Published online Jul 24, 2025. doi: 10.5306/wjco.v16.i7.107339
Revised: April 21, 2025
Accepted: June 3, 2025
Published online: July 24, 2025
Processing time: 115 Days and 20.2 Hours
Breast cancer is one of the most common malignancies worldwide and is a major cause of cancer-related mortality among women. Beyond tumor cells, the tumor microenvironment (TME) also plays an important role in cancer progression, therapy resistance, and metastasis. The TME is a complex ecosystem consisting of stromal and immune cells, extracellular matrix (ECM), and various signaling molecules that dynamically interact with tumor cells. Cancer-associated fibro
Core Tip: The tumor microenvironment (TME) in breast cancer is a complex and dynamic ecosystem consisting of immune cells, stromal components, and the extracellular matrix. This review investigates how key players in the TME, including cancer-associated fibroblasts, tumor-infiltrating lymphocytes, and macrophages, contribute to tumor progression, immune modulation, and metastasis. Understanding these interactions sheds light on the mechanisms driving breast cancer heterogeneity and disease progression.
- Citation: Çakal S, Er Urgancı B, Şimşek S. Key players in the breast cancer microenvironment: From fibroblasts to immune cells. World J Clin Oncol 2025; 16(7): 107339
- URL: https://www.wjgnet.com/2218-4333/full/v16/i7/107339.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i7.107339
Breast cancer is one of the most common types of cancer with approximately 2.3 million cases worldwide and is the fourth most common cause of cancer-related deaths. Breast cancer is not only the most commonly diagnosed cancer in women but is also the leading cause of cancer-related deaths among women.
Breast cancer is divided into three groups considering its pathological characteristics and invasiveness: Non-invasive
Invasive breast cancer is characterized by the spread of cancer cells from lobules or ducts into the surrounding stromal tissue. It is divided into two groups, namely, invasive ductal carcinoma and invasive lobular carcinoma. Invasive carcinomas tend to spread to other parts of the body, for example, they metastasize to lymph nodes or other organs[1]. A total of 90%-95% of all breast cancer cases are invasive.
Metastatic breast cancer, also known as stage 4 or advanced breast cancer, refers to cancer cells that have spread from the breast to other parts of the body. This spread can occur in nearby areas of the breast such as the axillary lymph nodes, as well as in distant areas such as the lung, liver, bone, and brain. Even if the primary tumor is removed, microscopic tumor cells or micrometastases can remain in the body, causing the cancer to recur and spread[1].
Molecular classification is the separation of invasive cancers by considering mRNA expression levels, regardless of the histological subtype. In their study conducted in 2000, Perou et al[2] divided 38 breast cancer cases into four groups: Luminal, human epidermal growth factor receptor 2 (HER2)-enriched, basal-like, and normal-like. Subsequently, the luminal group was divided into two subgroups: Luminal A and luminal B. The fifth subgroup, the claudin-low group, was discovered in 2007[3].
Luminal cancers, which are estrogen receptor-positive (ER+), constitute approximately 70% of breast cancer cases in Western populations and are primarily invasive in nature. Luminal A tumors are ER+ and/or progesterone receptor-positive (PR+) and HER2-negative (HER2-). In this subgroup, ER transcription factors activate genes whose expression is characteristic for the luminal epithelium in the breast ducts. It is a clinically low-grade and slow-progressing subtype with the best prognosis. Luminal A cancers constitute 40% of all breast cancer cases.
Luminal B subtype has a higher grade and worse prognosis and includes ER+ and PR+ and/or HER2+ breast cancers. In addition, the expression of genes associated with proliferation is high. Genes associated with luminal epithelial markers exhibit lower expression in luminal B and triple-negative breast cancers (TNBCs) compared to luminal A cancers. The luminal B subtype constitutes approximately 20% of all breast cancer cases.
Breast cancers where ER and PR are negative and HER2 is overexpressed, are characterized as HER2-positive breast cancer, which constitutes 10%-15% of breast cancer cases. In this subtype, the expression of proliferation-related genes is prominent, while expression of basal- and luminal-related genes is diminished. It progresses faster than luminal cancers and has a worse prognosis.
Basal-like/TNBC constitutes approximately 20% of breast cancer cases. TNBC is characterized by ER, PR, and HER2 negativity. TNBC is more common in women with the BReast CAncer gene 1 (BRCA1) mutation. It is usually more aggressive than other types and forms higher-grade tumors. A total of 11%-16% of patients with TNBC have BRCA mutations, and approximately 80% of those harboring the BRCA1 mutation are TNBC[1,4,5].
Two hypotheses have been put forward regarding the origin of breast cancer; the cancer stem cell hypothesis, and the clonal evolution hypothesis. Even though both hypotheses agree that tumors originate from a single cell with multiple mutations and unlimited proliferative potential, there are significant differences between the two models. Specifically, the two models explain tumor heterogeneity by different mechanisms, while the cancer stem cell hypothesis argues that it is the result of normal differentiation, the clonal evolution hypothesis argues that it originates from competition between tumor cells of different phenotypes.
As emphasized in the cancer stem cell hypothesis, differentiated cancer cells originate from cancer stem cells and lack the capacity for self-renewal. Therefore, only the cancer stem cell can accumulate additional genetic changes that can elevate tumor progression and drug resistance.
In the clonal evolution hypothesis, the phenotypes of the tumor cell are determined by considering the origin of the cell type that initiates the tumor, the genetic and epigenetic changes acquired, and the combination of paracrine signals from surrounding cells. The phenotypes of the cell are not constant and may change as the tumor develops. All tumor cells can regenerate themselves by undergoing cell division, so they all have the potential to contribute to tumor progression and drug resistance[6].
The metastasis of breast cancer to distant organs is one of the leading causes of breast cancer-related deaths. This is because surgical procedures are difficult to perform after metastasis occurs and there is no effective drug that can be used to treat metastatic breast cancer[7].
Similar to other cancers, breast cancer metastasis begins with local invasion. This is followed by dissemination, survival in circulation, colonization in distant organs, extravasation, and finally the formation of micrometastases and macrometastases. Each of these steps requires the functions of various genes. Breast cancer commonly metastasizes to the bone, lung, liver, and brain. Studies reported that metastatic breast cancer cells exhibit distinct molecular and cellular characteristics compared to their primary counterparts. Moreover, studies have also revealed that different sets of genes play a role in different organ metastases. This is because, when metastatic cancer cells reach the secondary organ, they must develop a different microenvironment specific to each organ in order to survive. The expression of these gene sets in the primary tumor can be used as prognostic markers in predicting the risk of metastasis[7].
The tumor microenvironment (TME) plays a critical role in the growth of cancer cells, as well as the metastasis process. This environment incorporates the dynamic interactions of tumor cells with stromal cells, immune cells, vascular systems, and the extracellular matrix (ECM). During the metastasis process, tumor cells separate from the primary tumor and invade the tissues around them and enter the blood or lymph circulation. These cells are transported to distant organs, where they form new tumor colonies.
During metastasis, the TME orchestrates a range of molecular pathways and cellular mechanisms that support tumor cell dissemination and colonization. For example, factors such as transforming growth factor beta (TGF-β) stimulate the epithelial-mesenchymal transition, allowing tumor cells to gain mobility. Hypoxia conditions promote the formation of blood vessels and the remodeling of the ECM, while increasing the invasiveness of tumor cells through hypoxia-inducible factors. In addition, cells such as tumor-associated fibroblasts and macrophages create a conducive environment for metastasis. These mechanisms indicate that TME is not only a passive supporter but an active regulator of the metastasis process[8].
About 10%-15% of patients with breast cancer develop metastasis to distant organs within 3 years after the detection of the primary tumor. However, the formation of micrometastases in distant regions is also common 10 years after the initial diagnosis. For this reason, patients with breast cancer have a lifetime risk of developing metastasis[1]. A prominent feature of cancer metastasis is that many tumor types preferentially tend to colonize certain tissues or organs. Breast cancer also exhibits an organ-specific pattern of spread and preferably metastasizes to bone (47%-60%), liver (19%-20%), lung (16%-34%), and brain (10%-16%)[1].
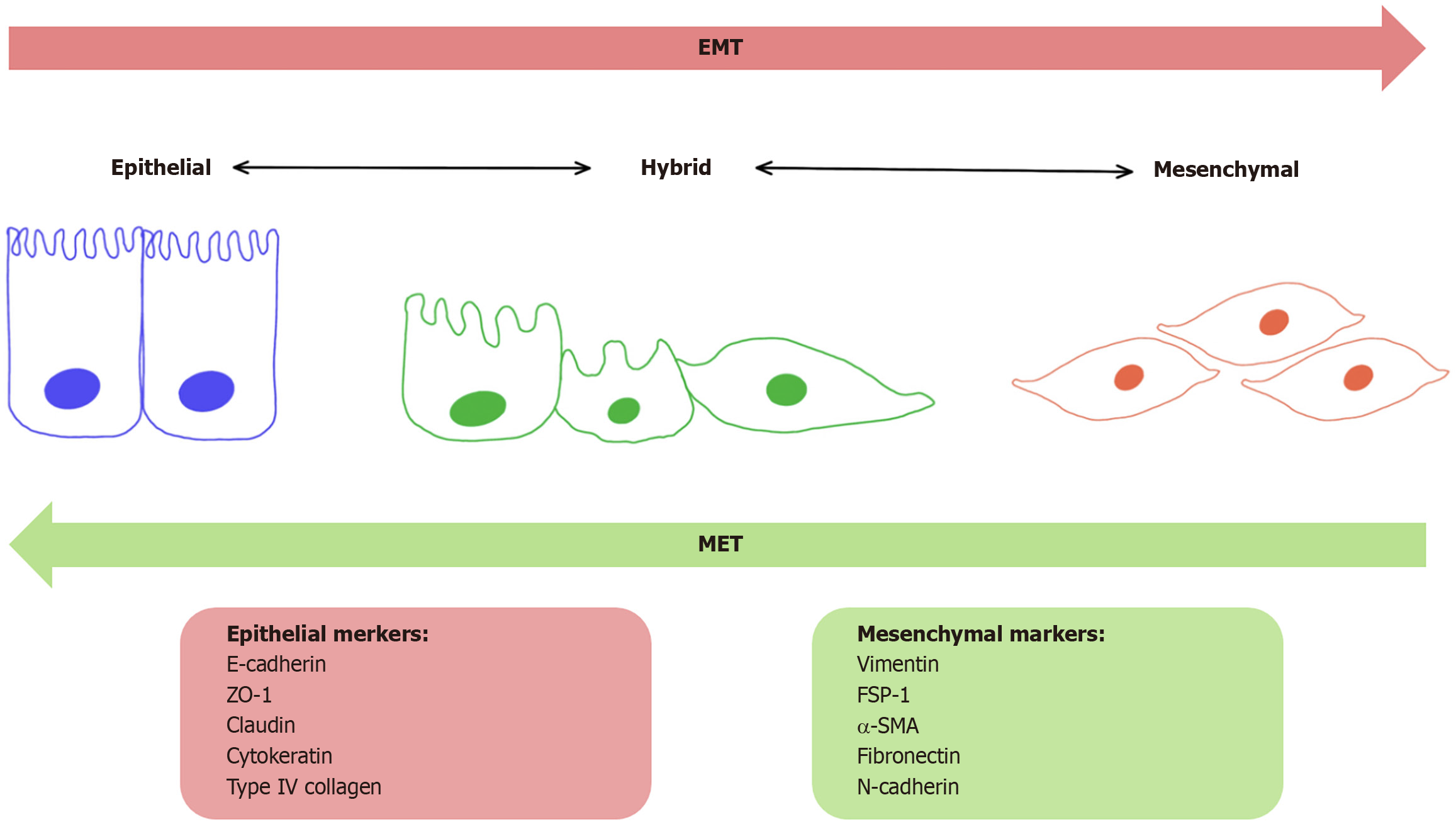
Invasion of breast cancer cells into surrounding tissue is the first step of the metastatic process and involves migration and changes in the diversity of cell adhesion molecules. One of the most studied mechanisms leading to local invasion is epithelial mesenchymal transformation. The epithelial-to-mesenchymal transition (EMT) process is characterized by the loss of cell-cell adhesion between epithelial cells and the acquisition of mesenchymal features (Figure 1). EMT can be initiated by the activation of pathways such as TGF-β, WNT, and Notch, which are important in the cell. Some studies reported that the stromal cells (e.g., cancer-associated fibroblasts [CAFs] or myeloid-derived suppressor cells) that con
The spread of cancer cells is a very late stage of cancer progression. However, some studies showed that the spread can also occur at an early stage. Breast cancer cells can spread through lymphatic and/or the blood vessel. Unlike blood vessels, lymphatic vessels lack tight interendothelial connections and have an intermittent basement membrane, making it easier for cancer cells to enter the lymphatic circulation. Accordingly, the first metastasis site of breast cancer is lymph nodes and increases the risk of distant metastasis. In addition, lymph node metastasis is an important marker for breast cancer staging.
Intravasation into the blood vessel requires the support of several non-cancerous cells, including perivascular macro
Once in circulation, these cells are referred to as circulating tumor cells (CTCs) and encounter several critical challenges including anoikis, immune-mediated clearance, and mechanical stress. Breast cancer cells have developed various mecha
Every organ creates different barriers when CTCs manage to attach to distant organs. One of these barriers is the different structures in the arrangement of the vessels. For example, lung capillaries are very tight against the infiltration capabi
After extravasation into the parenchyma tissue in the secondary organ, cancer cells need to establish colonies again to protect themselves from several obstacles that are specific to each organ. These barriers are: (1) Secretion of antimetastatic signals from stromal cells; (2) Attack of immune cells; and (3) The microenvironment, which is different from the primary site. To overcome them, breast cancer cells must be able to survive antimetastatic signals and make the microenvironment suitable for them to grow. Many studies revealed a variety of genes that contribute to organ-specific metastasis of breast cancer[7].
Breast cancer is a very complex disease in terms of its biology, morphology, and clinical history. The presence of heterogeneous subtypes is also one of the conditions that make breast cancer treatment difficult.
The immune system is a primary factor that plays a dual role in the development and progression of breast cancer. This is explained by the immune-editing mechanism: When the tumor is exposed to the pressures of the immune system, it can escape from the immune system by immune editing mechanisms. At this point, understanding what happens in the early stages of tumor development and progression will be useful in developing more effective therapy methods[9].
Until recently, it was thought that solid tumors such as breast cancer were not immunogenic, that is, they were not recognized by the immune system and thus did not trigger an immune response. However, in the light of recent studies, it was revealed that the TME contains a large number of immune cells and actively fights against cancer cells.
There are many heterogeneous cell populations in the microenvironment of breast cancer, including lymphocytes, macrophages, natural killer (NK) cells, dendritic cells (DCs), myeloid-derived suppressor cells, CAFs, and adipocytes. In addition, cytokines and ECM components are the primary components of the TME.
Breast cancer displays profound molecular heterogeneity, which is also mirrored in the complexity of its tumor microenvironment, particularly in terms of immune cell composition and function. Distinct phenotypic features of tumor-infiltrating T cells across different molecular subtypes reflect subtype-specific immune modulation. For example, diffe
This section examines the components of the breast cancer microenvironment.
TIL is a term used for all mononuclear cells in the tumor, and these cells include T lymphocytes, B lymphocytes, and plasma cells separated by their specific morphology. The presence of these cells in the tumor indicates an ongoing immune response to the tumor, and high TIL counts were associated with a good prognosis and better response to treatment[12]. These cells also play a critical role in regulating the immune response in the TME.
TILs have emerged as immunological biomarkers with subtype-specific prognostic significance in breast cancer. Extensive clinical studies and meta-analyses demonstrated that high TIL levels are strongly associated with improved response to neoadjuvant chemotherapy measured by pathological complete response, as well as better survival outcomes in TNBC and HER2+ subtypes[13]. In a large multicenter study carried out by Denkert et al[14], which included 3771 patients, every 10% increase in TIL levels was correlated with significantly longer disease-free and overall survival in TNBC. Similarly, in HER2+ breast cancer, TILs are predictive of treatment response and recurrence risk. Luminal-HER2- (hormone receptor-positive) tumors usually show low TIL levels. Interestingly, some studies associated high TIL infiltration in this subtype with worse outcomes. as reported in a meta-analysis carried out by Li et al[15], increased TILs had no significant impact on survival in patients with hormone receptor-positive breast cancer. In summary, while TILs serve as favorable prognostic markers in TNBC and HER2+ breast cancer, their role in hormone receptor-positive disease remains limited or potentially adverse. Therefore, the clinical application of TILs should be carefully tailored to the molecular subtype of the tumor.
TILs are categorized into various cell types: CD8+ cytotoxic T lymphocytes, CD4+ helper T lymphocytes, Treg regulator T lymphocytes, and tumor infiltrating B lymphocytes[11].
Tregs: Tregs in the TME are the cell type that can suppress the effective T-cell response and the activity of other immune cells and are the key regulators of immune tolerance and suppression of inflammatory responses under physiological conditions. The presence and increase in the number of Tregs in the breast cancer microenvironment was associated with the invasive phenotype. Tregs present in the tumor act as cells capable of inhibiting the activation and differentiation of CD4+ helper T cells and CD8+ cytotoxic T cells. They also contribute to tumor growth by secreting growth factors and interacting indirectly with fibroblast and endothelial cells. In addition, TILs secrete cytokines such as interleukin 2 (IL-2) that can affect the homeostasis and functions of immune effector cells such as NK cells in the TME.
Tregs are identified by the forkhead box P3 (Foxp3) transcription factor, which plays a very important role in maintaining their immunosuppressive properties. In the TME, Tregs secrete the immune-suppressing cytokines TGF-β, IL-10, and IL-35, limiting antitumor immune responses and promoting tumor escape from immunity. Continuous expression of Foxp3 was identified as a key factor enabling Tregs to maintain their full immunosuppressive capacity.
Tregs in the TME exert their effects through various mechanisms that suppress the immune system. These mechanisms include inhibiting the functions of immune cells through cytokine secretion (through molecules such as TGF-β, IL-10 and IL-35), reducing IL-2 levels by metabolic intervention and consequently inhibiting the growth of effector T cells, as well as increasing adenosine production by enzymes such as CD39 and CD73. Tregs also interact directly with DCs, weakening the antigen presentation capacity of these cells and thus suppressing the immune response[16].
CD4+ helper T cells: CD4+ T cells play important roles in the antitumor or protumor processes of the immune system in the TME. When these cells recognize the antigens that are bound to major histocompatibility complex (MHC) class II molecules on antigen-presenting cells (APCs) with T cell receptors, they can transform into Tregs or different T helper (Th) cell subgroups under the action of surrounding cytokines. This differentiation determines how the microenvi
After the Th cells transform into a subgroup, they regulate the immune system by direct communication or through the molecules they secrete. For example, CD4+ T cells provide strong activation of CD8+ T cells and the formation of immune memory. It also helps B cells produce antibodies and makes other cells of the immune system more effective during infection. These functions are critical in the progression or cessation of cancer. Recent studies also showed that some CD4+ T cells can directly kill tumor cells. Below are the functions of some helper T-cell subgroups[17].
Th1 cells: Th1 cells are particularly known to produce interferon gamma (IFN-γ), and this cytokine initiates a strong immune response against cancer cells. Th1 cells improve the capacity to kill tumor cells by increasing the activity of CD8+ T cells. It also regulates the antitumor responses of myeloid cells and can limit tumor growth by inhibiting angiogenesis. The production of IFN-γ and tumor necrosis factor alpha (TNF-α) by Th1 cells can cause cancer cells to stop the cell cycle (senescence), which inhibits the progression of cancer[17].
Th2 cells: Th2 cells are usually associated with the humoral immune response and activate B cells that produce antibodies. The role of Th2 cells in cancer is more complex because they have both anti-tumor and pro-tumor potential. Th2 cells produce cytokines such as IL-4 and IL-13, and these cytokines may promote tumor cell growth and metastasis in some types of cancer. However, in some cases, Th2 cells can inhibit the progression of cancer by enabling the activation of myeloid cells and attracting eosinophils into the TME[17].
Th17 cells: Th17 cells produce proinflammatory cytokines such as IL-17. The effects of Th17 cells on cancer are contradictory and can exhibit both anti-tumor and pro-tumor properties. Th17 cells can attract other immune cells to the tumor site, specifically targeting cancer cells located in the TME. However, in some types of cancer, Th17 cells can help cancer proliferate by increasing inflammation around the tumor[17].
CD8+ cytotoxic T cells: CD8+ T cells play a critical role in the TME in cancer immunity. These cells kill target cells through cytotoxic mechanisms and limit tumor growth by recognizing tumor cell-specific antigens presented by MHC class I molecules. Impairments or losses in the antigen presentation of tumor cells via MHC class I molecules may limit the recognition ability of CD8+ T cells. Furthermore, the immunosuppressive nature of the TME reduces both the activity and efficacy of CD8+ T cells. This is due to the genetic heterogeneity among stromal cells, secreted cytokines, and tumor cells. This repressor microenvironment not only inhibits the activation of CD8+ T cells but also negatively affects their differentiation into the cytotoxic T lymphocyte phenotype. Despite this, elevated CD8+ T-cell infiltration is often asso
CD8+ T cells recognize and destroy antigens specific to tumor cells. This process usually occurs through apoptosis mechanisms such as the perforin/granzyme and FAS/FASL pathways. In addition, CD8+ T cells regulate the immune response in the TME by secreting cytokines such as IFN-γ and TNF-α. However, the ability of CD8+ T cells to function effectively is often limited by the suppressive effects of the TME.
The immunosuppressive nature of TME limits the action of CD8+ T cells in several ways. Stromal cells produce immunosuppressive molecules and signals, preventing CD8+ T cells from penetrating tumors and forming an effective immune response. For example, CAFs limit the entry and activity of CD8+ T cells into the tumor by secreting factors such as TGF-β and C-X-C motif chemokine ligand 12 (CXCL12). It also suppresses the immune response by increasing the expression of immune checkpoint molecules (e.g., programmed cell death ligand 1 [PD-L1]). Tumor-associated macrophages, which often have the immunosuppressive M2 phenotype, also increase the expression of immune checkpoint molecules such as PD-L1 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). This supports tumor growth by restricting the functions of CD8+ T cells. Abnormal vessels formed in the TME prevent CD8+ T cells from accessing the tumor tissue. Molecules like vascular endothelial growth factor (VEGF) make this process worse.
Immune checkpoints such as PD-1, CTLA-4, and T-cell immunoglobulin domain and mucin domain-3 are important mechanisms that limit the activity of CD8+ T cells. The increase of immune checkpoint molecules in the TME renders CD8+ T cells functionally inactive. These cells cannot produce enough cytokines or effectively destroy tumor cells. Immunotherapies aim to reactivate CD8+ T cells and enable them to respond more effectively to tumor cells by targeting these immune checkpoints[18].
Tumor-infiltrating B lymphocytes: B cells in the TME play complex roles that can exhibit anti-tumor and pro-tumor effects. Tumor-infiltrating B cells (TIBs) modulate immune responses through mechanisms such as antibody production, antigen presentation, and cytokine release.
TIBs play important roles in antitumor immunity. It can bind to tumor cells through antibody production, forming antigen-antibody complexes and neutralizing tumor cells in this way. In addition, it can enable the destruction of tumor cells by triggering the antibody-dependent cellular cytotoxicity mechanism with complement system activation. In addition, B cells are potent antigen-serving cells. Activated by signals such as CD40 ligand and costimulatory molecules, B cells can present tumor antigens to T cells, activating both CD4+ helper T cells and CD8+ cytotoxic T cells. In addition, cytokines secreted by TIBs (e.g., IL-12, IFN-γ, TNF-α) supports the antitumor functions of T cells and NK cells.
The regulatory B cell (Breg) subgroup of TIBs exerts immunosuppressive effects. Bregs suppress T-cell activity and proliferation by producing immunosuppressive cytokines such as IL-10, TGF-β, and IL-35. It also strengthens tumor immune escape mechanisms by supporting the growth of Tregs. Bregs, which also have a direct effect on tumor cells, can activate tumor stem cells through chemokines such as CXCL13 and contribute to metastasis by promoting the EMT[19].
Macrophages are a member of the mononuclear phagocyte system and constitute the most common leukocyte population in breast tumors. Macrophages are divided into two functionally different types, M1 (antitumor) and M2 (protumor), by mechanisms regulated by signals from the microenvironment.
Active M1 macrophages express a wide range of proinflammatory genes. M2 macrophages, on the other hand, are induced by cytokines and express high levels of anti-inflammatory genes. The M1 macrophage population was shown to increase remarkably in the TME, where it is regulated by an immunomodulatory protein responsible for macrophage polarization and interaction. M2 macrophages were reported to promote metastasis of breast cancer cells both in vitro and in vivo[12].
M1 macrophages are macrophages that usually function as pro-inflammatory and tumor suppressors. They have a cytotoxic effect against cancer cells. These macrophages produce cytokines that activate the immune system (e.g., IL-1β, TNF-α, IL-12) and high levels of reactive oxygen species (ROS). These molecules can help kill cancer cells and prevent the spread of the tumor. M1 macrophages also prevent cancer cells from avoiding immune cells and form an important line of defense in the fight against tumor. These properties of M1 macrophages suggest that these cells may play an active role in cancer treatment. However, these macrophages are usually present at low levels in the TME.
On the other hand, M2 macrophages exhibit anti-inflammatory and tumor-supporting properties. M2 macrophages release several factors that can promote tumor growth. These cells create an environment, in which the immune system is suppressed, and helps spread tumor cells. M2 macrophages also produce growth factors (e.g., VEGF and TGF-β) that promote the growth of cancer cells. These macrophages promote the process of angiogenesis in the TME. Furthermore, M2 macrophages create immunosuppression by allowing cancer cells to escape from immune cells. These characteristics contribute to the development of cancer resistance to treatment.
The functional differences that macrophages exhibit between M1 and M2 are also supported by the diversity in their metabolic pathways. M1 macrophages create an oxidative environment by using energy production pathways such as glycolysis and the pentose phosphate pathway. These metabolic profiles reinforce the cytotoxic properties of M1 macrophages because these processes increase the production of ROS.
However, M2 macrophages produce energy using metabolic pathways such as oxidative phosphorylation and fatty acid oxidation. M2 macrophages use an energy-based metabolism needed to suppress the immune response and repair tissues. These macrophages often use lower levels of glycolysis and rely instead on oxidative phosphorylation. These differences strengthen the tumor-supporting functions of M2 macrophages because oxidative phosphorylation aligns with metabolic pathways that promote tumor cell growth. In addition, M2 macrophages shape the metabolism of cancer, meeting the energy needs of tumor cells, which in turn accelerates tumor growth[12,20].
In a study investigating the effects of macrophages on TME, it was revealed that the transition from epithelium to mesenchymal in breast cancer cells is regulated by macrophage subtypes. In this study, it was reported that M2 macrophages support tumor growth, while M1 macrophages may contribute to the dormancy of metastatic breast cancer cells[21].
NK cells play a critical role against cancer, and constitute an important part of the immune system. In the TME, NK cells exert cytotoxic effects against cancer cells, mostly by secreting cytotoxic molecules and exerting death receptors. Among the molecules they secrete, perforin acts by opening pores on the membrane of cancer cells, allowing granzymes and other lytic molecules to enter the cell. These effects trigger apoptosis processes in the target cell. In addition, death receptors such as FASL and TNF-related apoptosis-inducing ligand also induce apoptosis by binding to cancer cells.
The effects of NK cells on cancer are not limited to cytotoxicity. These cells produce important cytokines that shape immune responses. For example, cytokines such as IFN-γ and TNF-α can directly inhibit the proliferation of cancer cells, disrupt cell metabolism, and accelerate apoptosis processes. IFN-γ can weaken the survival ability of tumor cells by targeting tumors, as well as strengthen the immune response by interacting with other cells in the immune system.
In addition, NK cells direct T-cell responses by interacting with DCs in the cancer microenvironment. This interaction allows the anti-tumor activities of T cells to increase. NK cells were also reported to promote the M1 polarization of macrophages and shape adaptive immune responses in the regulation of the immune response.
However, the effects of NK cells on the tumor are often limited because many types of cancer have developed several strategies to evade the activity of NK cells. These escape mechanisms include the inability of NK cells to penetrate the tumor, the alteration of the immune genetic structures of cancer cells, and the inhibition of NK cells by immunosuppressive cells in the TME. Cancer cells can block the action of NK cells by reducing the ligands of activator receptors on their surface that can activate the immune system, or by increasing the ligands of inhibitor receptors. Furthermore, the TME is rich in repressor cells such as Tregs, tumor-associated macrophages, and myeloid-derived repressor cells (MDSCs), which significantly reduces the activity of NK cells[5,22].
DCs play a role in both natural and adaptive immune responses as the immune system’s strongest antigen-serving cells. In the antitumor immune response, DCs present tumor antigens to T cells in lymph nodes. DCs can cross-present antigens unlike other antigen-serving cells. In other words, they can present exogenous antigens in a way that activates CD8+ cytotoxic T cells. This feature is critical in tumor immunity, cytotoxic T cell activation, and destroying tumor cells.
DC are divided into subtypes such as plasmacytoid DC, conventional DC (cDC1 and cDC2), and monocyte-derived DC (moDC). The roles of these subtypes in the TME differ. Plasmacytoid DCs play an important role, especially in the production of type I IFN, and regulate the immune response. However, the TME usually creates immune tolerance by suppressing these cells. cDC1 subtype triggers a strong antitumor response by activating CD8+ T cells and is often associated with good prognosis. cDC2 supports different aspects of the immune system by activating CD4+ T cells. moDCs that occur in inflammatory states can play both supportive and suppressive roles in the immune response.
The TME can alter the functions of DC to suppress the immune response. For example, molecules such as tumor-derived prostaglandin E2, IL-6, and TGF-β may inhibit the maturation of DCs and their capacity to present antigens. This may weaken the immune response and continued tumor growth.
The TME promotes immunosuppression by inducing tolerogenic differentiation in DC. However, some DC subtypes, especially cDC1, play a critical role in tumor immunity. cDC1 cells capture apoptotic tumor cells and transport them to lymph nodes, activating CD8+ T cells through cross-presentation. This process is one of the necessary processes to create an effective antitumor immune response[23,24].
MDSCs are cells that help tumors escape from the immune system and progress, by exerting suppressive effects on the immune system. In particular, they suppress immune cells such as T cells and NK cells. These cells play a multifaceted suppressive role, targeting both natural and adaptive components of the immune system.
MDSCs are divided into two main subgroups, namely granulocytic (PMN-MDSC) and monocytic (M-MDSC). PMN-MDSCs often suppress immune cells in both antigen-specific and nonspecific ways through molecules such as nitric oxide and cytokines, while antigen-specific suppression is based on the production of ROS.
MDSCs’ suppression mechanisms include the production of nitric oxide and ROS that inhibit T cell activity, the consumption of amino acids (e.g., L-arginine and cysteine) necessary for the activation of T cells, the secretion of suppre
The TME also regulates the development of MDSCs and immune suppression functions. Factors in this microenvironment, such as tumor cells, stromal cells, hypoxia, and metabolic changes, increase the suppressive capacity of MDSCs. In addition, tumor-derived exomes (small extracellular vesicles) were also observed to increase the number of MDSCs and strengthen their suppression mechanisms[25].
Cell types other than immune cells in the microenvironment of solid tumors are called stromal cells. Stromal cells in the breast cancer microenvironment are endothelial cells, CAF, and adipocytes. Other factors affecting tumor development are divided into two as ECM and cytokines. In this section, stromal cells and other factors will be examined.
Endothelial cells: Endothelial cells, key stromal components in tumors, drive angiogenesis and form the lining of tumor vasculature. This feature of cancer is critical for the development and progression of primary breast cancer. These cells, located on the inner surface of blood vessels, control the passage of both molecules and cells into the tumor environment and contribute to the regulation of antitumoral responses by interacting with immune system cells.
Angiogenesis provides the extravasation of tumor cells into the systemic circulation, intravasation into secondary or distant regions, and the supply of oxygen and nutrients necessary for successful metastatic growth. Growth factors are regulated by many factors, such as cytokines and hypoxia.
Tumor vessels have an abnormal structure; they are usually irregular, permeable, immature and do not contain perivascular cells (pericytes) necessary for vascular maturation. These differences at the structural, genetic, and functional levels place them in a position to promote tumor growth and metastasis. For example, tumor-associated endothelial cells have a high proliferative potential and contribute to tumor progression by activating immunosuppressive mechanisms. In addition, these cells establish structures in the TME that help rearrange immune system cells.
Endothelial cells in the TME also act as “gatekeepers” for immune system cells. They regulate the entry of immune cells into the tumor and can help the tumor escape from the immune system by suppressing the activity of some cells
CAFs: CAFs are identified as the main regulators in the breast cancer TME. Tumor cells continuously associate with stromal cells, such as CAFs, to support survival, growth, and metastasis. CAFs are one of the most concentrated components in the TME and are prominent cells not only for their physical proximity to the tumor but also for their direct function in supporting tumor development. These fibroblasts are formed through the conversion of fibroblasts or mesenchymal stem cells of tissues that are normally at rest into CAFs by factors secreted by tumor cells. Once activated in the TME, CAFs play an effective role in processes such as tumor cell survival, spread, immune suppression, and therapy resistance.
Even though the exact origin of CAFs in breast cancer is not clear, it is thought that they may vary in different breast cancer subtypes or during tumor progression. CAFs not only contribute to the shaping of the TME but also facilitate the escape of the tumor from the immune system by suppressing immune responses. Furthermore, the ECM and remodeling enzymes produced by CAFs support the spread of tumor cells by altering the mechanical properties of the TME. The immune system regulatory effects of CAFs usually occur upon the release of immunosuppressive cytokines or ECM structures that limit the access of immune cells into the tumor[27].
Fibroblasts associated with breast cancer promote tumorigenic progression of both pre-malignant and malignant cells. They stimulate tumor cell proliferation, angiogenesis, invasion, and tumor growth by secreting growth factors and cytokines. It was also reported that CAFs induce epithelial-mesenchymal transition and exert tumor-suppressing effects[12].
Cancer-associated adipocytes: Cancer-associated adipocytes (CAAs), first described by Dirat et al[28] in 2011, are adipocytes that change their properties as a result of interaction with tumor cells. Since breast tissue is also very rich in adipocytes, CAAs are known to play an important role in the development and progression of breast cancer.
Studies on the effects of CAAs in the TME reported that these cells are not only passive supporting structures but are active regulators. Particularly in the context of breast cancer, metabolic and biochemical interactions of CAAs with tumor cells are among the main determinants of tumor progression.
CAAs, through the release of inflammatory cytokines such as IL-6 and TNF-α, foster a pro-inflammatory TME. This process increases the proliferation, angiogenesis, and metastasis potential of cancer cells, while time suppressing immune system responses at the same, facilitating tumor progression. In addition, CAAs transfer free fatty acids and other energy sources to tumor cells, reprogramming the energy metabolism of these cells and making them more resistant to environmental stresses. This metabolic adaptation both accelerates tumor growth and contributes to the development of resistance to treatment.
Exosomes and microRNAs secreted by CAAs are critical components that regulate intercellular communication. In particular, microRNAs create a microenvironment that meets the metabolic needs of tumor cells and supports them to acquire an aggressive phenotype. In cases such as obesity, these effects of CAAs increase and are associated with a worse prognosis[29,30].
ECM: The ECM is one of the key components of the TME and plays a central role in cancer development, progression, and metastasis. ECM is a complex molecular network of collagens, fibronectin, laminins, glycoproteins, and polysaccharides that not only provide structural support but also provide biochemical and mechanical signals that regulate the cells’ behavior such as survival, proliferation, differentiation, and invasion. The breast ECM was described as a force that drives the development and differentiation of breast tissue. However, changes in the structural and biochemical pro
Desmoplasia, marked by ECM stiffening and collagen accumulation, represents a hallmark alteration of the TME during progression. Cross-linking and linearization of collagen fibers affects the behavior of cells by altering the mechanical properties of the ECM. These mechanical changes trigger mechanosignalization in tumor cells, leading to upregulation and clustering of integrins. This process both facilitates tumor formation and maintains the proliferative capacity of advanced tumor cells. Furthermore, ECM stiffness contributes to the progression of cancer by increasing the activation of oncogenic microRNAs and invasive pathways.
ECM affects not only the behavior of tumor cells, but also the immune microenvironment. In a dense collagen microenvironment, cytokine expression increases, which alters tumor-associated phenotypes of immune cells.
Angiogenesis is also closely related with the mechanical properties of the ECM. The dense and rigid ECM affects vascular remodeling and the structural properties of the blood vessels within the tumor. The increased vascular density within invasive ductal carcinoma in situ and the stiffer and thinner blood vessels in the tumor nucleus provide important evidence regarding how the ECM promotes tumor invasion. Furthermore, ECM can facilitate the spread of tumor cells by increasing stiffness, endothelial permeability, and leukocyte migration[12,31,32].
Cytokines: Cytokines within the breast TME play important roles in facilitating bidirectional communication between immune cells and tumor cells, thereby regulating tumor development, invasion, metastasis, and the immune response. Leptin, an adipokine strongly associated with obesity, not only regulates energy homeostasis under physiological conditions but also supports tumor cell proliferation, migration, angiogenesis, and EMT. Leptin can also induce IL-8 production, contributing to a pro-inflammatory microenvironment. TNF-α, a potent pro-inflammatory cytokine secreted by macrophages and T cells, enhances survival signaling in tumor cells through the activation of nuclear factor kappa-B and mitogen-activated protein kinase pathways, promotes EMT and cell motility, and facilitates metastasis. It also increases aromatase activity, thus promoting estrogen production and the growth of hormone receptor-positive tumors[11,33].
Other cytokines, including IL-1β, IL-6, and IL-8, secreted by both tumor and immune cells further support tumor progression. IL-1β, particularly produced by macrophages and tumor-associated fibroblasts, induces COX2 activity, facilitating tumor invasion. IL-6 acts mainly through signal transducers and activators of transcription 3 signaling to promote cell cycle progression, apoptosis resistance, and stem-like tumor cell phenotypes. It also impairs T cell activation, contributing to an immunosuppressive TME. IL-8 recruits neutrophils and macrophages, alters immune responses, and enhances tumor cell migration, invasion, and angiogenesis[33].
IL-17 and IL-23, secreted predominantly by Th17 cells, amplify pro-tumor inflammation, activate immunosuppressive cells, and promote metastasis. On the other hand, cytokines such as IL-2, IL-12, and IFN-γ exert antitumor effects by increasing immune responses. IL-2 supports cytotoxic T cell activation, IL-12 promotes Th1 differentiation and IFN-γ production, and IFN-γ directly suppresses tumor cells while enhancing tumor-specific T cell responses. However, under certain conditions, the same cytokines may be co-opted by tumors to suppress immunity or evade immune detection[33].
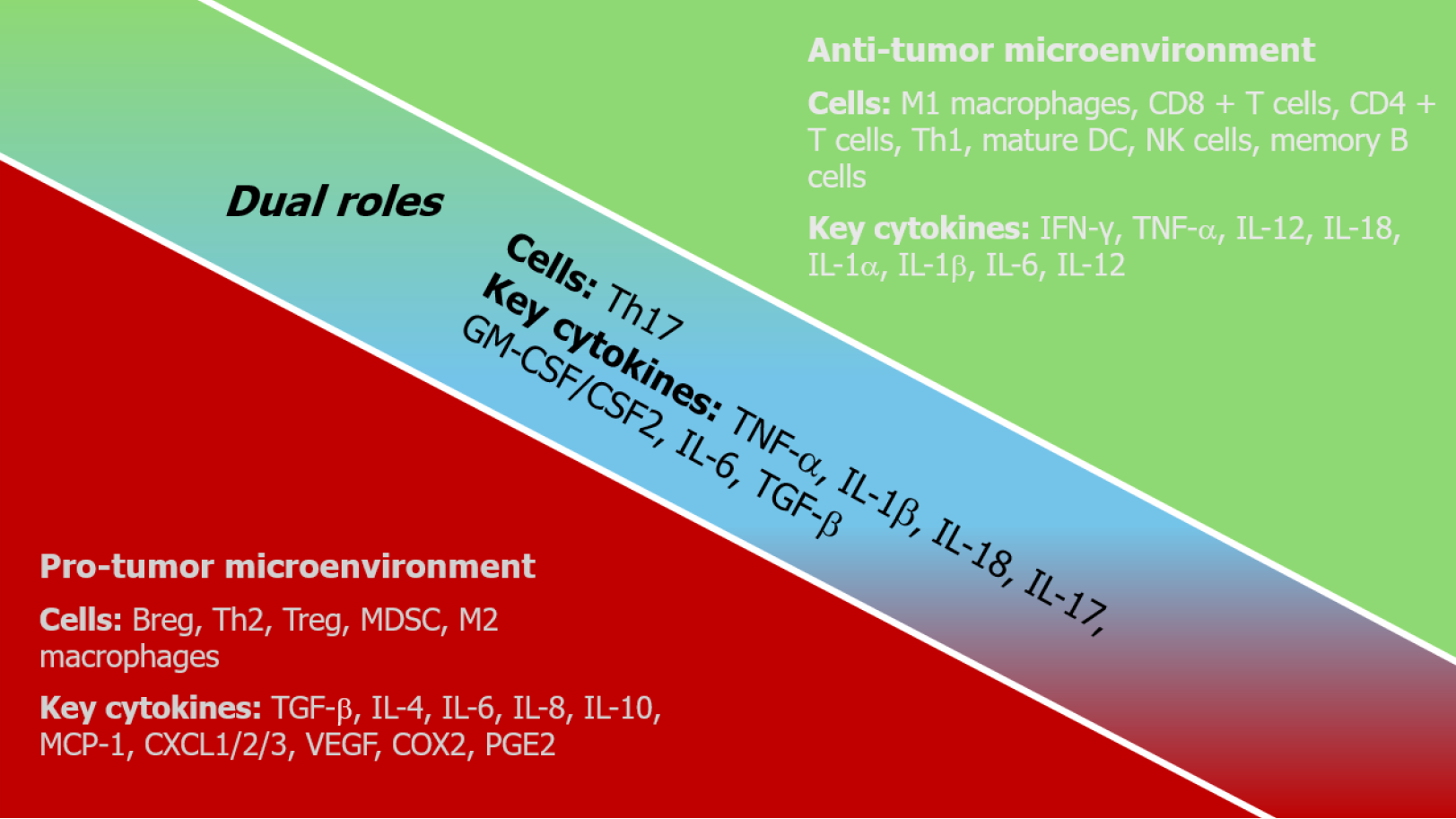
Therefore, the roles of cytokines in the TME are highly dynamic and context-dependent, influenced by cell type, concentration, timing, and surrounding immune components (Figure 2). Their functions as either tumor-promoting or tumor-suppressing factors are shaped by the complexity of intercellular communication. Understanding these interac
Recent technological advancements have significantly impacted research on the TME, a complex structure composed of cellular and acellular components that drive cancer progression and therapeutic response. The increasing realization of the TME’s significance in cancer biology has shifted cancer research from a cancer-centric model to one that considers the TME[34,35].
Spatial profiling technologies are powerful tools to visualize and systematically analyze the physical localization of TME components. These methodologies allow for the measurement of numerous proteins and transcripts within tissue samples while preserving tissue architecture[35].
Information derived from spatial profiling data reveals the intricate interplay between tumor genetics and the TME. For instance, the combination of single-cell RNA sequencing and spatial transcriptomics revealed the co-localization of tumor cells with an IFN response signature alongside T cells and macrophages[35].
Protein array technology offers another significant approach for understanding the TME by enabling the parallel detection of hundreds of proteins in small biological samples. Protein levels can more accurately reflect physiological and pathological states when compared to mRNA levels alone[34].
In conclusion, both spatial profiling technologies and protein arrays offer complementary and powerful methods for investigating the complexity and heterogeneity of the TME. The detailed insights gained from these approaches hold significant potential for improving cancer diagnosis, prognosis, and the development of more effective therapies.
Three-dimensional (3D) cell cultures are increasingly recognized as powerful in vitro tools for modeling the TME, offering advantages over traditional 2D cultures by better recapitulating the complexity of tumors. These models bridge the gap between 2D systems and in vivo animal models[36].
Advantages of 3D cultures include a more accurate reproduction of cell polarity and shape, tissue stiffness, oxygen and nutrient diffusion gradients, and cell-cell/cell-ECM interactions, which are often lacking in 2D cultures. For example, a 3D platform was used to show that the hypoxic core within a spheroid can significantly modulate drug uptake compared to 2D monolayers. Furthermore, MCF-7 breast cancer cells cultured in 3D collagen scaffolds displayed different morphologies and continued to proliferate for a longer duration compared to 2D cultures. The architecture of 3D models allows for the formation of distinct layers with varying proliferation rates, senescence, and hypoxia, mirroring the in vivo conditions in solid tumors. This complex organization affects drug efficacy, with studies reporting different responses to chemotherapeutic agents in 3D vs 2D cultures[37].
3D cultures are useful for drug discovery and testing in more physiologically relevant contexts, including evaluating chemoresistance and the efficacy of immunotherapies.
Immune checkpoint blockade (ICB) is currently the most widely used form of immunotherapy in clinical practice. In breast cancer, only therapies targeting the PD-1/PD-L1 axis have received clinical approval. Response to ICB is generally higher in tumors with an immune-enriched microenvironment, characterized by high levels of tumor-infiltrating lymphocytes and immune checkpoint expression[10].
In triple-negative breast cancer, pembrolizumab and atezolizumab are the only approved ICB agents. In metastatic TNBC, a significant clinical benefit from ICB requires the presence of PD-L1 expression. The KEYNOTE-355 trial established a global standard for first-line treatment of PD-L1-positive metastatic TNBC[38]. Following the IMpassion130 trial, the combination of atezolizumab with nab-paclitaxel was approved in some countries other than the United States. As a first-line treatment for PD-L1-positive metastatic TNBC; however, the Food and Drug Administration later withdrew this approval due to limitations in the IMpassion130 and IMpassion131 studies[39,40].
Pembrolizumab and atezolizumab show limited efficacy as monotherapy; thus, their combination with chemotherapy is necessary for achieving clinically significant outcomes. The KEYNOTE-355 trial suggested that reducing tumor burden may be more critical than the specific type of chemotherapy used[41]. Furthermore, retrospective analyses from the KEYNOTE-086 trial have shown that PD-L1 positivity, CD8+ T cell infiltration, stromal TILs, T cell-inflamed gene expression profiles, and high tumor mutational burden are associated with improved clinical outcomes with pembro
The typically high TIL infiltration observed in TNBC tumors supports the rationale for targeting the PD-1/PD-L1 axis in this subtype. Other checkpoint inhibitors targeting CTLA-4 and LAG3 are still under investigation[10].
In early-stage TNBC, current PD-L1 immunohistochemistry assays offer prognostic information but are not predictive of treatment benefit. However, in KEYNOTE-522, IMpassion031, and GeparNuevo trials, the combination of ICB with neoadjuvant chemotherapy improved pathological complete response rates in both PD-L1-positive and -negative tumors. Based on the findings reported in KEYNOTE-522, adjuvant ICB following neoadjuvant chemotherapy has become the new standard of care for stage II and III TNBC[10,43].
Adoptive cell therapy is a form of immunotherapy that is currently under intense investigation and showed significant efficacy in certain cancer types. Among these approaches, chimeric antigen receptor (CAR) T cell therapy emerged as the most advanced and transformative, particularly in the treatment of hematologic malignancies. Building on this success, efforts are underway to adapt this strategy to solid tumors. For CAR T cell therapy to be effective in breast cancer, it is important to identify a suitable and targetable tumor antigen. Ongoing trials are investigating CAR T cells directed against frequently expressed breast cancer antigens. However, to date, therapeutic efficacy in solid tumors remains limited compared to that achieved in hematologic cancers[10,44].
In addition to CAR T cells, the therapeutic potential of adoptive transfer of tumor-infiltrating lymphocytes has also been investigated. A phase II pilot clinical trial addressed the use of mutation-reactive TILs isolated from patients with metastatic breast cancer, administered in combination with a short course of pembrolizumab. Most patients developed immune responses against the targeted mutations, suggesting that TIL therapy may represent a promising personalized immunotherapy approach[45]. However, this strategy remains highly labor-intensive and costly, limiting its broader clinical use.
Breast cancer, with its heterogeneous structure and complex biology, poses significant challenges in terms of clinical treatment and prognosis. This mini review discusses the effects of various components of the breast cancer microenvironment on cancer development, metastasis, and response to treatment. The TME is a dynamic environment consisting of immune cells, CAF, adipocytes, and various molecules that direct the behavior of cancer cells. In particular, the presence of immune cells such as TILs and Tregs can influence the stage of the tumor and its response to treatment, while CAF and other stromal cells in the microenvironment promote cancer growth and metastasis. In recent years, a better under
| 1. | Feng Y, Spezia M, Huang S, Yuan C, Zeng Z, Zhang L, Ji X, Liu W, Huang B, Luo W, Liu B, Lei Y, Du S, Vuppalapati A, Luu HH, Haydon RC, He TC, Ren G. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018;5:77-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 796] [Cited by in RCA: 834] [Article Influence: 104.3] [Reference Citation Analysis (0)] |
| 2. | Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lønning PE, Børresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10665] [Cited by in RCA: 11139] [Article Influence: 428.4] [Reference Citation Analysis (12)] |
| 3. | Herschkowitz JI, Simin K, Weigman VJ, Mikaelian I, Usary J, Hu Z, Rasmussen KE, Jones LP, Assefnia S, Chandrasekharan S, Backlund MG, Yin Y, Khramtsov AI, Bastein R, Quackenbush J, Glazer RI, Brown PH, Green JE, Kopelovich L, Furth PA, Palazzo JP, Olopade OI, Bernard PS, Churchill GA, Van Dyke T, Perou CM. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8:R76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 971] [Cited by in RCA: 930] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 4. | Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A. Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies-An Updated Review. Cancers (Basel). 2021;13:4287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 960] [Cited by in RCA: 1015] [Article Influence: 203.0] [Reference Citation Analysis (0)] |
| 5. | Abdel-Latif M, Youness RA. Why natural killer cells in triple negative breast cancer? World J Clin Oncol. 2020;11:464-476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Polyak K. Breast cancer: origins and evolution. J Clin Invest. 2007;117:3155-3163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 439] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 7. | Kim MY. Breast Cancer Metastasis. Adv Exp Med Biol. 2021;1187:183-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 8. | Biray Avci C, Goker Bagca B, Nikanfar M, Takanlou LS, Takanlou MS, Nourazarian A. Tumor microenvironment and cancer metastasis: molecular mechanisms and therapeutic implications. Front Pharmacol. 2024;15:1442888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 9. | Semiglazov V, Tseluiko A, Kudaybergenova A, Artemyeva A, Krivorotko P, Donskih R. Immunology and immunotherapy in breast cancer. Cancer Biol Med. 2022;19:609-618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Harris MA, Savas P, Virassamy B, O'Malley MMR, Kay J, Mueller SN, Mackay LK, Salgado R, Loi S. Towards targeting the breast cancer immune microenvironment. Nat Rev Cancer. 2024;24:554-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 165] [Reference Citation Analysis (0)] |
| 11. | Kotsifaki A, Alevizopoulos N, Dimopoulou V, Armakolas A. Unveiling the Immune Microenvironment's Role in Breast Cancer: A Glimpse into Promising Frontiers. Int J Mol Sci. 2023;24:15332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Mittal S, Brown NJ, Holen I. The breast tumor microenvironment: role in cancer development, progression and response to therapy. Expert Rev Mol Diagn. 2018;18:227-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 13. | Ciarka A, Piątek M, Pęksa R, Kunc M, Senkus E. Tumor-Infiltrating Lymphocytes (TILs) in Breast Cancer: Prognostic and Predictive Significance across Molecular Subtypes. Biomedicines. 2024;12:763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 14. | Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen F, Furlanetto J, Schmitt WD, Blohmer JU, Karn T, Pfitzner BM, Kümmel S, Engels K, Schneeweiss A, Hartmann A, Noske A, Fasching PA, Jackisch C, van Mackelenbergh M, Sinn P, Schem C, Hanusch C, Untch M, Loibl S. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 1564] [Article Influence: 173.8] [Reference Citation Analysis (0)] |
| 15. | Li S, Zhang Y, Zhang P, Xue S, Chen Y, Sun L, Yang R. Predictive and prognostic values of tumor infiltrating lymphocytes in breast cancers treated with neoadjuvant chemotherapy: A meta-analysis. Breast. 2022;66:97-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 16. | Li C, Jiang P, Wei S, Xu X, Wang J. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol Cancer. 2020;19:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 352] [Cited by in RCA: 641] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 17. | Liudahl SM, Coussens LM. To Help or To Harm. Immunology. 2018. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Xie Q, Ding J, Chen Y. Role of CD8(+) T lymphocyte cells: Interplay with stromal cells in tumor microenvironment. Acta Pharm Sin B. 2021;11:1365-1378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 19. | Guo FF, Cui JW. The Role of Tumor-Infiltrating B Cells in Tumor Immunity. J Oncol. 2019;2019:2592419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Sadhukhan P, Seiwert TY. The role of macrophages in the tumor microenvironment and tumor metabolism. Semin Immunopathol. 2023;45:187-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 21. | Yang M, Ma B, Shao H, Clark AM, Wells A. Macrophage phenotypic subtypes diametrically regulate epithelial-mesenchymal plasticity in breast cancer cells. BMC Cancer. 2016;16:419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Guillerey C. NK Cells in the Tumor Microenvironment. Adv Exp Med Biol. 2020;1273:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 23. | Kim CW, Kim KD, Lee HK. The role of dendritic cells in tumor microenvironments and their uses as therapeutic targets. BMB Rep. 2021;54:31-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 24. | Verneau J, Sautés-Fridman C, Sun CM. Dendritic cells in the tumor microenvironment: prognostic and theranostic impact. Semin Immunol. 2020;48:101410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 25. | Yang Y, Li C, Liu T, Dai X, Bazhin AV. Myeloid-Derived Suppressor Cells in Tumors: From Mechanisms to Antigen Specificity and Microenvironmental Regulation. Front Immunol. 2020;11:1371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 203] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 26. | Leone P, Malerba E, Susca N, Favoino E, Perosa F, Brunori G, Prete M, Racanelli V. Endothelial cells in tumor microenvironment: insights and perspectives. Front Immunol. 2024;15:1367875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 62] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 27. | Houthuijzen JM, Jonkers J. Cancer-associated fibroblasts as key regulators of the breast cancer tumor microenvironment. Cancer Metastasis Rev. 2018;37:577-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 164] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 28. | Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S, Garrido I, Escourrou G, Valet P, Muller C. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455-2465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 843] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 29. | Wu Q, Li B, Li Z, Li J, Sun S, Sun S. Cancer-associated adipocytes: key players in breast cancer progression. J Hematol Oncol. 2019;12:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 324] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 30. | Wu C, Dong S, Huang R, Chen X. Cancer-Associated Adipocytes and Breast Cancer: Intertwining in the Tumor Microenvironment and Challenges for Cancer Therapy. Cancers (Basel). 2023;15:726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 31. | Yang J, Bahcecioglu G, Zorlutuna P. The Extracellular Matrix and Vesicles Modulate the Breast Tumor Microenvironment. Bioengineering (Basel). 2020;7:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Closset L, Gultekin O, Salehi S, Sarhan D, Lehti K, Gonzalez-Molina J. The extracellular matrix - immune microenvironment crosstalk in cancer therapy: Challenges and opportunities. Matrix Biol. 2023;121:217-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 33. | Habanjar O, Bingula R, Decombat C, Diab-Assaf M, Caldefie-Chezet F, Delort L. Crosstalk of Inflammatory Cytokines within the Breast Tumor Microenvironment. Int J Mol Sci. 2023;24:4002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 153] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 34. | Huang W, Luo S, Burgess R, Yi YH, Huang GF, Huang RP. New Insights into the Tumor Microenvironment Utilizing Protein Array Technology. Int J Mol Sci. 2018;19:559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Elhanani O, Ben-Uri R, Keren L. Spatial profiling technologies illuminate the tumor microenvironment. Cancer Cell. 2023;41:404-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 288] [Reference Citation Analysis (0)] |
| 36. | Fontana F, Marzagalli M, Sommariva M, Gagliano N, Limonta P. In Vitro 3D Cultures to Model the Tumor Microenvironment. Cancers (Basel). 2021;13:2970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 37. | Hoarau-Véchot J, Rafii A, Touboul C, Pasquier J. Halfway between 2D and Animal Models: Are 3D Cultures the Ideal Tool to Study Cancer-Microenvironment Interactions? Int J Mol Sci. 2018;19:181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 338] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 38. | Cortes J, Rugo HS, Cescon DW, Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Perez-Garcia J, Iwata H, Masuda N, Torregroza Otero M, Gokmen E, Loi S, Guo Z, Zhou X, Karantza V, Pan W, Schmid P; KEYNOTE-355 Investigators. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. N Engl J Med. 2022;387:217-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 774] [Cited by in RCA: 718] [Article Influence: 179.5] [Reference Citation Analysis (0)] |
| 39. | Emens LA, Loi S. Immunotherapy Approaches for Breast Cancer Patients in 2023. Cold Spring Harb Perspect Med. 2023;13:a041332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 40. | Dixon-Douglas J, Loibl S, Denkert C, Telli M, Loi S. Integrating Immunotherapy Into the Treatment Landscape for Patients With Triple-Negative Breast Cancer. Am Soc Clin Oncol Educ Book. 2022;42:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 41. | Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Holgado E, Iwata H, Masuda N, Otero MT, Gokmen E, Loi S, Guo Z, Zhao J, Aktan G, Karantza V, Schmid P; KEYNOTE-355 Investigators. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 1241] [Article Influence: 206.8] [Reference Citation Analysis (0)] |
| 42. | Loi S, Salgado R, Schmid P, Cortes J, Cescon DW, Winer EP, Toppmeyer DL, Rugo HS, De Laurentiis M, Nanda R, Iwata H, Awada A, Tan AR, Sun Y, Karantza V, Wang A, Huang L, Saadatpour A, Cristescu R, Yearley J, Lunceford J, Jelinic P, Adams S. Association Between Biomarkers and Clinical Outcomes of Pembrolizumab Monotherapy in Patients With Metastatic Triple-Negative Breast Cancer: KEYNOTE-086 Exploratory Analysis. JCO Precis Oncol. 2023;7:e2200317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 43. | Liu J, Blake SJ, Yong MC, Harjunpää H, Ngiow SF, Takeda K, Young A, O'Donnell JS, Allen S, Smyth MJ, Teng MW. Improved Efficacy of Neoadjuvant Compared to Adjuvant Immunotherapy to Eradicate Metastatic Disease. Cancer Discov. 2016;6:1382-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 686] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 44. | Domínguez-Cejudo MA, Gil-Torralvo A, Cejuela M, Molina-Pinelo S, Salvador Bofill J. Targeting the Tumor Microenvironment in Breast Cancer: Prognostic and Predictive Significance and Therapeutic Opportunities. Int J Mol Sci. 2023;24:16771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 45. | Zacharakis N, Huq LM, Seitter SJ, Kim SP, Gartner JJ, Sindiri S, Hill VK, Li YF, Paria BC, Ray S, Gasmi B, Lee CC, Prickett TD, Parkhurst MR, Robbins PF, Langhan MM, Shelton TE, Parikh AY, Levi ST, Hernandez JM, Hoang CD, Sherry RM, Yang JC, Feldman SA, Goff SL, Rosenberg SA. Breast Cancers Are Immunogenic: Immunologic Analyses and a Phase II Pilot Clinical Trial Using Mutation-Reactive Autologous Lymphocytes. J Clin Oncol. 2022;40:1741-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 139] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/