Published online Feb 24, 2026. doi: 10.5306/wjco.v17.i2.113113
Revised: October 4, 2025
Accepted: December 23, 2025
Published online: February 24, 2026
Processing time: 175 Days and 13.8 Hours
Small bowel lymphatic malformations are rare benign tumors of the lymphatic system, accounting for < 1% of intra-abdominal lymphatic malformations. They pose diagnostic challenges due to nonspecific presentations and are often misdiagnosed. To analyze clinical features, management, and outcomes of small bowel lymphatic malformations in adults through a case report and scoping review. A 47-year-old female with chronic abdominal pain underwent laparoscopic resec
Core Tip: Small bowel lymphatic malformations are rare, benign lymphatic tumors that present diagnostic challenges in adults due to nonspecific symptoms and varied imaging appearances. This report combines a rare ileal lymphatic malformation case with a scoping review of 97 adult cases, highlighting that abdominal pain and gastrointestinal bleeding are the most common presentations. Computed tomography remains the mainstay for detection, but preoperative diagnosis is uncommon. Complete surgical excision, open or laparoscopic, is curative, with recurrence largely linked to incomplete removal. Heightened clinical suspicion, careful imaging interpretation, and radical resection are key to optimal outcomes.
- Citation: Khalayleh H, Bader R, Abu Arafeh M, Odeh Q, Rogalsky B, Imam R, Khalaileh A, Imam A. Small bowel lymphatic malformation: Clinical presentation and a comprehensive literature review. World J Clin Oncol 2026; 17(2): 113113
- URL: https://www.wjgnet.com/2218-4333/full/v17/i2/113113.htm
- DOI: https://dx.doi.org/10.5306/wjco.v17.i2.113113
Lymphatic malformations are rare congenital malformations of the lymphatic system, composed of thin-walled, multiloculated cysts that manifest as benign soft tissue tumors[1,2]. They are lesions of vascular origin with lymphatic differentiation, with approximately 95% occurring in the neck and axilla, while the remaining 5% are found in the chest and abdomen[3]. Histologically, lymphatic malformations are classified into macrocystic (cysts > 2 cm), microcystic (cysts < 2 cm), or mixed cystic types, with each subtype differing in clinical behavior and prognosis[2,3].
Abdominal lymphatic malformations are extremely rare, accounting for approximately 1 per 100000 hospital admis
The clinical presentation of lymphatic malformations varies widely. While some are incidentally discovered on imaging, others present with acute abdominal symptoms[7]. Most cases remain asymptomatic until the tumor is significant[7]. Mesenteric lymphatic malformations, in particular, can lead to complications such as intestinal obstruction or volvulus, which may result in infarction[4-6]. The insidious nature of intra-abdominal lymphatic malformations, combined with the spacious abdominal cavity, often leads to a delayed diagnosis[8]. Additionally, a female predilection has been reported, with a female-to-male ratio of 1:1[9,10]. It is also important to differentiate lymphatic malformations from complex lymphatic anomalies such as generalized lymphatic anomaly, kaposiform lymphangiomatosis, and central conducting lymphatic anomaly, as well as from intestinal lymphangiectasia, since these entities share overlapping features but differ in prognosis and management[11].
Surgical resection remains the treatment of choice for intra-abdominal lymphatic malformations. While the prognosis is generally favorable, increasing tumor size can make radical resection more difficult and increase the risk of local recurrence. Although successful complete removal has been reported, laparoscopy offers a promising alternative approach for the management of these benign tumors[8-11]. To our knowledge, there are limited scoping reviews that have been conducted specifically on mesenteric and small bowel lymphatic malformations. In this study, we report a case of a female patient with small bowel lymphatic malformation and perform a systematic review and analysis of the English literature.
A 47-year-old female patient with a past medical history significant for multiple unprovoked deep veins thromboses, four cesarean sections, inguinal hernia repair, right superficial parotidectomy, and two prior episodes of partial small bowel obstruction managed conservatively presented to our general surgical clinic in May 2021. She reported chronic abdominal pain persisting for five years without associated symptoms such as nausea, vomiting, or rectal bleeding. On clinical examination, the patient appeared hemodynamically stable, with normal vital signs. The abdominal examination was unremarkable, revealing a soft, non-tender, and non-distended abdomen. Laboratory investigations were within normal limits.
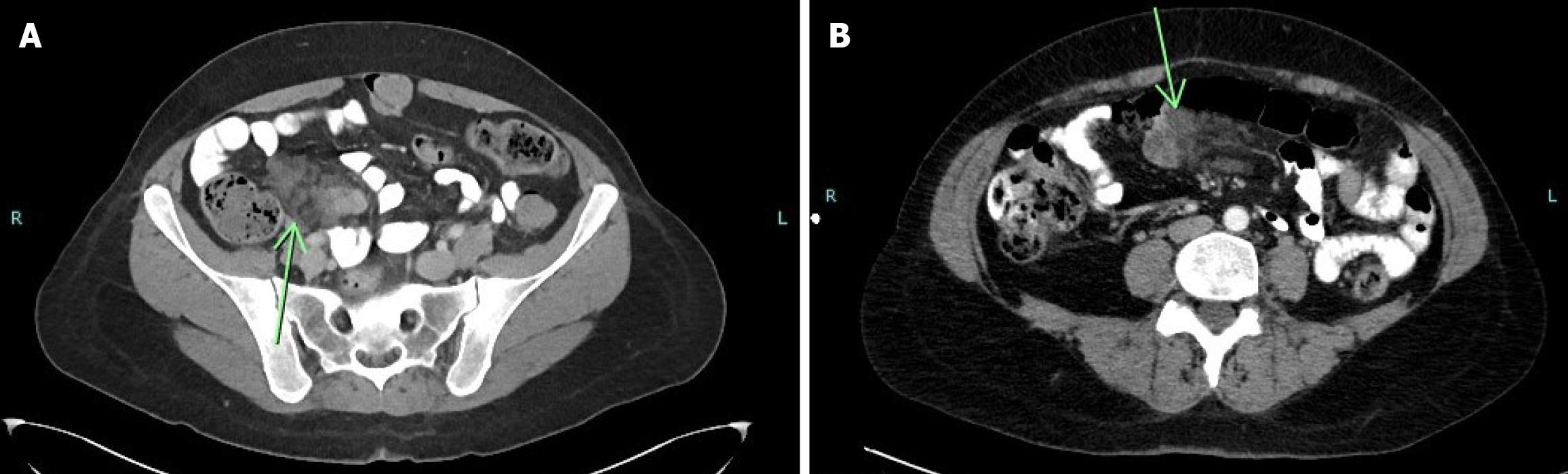
The initial computed tomography (CT) scan of the abdomen and pelvis, performed in August 2018, demonstrated segmental thickening of the distal small bowel wall, with surrounding fat stranding and a few enlarged lymph nodes. A repeat CT scan in November 2020 revealed a filling defect in the distal small intestine, along with persistent mesenteric fat stranding and multiple lymph nodes, raising a differential diagnosis of inflammatory enteritis vs a neoplastic process. Subsequent magnetic resonance imaging (MRI) of the abdomen identified cystic lesions in the small intestine within the left lower quadrant, raising suspicion for endometriosis. A capsule endoscopy was performed, revealing erythema of the ileal mucosa without additional specific findings. Upper and lower endoscopic evaluations, including gastroscopy and colonoscopy, were unremarkable. Given the persistence of symptoms and inconclusive imaging findings, a diagnostic laparoscopy was planned, with further surgical intervention contingent upon intraoperative findings (Figure 1).
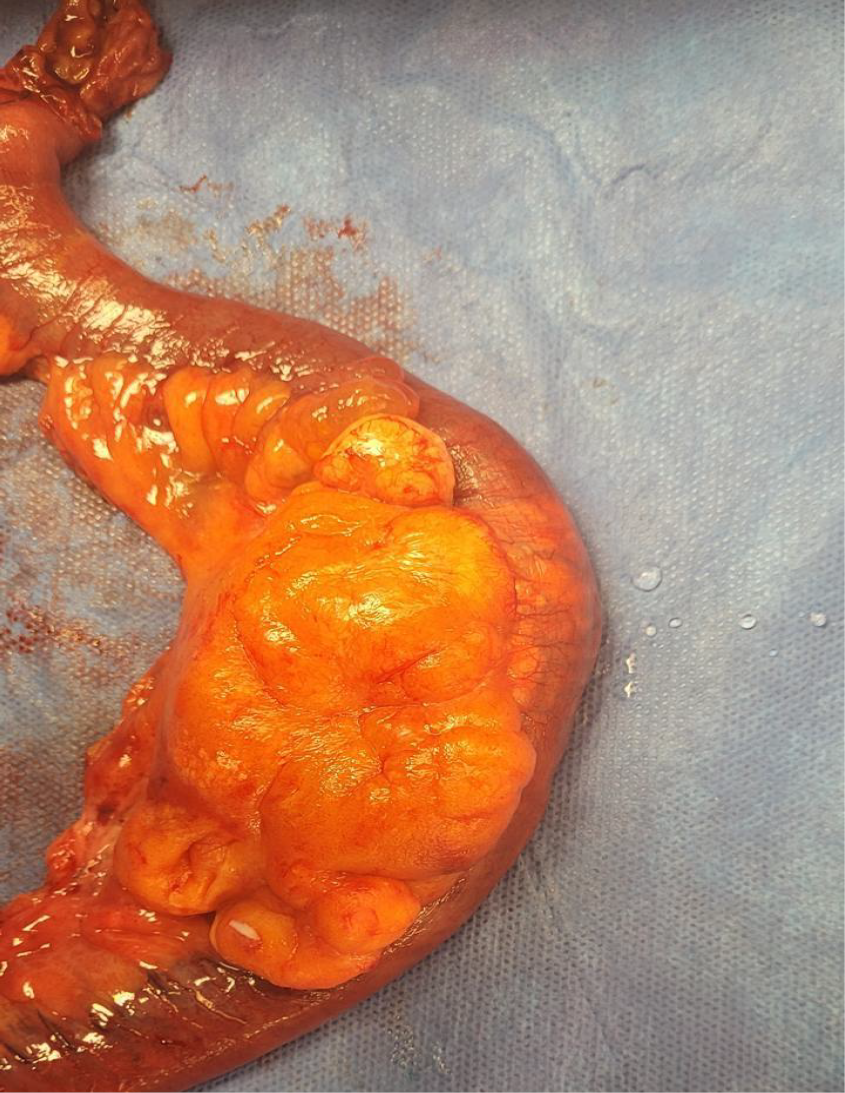
During the laparoscopy, the small and large intestines were systematically examined. Extensive adhesions were identified and subsequently lysed. A well-defined mass, measuring 8 cm × 6 cm × 3 cm, was observed 2.5 meters proximal to the ileocecal valve. Given its characteristics, en bloc resection of the mass along with a 22 cm segment of the small intestine was performed, followed by a primary end-to-end anastomosis (Figure 2).
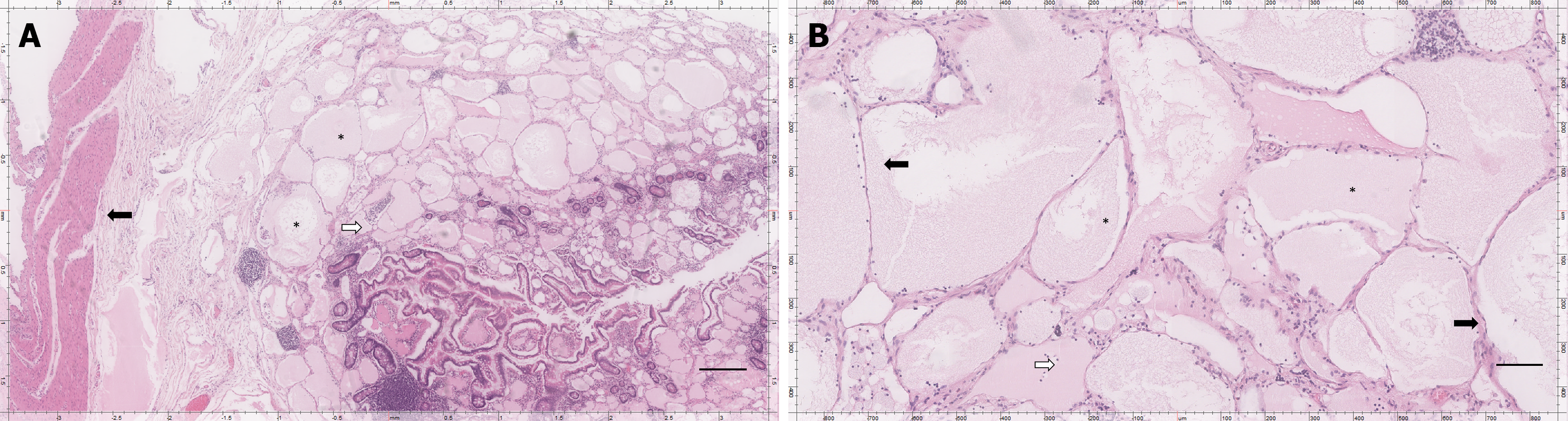
Gross examination of the resected specimen revealed a lobulated mass with multiple cystic spaces containing thick, white fluid. Histopathological analysis confirmed the diagnosis of mesenteric and small intestinal lymphatic malformation (Figure 3).
The patient experienced an uneventful early postoperative recovery and was discharged in stable condition on postope
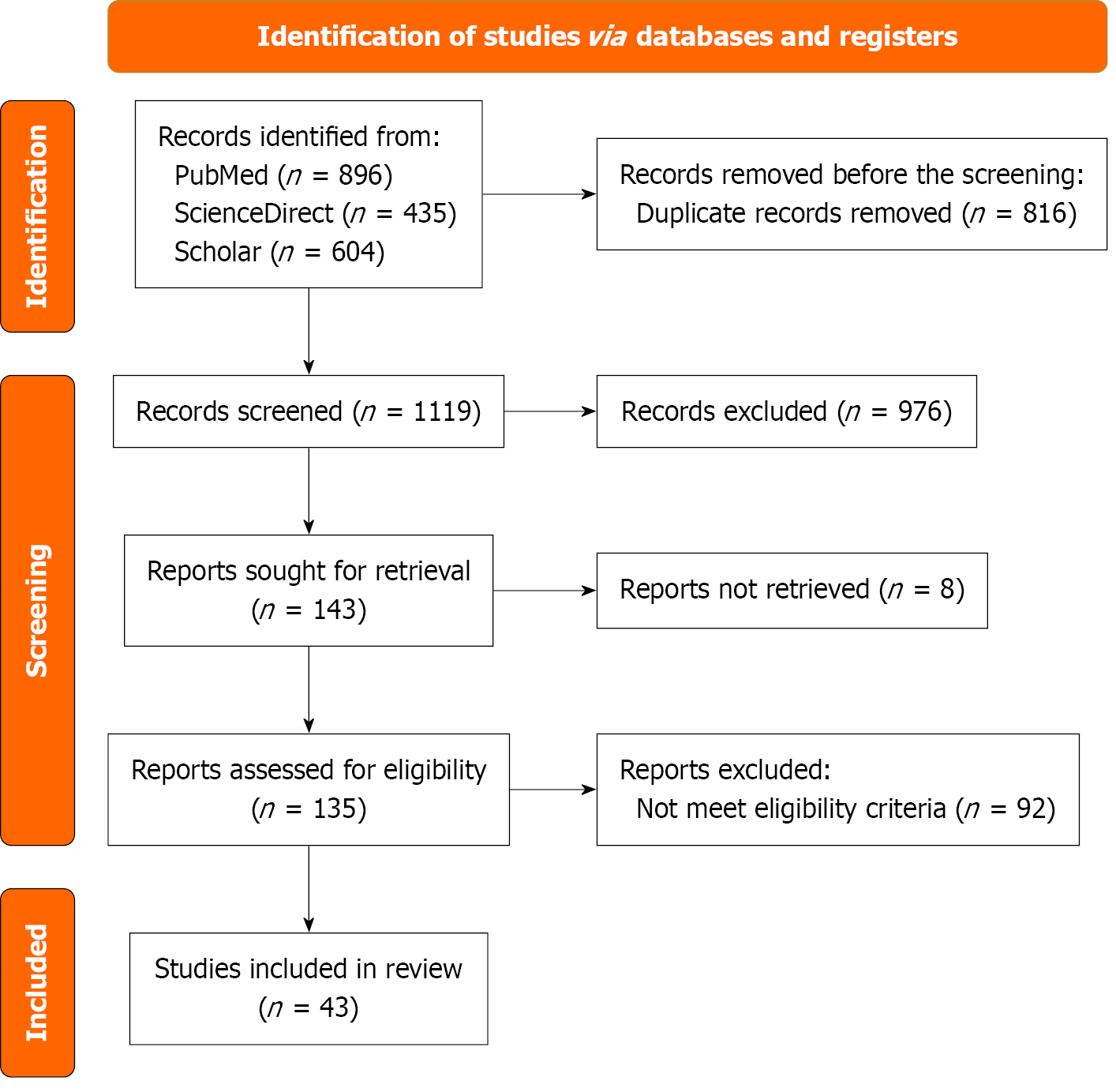
This scoping review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews guidelines to systematically map the existing literature on small bowel lymphatic malformation case reports and case series[12].
The literature search was conducted using three electronic databases: ScienceDirect, PubMed, and Google Scholar, covering all available literature from inception to August 30, 2024. The search strategy incorporated the following keywords and their synonyms: [(“lymphatic malformation” OR “cystic lymphangioma” OR “lymphangiomatous lesion” OR “mesenteric lymphangioma” OR “intestinal lymphatic malformation”) AND (“small intestine” OR “mesentery” OR “abdomen” OR “small bowel” OR “mesenteric” OR “intra-abdominal”) AND (“diagnosis” OR “surgery” OR “resection” OR “laparoscopy” OR “clinical presentation” OR “imaging” OR “histopathology”) AND (“case report” OR “case series”)]. Two researchers independently and concurrently performed the search. All titles, abstracts, and full texts were screened to identify eligible studies.
We included case reports and case series describing adult patients (≥ 18 years) diagnosed with small bowel lymphatic malformation. Articles were included if published in English. We excluded studies that contained duplicate data, did not report small bowel lymphatic malformations, or were published in languages other than English.
From each of the eligible studies, the following information was extracted: First author family name, publication year, gender, age, clinical presentation, duration of symptoms, workup and imaging done, management, location and size of the tumor, histology type, follow-up, and survival status of the patients.
Given the nature of the included studies (case reports and small series), formal meta-analysis, calculation of confidence intervals, and stratified statistical comparisons were not feasible. Data are presented as descriptive summaries of aggre
Our review included 97 patients from 43 studies (46% female, 54% male) with a mean age of 45.6 years (range: 18-76). Notable past histories included abdominal surgeries (e.g., hernia repairs, cholecystectomy; 28%), anemia (22%), and smoking (7%). The most common presentation was abdominal pain (74%), often localized to the epigastrium or lower quadrants, followed by gastrointestinal bleeding (melena/hematochezia; 39%), nausea/vomiting (30%), and asymptoma
| Ref. | Gender | Age (years) | Past history | Presentation | Duration of symptoms | Physical exam | Labs | Imaging modality | Site | Size (cm) | Multiplicity | Appearance on imaging |
| Akwei et al[46] | M | 62 | Inguinal hernia repair, hiatus hernia | Acute abdominal pain, nausea, vomiting | 36 hours | Soft distended abdomen, palpable tender mass; pyrexial (38.2 °C) | ↑Amylase (762 unit/dL), ↑ALT (191 IU/L), | CT | Proximal small bowel mesentery | 25 × 15 × 10 | Multiloculated | Large thin-walled multiloculated mass with chylous fluid |
| Al-Obeed and Abdulla[45] | M | 56 | Renal stones surgery, tympanoplasty | Epigastric pain, fullness, heartburn, constipation | 3 months | Soft/Lax abdomen; no tenderness/ | ↑WBC, | CT | Ileocecal valve | Not addressed | Not addressed | Small polypoidal mass |
| Barghash et al[44] | F | 26 | Post-abortion vaginal bleeding, smoking | Left-sided abdominal pain, distension | 24 hours | Distension; tenderness (left iliac fossa/flank) | Leukocytosis; normal LFTs/ | CT, MRI | Jejunal mesentery | Approximately 7 | Singular (lobulated) | Lobulated low-density mass; peripheral enhancement |
| Bucciero et al[43] | M | 28 | Not addressed | Weakness, melena, anemia (Hb 4 g/dL) | Not addressed | Not addressed | Severe anemia | CT | Jejunum | Not addressed | Not addressed | Inhomogeneous mass with stenosis |
| Cai et al[42] | F | 46 | Not addressed | Abdominal pain, melena, fatigue, shortness of breath | 3 months | Not addressed | Severe anemia (Hb approximately 90 g/L) | EGD, colonoscopy, CE, SBE | Proximal jejunum | 6 × 5 | Singular (multicystic) | Circumferential submucosal lesion; yellowish-white folds, bleeding points |
| Chae et al[40] | F | 37 | None | Severe abdominal pain, epigastralgia, fullness | Months (fullness); 2 weeks (pain) | Not addressed | Not addressed | CT | Jejunal mesentery | 9 × 7 | Multilocular | Hypodense well-capsulated cystic mass; whirling mesentery, proximal dilation |
| Chen et al[41] | F | 27 | None | Abdominal mass, epigastralgia, fullness after meals | Recent | Soft non-tender mass (upper left abdomen); normal bowel sounds | Normal tumor markers | US, MRI | Jejunal mesentery | 15 × 8 × 6 | Multilocular | Homogeneous mass with septa (MRI); multilocular cystic mass (US) |
| Chung et al[7] | M | 31 | None | Sudden severe abdominal pain, fever | Not addressed | Tenderness/ | Leukocytosis (14600/mm3), | CT | Jejunal mesentery | 8 × 6 × 6 | Not addressed | Low-density homogeneous oval; enhancing septum |
| Creger et al[39] | M | 76 | None | Incidental mass (asymptomatic) | Asymptomatic | 10-cm irregular soft mass | Aspiration cytology was negative for malignancy | CT | Jejunal mesentery | 9 × 6 (CT); 10 (exam) | Multiloculated | Multiloculated fluid-filled non-enhancing lesion |
| Cupido and Low[5] | F | 42 | Chronic iron deficiency anemia, menorrhagia | Menorrhagia, complex cystic mass | Several years | Not addressed | Not addressed | MRI | Small bowel mesentery | 17.6 × 6.8 × 8.7 | Multiloculated | Thin-walled multiloculated cyst; high T2/Low T1; no enhancement/solid components |
| Du et al[38] | M | 54 | Inguinal hernia surgery | Abdominal mass | 4 days | No positive signs | Normal blood/tumor markers (AFP, CEA, CA12-5, CA199) | US, CT | Jejunum/small bowel mesentery | 8 × 10 | Not addressed | Not addressed |
| Hwang et al[37] | F | 52 | Hysterectomy/salpingectomy | Abdominal mass | Not addressed | No positive signs | Normal blood/tumor markers (AFP, CA199, CA12-5) | US, CT | Small bowel mesentery | 5 × 5 | Not addressed | Irregular low-density mass |
| Ignjatovic et al[13] | M | 71 | Angina (5 years) | Rectal hard mass, hematochezia | Not addressed | Hard rectal mass | Normal labs | CT, PET/CT | Jejunum/mesentery | 8 × 6 | Nodular/multiloculated | Soft-tissue density; hazy attenuations |
| Honda et al[14] | M | 5-75 (range) | Not addressed | Abdominal pain, discomfort, nausea, vomiting | Approximately 6 months | Non-specific | Normal; tumor markers negative | US, MRI (inconclusive) | Ileal mesentery | 2 × 1.5 × 1.3 | Multiple locules | Lobulated cystic mass; milky fluid |
| Iwaya et al[36] | F | 31 | Not addressed | Profound anemia (Hb 53 g/dL) | Not addressed | Not addressed | Anemia; fecal occult blood+ | Barium radiography, CT, enteroscopy | Proximal jejunum | 3.5 × 3.0 | Multicystic | Filling defect (barium); lobulated mass (CT); yellowish-white submucosal tumor |
| Jang et al[35] | M | 70 | Hypertension | Iron deficiency anemia, dark stool | 3 years | Not addressed | Anemia | CE, DBE | Proximal small bowel | 2.0 × 1.7 × 1.2 | Singular | Raised granular lesion; white/thickened villi, oozing blood |
| Jayasundara et al[34] | M | 43 | Similar abdominal pain 20 years ago | Epigastric pain, nausea, vomiting | 20 days (between visits) | Epigastric tenderness/ | ↑BUN (42.4 mg/dL), | X-ray, CT | Pelvic cavity (small bowel mesentery) | 15 × 10 × 6 | Multiple locules | Cystic mass; homogeneous fluid; “beaked” small bowel (CT) |
| João et al[33] | F | 18 | None | Acute abdominal pain, vomiting | 4 hours | Tender (central/LUQ); early peritonism; temp 37.2 °C | Leukocytosis (13900/mm3), | CT | Jejunal mesentery | 10 × 10 × 9 | Two small polyps | Low-density mass encasing vessels |
| Khan et al[52] | F | 29 | None | Melena, severe anemia (Hb 55 g/dL) | Not addressed | Not addressed | Microcytic anemia (Hb 55 g/dL) | CE, enteroscopy | Proximal jejunum | 1.2 | Singular | Whitish “strawberry-like” mucosa, fresh blood |
| Konstantinidis et al[32] | F | 24 | None | Sudden right abdominal pain, nausea | Not addressed | Right abdominal tenderness | ↑WBC (15.1 L) | CT, endoscopy | Small bowel (mesentery) | 0.8-3.5 | Not addressed | Well-demarcated thin-walled oval mass |
| Kopáčová et al[51] | F | 31 | None | Acute right abdominal pain, nausea | Not addressed | Guarding, tenderness | Mild leukocytosis | US (initially negative) | Mid-ileum | Not addressed | Not addressed | Well-circumscribed whitish multicystic mass |
| Li et al[31] | M | 69 | GERD, peptic ulcer, hemorrhoids | Melena, symptomatic anemia | 6 months | Pale skin; melena | Anemia (Hb 60-8.5 g/dL) | CE, DBE | Small bowel | 0.4 × 0.6 | Singular | Nodular lesion (typical lymphatic malformation) |
| Lim et al[50] | M | 70 | Iron deficiency anemia | Anemia, dizziness, melena | 3 years | Dizziness, weakness | Hb 52 g/dL (pre-operative); Hb 104 g/dL (post-operative) | CE | Jejunum | < 1 | Multiple | Mucosal erosion, blood clot; multifocal erosions |
| Losanoff et al[29] | M | 35 | Not addressed | Painful abdominal mass | 4 weeks | Baseball-sized hard tender mass (RLQ); hypoactive bowel sounds | Normal | CT, MRI | Terminal ileum mesentery | 30 × 12 × 10 | Single (multiple cysts possible) | Large cyst; soft tissue mass |
| Mavrogenis et al[30] | M | 59 | Not addressed | Melena | Not addressed | Not addressed | Anemia | CE, DBE | Proximal jejunum | 3.5 × 7 | Singular | Polypoid lesion; whitish/red spots, spontaneous bleeding |
| Botey et al[49] | M | 52 | Not addressed | Diffuse abdominal pain, diarrhea, and fever | 3 days | Tympanic abdomen; diffuse pain, peritoneal irritation | ↑CRP (5.8 mg/dL) | CT | Proximal jejunum | 7.2 × 9.5 × 7.5 | Multilocular | Multilocular cystic mass; thin walls, higher density |
| Nakamura et al[28] | M | 60 | Dialysis | Obscure GI bleeding | Not addressed | Not addressed | Not addressed | EUS, DBE | Jejunum | < 1 | Not addressed | Hypoechoic mass with erosion/red sign |
| Stein et al[15] | M | 21 | Not addressed | GI bleeding, abdominal pain, weight loss | Months | Large nontender abdominal mass | Hb 70 g/dL (HCT 21%) | CT | Mesentery/duodenum | Not addressed | Not addressed | Large infiltrating spongy mass |
| Ong et al[27] | M | 28 | None | Recurrent abdominal pain | 18 months | Not addressed | Not addressed | CT, MRI | Distal small bowel mesentery/pelvis | Not addressed | Multicystic | Multicystic pelvic lesion |
| Rieker et al[4] | M | 61 | Not addressed | Abdominal pain (LLQ), fever to chills/ | Not addressed | Not addressed | Not addressed | US, CT | Ileal mesentery | 12 × 9 × 7 | Multicystic | Multicystic tumor; clear fluid |
| Rathod et al[26] | F | 18 | None | Asymptomatic (incidental) | Asymptomatic | Normal | Normal | CT | Jejunal mesentery | 10.5 × 10.0 × 6.0 | Two cystic swellings | Large well-defined cystic mass; hyperdense fluid, thin capsule/septa |
| Rojas and Molina[25] | F | 71 | Cholecystec | Nausea, lower abdominal pain, palpable mass | 1 month | Palpable lower abdominal mass | Normal | CT | Small bowel mesentery | 9 × 7 × 4 | Singular | Mesenteric mass |
| Lin et al[24] | F | 38 | Not addressed | Melena, weakness | 3 months (melena); 10 days (weakness) | Anemic appearance | Hb 74 g/L; albumin 20.9 g/L | CT | Fundus, peripancreatic, mesenteric, retroperitoneal, spleen | Largest 6.2 | Multiple | Multiple small cystic lesions; no enhancement |
| Safatle-Ribeiro et al[23] | M | 30 | Pulmonary tuberculosis | Recurrent melena | 14 years | Not addressed | Anemia | SBFT, DBE | Jejunum | 15 (specimen) | Diffuse | Irregular mucosa; diffuse thickening/nodularity |
| Samuelson et al[22] | M | 70 | ADPKD, CHF, valvular cardiomyopathy | Abdominal pain, nausea, emesis, weight loss, hematemesis | Not addressed | Not addressed | Iron deficiency | CT | Jejunum | 2.5 | Two lymphatic malformations | “Target sign” (intussusception) |
| Tang et al[47] | F | 38 | None | Recurrent melena, anemia | Not addressed | 1-cm oozing polypoid lesion | Anemia | CE | Proximal-mid small bowel | 1 | Singular | White-yellow “strawberry” mucosal pattern |
| Teng et al[21] | F | 55 | None | Right upper abdominal discomfort | 2 months | Mild RUQ tenderness | Normal | CT | Jejunum | 3 × 3; 2 × 2 | Two | Space-occupying lesion; calcium deposition, enhancement |
| Tomizawa et al[20] | F | 46 | Hypothyroi | Symptomatic anemia | 1 year | Pallor | Hb 6 g/dL; iron deficiency | CT enterography | Small bowel mesentery | 8.5 × 6 × 4 | Lobulated | Large cystic lobulated mass |
| Torashima et al[19] | F | 31 | Appendectomy (age 14) | Upper abdominal pain (pregnancy) | 24 weeks (gestation) | Abdominal distention, LUQ tenderness | WBC 7600/mm3; Hb 105 g/dL; CRP 0.19 mg/dL | US, CT, MRI | Ileum | 19 × 8 × 5.5 | Multiloculated | Thin cyst wall; multiloculated cystic lesion |
| Wei et al[48] | M | 21 | Known lymphatic malformation | Vomiting, severe LUQ pain | 12 hours | Not addressed | Not addressed | CT | Jejunal mesentery | Not addressed | Not addressed | Low-attenuation mass; “swirl sign” (volvulus) |
| Yang et al[18] | F | 37 | None | GI hemorrhage, anemia, melena, dizziness | 2 weeks | Not addressed | Anemia | CE, CT angiography | Ileum | 6.5 × 4.5 × 0.9 | Clustered nodules | Cystic: Low T1/high T2 signal |
| Yavuz et al[17] | F:14 M:8 | 40.68 (mean) | Not addressed | Abdominal pain (12), distension (6), mass (4), nausea/vomiting (3) | Not addressed | Palpable mass (4), distension (6) | Not addressed | US, CT | Small intestine (18), colon (4) | 10.04 (mean; range 2-27) | Not addressed | Not specified |
| Yeh et al[16] | M:22 F:12 | 50 (median) | Varied (e.g., choriocarcinoma, gastric cancer) | OGIB (25), abdominal pain (9) | > 3 years (some) | Not addressed | Anemia (OGIB: Hb 63 g/dL) | CT, DBE | Small bowel | Not addressed | Not addressed | Varied (e.g., mass, angiodysplasia) |
Physical examination revealed abdominal tenderness (65%), palpable masses (28%), distension (22%), or guarding (13%). Laboratory findings highlighted anemia (hemoglobin < 8 g/dL in 52%), leukocytosis (33%), and elevated amylase/C-reactive protein (17%). Imaging modalities predominantly used CT (87%), supplemented by MRI (26%), ultrasound (22%), and endoscopy (capsule endoscopy/double balloon enterostomy; 39%). Lesions were primarily located in the jejunal mesentery (52%) or ileum (26%), with a mean size of 9.2 cm (range: 0.4-30 cm). Most were multiloculated cystic masses (78%), appearing as thin-walled, fluid-filled lesions with septations (CT/MRI). Multiplicity was noted in 24% of cases (Table 2).
| Ref. | Management (surgery/endoscopy) | Histopathology findings | Type | Complications (post-operative) | Follow-up duration | Recurrence |
| Akwei et al[46] | Surgical resection (incomplete) | Complex multiloculated lesion; flattened cells, vascular channels, lymphoid aggregates | Cystic | Infection, pelvic collection (E. coli), required CT-guided drain | 1 year | Intra-abdominal collection (1 month); no cyst recurrence |
| Al-Obeed and Abdulla[45] | Laparoscopic right hemicolectomy | Dilated lymphatic vessels | Cystic | None | Not addressed | Not addressed |
| Barghash et al[44] | Segmental bowel resection | Dilated thin-walled channels/cystic spaces; flat endothelial cells, lymphocytes, RBCs | Cystic | None | 8 weeks | Not specified (risk noted) |
| Bucciero et al[43] | Surgical resection (duodenum/jejunum) | Dilated lymphatic vessels; histiocytes, lymphangiectasia | Lymphatic malformation | Not addressed | Not addressed | Not addressed |
| Cai et al[42] | Surgical resection | Dilated lymphatic channels (mucosa/submucosa); simple lymphatic malformation with bleeding | Simple | None | Not specified | No melena recurrence |
| Chae et al[40] | Surgical resection | Multi-septate cystic masses; flat lymphatic endothelial cells | Cystic (macrocystic) | None | 3 months | None |
| Chen et al[41] | Laparotomy | Dilated lymphatic channels; factor VIII+, actin+, cytokeratin- | Cystic | None | 18 months | None |
| Chung et al[7] | Surgical excision | Dilated lymphatic spaces; collagenous stroma, flattened endothelial cells | Cystic | None | 9 months | None |
| Creger et al[39] | Laparoscopy to open small bowel resection | Lymphatic malformation; no malignancy | Cystic | None | 2 weeks | Not addressed |
| Cupido and Low[5] | Conservative (follow-up); surgery if symptomatic | Not specified (cystic lymphatic malformation) | Cystic | Not addressed | 13 months | Not addressed |
| Du et al[38] | Not specified | Haemolymphangioma (CD34+, D2-40+) | Cystic | None | 4 months | None |
| Hwang et al[37] | Laparoscopic resection | Haemolymphangioma (CD34+, D2-40+) | Cystic | None | 4 months | None |
| Ignjatovic et al[13] | Laparoscopic abdominal transanal resection + ileostomy | Dark red multiloculated cystic lesion; CD34+ endothelial cells | Cavernous | Not addressed | Not addressed | Not addressed |
| Honda et al[14] | Surgical resection | Cystic lymphatic malformation | Cystic | None | 1 year | None |
| Iwaya et al[36] | Surgical resection | Dilated lymphatic vessels; eosinophilic fluid/RBCs | Lymphatic malformation | Not addressed | Not addressed | Not addressed |
| Jang et al[35] | Laparoscopic small bowel resection | Dilated vascular channels; D2-40+ (lymphatic), CD31+ (blood) | Hemangiolymphangioma | Not addressed | Not addressed | Not addressed |
| Jayasundara et al[34] | Segmental resection + anastomosis | Lymphatic malformation | Cystic | Ischemic stricture | 20 days | Complete small bowel volvulus |
| João et al[33] | Surgical excision | Thin-walled lymphatic spaces; eosinophilic material, neutrophils, lymphocytes | Cystic | None | 6 months | Not addressed |
| Khan et al[52] | Endoscopic mucosal resection | Large dilated lymphatic channels | Cavernous | None | 2 months | None |
| Konstantinidis et al[32] | Diagnostic laparoscopy to laparotomy (intussusception) | Cystic lymphatic malformation (irregular cysts) | Cystic | None | 2 weeks | Not addressed |
| Kopáčová et al[51] | Laparoscopic resection | Multicyclic lymphatic malformation; markedly dilated channels | Cystic | None | Not addressed | Not addressed |
| Li et al[31] | Endoscopic resection (DBE) | Small-bowel lymphatic malformation | Cystic | None | 4 months | Not addressed |
| Lim et al[50] | Surgical resection | Dilated lymphatics; thrombus/hemorrhage | Lymphatic malformation | Chronic anemia/melena (pre-operative); resolved post-op | 3 months | None |
| Losanoff et al[29] | Exploratory laparotomy | Cystic mass; attenuated endothelium, smooth muscle, lymphocytes | Cystic | Hemorrhage, infection, lymphatic fistula (risk) | Not addressed | 0%-100% (risk) |
| Mavrogenis et al[30] | Single-port laparoscopy | Mixed cavernous hemangioma-lymphatic malformation | Mixed | Not addressed | Not addressed | Not addressed |
| Botey et al[49] | Emergency laparotomy to bowel resection | Mesenteric lymphatic malformation; D2-40+ | Cystic | Not addressed | Not addressed | Not addressed |
| Nakamura et al[28] | Endoscopic mucosal resection | Lymphatic malformation | None | Not addressed | Not addressed | |
| Stein et al[15] | Open biopsy to alcohol ablation | Microcystic lymphatic malformation (small lymphatic channels) | Microcystic | Mild pain/fever (alcohol ablation) | 18 months | None (no bleeding recurrence) |
| Ong et al[27] | Not addressed | Not addressed | Not addressed | Not addressed | Not addressed | Not addressed |
| Rieker et al[4] | Complete excision | Dilated lymphatic channels; factor VIII+, Ulex europaeus+ | Cystic | None (initial); readmitted at 6 months | 6 months (readmission) | Possible (incomplete removal risk) |
| Rathod et al[26] | Surgical excision | Smooth capsulated cysts; chalky white fluid | Cystic | None | Not addressed | Not addressed |
| Rojas and Molina[25] | Laparotomy | Encapsulated fat; dilated lymph vessels | Cavernous | None | Not specified | None (incomplete resection risk) |
| Lin et al[24] | Exploratory laparotomy | Dilated lymphatic channels; D2-40+ | Cystic | Not addressed | Not addressed | Not addressed |
| Safatle-Ribeiro et al[23] | Surgical resection | Diffuse dilated lymphatic vessels | Cystic | Bleeding/wall thickness | 42 months | None |
| Samuelson et al[22] | Not addressed | Not addressed | Not addressed | Cachexia, multiple cancers | Not addressed | Not addressed |
| Tang et al[47] | Laparoscopic segmental resection | Cavernous lymphatic malformation | Cavernous | Not addressed | Not specified (bleeding/anemia resolved) | Not addressed |
| Teng et al[21] | Excisional surgery | Chronic inflammation; low-grade neoplasia | Hemolymphangioma | None | 6 months | None |
| Tomizawa et al[20] | Exploratory laparotomy | Cystic spaces with hemorrhage; D2-40+ | Lymphatic malformation | Not addressed | Not specified | Not addressed |
| Torashima et al[19] | Laparotomy to bowel resection | Multicyclic spaces; attenuated endothelium, proteinaceous fluid | Cystic | None | 18 months | None |
| Wei et al[48] | Emergency laparotomy to bowel resection | Dilated thin-walled channels; full-thickness bowel involvement | Lymphatic malformation | None | 3 months | None |
| Yang et al[18] | Laparoscopic-assisted resection | Diffuse proliferative blood/Lymphatic vessels | Hemolymphangioma | Not addressed | 1 year | Not addressed |
| Yavuz et al[17] | Enucleation (10), bowel resection (4), laparoscopic excision (4), hemicolectomy (3) | Simple cyst (17), lymphatic malformation (4), adenocarcinoma (1) | Cystic (simple/Lymphangioma) | SSI (3), anastomosis leak (1) | Not addressed | Not addressed |
| Yeh et al[16] | LABS (27), converted laparotomy (6) | Lymphatic malformation (1 case) | Lymphatic malformation | Transient fever/abdominal pain (tattoo leak) | 14 ± 3 months | Symptomatic recurrence (2) |
Surgical resection was the primary treatment (91%): (1) Segmental bowel resection (65%) for large or symptomatic masses; (2) Laparoscopic excision (26%) for accessible lesions; and (3) Enucleation (9%) for well-defined cysts. Endoscopic resection (9%) was reserved for small, bleeding submucosal lesions (e.g., endoscopic mucosal resection for lymphatic malformations < 2 cm). Incomplete resection occurred in 7% due to adhesions or pancreatic involvement.
Histopathology confirmed cystic lymphatic malformation (76%), cavernous (13%), or hemolymphangioma (11%). Postoperative complications occurred in 24%: Infections (13%), anastomotic leaks (4%), and transient pain/fever (7%). Median follow-up was 12 months (range: 3-42 months). Recurrence was observed in 11% (e.g., intra-abdominal collec
A total of 43 studies (41 case reports and 2 case series) were appraised using the Joanna Briggs Institute critical appraisal tools. The quality of reporting was variable across the domains. Patient demographics were fully described in 18 studies (41.9%), partially in 10 (23.3%), and omitted in 15 (34.9%). A clear clinical history and timeline were provided in 17 studies (39.5%). The patient’s condition at presentation was the most consistently reported item, with 34 studies (79.1%) providing full details and 1 (2.3%) providing partial details; however, 8 studies (18.6%) did not address it. Diagnostic tests were clearly described in 34 studies (79.1%), while interventions and post-intervention clinical conditions were detailed in 33 (76.7%) and 30 (69.8%) studies, respectively. Adverse events were documented in only a single study (2.3%). Takeaway lessons were included in 29 studies (67.4%). This assessment highlights significant heterogeneity in reporting standards, particularly concerning demographic details, clinical timelines, and outcomes.
Among the 97 cases, only 9 (9.3%) reported specific immunohistochemical markers. These included lymphatic markers (D2-40 in 5 cases), vascular markers (CD31 in 1 case, CD34 in 3 cases, factor VIII in 2 cases), and Ulex europaeus in 1 case. No cases reported using more specific lymphatic markers such as prospero homeobox 1 or vascular endothelial growth factor receptor 3.
Small bowel lymphatic malformations are exceptionally rare in adults, accounting for less than 1% of all lymphatic malformations and approximately 1 in 100000 hospital admissions. Our review, which included 46% female patients with a mean age of 45.6 years, aligns with the reported female predominance (female to male ratio of 1:1). Although traditionally considered congenital, secondary triggers such as abdominal surgery (noted in 28% of cases), inflammation, or trauma may contribute to the development of these lesions in adults[53-56].
Clinically, abdominal pain was the most prevalent symptom, reported in 74% of cases. This pain was often acute due to complications such as bowel obstruction, including volvulus resulting from mass effect, or chronic in nature from the lesion's insidious growth. Gastrointestinal bleeding occurred in 39% of cases and typically arose from erosion into blood vessels or mucosal ulceration[38,54,57]. In terms of diagnostics, CT scans were the most commonly used imaging modality, applied in 87% of cases. They revealed characteristic multiloculated, fluid-attenuated masses with septations. MRI, which was underutilized (26%), offered enhanced characterization of complex cysts, particularly through T2-hyperintensity signals. Capsule or balloon-assisted enterostomy was valuable in detecting bleeding or submucosal lesions in 39% of cases, though its sensitivity for mesenteric tumors was limited. Anemia, defined by hemoglobin levels below 8 g/dL, was common in bleeding cases (52%), while tumor markers such as carcinoembryonic antigen were typically within normal limits[53,56].
Surgical resection emerged as the gold standard treatment in 91% of cases. Segmental resection, performed in 65% of these, was preferred for large or symptomatic masses to ensure complete excision. Laparoscopic approaches, used in 26%, offered the benefits of minimally invasive surgery and shorter recovery times but often required conversion to open surgery in complex cases involving adhesions. Endoscopic resection proved effective for small submucosal lesions under 2 cm (9% of cases), although bleeding risks limited its broader applicability[38,56]. Postoperative complications were observed in 24% of patients, including surgical site infections in 13% and anastomotic leaks in 4%. Recurrence occurred in 11% of cases and was primarily associated with incomplete resection, particularly when the lesion was adherent to surrounding structures such as the pancreas. Notably, there were no recurrences following complete excision[54,56]. Histopathologically, cystic lymphatic malformation was the most common variant, seen in 76% of cases, and characterized by dilated lymphatic channels lined by endothelial cells. Hemolymphangioma, a mixed vascular and lymphatic form, was identified in 11% and required immunohistochemical confirmation using markers such as CD31, CD34, and D2-40.
However, several important limitations must be acknowledged. First, the included studies consisted predominantly of case reports with significant heterogeneity in reporting standards, particularly concerning demographic details, clinical timelines, and outcomes. Second, as a scoping review of predominantly low-level evidence, formal meta-analysis, calculation of confidence intervals, and stratified statistical comparisons were not methodologically appropriate; our findings should be interpreted as descriptive summaries rather than statistical inferences. Third, immunohistochemical characterization was inconsistently reported, with only 9.3% of cases documenting specific markers, and none reporting contemporary lymphatic markers such as prospero homeobox 1 or vascular endothelial growth factor receptor 3. Finally, publication bias likely led to overrepresentation of symptomatic or complicated cases, while the median follow-up of 12 months may be insufficient to capture late recurrences.
In summary, small bowel lymphatic malformations, despite their benign nature, can cause significant morbidity due to mass effect, bleeding, or obstruction. Our case and scoping review underscore key insights. Diagnosis relies on a high index of suspicion, with CT or MRI identifying multiloculated cysts and endoscopy providing supplementary evaluation for mucosal involvement. Management is centered on complete surgical resection, either open or laparoscopic, as it remains definitive. Endoscopic resection should be reserved for small, easily accessible lesions. Prognosis is excellent following complete excision, with 93% symptom resolution and recurrence primarily resulting from residual disease. Future efforts should focus on standardized reporting practices and long-term surveillance to better inform treatment strategies. Clinically, surgeons should maintain a high suspicion for lymphatic malformations when encountering cystic abdominal masses and prioritize radical excision to prevent complications.
| 1. | Levy AD, Cantisani V, Miettinen M. Abdominal lymphangiomas: imaging features with pathologic correlation. AJR Am J Roentgenol. 2004;182:1485-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Miceli A, Stewart KM. Lymphangioma. 2023 Aug 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 3. | de Perrot M, Rostan O, Morel P, Le Coultre C. Abdominal lymphangioma in adults and children. Br J Surg. 1998;85:395-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 110] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Rieker RJ, Quentmeier A, Weiss C, Kretzschmar U, Amann K, Mechtersheimer G, Bläker H, Herwart OF. Cystic lymphangioma of the small-bowel mesentery: case report and a review of the literature. Pathol Oncol Res. 2000;6:146-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Cupido BD, Low G. Incidental Cystic Lymphangioma of the Small Bowel Mesentery. J Clin Imaging Sci. 2015;5:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Engin G, Asoglu O, Kapran Y, Mert G. A gastrointestinal stromal tumor with mesenteric and retroperitoneal invasion. World J Surg Oncol. 2007;5:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Chung JC, Song OP. Cystic lymphangioma of the jejunal mesentery presenting with acute abdomen in an adult. Can J Surg. 2009;52:E286-E288. [PubMed] |
| 8. | Maghrebi H, Yakoubi C, Beji H, Letaief F, Megdich S, Makni A, Boukriba S, Frikha W, Ayadi M, Kacem M. Intra-abdominal cystic lymphangioma in adults: A case series of 32 patients and literature review. Ann Med Surg (Lond). 2022;81:104460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 9. | Parker DR, Kiely P, Smith R. Complete resection of a massive mesenteric lymphangioma in an adult. BMJ Case Rep. 2020;13:e233714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Chukwubuike KE. Incidence of appendices of the testis and epididymis in children who underwent groin/scrotal surgeries in a tertiary hospital in Enugu, Nigeria. Arch Clin Gastroenterol. 2021;7:018-020. [DOI] [Full Text] |
| 11. | Lee HH, Lee SY. Case report of solitary giant hepatic lymphangioma. Korean J Hepatobiliary Pancreat Surg. 2016;20:71-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 12. | Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, Hempel S, Akl EA, Chang C, McGowan J, Stewart L, Hartling L, Aldcroft A, Wilson MG, Garritty C, Lewin S, Godfrey CM, Macdonald MT, Langlois EV, Soares-Weiser K, Moriarty J, Clifford T, Tunçalp Ö, Straus SE. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22118] [Cited by in RCA: 22416] [Article Influence: 2802.0] [Reference Citation Analysis (1)] |
| 13. | Ignjatovic I, Milosavljevic V, Tadic B, Grubor N, Matic S. Lymphangioma of the Small Intestine Case Report and Review of the Literature. Serb J Exp Clin Res. 2019;20:357-360. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Honda K, Ihara E, Ochiai T, Matsumoto M, Matsuda H, Nakashima A, Harada N, Kabemura T. Lymphangioma of small intestine. Gastrointest Endosc. 2003;58:574-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Stein M, Hsu RK, Schneider PD, Ruebner BH, Mina Y. Alcohol ablation of a mesenteric lymphangioma. J Vasc Interv Radiol. 2000;11:247-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Yeh TS, Liu KH, Su MY, Lin CH, Chiu CT, Tseng JH. Laparoscopically assisted bowel surgery in an era of double-balloon enteroscopy: from inside to outside. Surg Endosc. 2009;23:739-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Yavuz Y, Varman A, Şentürk ÜM, Kafadar MT. Mesenteric Cyst in 22 Cases. J Gastrointest Cancer. 2021;52:993-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Yang SX, Zhou YH, Zhang J, Miao L, Zhong JW, Wang WX, Xu CL, Cai ZZ, Lu GR. Haemorrhagic ileal haemolymphangioma: a case report and review of the literature. J Int Med Res. 2021;49:300060520986677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Torashima Y, Yamaguchi J, Taniguchi K, Fujioka H, Shimokawa I, Izawa K, Kanematsu T. Surgery for ileal mesenteric lymphangioma during pregnancy: case report and review of the literature. J Gastrointest Surg. 2004;8:616-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Tomizawa Y, Garner K, Sohnen A. Lymphangioma of the small bowel mesentery: a rare intra-abdominal tumor causing anemia. Clin Gastroenterol Hepatol. 2013;11:e57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Teng Y, Wang J, Xi Q. Jejunal hemolymphangioma: A case report. Medicine (Baltimore). 2020;99:e18863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Samuelson H, Giannotti G, Guralnick A. Jejunal lymphangioma causing intussusception in an adult: An unusual case with review of the literature. Ann Med Surg (Lond). 2018;34:39-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Safatle-Ribeiro AV, Iriya K, Couto DS, Kawaguti FS, Retes F, Ribeiro U Jr, Sakai P. Secondary lymphangiectasia of the small bowel: utility of double balloon enteroscopy for diagnosis and management. Dig Dis. 2008;26:383-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Lin RY, Zou H, Chen TZ, Wu W, Wang JH, Chen XL, Han QX. Abdominal lymphangiomatosis in a 38-year-old female: case report and literature review. World J Gastroenterol. 2014;20:8320-8324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Rojas CL, Molina GA. Lymphangioma cavernous of the small bowel mesentery, an infrequent cause of acute abdomen in adult. J Surg Case Rep. 2018;2018:rjy018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Rathod J, Patel S, Upadhyay HM. Cystic lymphangioma of the jejunal mesentery in a young adult: a rare case report. Int Surg J. 2020;7:3831. [DOI] [Full Text] |
| 27. | Ong D, Cribb B, Marshall-Webb M, Yong J. Rare cystic mesenteric mass of the small bowel: mesenteric lymphangioma. ANZ J Surg. 2021;91:E417-E418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 28. | Nakamura M, Hirooka Y, Watanabe O, Yamamura T, Nagura A, Ando T, Goto H. Submucosal tumor in the small bowel resected by EMR at double-balloon endoscopy. Gastrointest Endosc. 2015;81:1024-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Losanoff JE, Richman BW, El-Sherif A, Rider KD, Jones JW. Mesenteric cystic lymphangioma. J Am Coll Surg. 2003;196:598-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 130] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Mavrogenis G, Coumaros D, Lakhrib N, Renard C, Bellocq JP, Leroy J. Mixed cavernous hemangioma-lymphangioma of the jejunum: detection by wireless capsule endoscopy. Endoscopy. 2011;43 Suppl 2 UCTN:E217-E218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Li F, Osuoha C, Leighton JA, Harrison ME. Double-balloon enteroscopy in the diagnosis and treatment of hemorrhage from small-bowel lymphangioma: a case report. Gastrointest Endosc. 2009;70:189-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Konstantinidis K, Theodoropoulos GE, Sambalis G, Georgiou M, Vorias M, Anastassakou K. Cystic lymphangioma of the small bowel in a woman at 15 weeks' gestation: laparoscopic approach. Surg Laparosc Endosc Percutan Tech. 2005;15:244-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | João M, Gravito-Soares E, Lopes S, Amaro P. Jejunal cavernous lymphangioma: successful endoscopic treatment of a rare cause of small bowel bleeding. Ann Gastroenterol. 2021;34:891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Jayasundara J, Perera E, Chandu de Silva MV, Pathirana AA. Lymphangioma of the jejunal mesentery and jejunal polyps presenting as an acute abdomen in a teenager. Ann R Coll Surg Engl. 2017;99:e108-e109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Jang JH, Lee SL, Ku YM, An CH, Chang ED. Small bowel volvulus induced by mesenteric lymphangioma in an adult: a case report. Korean J Radiol. 2009;10:319-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Iwaya Y, Streutker CJ, Coneys JG, Marcon N. Hemangiolymphangioma of the small bowel: A rare cause of chronic anemia. Dig Liver Dis. 2018;50:1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Hwang SS, Choi HJ, Park SY. Cavernous mesenteric lymphangiomatosis mimicking metastasis in a patient with rectal cancer: a case report. World J Gastroenterol. 2009;15:3947-3949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Du Y, Zhang JN, Zhu LL, Wang Y, Li WP. Haemolymphangioma of the small bowel mesentery in adults: two case reports and a literature review. BMC Gastroenterol. 2021;21:273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (4)] |
| 39. | Creger PE, Harper C 3rd, Curry C, Kramer A. Resection of an Asymptomatic Lymphangioma in a 76-Year-Old Male. Cureus. 2021;13:e15577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 40. | Chae HD, Cho C, Kim I. Mesenteric Lymphangioma with Small Intestinal Volvulus in an Adult. J Gastrointest Dig Syst. 2017;7:2. [DOI] [Full Text] |
| 41. | Chen CW, Hsu SD, Lin CH, Cheng MF, Yu JC. Cystic lymphangioma of the jejunal mesentery in an adult: a case report. World J Gastroenterol. 2005;11:5084-5086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Cai JT, Chen JM, Zhang XM, Chen Y, Wei SM, Du Q, Xie CG. Education and Imaging. Gastrointestinal: small bowel lymphangioma diagnosed by single-balloon enteroscopy. J Gastroenterol Hepatol. 2012;27:1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 43. | Bucciero F, Marsico M, Galli A, Tarocchi M. Small bowel lymphangioma: A rare case of intestinal bleeding. Dig Liver Dis. 2015;47:815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Barghash M, Nassif S, Alkurdi Y, Mansour M. Mesenteric Lymphangioma Presenting With Small Bowel Volvulus in an Adult. Cureus. 2021;13:e16771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Al-Obeed OA, Abdulla MH. Lymphangioma of the ileocecal valve clinically masquerading as a submucosal small intestinal GIST: report of a case and literature review. Saudi J Gastroenterol. 2014;20:262-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | Akwei S, Bhardwaj N, Murphy PD. Benign mesenteric lymphangioma presenting as acute pancreatitis: a case report. Cases J. 2009;2:9328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Tang SJ, Bhaijee F. Small Bowel Lymphangioma. Video J Encycl GI Endosc. 2014;1:663-665. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Wei MY, Chua J, Cheng Y, Grossberg P. Small bowel volvulus in an adult with mesenteric lymphangioma and ascariasis. ANZ J Surg. 2018;88:E859-E860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Botey M, Muñoz-Ramos C, Bonfill J, Roura J, Torres M, Mocanu S, Arner T, Pérez G, Salvans F, García-San-Pedro Á. Acute abdomen for lymphangioma of the small bowel mesentery: a case report and review of the literature. Rev Esp Enferm Dig. 2015;107:39-40. [PubMed] |
| 50. | Lim DR, Kuk JC, Kim T, Shin EJ. Surgery of multiple lymphangioma in small bowel: a rare case report of chronic gastrointestinal bleeding. Ann Surg Treat Res. 2018;94:52-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 51. | Kopáčová M, Rejchrt S, Bureš J, Tachecí I. Small intestinal tumours. Gastroenterol Res Pract. 2013;2013:702536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Khan K, Kleess L, Ganga R, DePaz H, Santopietro R. Small bowel lymphangioma causing ileo-ileal intussusception in adults. Int J Surg Case Rep. 2017;41:469-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | Mehmedovic Z, Mehmedovic M, Custovic MK, Sadikovic A, Mekic N. A rare case of giant mesenteric cystic lymphangioma of the small bowel in an adult: A case presentation and literature review. Acta Gastroenterol Belg. 2016;79:491-493. [PubMed] |
| 54. | Kumar B, Bhatnagar A, Upadhyaya VD, Gangopadhyay AN. Small Intestinal Lymphangioma Presenting as an Acute Abdomen with Relevant Review of Literature. J Clin Diagn Res. 2017;11:PD01-PD02. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Chin CC, Shiau J, Luo CW, Hou MF. Lymphangioma of small bowel in adults: A rare cause of abdominal symptoms. Asian J Surg. 2023;46:863-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 56. | Wang ZZ, Shen LY, Zhou JJ, Tang JL, Ye LP, Shen CB, Li SW, Zhou XB. Clinical manifestation and treatment of small intestinal lymphangioma: A single center analysis of 15 cases. Front Med (Lausanne). 2022;9:975698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Campbell WJ, Irwin ST, Biggart JD. Benign lymphangioma of the jejunal mesentery: an unusual cause of small bowel obstruction. Gut. 1991;32:1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/