Published online Feb 24, 2025. doi: 10.5306/wjco.v16.i2.98812
Revised: October 13, 2024
Accepted: October 24, 2024
Published online: February 24, 2025
Processing time: 158 Days and 2 Hours
Through deeper understanding of targetable driver mutations in non-small-cell lung cancer (NSCLC) over the past years, some patients with driver mutations have benefited from the targeted molecular therapies. Although the anaplastic lymphoma kinase and BRAF mutations are not frequent subtypes in NSCLC, the availability of several targeted-drugs has been confirmed through a series of clinical trials. But little is clear about the proper strategy in rare BRAF G469A mutation, not to mention co-exhibition of anaplastic lymphoma kinase and BRAF G469A mutations, which is extremely rare in NSCLC.
We present a patient to stage IVA lung adenocarcinoma with coexisting echi
Due to the rarity of co-mutations, the case not only enriches the limited literature on NSCLC harbouring BRAF G469A and echinoderm microtubule associated protein like-4 mutations, but also suggests the efficacy and safety of specific multiple-drug therapy in such patients.
Core Tip: We present a non-small-cell lung cancer patient with rarely co-occurring echinoderm microtubule associated protein like-4 rearrangement and BRAF G469A mutation. The patient suffered from anaplastic lymphoma kinase tyrosine kinase inhibitors resistance and adverse events. It’s a question whether the process of reducing dosage of alectinib was easier to cause resistance. Although we were unable to assess the correlation between BRAF G469A mutation and anaplastic lymphoma kinase tyrosine kinase inhibitors resistance, the safety and efficacy of combination therapy to multiple driver oncogenes are important in clinic.
- Citation: Guo X, Liu Y, Wang YT, Liu K, Ding H. Combined BRAF G469A mutation and echinoderm microtubule associated protein like-4-anaplastic lymphoma kinase rearrangement with resistance: A case report and review of literature. World J Clin Oncol 2025; 16(2): 98812
- URL: https://www.wjgnet.com/2218-4333/full/v16/i2/98812.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i2.98812
Non-small-cell lung cancer (NSCLC) as the major histology of lung cancer traditionally leads serious prognosis and cancer death, due to late diagnosis at an advanced stage and absence of effective systemic treatments[1]. With deeper cognition about molecular mechanisms, NSCLC was closely related to specific genetic mutations resulting into target medical therapy. Undoubtedly, the mutation of the epidermal growth factor receptor as the most common oncogenic driver approximately appears in 50% of Asian patients with NSCLC[2]. In addition, the rearrangement of anaplastic lymphoma kinase (ALK) is detectable in 2%-7% of NSCLC patients, who may get clinical benefit from ALK tyrosine kinase inhibitors (ALK-TKIs)[3]. Among other types of unusual mutations, BRAF harbor a mutation in 1%-4% of all NSCLC, particularly in adenocarcinomas[4]. The rearrangement of ALK is frequently found in non-smokers of NSLCL, while BRAF mutation is associated with new or past smoking[5]. Unfortunately, the published literature on co-occurring BRAF mutation and ALK rearrangement leading to target drug adjustment in NSCLC is limited. Here, we presented a patient of NSCLC with co-mutations of ALK and BRAF genes displayed target drug resistance and adverse events of combination therapy.
A 62-year-old female patient without smoking history visited our hospital with cough, chest pain and dyspnea for 1 week. Adenocarcinoma was confirmed by pathological examinations of exfoliated cells in pleural effusion via closing thoracic drainage. In addition, positron emission tomography-computed tomography revealed multiple bilateral pulmonary foci, especially in the upper lobe of the right lung paralleling the hilar lymph metastasis.
A 62-year-old female patient without smoking history visited our hospital with cough, chest pain and dyspnea for 1 week.
She had no past disease.
There is none family history.
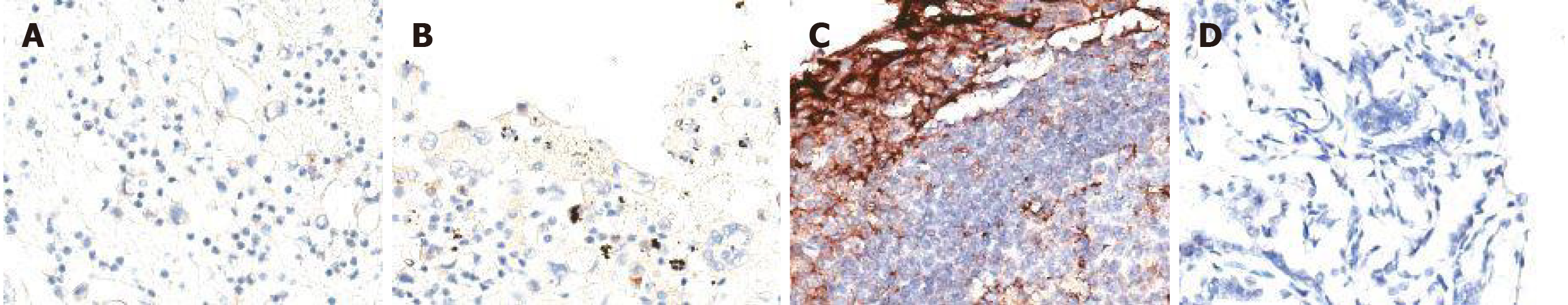
The next-generation sequencing (NGS) assay was performed on RNA isolated from the liquid biopsy, displaying ALK rearrangement harboring type of echinoderm microtubule associated protein like-4 (EML4-ALK) (MAF = 6.72%) and missense mutation of TP53 on p.P152L site (MAF = 2.25%). In order to identify the immunotherapeutic response in clinic, immunohistochemistry was used to analyze programmed cell death protein ligand 1 (PD-L1) expression and found non-stained PD-L1 with 0% in tumor proportion score and combined positive score (Figure 1). The data suggested that she limited beneficial to programmed death 1 blockade immunotherapy.
To accurately and timely evaluate the efficacy of treatment, multicriteria monitors on sensitive biomarkers of NSCLC as carbohydrate antigen 125 and carcinoembryonic antigen in blood per month were used, which was shown in Table 1.
| Nov 2020 | Dec 2020 | Jan 2021 | Feb 2021 | Mar 2021 | Apr 2021 | May 2021 | Jun 2021 | Jul 2021 | Aug 2021 | Sep 2021 | Oct 2021 | Nov 2021 | Dec 2021 | Jan 2022 | Feb 2022 | Mar 2022 | Apr 2022 | |
| WBC (109/L) | 6.7 | 7.3 | 4.4 | 3.7 | 6.8 | 7.2 | 6.1 | 5.8 | 5.3 | 5.4 | 6.4 | 8.2 | 9 | 19.2 | 4.7 | 5.9 | 4.1 | 3 |
| Hb (g/L) | 138 | 127 | 120 | 115 | 117 | 115 | 108 | 112 | 117 | 106 | 98 | 113 | 95 | 102 | 89 | 95 | 97 | 87 |
| Plt (109/L) | 253 | 267 | 223 | 213 | 242 | 223 | 267 | 252 | 265 | 252 | 301 | 296 | 432 | 392 | 423 | 456 | 349 | 297 |
| ALT (IU/L) | 30.4 | 54.3 | 103.8 | 25.3 | 35.2 | 19.6 | 26.1 | 19.7 | 21.4 | 27.9 | 14.3 | 15.1 | 13.1 | 16.6 | 187.9 | 12.1 | 19.8 | 17.1 |
| AST (IU/L) | 33.2 | 43.2 | 61.9 | 57.8 | 27.3 | 32.9 | 28.2 | 33.3 | 32.9 | 41.1 | 23.7 | 23.2 | 18.9 | 20.3 | 153.7 | 25.2 | 25.7 | 24.5 |
| CEA (U/mL) | 58.8 | 16.4 | 3.7 | 4.6 | 5.2 | 4.2 | 3.8 | 1 | 2.1 | 1.5 | 1.7 | 1.4 | 65.8 | 3.1 | 3.3 | 2 | 7.2 | 3.95 |
| CA125 (ng/mL) | 9.3 | 3.5 | 4.2 | 15.6 | 6.9 | 7.2 | 5.4 | 5.1 | 9.6 | 23.9 | 46.6 | 35 | 495.5 | 104.6 | 35.1 | 14.7 | 1.6 | 2.74 |
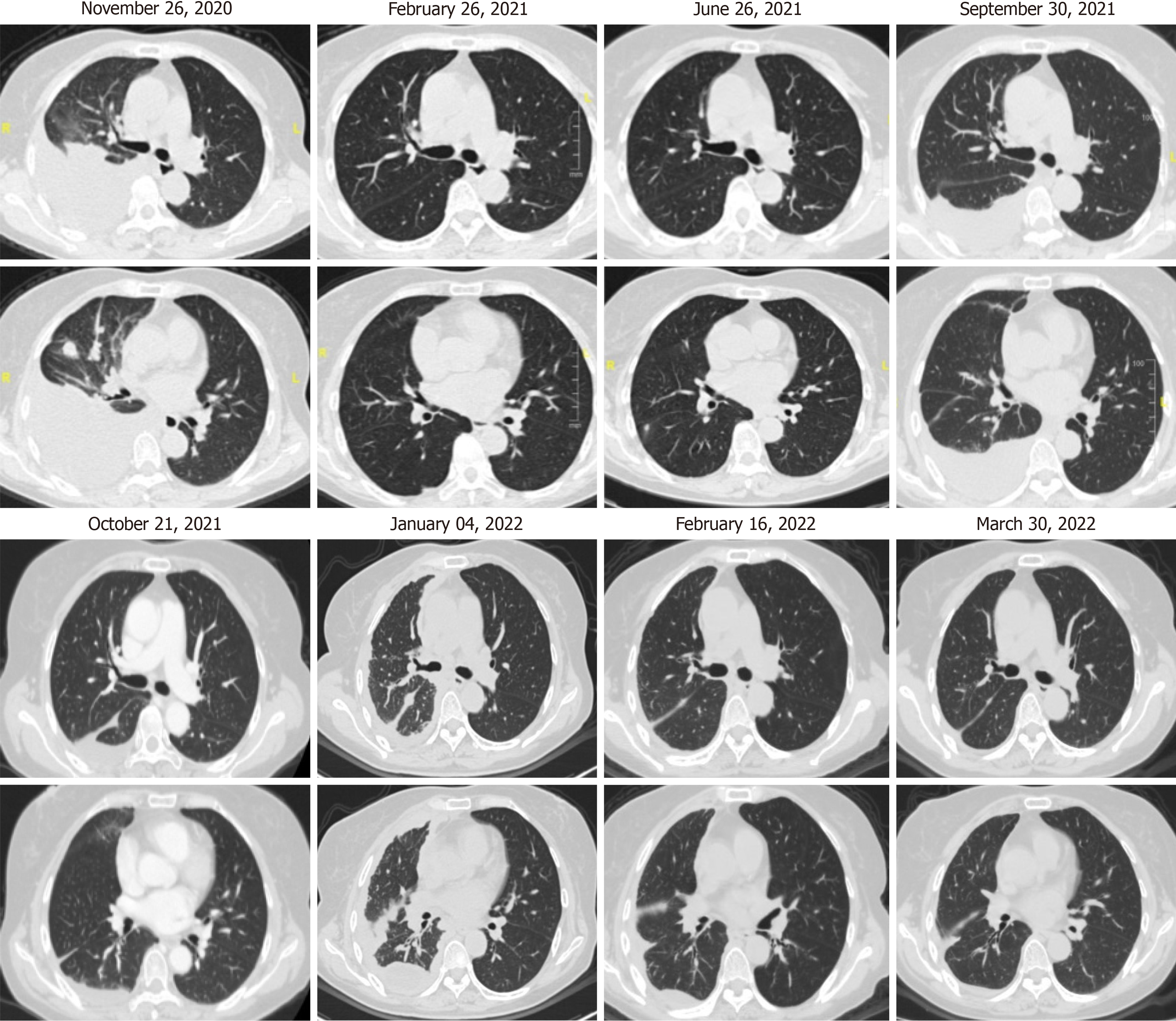
Chest computed tomography for the lung lesion absorption was routinely investigated per 3 months (Figure 2).
The clinical stage of NSCLC was IVA (cT2N1M1a).
Afterward, she received alectinib 600 mg twice daily as the first-line therapy with the progression-free survival (PFS) of 3 months. To accurately and timely evaluate the efficacy of treatment, multicriteria monitors on sensitive biomarkers of NSCLC as carbohydrate antigen 125 and carcinoembryonic antigen in blood per month were used, which was shown in Table 1. Chest computed tomography for the lung lesion absorption was routinely investigated per 3 months (Figure 2). Although alectinib was confirmed to be more useful anti-ALK therapeutic agent, its adverse events also deserved more attention[6]. Routine blood examination, liver and function were monitored every month. For 88 days after alectinib administration, the patient was admitted to hospital again for unbearable vomiting, excessive fatigue and muscle soreness. We checked and found the significant dysfunction of liver, as unintended high levels of alanine transaminase and aspartate transaminase in Table 1. The adverse events of alectinib were never released by routine drugs for protecting the liver. Then, we had to adjust the dosage to alectinib at 450 mg twice a day a tentative reduction. For more than 7 months in a continuous stable with treatment, she was admitted to hospital for increased right pleural effusion. Mali
After rigorous contemplation and following the desires of patient, the combination treatment consisted ceritinib (750 mg once daily), anlotinib (12 mg once daily) and chemotherapy with pemetrexed and carboplatin. After 2 courses of treatment, clinical symptoms relieved and MPE tended to be stable.
With the increasing availability of targeted anti-cancer medications in recent years, certain individuals with NSCLC who possess certain mutations may benefit in terms of prognosis and clinical aspects. Once inevitable drug-resistant has evolved, there’s little therapy alternatives. As far as we are aware, no reports exist of co-occurring EML4-ALK rearrangement and BRAF G469A mutation in NSCLC. There is currently minimal expertise selecting medications for these people. Here, we describe a unique case of adenocarcinoma patient with a multi-gene mutation and a complex medication regimen that is generating side effects.
When adenocarcinoma histology was seen in younger non-smokers, ALK rearrangement as diamond mutation was commonly observed. Since EML4-ALK fusion in NSCLC was widely accepted[7], several clinical trials confirmed the increased PFS and overall survival in NSCLC patients with ALK-TKI treatment[8-10]. Crizotinib, the first-generation ALK-TKI, was allowed to treat NSCLC with ALK rearrangement and significantly increased 8-11 months PFS compared with chemotherapy[10,11]. On the other side, the clinical progression and brain metastasis in NSCLC patients with ALK-positive especially were developed during the first year of crizotinib use[12]. The reasons were blamed on resistance to crizotinib and low concentration of drug in the central nervous system[6]. Adverse events of crizotinib as visual im
As a rare mutation, BRAF-positive NSCLC patients tend to be smokers and adenocarcinoma[22]. One known risk factor for lung cancer is smoking. It is true that the mortality rate from lung cancer can be lowered by quitting smoking. Certain compounds have been proposed as mutagens when inhaled. It has been established that smoking causes gene mutations, which make treatment more complex and the prognosis poorer[5,22]. The most type of BRAF mutations is V600E, while G469A accounts for nearly 23%[23]. From investigation in 2001 NSCLC cases, Kinno et al[24] confirmed the low frequency of BRAF mutation in an Asian cohort. In the previous study, the combination between dabrafenib and vemurafenib contributed an improved objective response rate of 33%-42% to BRAF V600E mutant metastatic NSCLC[25]. Notwithstanding the benefit to target therapy is attractive, the valuable information is limited for BRAF-mutated NSCLC on non-V600E, especially G469A. Although Negrao et al[26] revealed the meaningful sensitivity of human lung cancer cells with BRAF G469 mutant to trametinib plus or minus dabrafenib, the United States Food and Drug Administration approved dabrafenib plus trametinib for BRAF V600E mutant NSCLC, but not for patients with BRAF G469A mutation[27]. An in vitro study also suggested that trametinib or erlotinib significantly suppressed the growth of lung tumoroid line with BRAF G469A mutation[28]. Based on the preclinical results, some scholars considered the possibility of similar effects with trametinib alone to BRAF G469A mutation NSCLC. However, it’s not enough and needs more data to support. There’s a case report of a patient with NSCLC and the BRAF G469R mutation who showed a dramatic response to sorafenib[29]. Unfortunately, sorafenib has not been allowed to treat NSCLC by now. In our case, the second NGS confirmed the co-occurring ALK-EML4 and BRAF G469 mutations. Considering the positive effects of alectinib at 600 mg twice daily and adverse events of combination therapy, we commanded alectinib plus dabrafenib as the first choice in this case. The clinical value of anti-tumor progress was appeared for a while. Once severe adverse events and advanced MPE popped up again, we tented to use ceritinib, anlotinib and chemotherapy together under complex consideration including trametinib unobtainable. As a multi-target TKI, anlotinib mainly acts anti-tumor effects through anti-vascular endothelial growth factor, anti-VEGF biology[30,31]. And anlotinib as third-line drug was approved to treat advance NSCLC. Combination therapy significantly alleviated the progress of cancer and adverse events. The curative effects and safety need to be further investigated.
The potential impacts on target drug choice and response in co-exhibition of activating mutations should not be neglected. There may be internal roles on resistant pathways in a rare coexistence of BRAF G469A mutation and EML4-ALK rearrangement. Alrifai et al[32] reported a patient with squamous cell carcinoma harbouring both BRAF V600E mutation and an ALK rearrangement exhibited resistance to crizotinib. Another study on a patient of ALK rearranged paralleling BRAF V600E mutation lung adenocarcinoma with acquired resistance to crizotinib indicated that BRAF V600E mutation might be responsible for ALK-TKI resistance[33]. Furthermore, BRAF G469A mutation was confirmed to be an activator of resistance to osimertinib in a vitro study[34]. Although BRAF-mutant NSCLCs were reported to have a better response to immune-checkpoint inhibitors, but the specific G469A subgroup was not enrolled[35]. Due to no PD-L1 expression in our case, the immune-checkpoint inhibitors will not the better choice.
Here, we report a patient of NSCLC harboring co-occurring BRAF G469A and EML4-ALK mutations in twisting process of complicated drug therapy. Although we were unable to assess the correlation between BRAF G469A mutation and ALK-TKI resistance, the safety and efficacy of combination therapy to multiple driver oncogenes are important in clinic. Future studies are warranted to validate the mechanisms of drug resistance on patients with multiple gene co-mutation, in order to benefit from targeted treatment.
| 1. | Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1607] [Cited by in RCA: 3396] [Article Influence: 424.5] [Reference Citation Analysis (0)] |
| 2. | Ye L, Chen X, Zhou F. EGFR-mutant NSCLC: emerging novel drugs. Curr Opin Oncol. 2021;33:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Golding B, Luu A, Jones R, Viloria-Petit AM. The function and therapeutic targeting of anaplastic lymphoma kinase (ALK) in non-small cell lung cancer (NSCLC). Mol Cancer. 2018;17:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 202] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 4. | Sheikine Y, Pavlick D, Klempner SJ, Trabucco SE, Chung JH, Rosenzweig M, Wang K, Velcheti V, Frampton GM, Peled N, Murray M, Chae YK, Albacker LA, Gay L, Husain H, Suh JH, Millis SZ, Reddy VP, Elvin JA, Hartmaier RJ, Dowlati A, Stephens P, Ross JS, Bivona TG, Miller VA, Ganesan S, Schrock AB, Ou SI, Ali SM. BRAF in Lung Cancers: Analysis of Patient Cases Reveals Recurrent BRAF Mutations, Fusions, Kinase Duplications, and Concurrent Alterations. JCO Precis Oncol. 2018;2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Rittberg R, Banerji S, Green S, Qing G, Dawe DE. Immunotherapy Benefit in a Patient With Non-Small Cell Lung Cancer and a Rare BRAF Mutation. Cureus. 2020;12:e11224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Yang YL, Xiang ZJ, Yang JH, Wang WJ, Xiang RL. Effect of alectinib versus crizotinib on progression-free survival, central nervous system efficacy and adverse events in ALK-positive non-small cell lung cancer: a systematic review and meta-analysis. Ann Palliat Med. 2020;9:1782-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K, Hatanaka H, Bando M, Ohno S, Ishikawa Y, Aburatani H, Niki T, Sohara Y, Sugiyama Y, Mano H. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3816] [Cited by in RCA: 4167] [Article Influence: 219.3] [Reference Citation Analysis (0)] |
| 8. | Kim DW, Mehra R, Tan DSW, Felip E, Chow LQM, Camidge DR, Vansteenkiste J, Sharma S, De Pas T, Riely GJ, Solomon BJ, Wolf J, Thomas M, Schuler M, Liu G, Santoro A, Sutradhar S, Li S, Szczudlo T, Yovine A, Shaw AT. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016;17:452-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 375] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 9. | Shaw AT, Gandhi L, Gadgeel S, Riely GJ, Cetnar J, West H, Camidge DR, Socinski MA, Chiappori A, Mekhail T, Chao BH, Borghaei H, Gold KA, Zeaiter A, Bordogna W, Balas B, Puig O, Henschel V, Ou SI; study investigators. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol. 2016;17:234-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 522] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 10. | Ando K, Akimoto K, Sato H, Manabe R, Kishino Y, Homma T, Kusumoto S, Yamaoka T, Tanaka A, Ohmori T, Sagara H. Brigatinib and Alectinib for ALK Rearrangement-Positive Advanced Non-Small Cell Lung Cancer With or Without Central Nervous System Metastasis: A Systematic Review and Network Meta-Analysis. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, Ou SH, Dezube BJ, Jänne PA, Costa DB, Varella-Garcia M, Kim WH, Lynch TJ, Fidias P, Stubbs H, Engelman JA, Sequist LV, Tan W, Gandhi L, Mino-Kenudson M, Wei GC, Shreeve SM, Ratain MJ, Settleman J, Christensen JG, Haber DA, Wilner K, Salgia R, Shapiro GI, Clark JW, Iafrate AJ. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693-1703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3613] [Cited by in RCA: 3571] [Article Influence: 223.2] [Reference Citation Analysis (0)] |
| 12. | Costa DB, Shaw AT, Ou SH, Solomon BJ, Riely GJ, Ahn MJ, Zhou C, Shreeve SM, Selaru P, Polli A, Schnell P, Wilner KD, Wiltshire R, Camidge DR, Crinò L. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol. 2015;33:1881-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 506] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 13. | Camidge DR, Dziadziuszko R, Peters S, Mok T, Noe J, Nowicka M, Gadgeel SM, Cheema P, Pavlakis N, de Marinis F, Cho BC, Zhang L, Moro-Sibilot D, Liu T, Bordogna W, Balas B, Müller B, Shaw AT. Updated Efficacy and Safety Data and Impact of the EML4-ALK Fusion Variant on the Efficacy of Alectinib in Untreated ALK-Positive Advanced Non-Small Cell Lung Cancer in the Global Phase III ALEX Study. J Thorac Oncol. 2019;14:1233-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 355] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 14. | Tamura T, Kiura K, Seto T, Nakagawa K, Maemondo M, Inoue A, Hida T, Yoshioka H, Harada M, Ohe Y, Nogami N, Murakami H, Kuriki H, Shimada T, Tanaka T, Takeuchi K, Nishio M. Three-Year Follow-Up of an Alectinib Phase I/II Study in ALK-Positive Non-Small-Cell Lung Cancer: AF-001JP. J Clin Oncol. 2017;35:1515-1521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Gainor JF, Sherman CA, Willoughby K, Logan J, Kennedy E, Brastianos PK, Chi AS, Shaw AT. Alectinib salvages CNS relapses in ALK-positive lung cancer patients previously treated with crizotinib and ceritinib. J Thorac Oncol. 2015;10:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 16. | Wood DE, Kazerooni EA, Baum SL, Eapen GA, Ettinger DS, Hou L, Jackman DM, Klippenstein D, Kumar R, Lackner RP, Leard LE, Lennes IT, Leung ANC, Makani SS, Massion PP, Mazzone P, Merritt RE, Meyers BF, Midthun DE, Pipavath S, Pratt C, Reddy C, Reid ME, Rotter AJ, Sachs PB, Schabath MB, Schiebler ML, Tong BC, Travis WD, Wei B, Yang SC, Gregory KM, Hughes M. Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:412-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 441] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 17. | Nakagawa K, Hida T, Nokihara H, Morise M, Azuma K, Kim YH, Seto T, Takiguchi Y, Nishio M, Yoshioka H, Kumagai T, Hotta K, Watanabe S, Goto K, Satouchi M, Kozuki T, Koyama R, Mitsudomi T, Yamamoto N, Asakawa T, Hayashi M, Hasegawa W, Tamura T. Final progression-free survival results from the J-ALEX study of alectinib versus crizotinib in ALK-positive non-small-cell lung cancer. Lung Cancer. 2020;139:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 18. | Xia B, Nagasaka M, Zhu VW, Ou SI, Soo RA. How to select the best upfront therapy for metastatic disease? Focus on ALK-rearranged non-small cell lung cancer (NSCLC). Transl Lung Cancer Res. 2020;9:2521-2534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Solomon BJ, Besse B, Bauer TM, Felip E, Soo RA, Camidge DR, Chiari R, Bearz A, Lin CC, Gadgeel SM, Riely GJ, Tan EH, Seto T, James LP, Clancy JS, Abbattista A, Martini JF, Chen J, Peltz G, Thurm H, Ou SI, Shaw AT. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018;19:1654-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 582] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 20. | Kim ES, Barlesi F, Mok T, Ahn MJ, Shen J, Zhang P, Ou SI. ALTA-2: Phase II study of brigatinib in patients with ALK-positive, advanced non-small-cell lung cancer who progressed on alectinib or ceritinib. Future Oncol. 2021;17:1709-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Murray BW, Zhai D, Deng W, Zhang X, Ung J, Nguyen V, Zhang H, Barrera M, Parra A, Cowell J, Lee DJ, Aloysius H, Rogers E. TPX-0131, a Potent CNS-penetrant, Next-generation Inhibitor of Wild-type ALK and ALK-resistant Mutations. Mol Cancer Ther. 2021;20:1499-1507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 22. | Baik CS, Myall NJ, Wakelee HA. Targeting BRAF-Mutant Non-Small Cell Lung Cancer: From Molecular Profiling to Rationally Designed Therapy. Oncologist. 2017;22:786-796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 23. | Carter J, Tseng LH, Zheng G, Dudley J, Illei P, Gocke CD, Eshleman JR, Lin MT. Non-p.V600E BRAF Mutations Are Common Using a More Sensitive and Broad Detection Tool. Am J Clin Pathol. 2015;144:620-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Kinno T, Tsuta K, Shiraishi K, Mizukami T, Suzuki M, Yoshida A, Suzuki K, Asamura H, Furuta K, Kohno T, Kushima R. Clinicopathological features of nonsmall cell lung carcinomas with BRAF mutations. Ann Oncol. 2014;25:138-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 25. | Mazieres J, Cropet C, Montané L, Barlesi F, Souquet PJ, Quantin X, Dubos-Arvis C, Otto J, Favier L, Avrillon V, Cadranel J, Moro-Sibilot D, Monnet I, Westeel V, Le Treut J, Brain E, Trédaniel J, Jaffro M, Collot S, Ferretti GR, Tiffon C, Mahier-Ait Oukhatar C, Blay JY. Vemurafenib in non-small-cell lung cancer patients with BRAF(V600) and BRAF(nonV600) mutations. Ann Oncol. 2020;31:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 26. | Negrao MV, Raymond VM, Lanman RB, Robichaux JP, He J, Nilsson MB, Ng PKS, Amador BE, Roarty EB, Nagy RJ, Banks KC, Zhu VW, Ng C, Chae YK, Clarke JM, Crawford JA, Meric-Bernstam F, Ignatius Ou SH, Gandara DR, Heymach JV, Bivona TG, McCoach CE. Molecular Landscape of BRAF-Mutant NSCLC Reveals an Association Between Clonality and Driver Mutations and Identifies Targetable Non-V600 Driver Mutations. J Thorac Oncol. 2020;15:1611-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 27. | Odogwu L, Mathieu L, Blumenthal G, Larkins E, Goldberg KB, Griffin N, Bijwaard K, Lee EY, Philip R, Jiang X, Rodriguez L, McKee AE, Keegan P, Pazdur R. FDA Approval Summary: Dabrafenib and Trametinib for the Treatment of Metastatic Non-Small Cell Lung Cancers Harboring BRAF V600E Mutations. Oncologist. 2018;23:740-745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 180] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 28. | Yokota E, Iwai M, Yukawa T, Yoshida M, Naomoto Y, Haisa M, Monobe Y, Takigawa N, Guo M, Maeda Y, Fukazawa T, Yamatsuji T. Clinical application of a lung cancer organoid (tumoroid) culture system. NPJ Precis Oncol. 2021;5:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 29. | Sereno M, Moreno V, Moreno Rubio J, Gómez-Raposo C, García Sánchez S, Hernández Jusdado R, Falagan S, Zambrana Tébar F, Casado Sáenz E. A significant response to sorafenib in a woman with advanced lung adenocarcinoma and a BRAF non-V600 mutation. Anticancer Drugs. 2015;26:1004-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Shen G, Zheng F, Ren D, Du F, Dong Q, Wang Z, Zhao F, Ahmad R, Zhao J. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 485] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 31. | Chu T, Zhong R, Zhong H, Zhang B, Zhang W, Shi C, Qian J, Zhang Y, Chang Q, Zhang X, Dong Y, Teng J, Gao Z, Qiang H, Nie W, Zhao Y, Han Y, Chen Y, Han B. Phase 1b Study of Sintilimab Plus Anlotinib as First-line Therapy in Patients With Advanced NSCLC. J Thorac Oncol. 2021;16:643-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 168] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 32. | Alrifai D, Popat S, Ahmed M, Gonzalez D, Nicholson AG, Parcq Jd, Benepal T. A rare case of squamous cell carcinoma of the lung harbouring ALK and BRAF activating mutations. Lung Cancer. 2013;80:339-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Sui A, Song H, Li Y, Guo L, Wang K, Yuan M, Chen R. BRAF V600E mutation as a novel mechanism of acquired resistance to ALK inhibition in ALK-rearranged lung adenocarcinoma: A case report. Medicine (Baltimore). 2021;100:e24917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | La Monica S, Minari R, Cretella D, Bonelli M, Fumarola C, Cavazzoni A, Galetti M, Digiacomo G, Riccardi F, Petronini PG, Tiseo M, Alfieri R. Acquired BRAF G469A Mutation as a Resistance Mechanism to First-Line Osimertinib Treatment in NSCLC Cell Lines Harboring an EGFR Exon 19 Deletion. Target Oncol. 2019;14:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 35. | Guisier F, Dubos-Arvis C, Viñas F, Doubre H, Ricordel C, Ropert S, Janicot H, Bernardi M, Fournel P, Lamy R, Pérol M, Dauba J, Gonzales G, Falchero L, Decroisette C, Assouline P, Chouaid C, Bylicki O. Efficacy and Safety of Anti-PD-1 Immunotherapy in Patients With Advanced NSCLC With BRAF, HER2, or MET Mutations or RET Translocation: GFPC 01-2018. J Thorac Oncol. 2020;15:628-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 229] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/