that the level of miR-210 expression was negatively correlated with a poorly differentiated pathological type and rate of local control after radiotherapy. Increased miR-210 expression reversed the radioresistance of stem cell-like cells of ESCC by decreasing the ATM and DNA-PKcs-dependent cell cycle arrest and the rates of DSB repair and stem cell proliferation. The mechanisms underlying these processes must be determined in future investigations.
We are grateful to all of the technicians for their assistance with the radiation-treatment experiments, to Prof. Chen for the use of the Translational Medicine Centre laboratory and to Prof. Wang for help with the statistical analyses.
The mechanism underlying tumour resistance to radiotherapy is unclear. There is a growing body of evidence showing that microRNAs are involved in the regulation of multiple cellular pathways that are associated with radiation resistance. A number of miR-210 target genes that have been identified are involved in the cell cycle, DNA repair, vascular generation and tumour stem cell survival, most of which are important processes in the development of radioresistance.
MiR-210 appears to be involved in radiosensitivity of tumour cells. However, there is no evidence that miR-210 affects the radiosensitivity of oesophageal squamous cell carcinoma (ESCC) cells. In this study, the authors demonstrated that miR-210 may affect the prognosis of radiotherapy and participate in the regulation of radioresistance in ESCC cells.
Recent reports have highlighted the importance of miR-210 expression in hepatomas and lung cancer. This is the first study to report that miR-210 is expressed at a low level in ESCC and that the level of its expression is correlated with the prognosis of ESCC patients treated with radiotherapy. Furthermore, the results of the authors in vitro studies suggested that this miRNA may be an important regulator of radioresistance in ESCC.
By demonstrating that miR-210 regulates the radioresistance of ESCC cells, this study presents a novel target for reversing the radioresistance in relapsed ESCC.
The radioresistance that develops during radiotherapy leads to an increased number of cancer stem cells, which appear to be concentrated by therapeutic selection or radiation induction. Radioresistant cells are also called cancer stem-like cells. These cells have been found to have a distinctive cell cycle distribution pattern and a high efficient mechanism of DNA double-strand break repair.
It is meaningful in that miR-210 is explored in ESCC cells with radiation resistance.
| 1. | Kim HW, Haider HK, Jiang S, Ashraf M. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J Biol Chem. 2009;284:33161-33168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 273] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 2. | Yang W, Sun T, Cao J, Liu F, Tian Y, Zhu W. Downregulation of miR-210 expression inhibits proliferation, induces apoptosis and enhances radiosensitivity in hypoxic human hepatoma cells in vitro. Exp Cell Res. 2012;318:944-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Redova M, Poprach A, Besse A, Iliev R, Nekvindova J, Lakomy R, Radova L, Svoboda M, Dolezel J, Vyzula R. MiR-210 expression in tumor tissue and in vitro effects of its silencing in renal cell carcinoma. Tumour Biol. 2013;34:481-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Wang F, Xiong L, Huang X, Zhao T, Wu LY, Liu ZH, Ding X, Liu S, Wu Y, Zhao Y. miR-210 suppresses BNIP3 to protect against the apoptosis of neural progenitor cells. Stem Cell Res. 2013;11:657-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Yang W, Wei J, Sun T, Liu F. Effects of knockdown of miR-210 in combination with ionizing radiation on human hepatoma xenograft in nude mice. Radiat Oncol. 2013;8:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Grosso S, Doyen J, Parks SK, Bertero T, Paye A, Cardinaud B, Gounon P, Lacas-Gervais S, Noël A, Pouysségur J. MiR-210 promotes a hypoxic phenotype and increases radioresistance in human lung cancer cell lines. Cell Death Dis. 2013;4:e544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 190] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 7. | Bueno RC, Canevari RA, Villacis RA, Domingues MA, Caldeira JR, Rocha RM, Drigo SA, Rogatto SR. ATM down-regulation is associated with poor prognosis in sporadic breast carcinomas. Ann Onco. 2014;25: 69-75. [PubMed] |
| 8. | Zhang Z, Wang CZ, Du GJ, Qi LW, Calway T, He TC, Du W, Yuan CS. Genistein induces G2/M cell cycle arrest and apoptosis via ATM/p53-dependent pathway in human colon cancer cells. Int J Oncol. 2013;43:289-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 9. | Zhou Y, Wan G, Spizzo R, Ivan C, Mathur R, Hu X, Ye X, Lu J, Fan F, Xia L. miR-203 induces oxaliplatin resistance in colorectal cancer cells by negatively regulating ATM kinase. Mol Oncol. 2014;8:83-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Winnicki K. ATM/ATR-dependent Tyr15 phosphorylation of cyclin-dependent kinases in response to hydroxyurea in Vicia faba root meristem cells. Protoplasma. 2013;250:1139-1145. [PubMed] [DOI] [Full Text] |
| 11. | Lahm A, Uhl M, Lehr HA, Ihling C, Kreuz PC, Haberstroh J. Photoshop-based image analysis of canine articular cartilage after subchondral damage. Arch Orthop Trauma Surg. 2004;124:431-436. [PubMed] |
| 12. | Qiu S, Lin S, Hu D, Feng Y, Tan Y, Peng Y. Interactions of miR-323/miR-326/miR-329 and miR-130a/miR-155/miR-210 as prognostic indicators for clinical outcome of glioblastoma patients. J Transl Med. 2013;11:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Satzger I, Mattern A, Kuettler U, Weinspach D, Voelker B, Kapp A, Gutzmer R. MicroRNA-15b represents an independent prognostic parameter and is correlated with tumor cell proliferation and apoptosis in malignant melanoma. Int J Cancer. 2010;126:2553-2562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Zhang Z, Sun H, Dai H, Walsh RM, Imakura M, Schelter J, Burchard J, Dai X, Chang AN, Diaz RL. MicroRNA miR-210 modulates cellular response to hypoxia through the MYC antagonist MNT. Cell Cycle. 2009;8:2756-2768. [PubMed] |
| 15. | McCormick RI, Blick C, Ragoussis J, Schoedel J, Mole DR, Young AC, Selby PJ, Banks RE, Harris AL. miR-210 is a target of hypoxia-inducible factors 1 and 2 in renal cancer, regulates ISCU and correlates with good prognosis. Br J Cancer. 2013;108:1133-1142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 16. | Takikawa T, Masamune A, Hamada S, Nakano E, Yoshida N, Shimosegawa T. miR-210 regulates the interaction between pancreatic cancer cells and stellate cells. Biochem Biophys Res Commun. 2013;437:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Papaconstantinou IG, Manta A, Gazouli M, Lyberopoulou A, Lykoudis PM, Polymeneas G, Voros D. Expression of microRNAs in patients with pancreatic cancer and its prognostic significance. Pancreas. 2013;42:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 18. | Liu Y, Han Y, Zhang H, Nie L, Jiang Z, Fa P, Gui Y, Cai Z. Synthetic miRNA-mowers targeting miR-183-96-182 cluster or miR-210 inhibit growth and migration and induce apoptosis in bladder cancer cells. PLoS One. 2012;7:e52280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Toyama T, Kondo N, Endo Y, Sugiura H, Yoshimoto N, Iwasa M, Takahashi S, Fujii Y, Yamashita H. High expression of microRNA-210 is an independent factor indicating a poor prognosis in Japanese triple-negative breast cancer patients. Jpn J Clin Oncol. 2012;42:256-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Hong L, Yang J, Han Y, Lu Q, Cao J, Syed L. High expression of miR-210 predicts poor survival in patients with breast cancer: a meta-analysis. Gene. 2012;507:135-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Võsa U, Vooder T, Kolde R, Vilo J, Metspalu A, Annilo T. Meta-analysis of microRNA expression in lung cancer. Int J Cancer. 2013;132:2884-2893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 22. | Mathé EA, Nguyen GH, Bowman ED, Zhao Y, Budhu A, Schetter AJ, Braun R, Reimers M, Kumamoto K, Hughes D. MicroRNA expression in squamous cell carcinoma and adenocarcinoma of the esophagus: associations with survival. Clin Cancer Res. 2009;15:6192-6200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 309] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 23. | Tsuchiya S, Fujiwara T, Sato F, Shimada Y, Tanaka E, Sakai Y, Shimizu K, Tsujimoto G. MicroRNA-210 regulates cancer cell proliferation through targeting fibroblast growth factor receptor-like 1 (FGFRL1). J Biol Chem. 2011;286:420-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 24. | Nakada C, Tsukamoto Y, Matsuura K, Nguyen TL, Hijiya N, Uchida T, Sato F, Mimata H, Seto M, Moriyama M. Overexpression of miR-210, a downstream target of HIF1α, causes centrosome amplification in renal carcinoma cells. J Pathol. 2011;224:280-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Giannakakis A, Sandaltzopoulos R, Greshock J, Liang S, Huang J, Hasegawa K, Li C, O’Brien-Jenkins A, Katsaros D, Weber BL. miR-210 links hypoxia with cell cycle regulation and is deleted in human epithelial ovarian cancer. Cancer Biol Ther. 2008;7:255-264. [PubMed] |
| 26. | Chan SY, Loscalzo J. MicroRNA-210: a unique and pleiotropic hypoxamir. Cell Cycle. 2010;9:1072-1083. [PubMed] |
| 27. | Hu YC, Hsieh BS, Cheng HL, Huang LW, Huang TC, Huang IY, Chang KL. Osteoblasts survive the arsenic trioxide treatment by activation of ATM-mediated pathway. Biochem Pharmacol. 2013;85:1018-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Martín M, Terradas M, Tusell L, Genescà A. ATM and DNA-PKcs make a complementary couple in DNA double strand break repair. Mutat Res. 2012;Epub ahead of print. [PubMed] |
| 29. | Yan D, Ng WL, Zhang X, Domingues MA, Caldeira JR, Rocha RM, Drigo SA, Rogatto SR. Targeting DNA-PKcs and ATM with miR-101 sensitizes tumors to radiation. PLoS One. 2010;5:e11397. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 30. | Lindenbergh-van der Plas M, Martens-de Kemp SR, de Maaker M, van Wieringen WN, Ylstra B, Agami R, Cerisoli F, Leemans CR, Braakhuis BJ, Brakenhoff RH. Identification of lethal microRNAs specific for head and neck cancer. Clin Cancer Res. 2013;19:5647-5657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
P- Reviewer: Kim SB S- Editor: Ji FF L- Editor: Wang TQ E- Editor: Liu SQ