Published online May 24, 2025. doi: 10.5306/wjco.v16.i5.104762
Revised: March 4, 2025
Accepted: April 22, 2025
Published online: May 24, 2025
Processing time: 133 Days and 0.7 Hours
Hepatocellular carcinoma (HCC) with advanced features such as Budd-Chiari syndrome, chronic liver failure and multiple intrahepatic metastases poses significant therapeutic challenges. Yttrium-90 (90Y) radioembolization is a locoregional treatment option with potential benefits in such complex cases. This case report explores the application of 90Y radioembolization in combination with systemic therapies, highlighting its potential role in managing advanced HCC.
A 51-year-old male presented with HCC characterized by massive intrahepatic lesions, multiple metastases, and chronic liver failure secondary to Budd-Chiari syndrome. The patient underwent 90Y radioembolization following hepatic arterial infusion chemotherapy and was subsequently combined with lenvatinib. Post-treatment follow-up revealed a significant reduction in tumor size, with the maximum diameter decreasing from 142.45 mm to 73.16 mm over six months. Liver function improved from Child-Pugh class B to A. However, new intrahepatic lesions emerged at ten months, and liver function deteriorated to Child-Pugh class C. The patient survived for 18 months after initial diagnosis.
Yttrium-90 radioembolization combined with systemic therapies demonstrated significant tumor regression and temporary liver function improvement in a patient with advanced HCC, suggesting its potential as a treatment option in complex cases.
Core Tip: This case report highlights the successful combination of yttrium-90 radioembolization therapy and lenvatinib in treating a patient with advanced hepatocellular carcinoma characterized by large tumors, multiple intrahepatic metastases, and Budd-Chiari syndrome complicated by chronic hepatic failure. Despite the complexity of the case, this treatment approach effectively reduced tumor burden, improved liver function, and significantly prolonged the patient’s survival, offering a promising therapeutic option for similar challenging scenarios.
- Citation: Shao MH, Tan BB, Chen HL, Zhang H. Yttrium-90 radioembolization for advanced hepatocellular carcinoma with Budd-Chiari syndrome: A case report. World J Clin Oncol 2025; 16(5): 104762
- URL: https://www.wjgnet.com/2218-4333/full/v16/i5/104762.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i5.104762
The prognosis of hepatocellular carcinoma (HCC) is influenced by both the tumor’s responsiveness to treatment and the patient’s hepatic functional reserve[1]. Managing HCC in the context of chronic hepatic failure necessitates a delicate balance between halting disease progression and preserving liver function. Selective internal radiation therapy, also known as radioembolization, employs yttrium-90 (90Y) microspheres and offers low risks of toxicity and liver function impairment. This therapy can be tailored to different therapeutic goals[2]. This case report describes the use of 90Y radioembolization to treat a patient with massive HCC lesions, multiple intrahepatic metastases, and Budd-Chiari syndrome (BCS) complicated by chronic hepatic failure. This treatment significantly prolonged the patient’s survival and improved quality of life.
Intermittent swelling and pain in the upper right abdomen for 2 months. Liver tumor was found in 1 month.
Two months ago, the patient presented with right upper quadrant abdominal distension and pain without obvious inducement. There were no associated symptoms of fever, jaundice, nausea, vomiting, diarrhea, or symptoms of acid reflux. One month earlier, the patient had been diagnosed with HCC at another hospital, he was also instructed to take lenvatinib and hepatoprotective drugs (glutathione and S-adenosyl-L-methionine-1,4-butane-disulfonate). Since first developing the condition, with poor sleep quality, listlessness, reduced appetite, and weight loss of 2 kg.
Four years prior to admission, the patient was diagnosed with BCS (mixed type) and liver cirrhosis occurred to bilateral lower extremity edema. Then underwent inferior vena cava stent placement, alleviated symptoms. The patient has no history of hepatitis B, hepatitis C, tuberculosis, hypertension, diabetes mellitus, thyroid disease, chronic obstructive pulmonary disease, or cardiovascular disease. Additionally, there is no reported history of neurological disorders or significant trauma.
The patient works as a rural physician, has no history of smoking and alcohol, and there is no history of infectious or hereditary diseases in the family.
On admission, the patient appeared normal and in no acute distress. His vital signs were stable, Eastern Cooperative Oncology Group performance status score was 0, A palpable mass measuring approximately 8 cm × 10 cm was detected below the xiphoid process. The mass had poor mobility, was hard in consistency, and exhibited slight tenderness on palpation without rebound tenderness. No manifestations of hepatic encephalopathy.
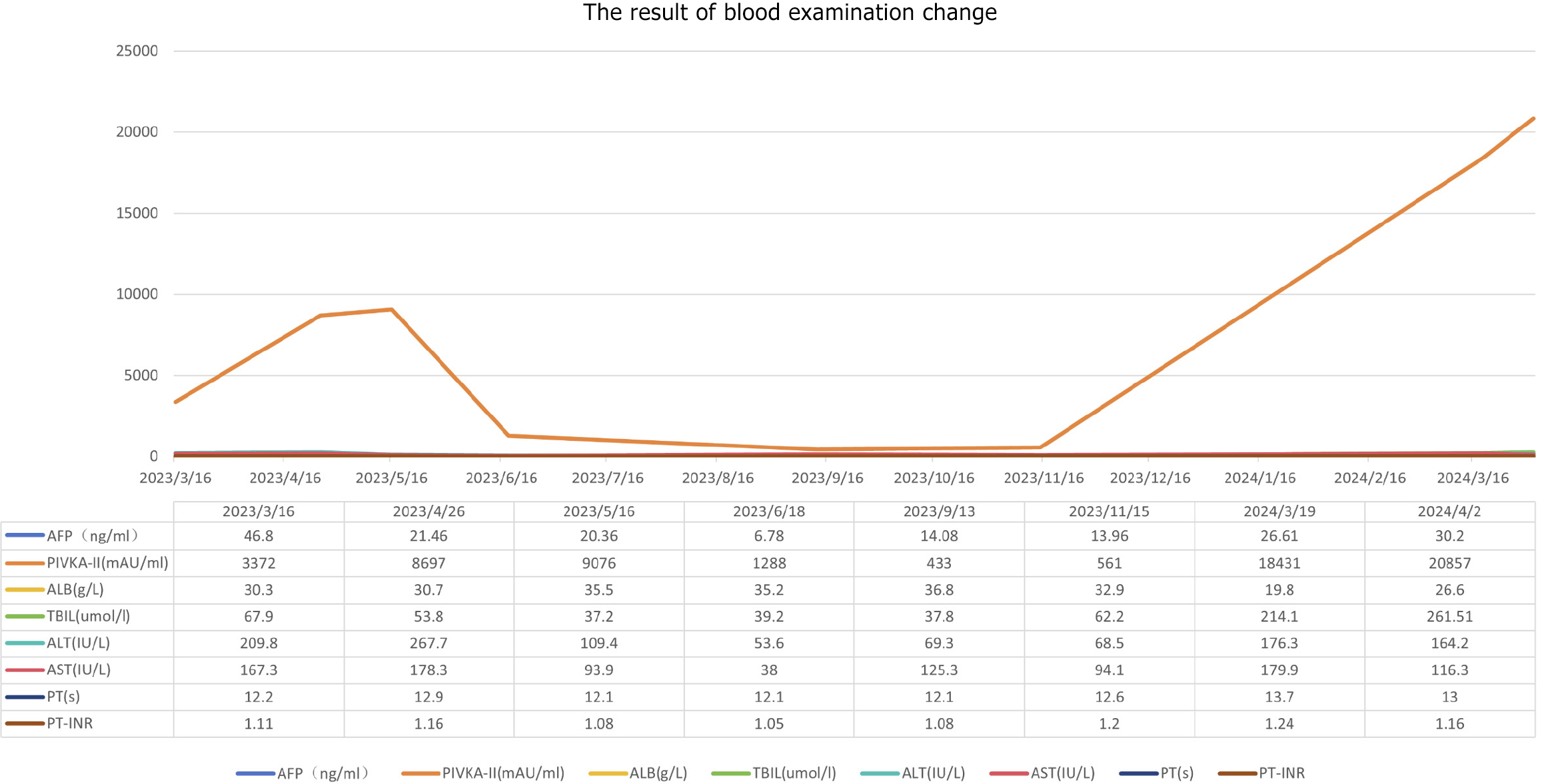
Blood tests revealed chronic hepatic failure. Routine blood tests, coagulation function is normal, white blood cells (3.28 × 109/L), red blood cells (4.03 × 1012/L), blood platelet (67 × 109/L), hemoglobin (127 g/L), alanine aminotransferase (209.80 IU/L), aspartate aminotransferase (167.30 IU/L), gamma-glutamyl transpeptidase (106.40 IU/L), albumin (30.30 g/L), total bilirubin (67.9 μmol/L), direct bilirubin (27.01 μmol/L), alpha fetoprotein (AFP, 46.8 ng/mL), and protein induced by vitamin K absence or antagonist-II (PIVKA-II, 3372.00 mAU/mL). The Child-Pugh class was B. The tests for hepatitis B and hepatitis C were both negative.
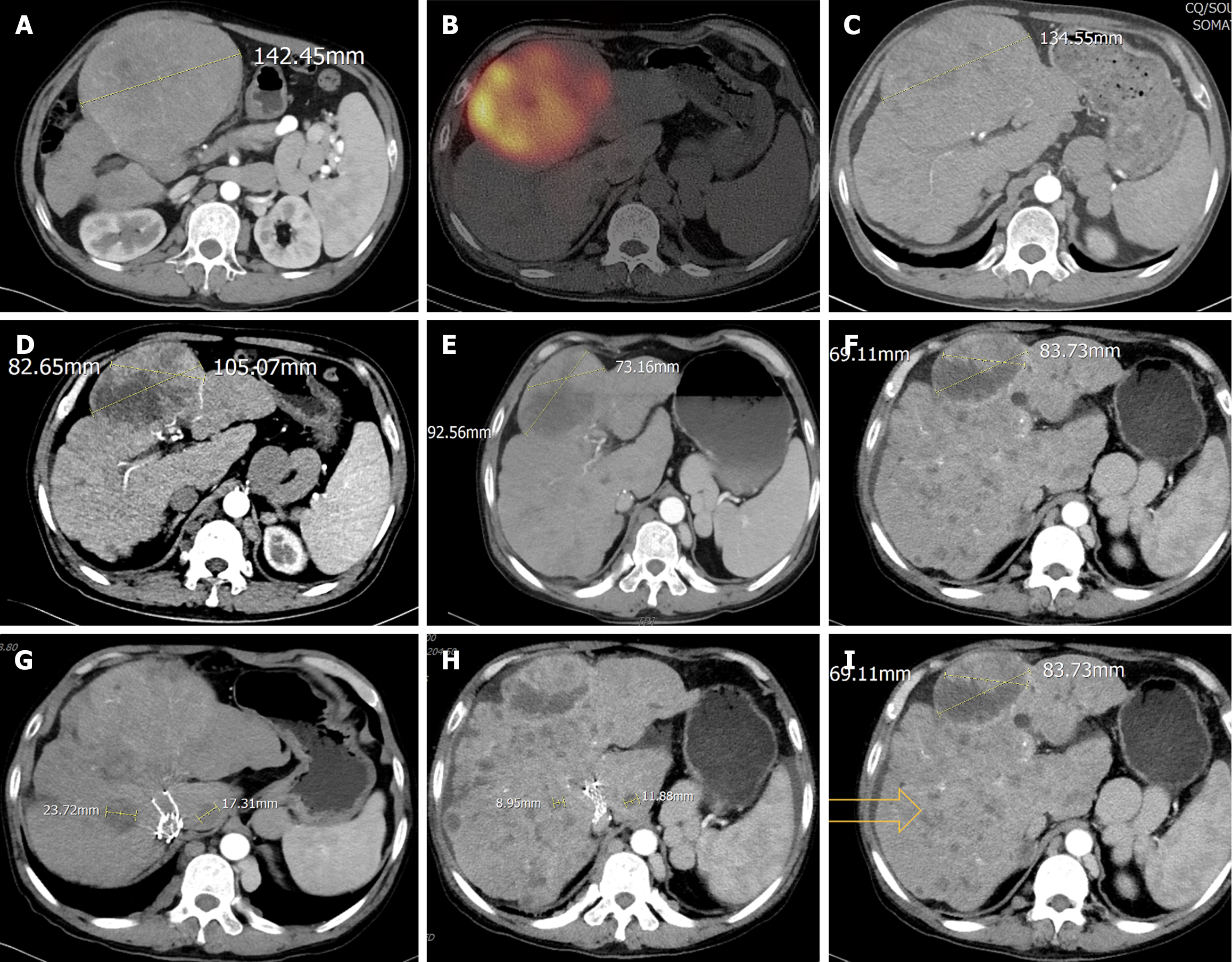
The current imaging assessment with enhanced computed tomography (CT) revealed ascites and exophytic massive HCC lesions located at the junction of the left and right liver lobes and multiple intrahepatic metastases (Figure 1), with a maximum tumor diameter [Barcelona Clinic Liver Cancer (BCLC) B] of 142.45 mm (Figure 1A).
The Departments of Radiology, Hepatology, Nutrition, Oncology, and Nuclear Medicine, in conjunction with Hepatobiliary Intervention, convened to formulate a comprehensive treatment plan for the patient. The patient is diagnosed with giant HCC with multiple intrahepatic metastases, rendering surgical intervention infeasible. Given the favorable tumor response and low incidence of adverse events associated with 90Y radioembolization, this modality was considered for its potential to prolong the patient’s life and enhance quality of life. However, despite the typical indication criteria of 90Y radioembolization requiring a total bilirubin level below 34.2 μmol/L[3], the multidisciplinary tumor board recommended proceeding with this treatment after thorough deliberation. Prior to the procedure, a Technetium-99m-labeled macroaggregated albumin (Tc99m-MAA) perfusion test revealed a high hepatic-pulmonary shunt fraction of 13.8%, which significantly increased the risk of postoperative radiation pneumonitis. Therefore, it was advised that the patient first undergo hepatic arterial infusion chemotherapy using the FOLFOX regimen to control disease progression. Considering the patient’s hepatic insufficiency, lenvatinib targeted therapy was temporarily discontinued, and the patient was advised to continue with hepatoprotective and nutritional support therapies.
The patient was diagnosed giant HCC with multiple intrahepatic metastases (BCLC B), BCS (mixed type), liver cirrhosis and hepatic insufficiency (Child-Pugh B).
The patient initially presented to our hospital on March 8, 2023. Upon admission, hepatoprotective therapy (glutathione and S-adenosyl-L-methionine-1,4-butane-disulfonate) and nutritional support were continued. On March 22, 2023, a Tc99m-MAA perfusion test was performed to evaluate the hepatic vascular architecture. Following a multidisciplinary discussion, lenvatinib was temporarily discontinued due to its potential impact on hepatic function and tumor perfusion. Hepatic arterial infusion chemotherapy was then initiated as an alternative therapeutic strategy. On May 13, 2023, Tc99m-MAA perfusion test was conducted again, the middle hepatic artery was mapped, confirming the absence of extrahepatic uptake, revealing a hepatic-pulmonary shunt fraction of 9.5%, which met the criteria for 90Y radioembolization. Subsequently, on May 25, 2023, the patient underwent 90Y radioembolization targeting the largest intrahepatic lesion (Figure 1B). For 90Y radioembolization with resin microspheres (SIR-Spheres®, Sirtex Medical), the prescribed activity was 3.4 GBq, with estimated tumor and lung absorbed doses of 140 Gy and 15.4 Gy, respectively. Single-photon emission CT/CT examination after 90Y injection revealed good deposition in the target tumor. This minimally invasive procedure delivers high doses of radiation directly to the tumor while sparing surrounding healthy tissue.
Post-treatment, the patients’ liver function improved to Child-Pugh class A, reflecting enhanced hepatic functional reserve. On May 14, 2023, a contrast-enhanced CT scan demonstrated a significant reduction in the maximum diameter of the tumor to 134.55 mm (Figure 1C), along with decreased tumor enhancement, indicating effective treatment response, PIVKA-II was 8697 mAU/mL, and AFP was 21.46 ng/mL. Given the improved clinical status, lenvatinib targeted therapy was reinitiated to further control disease progression. Hepatoprotective and nutritional support therapies were continued to maintain liver function and overall patient well-being.
One month after the procedure, the patient’s PIVKA-II level decreased to 1288 mAU/mL, and AFP level was 6.78 ng/mL (Figure 2). Liver function remained classified as Child-Pugh class A. The patient continued lenvatinib targeted therapy at a dose of 8 mg/day, along with hepatoprotective measures and nutritional support to promote liver function recovery. In the fourth month post-treatment (September 14, 2023), follow-up contrast-enhanced CT demonstrated extensive necrosis of the target lesion, with non-target intrahepatic lesions showing neither progression nor regression (Figure 1D). According to the modified Response Evaluation Criteria in Solid Tumors, the response was classified as partial response. At this time, PIVKA-II was 433 mAU/mL, AFP was 14.8 ng/mL (Figure 2), and liver function remained Child-Pugh class A. The patient was advised to consider liver transplantation but declined the suggestion.
By the sixth month post-treatment (November 2023), the patient developed scleral icterus. Contrast-enhanced CT revealed continued regression of the target lesion but little enlargement of non-target intrahepatic lesions (Figure 1E). PIVKA-II increased to 1153 mAU/mL, AFP was 22.85 ng/mL, and liver function deteriorated to Child-Pugh class B, with total bilirubin elevated to 62.2 μg/mL. Consequently, lenvatinib targeted therapy was discontinued, and the patient applicated with artificial extracorporeal liver support and continued with hepatoprotective measures, nutritional support. In the tenth month post-treatment (March 2024), follow-up CT showed continued shrinkage of the target lesion in the left medial lobe and the diameter of measurable non-target lesions reductions, but revealed multiple new small nodules in the remaining liver (Figure 1F and I). Additionally, pleural and peritoneal effusions were detected. At this time, PIVKA-II was 18431 mAU/mL, AFP was 26.61 ng/mL, and liver function further deteriorated to Child-Pugh class C, Eastern Cooperative Oncology Group performance status score was 1. The patient requested palliative care and passed away on August 18, 2024.
Hepatic functional reserve is an important determinant of long-term survival in patients with HCC[4]. In this case, BCS led to cirrhosis, which subsequently caused chronic hepatic decompensation. Additionally, the patient presented with multiple intrahepatic tumor lesions, among which the largest tumor, located in the left medial lobe, exhibited exophytic growth with a large volume and significant mass effect, posing a high risk of tumor rupture and hemorrhage. According to the BCLC staging system, the patient was classified as stage B[5]. However, the patient’s fragile hepatic function, complex clinical condition, and limited treatment options posed significant challenges. Traditional transcatheter arterial chemoembolization may bring serious complications, and the patient was at high risk of developing acute liver failure following such procedures[6].
90Y radioembolization has emerged as a promising therapeutic option with a lower incidence of adverse events and complications compared to conventional treatments[7]. Moreover, 90Y radioembolization has been shown to promote hepatic regeneration[8]. Although 90Y radioembolization is recommended for palliative treatment of unresectable HCC, there are limited reports on its use in such complex cases. After multidisciplinary team discussion, 90Y radioembolization was deemed beneficial for prolonging the patient’s survival and improving quality of life. Targeted and immune therapies are indicated for patients with BCLC stage B and C HCC[9]. Studies have demonstrated that external beam radiotherapy combined with targeted and immune therapies can enhance tumor response rates[10]. However, reports on the combination of 90Y radioembolization with targeted or immune therapies are scarce, and no randomized controlled trials have been conducted. Given the patient’s poor hepatic reserve, the efficacy and safety of combination therapies remain uncertain. However, considering the high risk of disease progression due to multiple intrahepatic lesions, the patient provided informed consent after thorough deliberation and opted for 90Y radioembolization. Fortunately, the patient’s hepatic function improved following supportive care and nutritional support, and after comprehensive assessment, the patient was deemed eligible for 90Y radioembolization. Subsequently, the patient initiated lenvatinib targeted therapy.
During follow-up, the patient did not experience significant adverse reactions or complications. Imaging studies revealed a gradual reduction in the largest tumor lesion (i.e., the target lesion) and varying degrees of disease control in non-target lesions. This therapeutic effect was likely attributable to the synergistic action of 90Y radioembolization and lenvatinib targeted therapy. According to the Hangzhou criteria, the patient was considered a suitable candidate for liver transplantation, which could potentially offer survival benefits[11]. However, the patient declined this option. Unfortunately, at the sixth month post-treatment, the patient developed acute hepatic decompensation, which was likely related to the underlying cirrhosis and poor hepatic reserve, and possibly associated with lenvatinib use[12]. Consequently, lenvatinib was discontinued. New intrahepatic lesions emerged during follow-up, the target lesion continued to shrink, and the patient’s hepatic function deteriorated progressively, with a Child-Pugh score deteriorating to class C. The sustained relief of the target lesion verified the therapeutic effectiveness of 90Y radioembolization. The observed tumor progression in this case may imply the refractory nature and intrinsic heterogeneity characteristic of HCC. Meanwhile, the patient with pre-existing hepatic insufficiency, progressive deterioration of hepatic functional capacity, and the potential emergence of resistance mechanisms to molecularly targeted therapies[13].
Ultimately, the patient opted for palliative care. Previous studies have shown that 90Y radioembolization for unresectable HCC can achieve a median overall survival of 7 to 17 months[14]. Despite the patient’s severe clinical condition at presentation, the total survival duration reached 18 months. Although the patient achieved a relatively prolonged survival, treatment options for such complex cases remain limited, and further exploration of more rational and effective therapeutic strategies is urgently needed. 90Y radioembolization is widely recognized for its high efficacy and low incidence of adverse events. However, more research is required to identify appropriate patient populations for combination or monotherapy with 90Y radioembolization.
In conclusion, this case study supports our hypothesis that, in appropriately selected patients, the combination of 90Y radioembolization with systemic treatments is a promising and reasonable therapeutic option for patients with poor prognoses and can provide durable antitumor activity, promising long-term outcomes, and improved quality of life.
The authors would like to thank Dr. Li, Department of Radiology, for his invaluable assistance with the radiological assessments. We also extend our gratitude to Dr. Pan, Department of Nuclear Medicine, for his help us to in calculating the dose of yttrium-90. Their contributions were instrumental in the preparation of this manuscript.
| 1. | Helmberger T, Golfieri R, Pech M, Pfammatter T, Arnold D, Cianni R, Maleux G, Munneke G, Pellerin O, Peynircioglu B, Sangro B, Schaefer N, de Jong N, Bilbao JI; On behalf of the CIRT Steering Committee; On behalf of the CIRT Principal Investigators. Clinical Application of Trans-Arterial Radioembolization in Hepatic Malignancies in Europe: First Results from the Prospective Multicentre Observational Study CIRSE Registry for SIR-Spheres Therapy (CIRT). Cardiovasc Intervent Radiol. 2021;44:21-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 2. | Hatzidakis A, Müller L, Krokidis M, Kloeckner R. Local and Regional Therapies for Hepatocellular Carcinoma and Future Combinations. Cancers (Basel). 2022;14:2469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Rivera L, Giap H, Miller W, Fisher J, Hillebrand DJ, Marsh C, Schaffer RL. Hepatic intra-arterial infusion of yttrium-90 microspheres in the treatment of recurrent hepatocellular carcinoma after liver transplantation: a case report. World J Gastroenterol. 2006;12:5729-5732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 2010;7:448-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 979] [Cited by in RCA: 1067] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 5. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 3089] [Article Influence: 772.3] [Reference Citation Analysis (61)] |
| 6. | Bargellini I, Lorenzoni V, Lorenzoni G, Scalise P, Andreozzi G, Bozzi E, Giorgi L, Cervelli R, Scandiffio R, Perrone O, Meccia DV, Boccuzzi A, Daviddi F, Cicorelli A, Lunardi A, Crocetti L, Turchetti G, Cioni R. Duration of response after DEB-TACE compared to lipiodol-TACE in HCC-naïve patients: a propensity score matching analysis. Eur Radiol. 2021;31:7512-7522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Chow R, Simone CB 2nd, Jairam MP, Swaminath A, Boldt G, Lock M. Radiofrequency ablation vs radiation therapy vs transarterial chemoembolization vs yttrium 90 for local treatment of liver cancer - a systematic review and network meta-analysis of survival data. Acta Oncol. 2022;61:484-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Sidali S, Trépo E, Sutter O, Nault JC. New concepts in the treatment of hepatocellular carcinoma. United European Gastroenterol J. 2022;10:765-774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 9. | Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15:599-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1487] [Cited by in RCA: 1458] [Article Influence: 182.3] [Reference Citation Analysis (0)] |
| 10. | Yu Q, Wang Y, Ungchusri E, Patel M, Kumari D, Van Ha T, Pillai A, Liao CY, Ahmed O. Combination of transarterial radioembolization with atezolizumab and bevacizumab for intermediate and advanced staged hepatocellular carcinoma: A preliminary report of safety and feasibility. J Interv Med. 2023;6:187-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 11. | . Liver transplantation for hepatocellular carcinoma: Hangzhou experiences: Retraction. Transplantation. 2019;103:1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Huynh J, Cho MT, Kim EJ, Ren M, Ramji Z, Vogel A. Lenvatinib in patients with unresectable hepatocellular carcinoma who progressed to Child-Pugh B liver function. Ther Adv Med Oncol. 2022;14:17588359221116608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 13. | Yin Y, Feng W, Chen J, Chen X, Wang G, Wang S, Xu X, Nie Y, Fan D, Wu K, Xia L. Immunosuppressive tumor microenvironment in the progression, metastasis, and therapy of hepatocellular carcinoma: from bench to bedside. Exp Hematol Oncol. 2024;13:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 62] [Reference Citation Analysis (0)] |
| 14. | Di Federico A, Rizzo A, Carloni R, De Giglio A, Bruno R, Ricci D, Brandi G. Atezolizumab-bevacizumab plus Y-90 TARE for the treatment of hepatocellular carcinoma: preclinical rationale and ongoing clinical trials. Expert Opin Investig Drugs. 2022;31:361-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/