Published online Dec 10, 2014. doi: 10.5306/wjco.v5.i5.1078
Revised: October 10, 2014
Accepted: October 28, 2014
Published online: December 10, 2014
Processing time: 120 Days and 19.8 Hours
AIM: To evaluate the M1 and M2 monocyte phenotype in patients with non-small cell lung cancer (NSCLC) compared to controls. Also, to examine the expression of Th1 and Th2 cytokines in plasma of NSCLC vs controls.
METHODS: Freshly prepared peripheral blood mononuclear cells samples were obtained from patients with NSCLC (lung adenocarcinoma and squamous cell lung carcinoma) and from non-cancer controls. Flow cytometry was performed to investigate M1 and M2 phenotypes in peripheral monocytes (classical monocytes CD14+, CD45+ and CD16-) using conventional surface markers. Th1 and Th2 cytokine production was also analysed in the plasma using cytometric bead array technique.
RESULTS: There were no significant difference in expression of M1 (HLA-DR) and/or M2 markers (CD163 and CD36) markers on classical monocytes in patients with NSCLC compared to non-cancer controls. Expression of CD11b, CD11c, CD71 and CD44 was also shown to be similar in patients with NSCLC compared to non-cancer controls. Th1 and Th2 cytokines [interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-8, IL-10, IL-12 (p70), tumor necrosis factor (TNF)-α, TNF-β, and interferon-γ] analysis revealed no significant difference between patients with NSCLC and non-cancer controls.
CONCLUSION: This study shows no alteration in peripheral monocyte phenotype in circulating classical monocytes in patients with NSCLC compared to non-cancer controls. No difference in Th1 and Th2 cytokine levels were noted in the plasma of these patients.
Core tip: Monocytes perform a critical role in immune system and have similar phenotype as seen in M1 (classically activated) and M2 (alternatively activated) tumour-associated macrophage. Nevertheless, monocyte phenotypes in human lung cancer patients are not fully understood and further investigation are really needed. Our study examines the M1 and M2 monocyte phenotypes in patients with non-small lung carcinoma [non-small cell lung cancer (NSCLC)] compared to non-cancer controls. This study indicated that freshly isolated peripheral blood monocytes from patients with NSCLC do not show an altered phenotype and/or cytokines secretion. These outcomes might enhance the knowledge regarding the connections between monocyte-macrophage phenotype and tumour progression.
- Citation: Almatroodi SA, McDonald CF, Collins AL, Darby IA, Pouniotis DS. Blood classical monocytes phenotype is not altered in primary non-small cell lung cancer. World J Clin Oncol 2014; 5(5): 1078-1087
- URL: https://www.wjgnet.com/2218-4333/full/v5/i5/1078.htm
- DOI: https://dx.doi.org/10.5306/wjco.v5.i5.1078
Lung cancer is now one of the most common cancers in the world and, as the leading cause of cancer mortality, is responsible for about 1.4 million deaths worldwide annually[1]. Despite incremental advances in treatment strategies, the prognosis for lung cancer remains poor, with only 10%-15% of patients surviving five years or longer[1,2]. Further understanding of the immunology of lung cancer may enable the development of immune-modulatory strategies beyond those in current use, such as targeted monoclonal antibodies to specific cellular receptors.
Monocytes are an important part of the innate immune response to cancer. The notion that the immune system has a protective role in tumour development is well established[3], with recent work also suggesting a converse role in promoting tumour initiation and progression[4]. Previous studies looking at monocytes in a range of different cancer types have demonstrated conflicting results regarding monocyte phenotype and function in different cancer microenvironments. Studies in patients with lung, breast and other cancers have described hindered monocyte function[5,6], whereas Mariotta et al[7] suggested that non-small lung carcinoma (NSCLC) does not affect monocyte adherence and phagocytosis in lung cancer patients compared to healthy controls. Other studies demonstrated that monocytes are capable of both inhibiting and stimulating tumour growth[8].
Monocytes can be characterised into classical monocyte (pro-inflammatory) and non-classical monocyte (anti-inflammatory) phenotype, both of which have been detected in circulating peripheral blood mononuclear cells (PBMC)[9,10]. Monocytes are known to differentiate into tissue macrophages. Classical monocytes (CD14++/CD16-) identified to differentiate into M1 macrophage, while non-classical monocytes (CD14+/CD16++) differentiate into M2 macrophage[11]. M1 works as an antigen-presenting cell and has a vital role in immune activation and function[12]. In contrast, M2 is known to be associated with poor antigen presentation producing factors that suppress T cell proliferation and activity[12]. Although, the main source of tissue macrophages is classical monocytes, the majority of the macrophages within the tumour area have been identified as M2 macrophage[11]. However, a study has reported that classical monocytes can differentiate into M2 macrophage[13].
There is a controversy regarding monocyte differentiation and effect in tumour microenvironment. Therefore in this current study, freshly isolated un-stimulated classical monocytes were used to ascertain the phenotypic changes in patients with NSCLC compared to non-cancer controls. To the best of our knowledge this is the first study that has analysed classical monocytes from freshly isolated PBMC to give a better understanding of the NSCLC, monocyte phenotype and function and Th1/Th2 plasma expression.
Blood was obtained from 30 patients undergoing diagnostic bronchoscopy for investigation of NSCLC recruited through the Department of Respiratory and Sleep Medicine, Austin Health, Heidelberg, VIC, Australia. Twenty non-cancer control samples were obtained from subjects undergoing diagnostic bronchoscopy for investigation of breathlessness, or chronic cough and haemoptysis, or benign lung lesions. Ethics committee approval was received from Austin Health Ethics Committee, and informed consent of all participating subjects was obtained. Patient demographic information is presented in Table 1. Staging was applied in this study using the new TNM (tumour, node, metastases) staging system (7th edition) for lung cancer[14].
| n | Age (yr) | Gender | Smoking status | Stages | Subtypes | |
| Mean ± SD | M/F | N/Ex/S | I/II/III/IV | N/A/S | ||
| Control | 20 | 60.45 ± 19.31 | 10/10 | 7/11/2 | ||
| Cancer | 30 | 67.1 ± 10.68 | 17/13 | 3/16/11 | 8/3/7/12 | 9/11/10 |
Venous blood was collected in heparinised tubes and PBMC isolated by Ficoll-Paque density gradient centrifugation (GE Healthcare Bio-sciences, Uppsala, Sweden). Complete blood count (CBC) was completed for all blood samples using Beckman Coulter (AcT 5 blood differentiation, RMIT Hematology Department, Melbourne) (Fullerton, CA, United States). PBMC washed twice with PBS (phosphate buffered saline) and then resuspended in RPMI completed media (2% Hepes buffer, 0.1 mmol/L 2-mercaptoethanol, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mmol/L glutamine and 10% fetal calf serum) (Sigma, St. Louis, MO). All samples were stored at -80 °C until use.
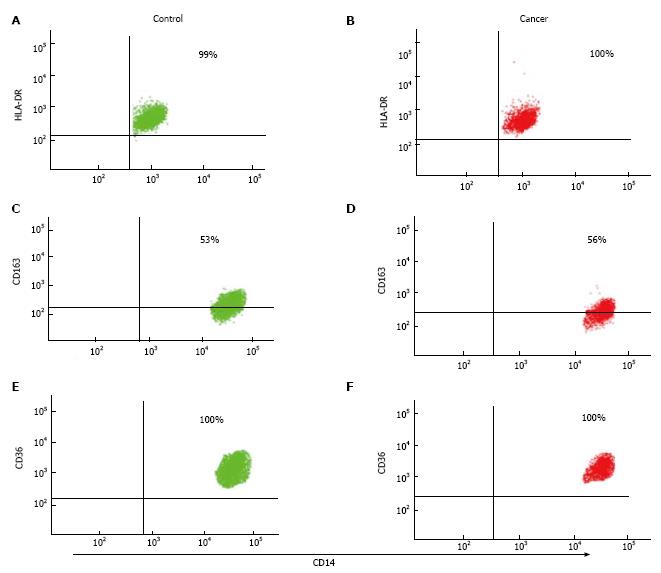
Flow cytometry was used to assess classical monocytes expression of CD14, CD45, CD71, CD11b, CD44, CD16 and CD11c (BD Pharmingen™, United States) as well as the M1 marker HLA-DR (BD Pharmingen™, United States) and the M2 markers CD163 and CD36 (BD Pharmingen™, United States). Cells were stained with antibodies directly conjugated to fluorescent probes for 30 min at 4 °C in 2% (w/v) BSA/PBS. Cells were washed and analyzed by flow cytometry (FACS Canto, BD Biosciences, San Jose, CA, United States). 106 cells were stained with various combinations of antibodies for 40 min in the dark on ice. Purity of classical monocytes was assessed according to CD14, CD45 and CD16 expression by flow cytometry. At least 5000 cells were collected and analysed (BD FACS Canto BD Biosciences, San Jose, CA, United States). All analysis was completed within the RMIT Flow Cytometry Facility, Bundoora, Melbourne. All quadrants were set up according to matched isotype control antibodies and all results are shown as surface expression (%SE) and mean fluorescence intensity (MFI).
The Human Th1/Th2 11plex Ready-to-Use FlowCytomix Multiplex (eBiosciences, United States) was applied to detect the level (pg/mL) of Th1/ Th2 cytokines including interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-8, IL-10, IL-12 (p70), tumor necrosis factor (TNF)-α, TNF-β, and interferon (IFN)-γ in plasma samples of patients with primary lung cancer and non-cancer controls, according to the manufacturer’s instructions (Human Th1/Th2 11plex RTU FlowCytomix Kit). Fluorescence was analysed using flow cytometry (FACS Canto, BD Biosciences, San Jose, CA, United States) and cytokine levels were determined using the BMS FlowCytomix Software manual within the RMIT Flow Cytometry Facility, Bundoora, Melbourne.
Experiments were performed in triplicate. Mean values ± standard error (SEM) were compared using the one way ANOVA (GraphPad Prism 6) with P-values ≤ 0.05 was considered to be significant.
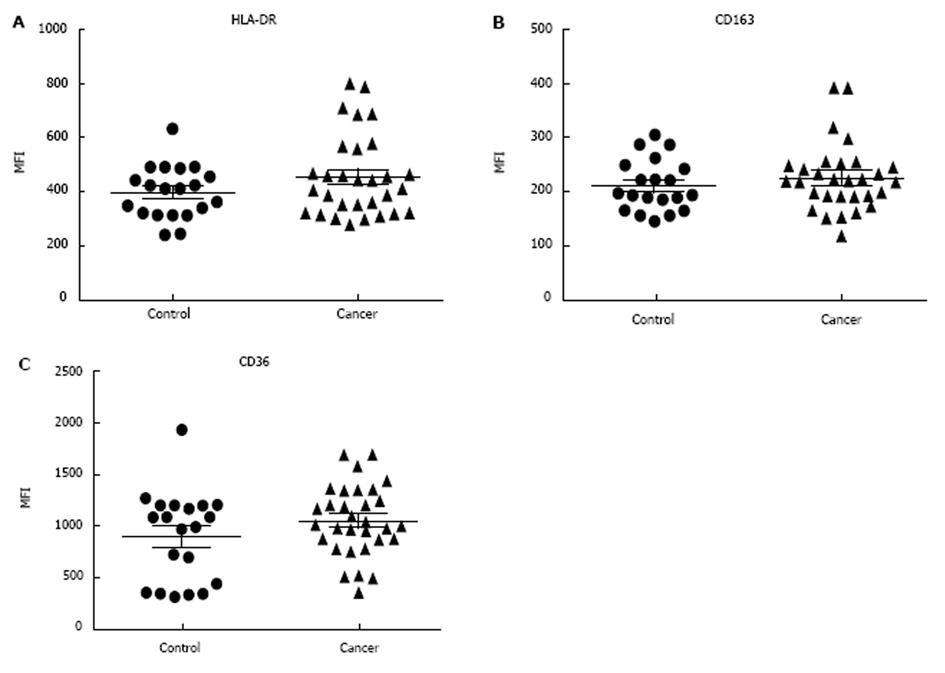
Underlying CBC was investigated in patient groups to verify that the patients has significant underlying medical condition e.g., infection, which could results in monocyte phenotype alteration. The total mean number of white blood cells (WBC) in NSCLC patients was significantly higher than non-cancer controls. The mean values of all WBC types (except basophile cells) and RBCs were higher in patients with NSCLC compared to non-cancer controls. However, the mean values of all WBC types and RBCs were within the normal range in both groups (Table 2). In flow cytometry results, there were no statistically significant differences in M1 marker (HLA-DR), M2 markers (CD163 and CD36) and (CD11c and CD44) in patients with NSCLC compared to non-cancer controls. Both %SE and MFI expression of surface markers showed similar values. CD11b and CD71 expression was also shown to be similar between patient groups.
| Groups | Total number | WBC | NE | LY | MO | EO | BA | RBC |
| Normal value | 4-11 × 10-9/L | 2-7.5 × 10-9/L | 1.5-4 × 10-9/L | 0.2-0.80 × 10-9/L | 0.04-0.40 × 10-9/L | 0.02-0.10 × 10-9/L | 3.80-6.50 × 10-9/L | |
| Control | 20 | 4.35 ± 1.82 | 2.11 ± 1.07 | 1.31 ± 0.47 | 0.23 ± 0.14 | 0.13 ± 0.06 | 0.38 ± 0.5 | 4.85 ± 0.91 |
| Cancer | 30 | 6.76 ± 3.9 | 3.4095 ± 3.46 | 1.68 ± 1.31 | 0.34 ± 0.22 | 0.2 ± 0.17 | 0.29 ± 0.2 | 5.04 ± 1.08 |
Classical monocytes were gated based on forward scatter (FSC) and side scatter (SSC) profiles within patient groups and based on the expression of CD14, CD45 and CD16 markers. Results show that there were no significant differences in %SE (P = 0.155) and MFI (P = 0.51) of HLA-DR (M1 marker) expression in patients with NSCLC compared to non-cancer controls. The expression of HLA-DR did not differ depending on tumour progression, as there were no significance differences between early and/or advanced lung cancer criteria (Figure 1). In addition, flow cytometry analysis showed there were no significant differences in the %SE (P = 0.505) and MFI (P = 0.39) of CD163 (M2 marker) in NSCLC patients compared to non-cancer controls. We also showed that there were no significant differences in the %SE (P = 0.160) and MFI (P = 0.17) of CD36 (M2 marker) staining between the two groups. Again, the expression of CD163 and CD36 showed no difference in patients with more advanced compared to early lung cancer (Figure 2).
The %SE and MFI of CD11b, CD71, CD11c and CD44 were similar between patient groups. The %SE and MFI of the myeloid marker CD11b marker was not different between cancer and non-cancer control subjects: %SE (P = 0.58), MFI (P = 0.61). Also CD71 results indicated that there were no significant differences in the %SE (P = 0.97) and MFI (P = 0.41) of the transferrin receptor marker in patients with NSCLC compared to non-cancer controls. CD11c results showed that there were no significant difference in the %SE (P = 0.93) and MFI (P = 0.20) of the CD11c in patients with NSCLC compared to non-cancer controls. In addition, CD44 results revealed that there were no significant differences in the %SE (P = 0.50) and MFI (P = 0.53) of the CD44 marker in NSCLC patients compared to non-cancer controls. The graphs (Figure 3) show MFI ± SEM of all the markers (CD11b, CD71, CD11c and CD44) in classical monocytes from non-cancer controls and cancer patients (%SE results not shown).
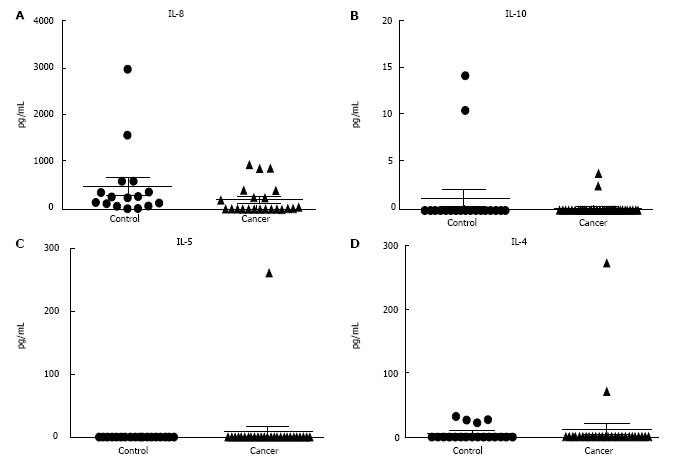
Cytokine analysis revealed no significant difference in Th1/Th2 cytokines plasma levels in patients with primary lung cancer compared to non-cancer controls (Figures 4 and 5). Monocytes are known to produce several cytokines, chemokines including IL-1β, IL-2, IL-4, IL-5, IL-8, IL-10, IL-12 (p70), TNF-α, TNF-β, and IFN-β. Cytokines were analysed in patients with NSCLC and non-cancer controls by CBA analysis. Cytokines that were detectable in patient plasma samples included Th1 cytokines TNF-α, TNF-β, IFN-β, IL-2, IL-12 (p70), IL-1β and Th2 cytokines IL-4 and IL-5 showed no significant differences between patients with NSCLC compared to non-cancer controls.
The concept that the immune system has a protective role in tumour development is well established[3]. Recent work has demonstrated that the immune system can both prevent tumour formation but potentially function to promote tumour initiation and progression[4]. In particular, immune cells such as blood monocytes can function differently depending on the cancer types[8]. While some studies indicated at monocyte function in cancer patients within normal range[7,15], other studies have shown impairment in monocyte function[6].
In this study we demonstrated that the phenotype of freshly blood classical monocytes from patients with NSCLC is not altered and does not show skewing from a pro-tumour (M1) to an anti-tumour (M2) phenotype. There were no significant differences in expression of M1 marker (HLA-DR), M2 markers (CD163 and CD36), CD11c and CD44 marker, myeloid marker CD11b and/or transferrin receptor CD71 in patients with NSCLC compared to non-cancer controls. In addition, there were no significant differences in the secretion of Th1/Th2 cytokines between NSCLC patients and non-cancer controls.
M1 phenotype was assessed here using HLA-DR. HLA-DR molecule plays a vital role in the immune response by regulating the interaction between antigen-presenting cells including monocytes[16,17]. It has been described as an M1 marker in the monocyte-macrophage system[18]. Some studies have reported reduced HLA-DR expression on blood monocytes in human cancers[5,19]. As well as examining M1 phenotype, M2 phenotype markers (CD163 and CD36) were used to investigate skewing from M1 to M2 markers on blood classical monocytes in patients with NSCLC. CD163 is a scavenger receptor that plays a major role in the anti-inflammatory response and has been identified as a M2 marker[20,21]. CD36 is also expressed on monocytes and is involved mainly in phagocytosis[21,22]. Sugai et al[23] studied the alteration of monocyte characteristics by examining the intracellular expression of IL-10 and IL-12 cytokines[23]. They found that patients with advanced gastric cancer had different monocyte phenotypic characteristics compared to those with early stage cancer and non-cancer control subjects[23]. In our study, there were no significant differences in expression of CD163 and CD36 between non-cancer controls and NSCLC patients. Also there was no apparent influence of tumour stage upon expression of these markers. These results when viewed in the context of previous studies raises questions regarding the impact of experimental design such as culturing and the use of molecules like lipopolysaccharide (LPS) on monocyte polarisation and function.
CD11b and CD11c are myeloid cell markers that are expressed on monocytes and macrophages[24]. CD11b has been shown to play a major role in many functions of myeloid cells including adhesion, migration, chemotaxis and phagocytosis[24-26]. In this study we therefore investigated the effect of NSCLC on the monocyte expression of CD11b and CD11c. There were no significant differences in expression of CD11b and CD11c in NSCLC patients compared to non-cancer controls. These results are consistent with Mariotta et al[7] study outcomes, which suggested NSCLC does not affect monocyte adherence and phagocytosis in lung cancer patients compared to healthy controls[7].
Another marker that was examined was transferrin receptor (CD71). CD71 is known to be associated with rapidly proliferation cells such as cancer cells and plays a major role in cell growth and DNA synthesis, proliferation and cell survival[27,28]. Increased CD71 expression has been demonstrated in cancer patients including lung cancer in lung tissue and BALF (bronchoalveolar lavage fluid) but not in blood serum[28,29]. Dowlati et al[28] investigated soluble CD71 in the serum of NSCLC patients. They verified no difference in the level of secreted CD71 in blood serum between NSCLC and control groups[28]. Similar to this outcome we demonstrated that there were no significant differences in surface expression of CD71 on classical monocytes in NSCLC patients compared to non-cancer controls.
CD44 expression was also investigated in this study as it has been suggested a potential marker of tumour initiation in lung cancer. Elevated CD44 expression has been observed in the serum of cancer patients such as gastric and renal cancer[30,31]. However, another study showed that NSCLC does not influence CD44 expression in the serum of NSCLC patients compared to benign lung disease[15]. Similarly, this study revealed no significant difference in surface expression of CD44 on classical monocytes in NSCLC patients compared to non-cancer controls.
The presence of cytokines is essential for immunity initiation. Th1 cells have been found to play a major role in anti-tumour immunity and stimulation of cell-mediated responses. Pro-inflammatory cytokines such as TNF-α and IFN-γ are known to stimulate Th1 cells. In contrast, Th2 cells are known to act as the helper cells that influence B-cell development and produce anti-inflammatory cytokines such as IL-4 and IL-10[32,33]. Analysis of Th1 and Th2 cytokines in the plasma revealed no differences in NSCLC patients in compared to non-cancer controls. Similarly, Gürsel et al[34] also observed no differences in TNF-α concentration between pleural effusion and serum in patients with cancer[34]. Although many studies have not looked at specific cytokine profiles in lung cancer, it has been shown that freshly prepared monocytes do not show any differences in pro-inflammatory and anti-inflammatory cytokine responses except IL-12 (p70) in endometrial cancer patients compared to controls[6].
Although the findings of this study are interesting, there are some limitations including an inability to compare results from subtypes within NSCLC grouping as all patients samples were lung adenocarcinoma and squamous cell lung carcinoma and no large cell lung carcinoma. Also the majority of samples were from patients with advanced stage disease, so we could not confidently address the question of whether the NSCLC influences monocyte function and polarisation changes with tumour progression. In future studies, examining monocyte polarisation and function in non-cancer controls vs lung cancer should be done on all monocytes subset by using freshly un-stimulated monocytes as well as cytokine treated monocytes at the same time to observe any variation that may occur. Also other lung cancer subtypes should be considered to inspect if they have any potential role in altering monocyte functions and phenotypes.
Our results demonstrate that freshly isolated peripheral blood monocytes from patients with NSCLC (lung adenocarcinoma and squamous cell lung carcinoma) do not show an altered phenotype and/or cytokines secretion. Therefore, these results suggest that peripheral classical monocytes are not altering into M2-like phenotype in bloodstream. More studies are needed to investigate the connections between monocyte-macrophage phenotype polarisation and tumour progression associated with lung cancer.
Lung cancer is a common cancer that has high incidence and death rates. Monocytes play an important role in the immune response against tumour cells including lung tumour cells. Diverse monocyte phenotypes were defined previously and they act differently to tumour cells.
The role of monocyte phenotype in human lung cancer patients is conflicting and still need further investigations. More studies are needed to investigate the connections between monocyte-macrophage phenotype polarisation and tumour progression associated with lung cancer.
Previous studies highlighted the importance of monocyte phenotype and function in patient with cancer including lung cancer. This is the first study that has broadly analysed classical monocytes to give a better understanding of the lung cancer, monocyte phenotype and some functions.
This study suggests that there is no monocyte-specific systemic impairment in patients with lung cancer (in particular, lung adenocarcinoma and squamous cell lung carcinoma subtypes).
Monocytes are immune cells that are known to play an important part of the innate immune response to cancer.
This is an interesting study. Good design and analysis. Good writing and focus.
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11889] [Article Influence: 792.6] [Reference Citation Analysis (6)] |
| 2. | Beadsmoore CJ, Screaton NJ. Classification, staging and prognosis of lung cancer. Eur J Radiol. 2003;45:8-17. [PubMed] |
| 3. | Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991-998. [PubMed] |
| 4. | Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3122] [Cited by in RCA: 4073] [Article Influence: 254.6] [Reference Citation Analysis (1)] |
| 5. | Vuk-Pavlović S, Bulur PA, Lin Y, Qin R, Szumlanski CL, Zhao X, Dietz AB. Immunosuppressive CD14+HLA-DRlow/- monocytes in prostate cancer. Prostate. 2010;70:443-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 6. | Brooks N, Stojanovska L, Grant P, Apostolopoulos V, McDonald CF, Pouniotis DS. Characterization of blood monocyte phenotype in patients with endometrial cancer. Int J Gynecol Cancer. 2012;22:1500-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Mariotta S, Aquilini M, Ricci A, Papale M, Pabani R, Sposato B, Mannino F. Changes in monocyte phagocyting activity after multi-agent chemotherapy in non-small cell lung cancer. Eur Rev Med Pharmacol Sci. 2002;6:67-73. [PubMed] |
| 8. | Mytar B, Baj-Krzyworzeka M, Majka M, Stankiewicz D, Zembala M. Human monocytes both enhance and inhibit the growth of human pancreatic cancer in SCID mice. Anticancer Res. 2008;28:187-192. [PubMed] |
| 9. | Satoh N, Shimatsu A, Himeno A, Sasaki Y, Yamakage H, Yamada K, Suganami T, Ogawa Y. Unbalanced M1/M2 phenotype of peripheral blood monocytes in obese diabetic patients: effect of pioglitazone. Diabetes Care. 2010;33:e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Garrett S, Dietzmann-Maurer K, Song L, Sullivan KE. Polarization of primary human monocytes by IFN-gamma induces chromatin changes and recruits RNA Pol II to the TNF-alpha promoter. J Immunol. 2008;180:5257-5266. [PubMed] |
| 11. | Lee HW, Choi HJ, Ha SJ, Lee KT, Kwon YG. Recruitment of monocytes/macrophages in different tumor microenvironments. Biochim Biophys Acta. 2013;1835:170-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Nagorsen D, Deola S, Smith K, Wang E, Monsurro V, Zanovello P, Marincola FM, Panelli MC. Polarized monocyte response to cytokine stimulation. Genome Biol. 2005;6:R15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Movahedi K, Laoui D, Gysemans C, Baeten M, Stangé G, Van den Bossche J, Mack M, Pipeleers D, In’t Veld P, De Baetselier P. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728-5739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 799] [Cited by in RCA: 970] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 14. | UyBico SJ, Wu CC, Suh RD, Le NH, Brown K, Krishnam MS. Lung cancer staging essentials: the new TNM staging system and potential imaging pitfalls. Radiographics. 2010;30:1163-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Takigawa N, Segawa Y, Mandai K, Takata I, Fujimoto N. Serum CD44 levels in patients with non-small cell lung cancer and their relationship with clinicopathological features. Lung Cancer. 1997;18:147-157. [PubMed] |
| 16. | Imamura Y, Yokoyama T, Takesue Y, Hiyama E, Sueda T. The TH1/TH2 ratio in patients with diminished expression of HLA-DR on monocytes. International Congress Series. 2003;1255:225-229. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Hiraki A, Kaneshige T, Kiura K, Ueoka H, Yamane H, Tanaka M, Harada M. Loss of HLA haplotype in lung cancer cell lines: implications for immunosurveillance of altered HLA class I/II phenotypes in lung cancer. Clin Cancer Res. 1999;5:933-936. [PubMed] |
| 18. | Lechner MG, Megiel C, Russell SM, Bingham B, Arger N, Woo T, Epstein AL. Functional characterization of human Cd33+ and Cd11b+ myeloid-derived suppressor cell subsets induced from peripheral blood mononuclear cells co-cultured with a diverse set of human tumor cell lines. J Transl Med. 2011;9:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 19. | Soares-Schanoski A, Jurado T, Córdoba R, Siliceo M, Fresno CD, Gómez-Piña V, Toledano V, Vallejo-Cremades MT, Alfonso-Iñiguez S, Carballo-Palos A. Impaired antigen presentation and potent phagocytic activity identifying tumor-tolerant human monocytes. Biochem Biophys Res Commun. 2012;423:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Lovren F, Pan Y, Quan A, Szmitko PE, Singh KK, Shukla PC, Gupta M, Chan L, Al-Omran M, Teoh H. Adiponectin primes human monocytes into alternative anti-inflammatory M2 macrophages. Am J Physiol Heart Circ Physiol. 2010;299:H656-H663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 21. | Moniuszko M, Kowal K, Rusak M, Pietruczuk M, Dabrowska M, Bodzenta-Lukaszyk A. Monocyte CD163 and CD36 expression in human whole blood and isolated mononuclear cell samples: influence of different anticoagulants. Clin Vaccine Immunol. 2006;13:704-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Huh HY, Pearce SF, Yesner LM, Schindler JL, Silverstein RL. Regulated expression of CD36 during monocyte-to-macrophage differentiation: potential role of CD36 in foam cell formation. Blood. 1996;87:2020-2028. [PubMed] |
| 23. | Sugai H, Kono K, Takahashi A, Ichihara F, Kawaida H, Fujii H, Matsumoto Y. Characteristic alteration of monocytes with increased intracellular IL-10 and IL-12 in patients with advanced-stage gastric cancer. J Surg Res. 2004;116:277-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Ahn GO, Tseng D, Liao CH, Dorie MJ, Czechowicz A, Brown JM. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc Natl Acad Sci USA. 2010;107:8363-8368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 305] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 25. | Cifarelli V, Libman IM, Deluca A, Becker D, Trucco M, Luppi P. Increased Expression of Monocyte CD11b (Mac-1) in Overweight Recent-Onset Type 1 Diabetic Children. Rev Diabet Stud. 2007;4:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Srivastava MK, Zhu L, Harris-White M, Kar UK, Huang M, Johnson MF, Lee JM, Elashoff D, Strieter R, Dubinett S. Myeloid suppressor cell depletion augments antitumor activity in lung cancer. PLoS One. 2012;7:e40677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 27. | Habashy HO, Powe DG, Staka CM, Rakha EA, Ball G, Green AR, Aleskandarany M, Paish EC, Douglas Macmillan R, Nicholson RI. Transferrin receptor (CD71) is a marker of poor prognosis in breast cancer and can predict response to tamoxifen. Breast Cancer Res Treat. 2010;119:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 170] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 28. | Dowlati A, Loo M, Bury T, Fillet G, Beguin Y. Soluble and cell-associated transferrin receptor in lung cancer. Br J Cancer. 1997;75:1802-1806. [PubMed] |
| 29. | Whitney JF, Clark JM, Griffin TW, Gautam S, Leslie KO. Transferrin receptor expression in nonsmall cell lung cancer. Histopathologic and clinical correlates. Cancer. 1995;76:20-25. [PubMed] |
| 30. | Guo YJ, Liu G, Wang X, Jin D, Wu M, Ma J, Sy MS. Potential use of soluble CD44 in serum as indicator of tumor burden and metastasis in patients with gastric or colon cancer. Cancer Res. 1994;54:422-426. [PubMed] |
| 31. | Kan M, Kanayama H, Naruo S, Tsuji M, Kojima K, Kurokawa Y, Kagawa S. Serological evaluation of soluble CD44 in renal cancer. Jpn J Cancer Res. 1996;87:1191-1194. [PubMed] [DOI] [Full Text] |
| 32. | Romagnani S. Biology of human TH1 and TH2 cells. J Clin Immunol. 1995;15:121-129. [PubMed] |
| 33. | Romagnani S. Th1 and Th2 in human diseases. Clin Immunol Immunopathol. 1996;80:225-235. [PubMed] [DOI] [Full Text] |
| 34. | Gürsel G, Gökçora N, Elbeg S, Samurkaşoğlu B, Ekim N. Tumor necrosis factor-alpha (TNF-alpha) in pleural fluids. Tuber Lung Dis. 1995;76:370-371. [PubMed] |
P- Reviewer: Cihan YB, Sugawara I S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ