Published online Jan 24, 2026. doi: 10.5306/wjco.v17.i1.111426
Revised: July 29, 2025
Accepted: December 11, 2025
Published online: January 24, 2026
Processing time: 205 Days and 10 Hours
The E3 ubiquitin ligase murine double minute 2 (MDM2) is a key negative regu
To evaluate the clinical, pathological, and prognostic significance of MDM2 expr
A retrospective analysis was conducted on 71 patients diagnosed with MM or related plasma cell disorders treated at the National Cancer Institute between 2018 and 2022. MDM2 protein expression was assessed using IHC on extramedullary lesion biopsy samples, employing the MDM2 (A.M.1) monoclonal antibody. Nuc
MDM2 expression was identified in 30% of patient samples. While no major differences were observed in baseline demographics, disease stage, or most laboratory values, serum albumin levels were significantly lower in MDM2-positive patients (P = 0.007). At 12 weeks, patients with MDM2-positive disease showed significantly poorer treatment responses based on International Myeloma Working Group criteria (P = 0.002), and early clinical response was moderately negatively correlated with MDM2 expression (Spearman’s P = 0.375, P = 0.001). This correlation was not observed at 24 weeks. Immunophenotypic analysis indicated that MDM2-positive plasma cells exhibited lower epithelial membrane antigen (P = 0.014) and higher CD45 expression (P = 0.039), suggesting altered differentiation. Kaplan-Meier survival analysis demonstrated a markedly shorter median RFS in the MDM2-positive group (22 months vs 68 months, P < 0.001), although no significant difference was found in overall sur
IHC-detected MDM2 overexpression identifies a distinct subset of plasma cell neoplasms characterized by reduced early treatment responsiveness and significantly shorter RFS. These findings support the potential of MDM2 as a prognostic biomarker for early relapse risk in MM. Incorporating MDM2 assessment into diagnostic and pro
Core Tip: This research explores the prognostic significance of murine double minute 2 (MDM2) expression in plasma cell neoplasms, with particular attention to plasmacytomas. The findings reveal a distinct subset of patients with MDM2 positivity who demonstrate poorer early treatment responses and markedly shorter relapse-free survival intervals. Incor
- Citation: Ebrahim NAA, Elfandy H, Arafat AMA, Darwish AD, Eltohamy MI. Evaluating murine double minute 2 status as a stratification tool for risk-adapted management in plasma cell neoplasms. World J Clin Oncol 2026; 17(1): 111426
- URL: https://www.wjgnet.com/2218-4333/full/v17/i1/111426.htm
- DOI: https://dx.doi.org/10.5306/wjco.v17.i1.111426
The E3 ubiquitin ligase murine double minute 2 (MDM2) plays a central role in regulating the tumor suppressor protein p53 by binding to its transactivation domain and targeting it for ubiquitin-dependent degradation. Under normal physiological conditions, this autoregulatory feedback loop ensures that p53 levels remain low. However, in response to cellular stress, MDM2 activity is suppressed, allowing p53 to accumulate and activate its downstream tumor-suppressive functions[1,2]. In many cancers, MDM2 is frequently overexpressed or amplified, effectively disabling p53’s tumor-suppressive activity even in cases where the TP53 gene remains unmutated. Additionally, accumulating evidence high
In the context of multiple myeloma (MM), elevated MDM2 expression has been consistently reported and appears to contribute to malignant plasma cell proliferation and survival. Prior research demonstrated abundant MDM2 protein levels in MM cell lines and plasma cell leukemia samples, contrasting with its low expression in normal bone marrow (BM) cells. Functional studies using antisense oligonucleotides to inhibit MDM2 in MM models resulted in cell cycle arrest at the G1 phase and induction of apoptosis, underscoring its role in promoting tumor cell viability[3,4]. Clinical gene expression datasets further reveal that MDM2 expression is significantly higher in MM patients compared to healthy individuals and tends to increase with more advanced stages according to the International Staging System (ISS) and revised ISS. Importantly, overexpression of MDM2 has been linked to disease progression in MM, with large-scale data indicating that patients with higher MDM2 levels experience more advanced disease at diagnosis, increased relapse rates, and shorter progression-free survival compared to those with lower MDM2 expression[3,4].
Given these findings, nuclear accumulation of MDM2 may serve as a potential biomarker for high-risk MM. However, the prognostic value of MDM2 protein expression detected by immunohistochemistry (IHC) in MM remains poorly defined. To explore this, we conducted a retrospective study involving 71 patients with MM or related plasma cell neo
This investigation was conducted as a retrospective study following approval from the Institutional Review Board of the National Cancer Institute. We retrospectively reviewed 71 patients diagnosed with MM or other plasma cell neoplasms, including solitary plasmacytoma and plasma cell leukemia, at the National Cancer Institute between January 2018 and December 2022. All diagnoses were established following the 5th edition WHO classification of hematolymphoid neo
Standardized case report forms were utilized in all data collection to ensure data integrity. The clinical data were abs
MDM2 expression was assessed on extramedullary lesions, which were biopsied for diagnosis and represent a more aggressive disease phenotype with distinct biological characteristics from BM-limited disease. Diagnostic tissue biopsy samples, previously preserved as formalin-fixed, paraffin-embedded blocks, were collected from the Oncologic Pathology Department for immunohistochemical analysis and sectioned at a thickness of 4 microns. Immunohistochemical staining for MDM2 protein was performed on the BenchMarkUltra system using a standardized avidin-biotin peroxidase method with a commercially sourced monoclonal antibody specific for MDM2 [MDM2 (A.M.1) monoclonal antibody]. The ≥ 1% threshold for MDM2 positivity was established using established precedent from prior research that analyzed oncogenic protein expression in neoplasms[6-8], where percentage cut-offs have been of clinical importance in MDM2 testing. Nuclear intensity of staining was analyzed by four blinded pathologists in a consensus fashion, and good concordance among pathologists in marking positive vs negative cases was obtained. The percentage of positively stained nuclei was documented, and cases were categorized into positive or negative groups for comparative analyses. To ensure the specificity of MDM2 expression, 10 control patients with normal BM samples free of hematologic malignancy were analyzed, and there was little nuclear staining for MDM2 in normal plasma cells (0%-1% positivity), validating the ≥ 1% threshold of abnormal overexpression.
Patients were divided into MDM2-positive and MDM2-negative cohorts for comparative evaluation. Continuous variables were reported as medians or means and compared using either the independent t-test or the Mann-Whitney U test, depending on data distribution. Categorical variables were summarized as n (%) and compared using χ2 or Fisher’s exact tests, as appropriate. The timing of remission was defined as achieving either complete remission or very good partial remission. Relapse-free survival (RFS) was defined as the interval from the time of best response, either complete or partial remission, to the first documented disease progression or relapse, with patients remaining in remission censored at last follow-up. OS was measured from the date of diagnosis to death from any cause or last clinical contact. Kaplan-Meier survival curves were constructed for both RFS and OS, with comparisons between MDM2-positive and MDM2-negative groups assessed via the log-rank test. The non-parametric correlation analyses were conducted using Kendall’s tau-b and Spearman’s rho coefficients. All statistical tests were two-tailed, and a P-value of less than 0.05 was considered statistically significant. Statistical analyses were conducted using SPSS version 28 software (IBM Corp., Armonk, NY, United States).
A total of 71 patients diagnosed with MM or plasma cell neoplasms were included in this retrospective analysis. Among these, 50 patients (70%) were classified as MDM2-negative, while 21 patients (30%) were MDM2-positive. Table 1 pro
| Parameter | MDM2-negative (n = 50) | MDM2-positive (n = 21) | Total (n = 71) |
| At diagnosis | |||
| Age (years) | 57 (34-89) (59.1 ± 11.5) | 58 (40-79) (58.5 ± 10.3) | 58 (34-89) (58.9 ± 11.1) |
| Calcium (mg/dL) | 10.6 (7.9-13.5) (10.9 ± 1.4) | 10.4 (8.0-12.9) (10.6 ± 1.2) | 10.5 (7.9-13.5) (10.8 ± 1.4) |
| Creatinine (mg/dL) | 1.1 (0.5-3.5) (1.5 ± 0.8) | 1.3 (0.7-4.0) (1.5 ± 0.8) | 1.2 (0.5-4.0) (1.5 ± 0.8) |
| β2-microglobulin (mg/L) | 4.9 (1.9-22.8) (6.6 ± 4.5) | 4.7 (2.1-15.3) (5.9 ± 3.4) | 4.9 (1.9-22.8) (6.4 ± 4.2) |
| LDH (U/L) | 278 (149-790) (321.3 ± 159.5) | 275 (188-450) (286.1 ± 85.4) | 276 (149-790) (310.9 ± 142.0) |
| Total protein (g/dL) | 10.2 (6.3-13.4) (10.4 ± 1.4) | 10.5 (7.0-12.9) (10.3 ± 1.4) | 10.4 (6.3-13.4) (10.4 ± 1.4) |
| Albumin (g/dL) | 4.1 (2.0-5.9) (3.9 ± 1.2) | 2.9 (2.0-5.3) (3.1 ± 0.9) | 3.7 (2.0-5.9) (3.7 ± 1.2) |
| BM plasma cells (%) | 20.5 (0-87) (28.2 ± 21.8) | 26 (7-80) (34.2 ± 23.2) | 22 (0-87) (30.0 ± 22.2) |
| Hemoglobin (g/dL) | 8.4 (5.0-12.0) (8.2 ± 1.8) | 8.0 (5.0-10.2) (8.1 ± 1.4) | 8.3 (5.0-12.0) (8.2 ± 1.7) |
| At 12 weeks | |||
| Hemoglobin (g/dL) | 10.0 (6.4-14.0) (9.7 ± 1.8) | 9.4 (5.4-11.5) (9.3 ± 1.4) | 10.0 (5.4-14.0) (9.6 ± 1.7) |
| β2-microglobulin (mg/L) | 2.3 (0.5-9.3) (2.9 ± 1.9) | 2.4 (0.9-9.6) (2.9 ± 1.8) | 2.3 (0.5-9.6) (2.9 ± 1.9) |
| Creatinine (mg/dL) | 8.9 (7.2-11.8) (8.9 ± 0.9) | 8.9 (6.3-10.1) (8.8 ± 0.8) | 8.9 (6.3-11.8) (8.9 ± 0.9) |
| Calcium (mg/dL) | 1.1 (0.6-5.6) (1.3 ± 0.8) | 1.3 (0.5-2.5) (1.3 ± 0.5) | 1.1 (0.5-5.6) (1.3 ± 0.7) |
| Total protein (g/dL) | 8.0 (5.1-12.8) (8.3 ± 1.6) | 8.0 (5.7-11.9) (8.4 ± 1.8) | 8.0 (5.1-12.8) (8.3 ± 1.6) |
Initial treatment modalities (Table 2) included chemotherapy (CTH), radiotherapy, and combined CTH and radiotherapy. CTH regimens predominantly consisted of cyclophosphamide, bortezomib, and dexamethasone (CyBorD) and vin
| Initial treatment type | Frequency | Percent (%) | Initial chemotherapy type | Frequency | Percent (%) |
| CTH | 62 | 87.3 | CyBorD | 37 | 52.1 |
| CTH and RTH | 8 | 11.3 | VAD | 29 | 40.8 |
| RTH | 1 | 1.4 | Daratumumab | 4 | 5.6 |
No statistically significant difference between the two groups regarding age (P = 0.83). gender distribution (P = 0.74), family history of malignancy (P = 0.251), and comorbidities (P = 0.143). The prevalence of comorbidities, including diabetes, hypertension, or both, was also similar between groups (P = 0.12). Additionally, no statistically significant associations were identified for MDM2 status and pathological fractures (P = 0.605), paraplegia (P = 0.521), plasmacytoma classification (P = 0.444), extramedullary plasmacytoma location (P = 0.538), Eastern Cooperative Oncology Group performance status (P = 0.423), M-protein type by immunofixation (P = 0.478), circulating clonal plasma cells (P = 0.518). According to the ISS, stage 3 disease predominated (41%) across the entire cohort, with a nonsignificant distribution between MDM2 categories (P = 0.118). Regarding other recognized high-risk factors, no statistical association between MDM2 expression and M-protein quantitation in serum (P = 0.159), elevated levels of β2-microglobulin (P = 0.97), elevated LDH (P = 0.88), or renal insufficiency (P = 0.47). The sole finding of statistical significance was association with low serum albumin (P = 0.007). BM assessment, including BM plasma cell percentage in aspirates and biopsy, overall marrow cellularity, and reticulin fibrosis grade as per WHO classification, did not reveal any significant differences between MDM2-positive and MDM2-negative cases. There were no substantial differences in the 12 and 24-week la
A significant inverse correlation was observed between MDM2 positivity and serum albumin levels at presentation (r =
At the 12-week evaluation, a statistically significant association was found between MDM2 status and IMWG response categories [Pearson’s χ2 (2) = 12.530, P = 0.002]. This result was supported by the likelihood ratio test [χ2 (2) = 13.541, P = 0.001] and the linear-by-linear association test, which revealed a significant linear trend across the ordered response categories [χ2 (1) = 10.897, P = 0.001]. Collectively, these analyses indicate that MDM2 positivity was associated with poorer clinical outcomes at this early stage of treatment. At the 24-week timepoint, although testing the association between MDM2 status and IMWG response categories did not reach conventional levels of statistical significance [χ2 (2) = 5.482, P = 0.065], it hinted at a potential trend. The likelihood ratio test approached significance [χ2 (2) = 6.140, P = 0.046], while the linear-by-linear association test did not indicate a significant trend [χ2 (1) = 0.473, P = 0.492]. These findings suggest that the prognostic impact of MDM2 status on response outcomes diminishes as treatment progresses. In addition, the association between MDM2 status and the timing of remission, defined as achieving either complete remission or very good partial remission, was evaluated using χ2 tests. No significant association was observed in this analysis [Pearson’s χ2 (64) = 68.600, P = 0.324], although the likelihood ratio test trended toward, but did not achieve, significance [χ2 (64) = 83.456, P = 0.052].
To further assess the relationship between MDM2 status and treatment response, non-parametric correlation analyses were conducted using Kendall’s tau-b and Spearman’s rho coefficients. At 12 weeks, a moderate, positive, and statistically significant correlation was observed (Kendall’s tau-b = 0.368, P = 0.002; Spearman’s P = 0.375, P = 0.001), indicating that patients with positive MDM2 status were more likely to experience less favorable responses, reflected by assignment to higher IMWG response categories. By 24 weeks, however, this relationship had substantially weakened and was no longer statistically significant (Kendall’s tau-b = 0.051, P = 0.653; Spearman’s P = 0.054, P = 0.657). Of particular importance, a strong, positive, and highly significant correlation persisted between IMWG response categories at 12 weeks and 24 weeks (Kendall’s tau-b = 0.627, P < 0.001; Spearman’s P = 0.678, P < 0.001), demonstrating that early treatment responses strongly predicted subsequent outcomes throughout the course of therapy. These findings highlight MDM2 status as a valuable early prognostic biomarker in MM. Its assessment appears to be particularly useful for pre
Interestingly, epithelial membrane antigen (EMA) expression differed markedly: 45% of MDM2-negative patients were EMA-positive, whereas none of the MDM2-positive patients exhibited EMA positivity, a difference that reached statistical significance (P = 0.014). Additionally, leukocyte common antigen expression patterns varied, with 36% of MDM2-positive cases demonstrating focal expression compared to 5.6% in MDM2-negative patients (P = 0.039). The observed EMA negativity and altered leukocyte common antigen (CD45) expression patterns in the MDM2-positive group reflect disrupted plasma cell differentiation and maturation programs with significant biological implications. EMA loss indi
Among cases for which there was accessible CD56 IHC (n = 60), CD56-negative cases showed a trend towards greater MDM2 positivity (42% vs 23%, P = 0.08), consistent with the known relationship between CD56 negativity and aggressive MM biology. Other studies incorporating comprehensive flow cytometry panels and measurement of MRD would provide valuable confirmation of MDM2’s prognostic significance relative to that of established biomarkers.
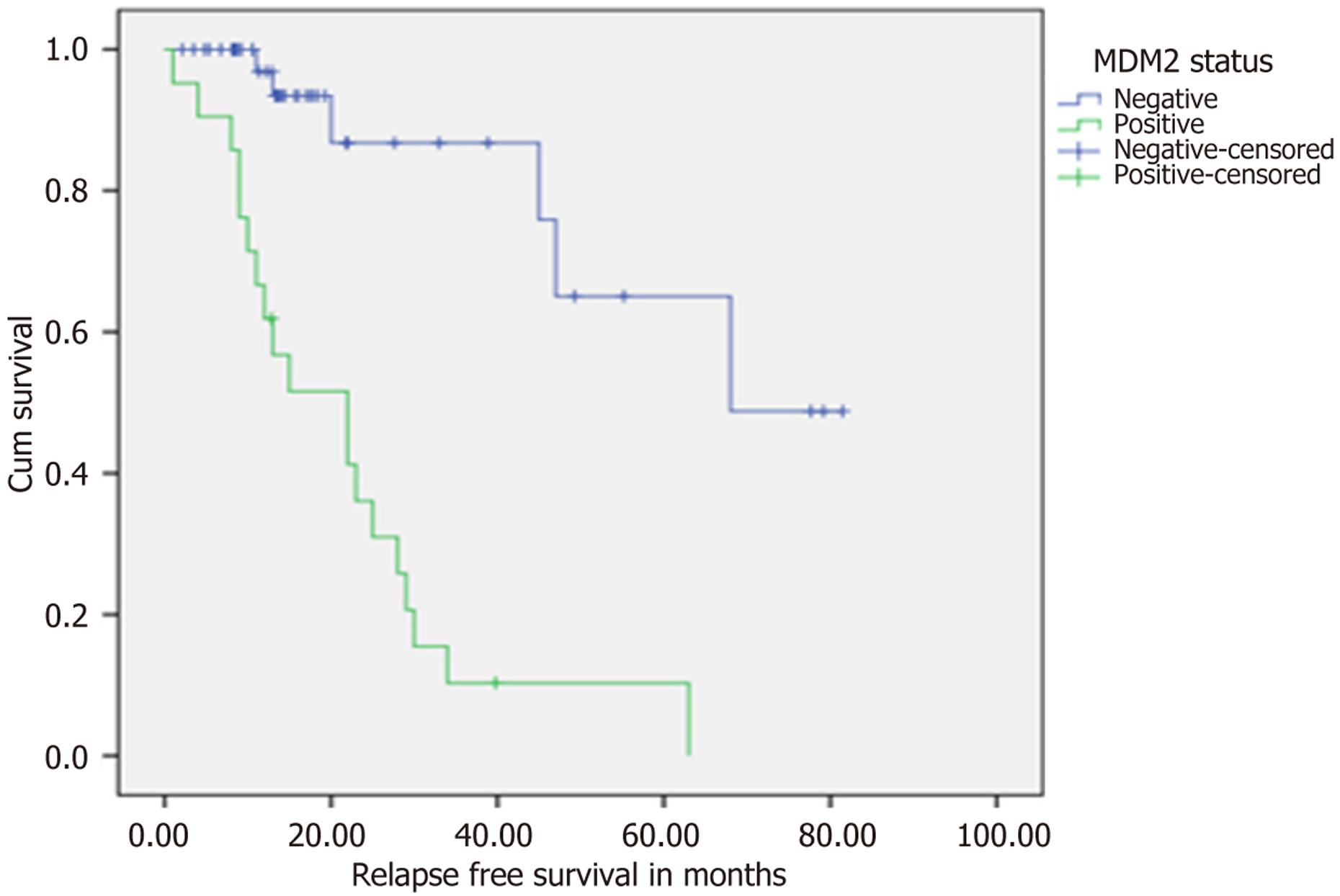
In this study, patients were followed for a period ranging from 1.64 months to 81.50 months, with an average follow-up duration of 25.54 ± 20.89 months. To investigate the prognostic relevance of MDM2 status, a Kaplan-Meier analysis assessed RFS in a cohort of 71 patients stratified according to MDM2 status (Table 3). The results demonstrated a clear and significant difference in outcomes between the groups. Patients without MDM2 expression achieved a notably higher median RFS compared to those with positive MDM2. The mean RFS values also favoured the MDM2-negative group. This difference was statistically significant. Additionally, a higher censoring rate was observed in the MDM2-negative group (88%), reflecting a greater proportion of patients remaining relapse-free or alive at last follow-up. These data collectively indicate that MDM2 status is a strong prognostic indicator for RFS in patients with MDM2 positivity, linked to significantly poorer outcomes.
| Parameter | MDM2-negative (n = 50) | MDM2-positive (n = 21) | P value |
| Median RFS (months) | 68 | 22 | < 0.001 |
| Mean RFS (months) | 62.93 | 21.98 | |
| Median OS (months) | Not reached1 | 35 (95%CI: 23-47) | 0.401 |
| Mean OS (months) | 54.8 (95%CI: 42-67.6) | 46.2 (95%CI: 33.9-58.5) |
Subsequently, the effect of MDM2 status on OS was examined through a separate Kaplan-Meier analysis within the same patient cohort. Among the 50 MDM2-negative patients, 10 deaths (20%) occurred, while 40 cases (80%) were censored. In comparison, 21 patients with MDM2 included 11 deaths (52.4%) and 10 censored cases (47.6%). The mean OS was higher in the MDM2-negative group than in the MDM2-positive group. However, the difference between the MDM2-positive and MDM2-negative groups did not reach statistical significance. These findings suggest that while MDM2 status is a significant prognostic factor for RFS in this setting, its effect on OS is less evident.
Notably, MDM2 positivity was also not associated with other recognized high-risk features like advanced ISS stage, elevated β2-microglobulin, or renal insufficiency. MDM2 expression ≥ 1% emerges as a highly significant independent risk factor for early relapse in MM patients. The adjusted odds ratio of 370.73 indicates an extraordinarily strong asso
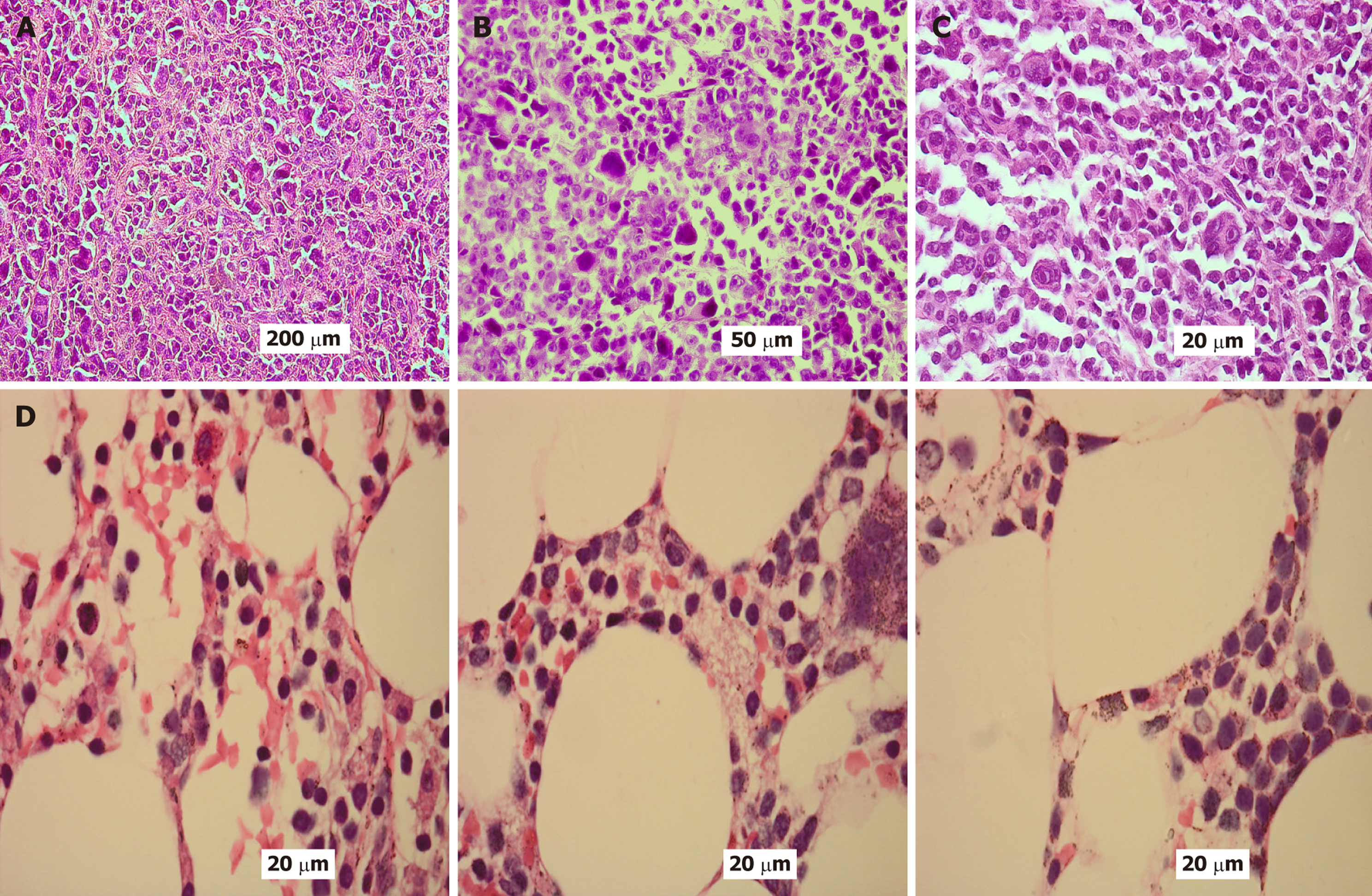
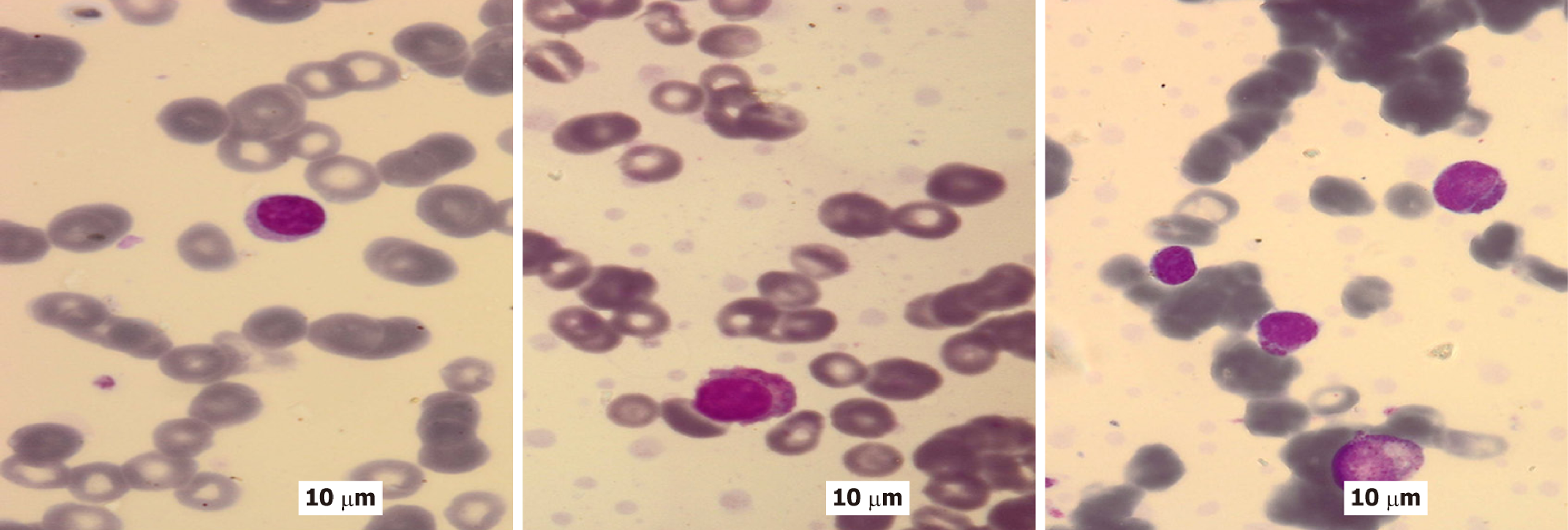
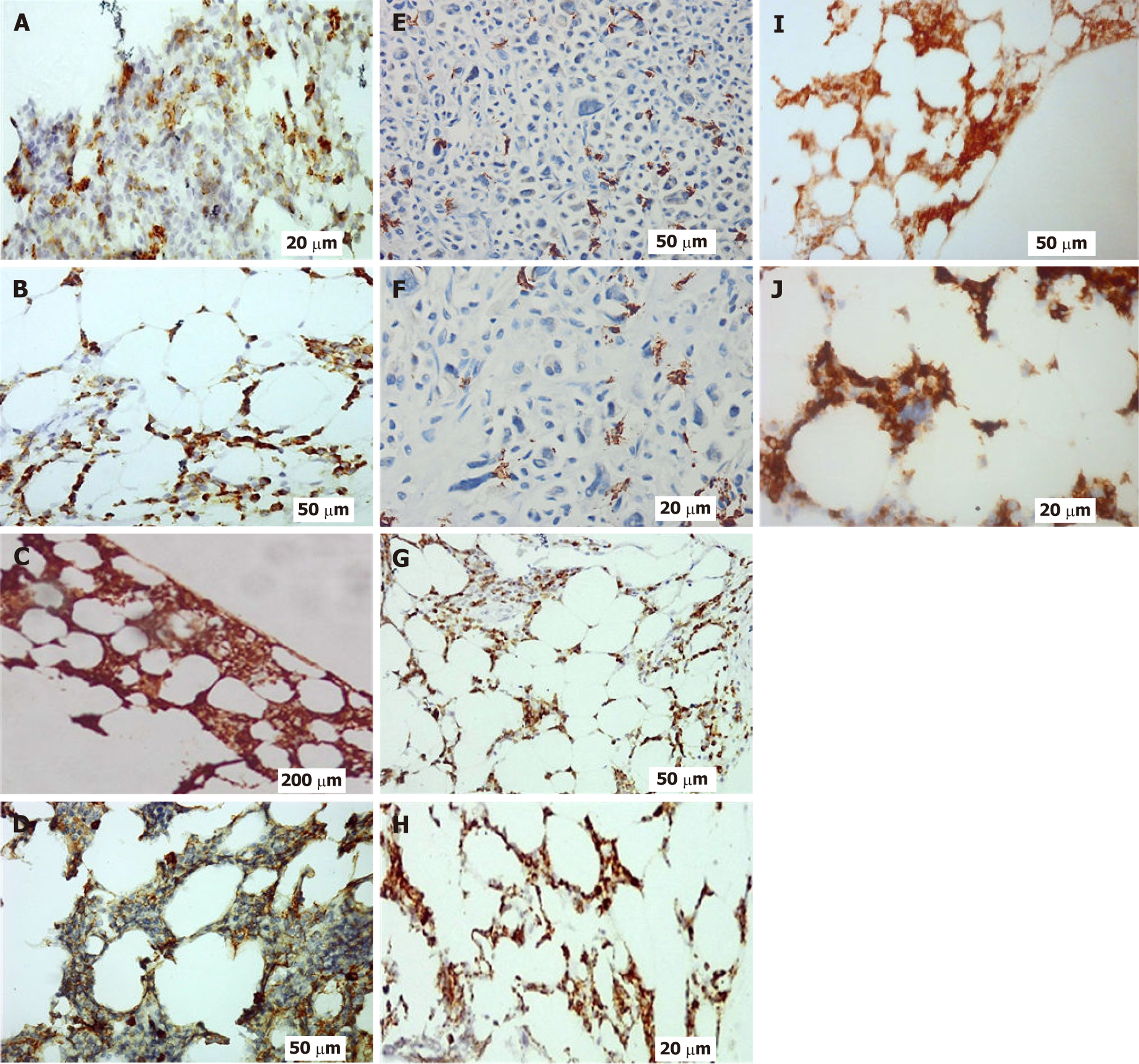
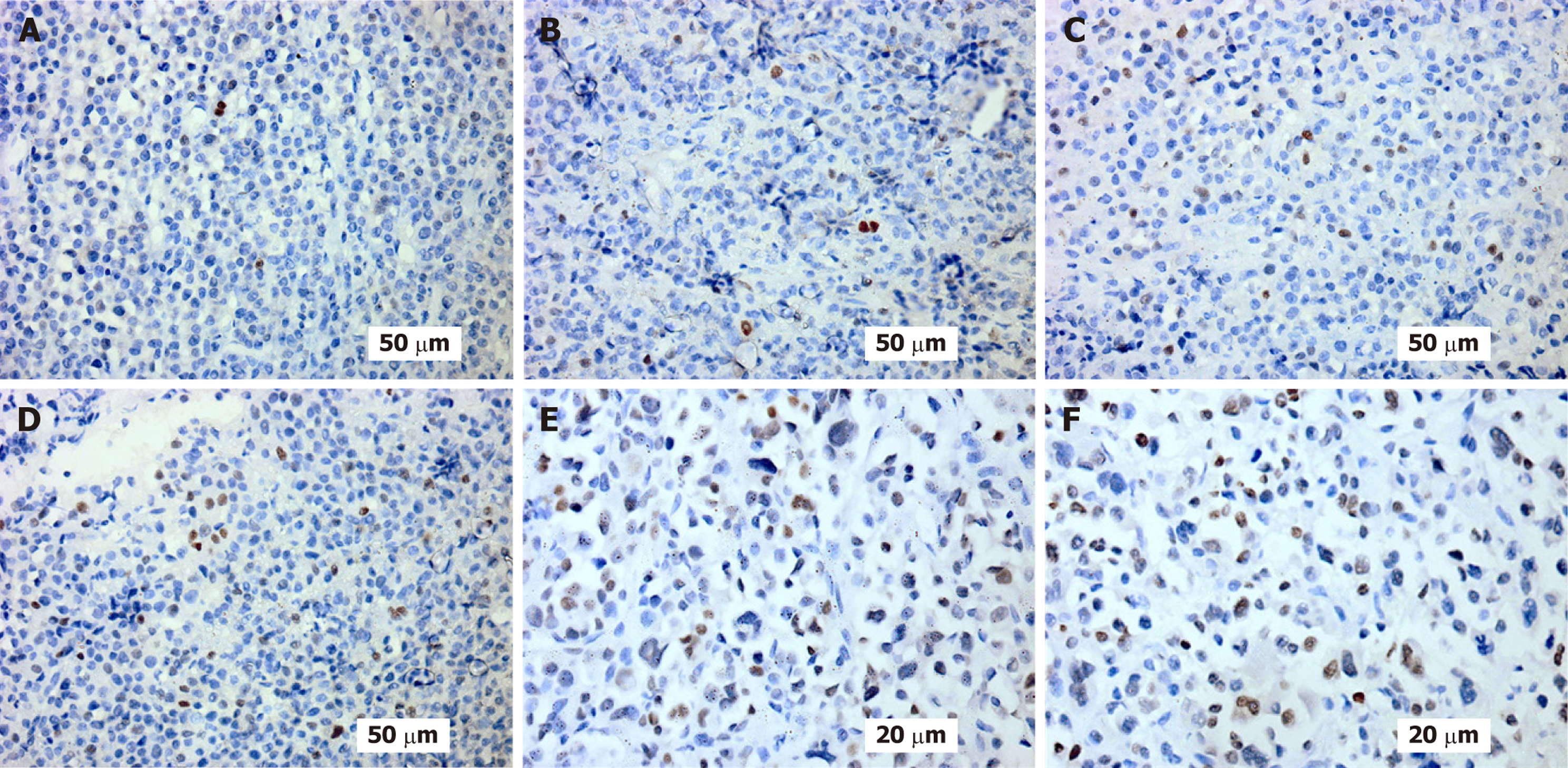
Figures 1-3 provide illustrative examples of the histopathological and immunophenotypic characteristics commonly observed in MM and related plasma cell disorders. Figure 1A-C displays a series of hematoxylin and eosin-stained sections at increasing magnifications, capturing extramedullary tissue infiltration by malignant plasma cells. Figure 1D focuses on BM involvement, showing dense intertrabecular infiltration by atypical plasma cells with hallmark morphological features. In Figure 2, peripheral blood smears reveal circulating neoplastic plasma cells alongside classic hema
In this 71-patient cohort of MM, we determined that nuclear MDM2 expression in ≥ 1% of myeloma cells was associated with significantly reduced RFS. Our findings demonstrate that MDM2-positive patients had significantly inferior median RFS of 22 months compared with MDM2-negative patients at 68 months (P < 0.001), as well as significantly inferior early treatment response at 12 weeks (P = 0.002). These results concur with new data from a series of recent works indicating the prognostic significance of the p53-MDM2 pathway in plasma cell malignancies. Our findings concur with previous works demonstrating the adverse prognosis of dysregulation of the p53 pathway in MM. Pruneri et al[12] had previously indicated that p53 nuclear accumulation, determined by IHC in BM biopsies, was significantly correlated with reduced survival, and its prognostic value held even after multivariate analysis. This is consistent with our finding that MDM2 overexpression, which functionally inactivates p53, is associated with unfavorable clinical outcomes. In the same vein, the systematic review by Flynt et al[1] also highlighted that TP53 dysregulation, specifically MDM2 overexpression, is a key mechanism of high-risk disease biology in MM and that patients have significantly reduced progression-free and OS[1]. The molecular basis for our results is underpinned by recent genomic analyses. Lv et al[13] revealed that MDM2 is a key member of p53-linked gene signatures implicated with adverse prognosis in MM and that overexpression of MDM2 has been linked with poorer OS in large patient cohorts. Moreover, studies have proven that overexpression of the MDM2 protein enhances the proliferative capability of MM cells by binding E2F-1, p53, and p21 to circumvent normal cell cycle checkpoints and promote cancer growth. Our observation that MDM2-positive patients had clear-cut immunophenotypic characteristics - i.e., diminished EMA expression (P = 0.014) and augmented CD45 expression (P = 0.039) - suggests that overexpression of MDM2 may be associated with less differentiated, more malignant plasma cell clones. This phenotypic pattern is consistent with the prediction that disruption of the p53 pathway underlies cancellation of normal differentiation programs and enhanced stem cell-like behavior, conferring resistance to therapy and early relapse. Our findings must be viewed against conflicting evidence regarding the prognostic function of MDM2 in hematologic malignancies. Conflicting evidence comes from the work of Elnenaei et al[14], in which they compared the amplification of the MDM2 gene and trisomy 12 in 48 patients with MM using fluorescent in situ hybridization. Contrary to our observations, they concluded that although MDM2 gene amplification was observed in 8% of the cases, it was not correlated with poor prognosis, response to therapy, survival, or event-free survival. The authors made the conclusion that “the presence of amplification or trisomy 12 did not appear to be related to an unfavorable prognosis”. This discrepancy highlights a key methodological observation: Our research quantified MDM2 protein expression by IHC, while Elnenaei et al[14] quantified gene amplification. The inability of gene amplification to correlate with clinical outcomes in their research suggests that MDM2 overexpression can be the result of mechanisms other than gene amplification, including more stable mRNA, increased protein translation, or reduced protein degradation. Actually, their study found that “the mechanism of this overexpression, as well as the role of MDM2 in MM, is not well established”. The broader MDM2 lite
Our findings have therapeutic implications for the clinical context in addition to prognostication and provide potential for therapeutic application. The p53-MDM2 pathway has been an encouraging target for therapy, with MDM2 anta
The absence of relevant OS differences despite shorter RFS in MDM2-positive patients should be accounted for by considering some underlying causes. The majority of patients in our series received effective salvage treatments in the setting of post-relapse, such as new drugs and autologous stem cell transplantation, which potentially counteracted survival differences. Furthermore, our median follow-up time of 25.54 months may have been too brief to detect long-term survival differences. This trend suggests that MDM2 would be involved primarily in early resistance to treatment and relapse kinetics, but later treatment may well overcome the initial biological disadvantage.
There are some limitations of the present study that bear mentioning. First, the single-center, retrospective study design limits generalizability and introduces potential selection bias. Second, our patient sample of 71 patients, while adequate for the aims of initial biomarker evaluation, must be validated in larger multicenter cohorts. Third, MDM2 assessment was performed on extramedullary lesions rather than BM samples and thus may not optimally represent the BM disease biology. Fourth, our follow-up time, while long enough to demonstrate significant differences in RFS, may not uncover the ultimate effect on OS, particularly given the enhanced survival benefit with more contemporary MM therapies. Fifth, we lacked complete information on some established prognostic markers, such as cytogenetic abnormalities and minimal residual disease status. Sixth, we did not obtain TP53 mutation status through screening, which could influence the functional importance of MDM2 overexpression. Finally, the observational nature of the study design precludes causal inferences between MDM2 expression and clinical outcomes.
This study demonstrates that overexpression of MDM2 protein in MM is associated with significantly reduced RFS, indicative of a more aggressive disease course. Immunohistochemical detection of MDM2 may offer prognostic value in identifying patients at higher risk of early relapse. Clinically, this biomarker could help guide risk-adapted management strategies, including closer monitoring or consideration of novel targeted therapies in MDM2-positive individuals. While our study shows the potential utility of MDM2 as a prognostic biomarker, validation by large, multicenter prospective trials is required before clinical use. Subsequent studies will need to specifically examine MDM2’s use in risk stratification models and its utility to inform therapeutic choices in risk-adapted therapies.
The authors sincerely acknowledge Cairo University for its institutional support, with special appreciation extended to the National Cancer Institute and the Department of Oncologic Pathology. Their expert insight, collaborative spirit, and access to critical research facilities significantly contributed to the successful completion of this work.
| 1. | Flynt E, Bisht K, Sridharan V, Ortiz M, Towfic F, Thakurta A. Prognosis, Biology, and Targeting of TP53 Dysregulation in Multiple Myeloma. Cells. 2020;9:287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 2. | Yao Y, Zhang Q, Li Z, Zhang H. MDM2: current research status and prospects of tumor treatment. Cancer Cell Int. 2024;24:170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 3. | Teoh G, Urashima M, Ogata A, Chauhan D, DeCaprio JA, Treon SP, Schlossman RL, Anderson KC. MDM2 protein overexpression promotes proliferation and survival of multiple myeloma cells. Blood. 1997;90:1982-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Faruq O, Zhao D, Shrestha M, Vecchione A, Zacksenhaus E, Chang H. Targeting an MDM2/MYC Axis to Overcome Drug Resistance in Multiple Myeloma. Cancers (Basel). 2022;14:1592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Quesnel B, Preudhomme C, Oscier D, Lepelley P, Collyn-d'Hooghe M, Facon T, Zandecki M, Fenaux P. Over-expression of the MDM2 gene is found in some cases of haematological malignancies. Br J Haematol. 1994;88:415-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Yoshida A, Ushiku T, Motoi T, Shibata T, Beppu Y, Fukayama M, Tsuda H. Immunohistochemical analysis of MDM2 and CDK4 distinguishes low-grade osteosarcoma from benign mimics. Mod Pathol. 2010;23:1279-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Turbin DA, Cheang MC, Bajdik CD, Gelmon KA, Yorida E, De Luca A, Nielsen TO, Huntsman DG, Gilks CB. MDM2 protein expression is a negative prognostic marker in breast carcinoma. Mod Pathol. 2006;19:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Dujardin F, Binh MB, Bouvier C, Gomez-Brouchet A, Larousserie F, Muret Ad, Louis-Brennetot C, Aurias A, Coindre JM, Guillou L, Pedeutour F, Duval H, Collin C, de Pinieux G. MDM2 and CDK4 immunohistochemistry is a valuable tool in the differential diagnosis of low-grade osteosarcomas and other primary fibro-osseous lesions of the bone. Mod Pathol. 2011;24:624-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 9. | Baldus SE, Palmen C, Thiele J. MUC1 (EMA) expressing plasma cells in bone marrow infiltrated by plasma cell myeloma. Histol Histopathol. 2007;22:889-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 10. | Kumar S, Rajkumar SV, Kimlinger T, Greipp PR, Witzig TE. CD45 expression by bone marrow plasma cells in multiple myeloma: clinical and biological correlations. Leukemia. 2005;19:1466-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Kumar S, Kimlinger T, Morice W. Immunophenotyping in multiple myeloma and related plasma cell disorders. Best Pract Res Clin Haematol. 2010;23:433-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Pruneri G, Carboni N, Baldini L, Intini D, Colombi M, Bertolini F, Valentini S, Maisonneuve P, Viale G, Neri A. Cell cycle regulators in multiple myeloma: prognostic implications of p53 nuclear accumulation. Hum Pathol. 2003;34:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Lv JT, Jiao YT, Han XL, Cao YJ, Lv XK, Du J, Hou J. Integrating p53-associated genes and infiltrating immune cell characterization as a prognostic biomarker in multiple myeloma. Heliyon. 2024;10:e30123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Elnenaei MO, Gruszka-Westwood AM, A'Hernt R, Matutes E, Sirohi B, Powles R, Catovsky D. Gene abnormalities in multiple myeloma; the relevance of TP53, MDM2, and CDKN2A. Haematologica. 2003;88:529-537. [PubMed] |
| 15. | Onel K, Cordon-Cardo C. MDM2 and prognosis. Mol Cancer Res. 2004;2:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Zhang X, Wen X, Peng R, Pan Q, Weng D, Ma Y, Zhang Y, Yang J, Men L, Wang H, Liang E, Wang C, Yang D, Zhang L, Zhai Y. A first-in-human phase I study of a novel MDM2/p53 inhibitor alrizomadlin in advanced solid tumors. ESMO Open. 2024;9:103636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/