Published online Feb 24, 2025. doi: 10.5306/wjco.v16.i2.98223
Revised: October 1, 2024
Accepted: November 12, 2024
Published online: February 24, 2025
Processing time: 173 Days and 19.6 Hours
Adrenocortical oncocytoma is a rare, mostly benign, nonfunctional tumor that is typically detected incidentally. Its diagnosis is challenging because of the absence of distinctive imaging characteristics, necessitating pathological validation.
We present a case report of a 35-year-old woman with an adrenal mass located on the left side, where endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) was performed after comprehensive diagnostic assessment. Our results are consistent with those of previously documented cases regarding tumor demo
For patients with adrenocortical oncocytoma, EUS-FNA can enables collection of preoperative tissue specimens leading to suitable treatment strategies.
Core Tip: The patient, a 35-year-old female, presented with an incidental finding of a left-sided adrenal mass. The nature of the mass remained uncertain following conventional imaging modalities and laboratory tests, leading us to the utilization of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA). The resultant pathological report confirmed the diagnosis of a nonfunctional adrenocortical oncocytoma, a rare and generally benign adrenal neoplasm. Based on this diagnosis, a tailored treatment plan was devised. This case highlights the utility of EUS-FNA in obtaining preoperative pathological confirmation, vital for customizing management strategies for individuals with nonfunctional adrenocortical oncocytomas.
- Citation: Chen H, Jing X. Individualized treatment guided by endoscopic ultrasound-guided fine-needle aspiration for adrenocortical oncocytoma: A case report. World J Clin Oncol 2025; 16(2): 98223
- URL: https://www.wjgnet.com/2218-4333/full/v16/i2/98223.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i2.98223
Adrenocortical oncocytoma is an uncommon variant of adrenal tumor. These tumors are generally non-functioning, indicating that they do not secrete excessive hormones, and are frequently discovered incidentally during imaging tests for other medical reasons[1].
Because these tumors lack characteristic imaging features, imaging alone is insufficient to diagnose and predict the nature of the lesion. Previous studies using fluorine-18-fluorodeoxyglucose (18F-FDG) positron emission tomography have shown that neoplastic tissues often exhibit elevated uptake. However, high uptake may lead to false positive results due to the abundance of mitochondria in tumor cells[2,3]. In summary, diagnosing adrenocortical oncocytoma is challenging. Additionally, due to their rarity, there is limited experience in managing these tumors. Traditionally, diagnosis is confirmed through pathological examination after surgical removal of the affected tissue. However, obtaining a preoperative pathological diagnosis could aid in customizing treatment and potentially avoiding unnecessary surgery.
Endoscopic ultrasound (EUS) has emerged as a pivotal tool for the assessment of abdominal diseases. It is more advantageous than computed tomography (CT)/magnetic resonance (MR) for elucidating nuanced interactions between neoplasms and adjacent anatomical structures. EUS-guided fine-needle aspiration (EUS-FNA) is a minimally invasive technique that allows for sampling deep-seated lesions for histological examination[4]. It is widely used for evaluating pancreatic and mediastinal masses. The use of EUS-FNA to diagnose adrenal masses is common, with a high rate of diagnostic positivity and a low incidence of adverse events such as hemorrhage and metastasis along the needle tract. A retrospective study of 416 EUS-FNA procedures for adrenal lesions, observed only one instance of significant bleeding, reaffirming the safety profile of the method[5]. Preoperative pathology can be used to identify benign and malignant tumors of the adrenal gland and tumors of other origins, such as adrenal metastases, adrenal hemangiomas, and adrenal lymphomas, which are essential for determining the next steps in disease management.
A thorough literature review of this rare oncological entity has revealed that most cases culminate in surgical excision following lesion detection. Only one case achieved a preoperative pathological diagnosis via EUS-FNA[6]. However, specifics regarding the EUS findings and aspiration technique were not delineated, representing a knowledge gap that could enhance clinical practice. In our case, we provide a detailed description of the method, which we believe can offer new insights for diagnosing and managing this disease.
In this report, we present the case of a 35-year-old diagnosed with non-functioning adrenocortical oncocytoma. Using EUS-FNA, we successfully established a preoperative pathological diagnosis, subsequently confirming the intermediate malignancy risk of the lesion and providing tailored therapeutic interventions.
A 35-year-old woman was hospitalized after an occupying lesion was detected during a routine examination. She exhibited no symptoms typically associated with adrenal dysfunction such as headaches, flushing, palpitations, hypertension, hirsutism, or changes in body weight.
CT scan conducted at a local medical facility indicated the presence of an occupying mass in the pancreatic tail, raising the suspicion of a pancreatic tumor. Consequently, the patient sought a consultation at our institution for a more precise diagnosis and subsequent treatment.
The patient reported being in good health before the current episode.
Both her personal and familial medical history were free of significant findings.
The patient's blood pressure was 138/70 mmHg, with no observable positive signs.
Laboratory tests (CEA, CA19-9, fasting glucose, etc.) revealed no significant abnormalities.
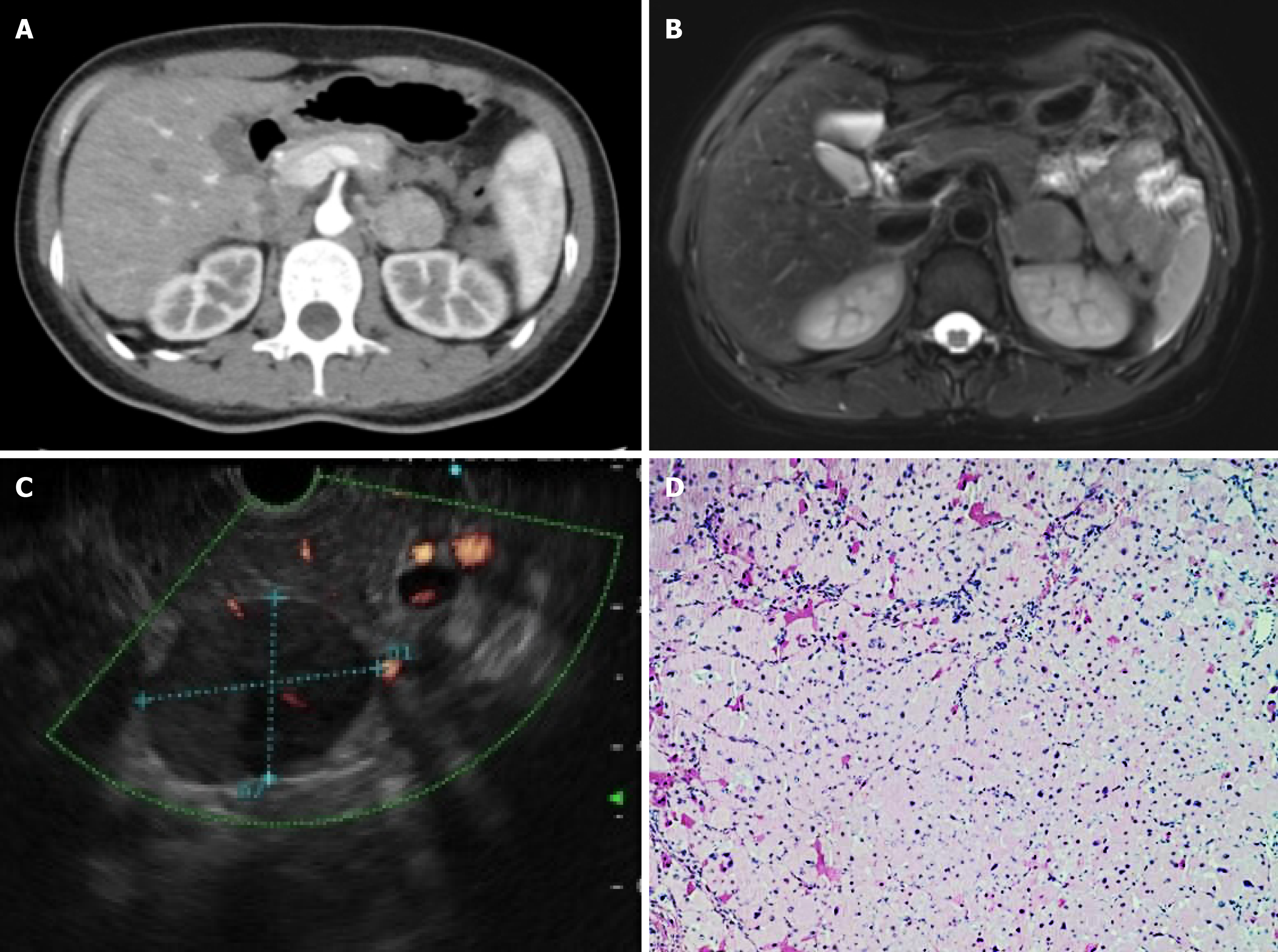
Enhanced CT scans revealed a nodular soft tissue shadow located in the lower section of the pancreas, measuring approximately 3.0 cm × 2.7 cm. The lesion exhibited a heterogeneous density with enhanced contrast uptake, which was particularly pronounced during the arterial phase relative to the portal and delayed phases (Figure 1A). Enhanced MR imaging revealed iso-T1 and moderate to high T2-weighted imaging of a nodular mass located in the lower part of the pancreas body, approximately 3.1 cm × 2.7 cm in size, with no significant high diffusion weighted imaging signal. The lesion was noted to have more obvious arterial phase enhancements and surrounding enhancements surrounding it, with a clear border (Figure 1B). Both enhanced CT and MR scans indicated that the tumor could be a retroperitoneal tumor, with a neurogenic tumor being the most likely diagnosis.
EUS-FNA was performed to clearly understand the nature of the lesion. Upon observing the endoscopy, a 3.5 cm × 2.6 cm hypoechoic mass was found behind the pancreatic body-tail junction, featuring heterogeneously internal echogenicity and a clear border. No enlarged lymph nodes were observed (Figure 1C). Color Doppler test revealed a rich blood flow signal. EUS-FNA was performed using a 22-gauge COOK needle. While applying negative pressure using a 5-mL syringe and monitoring the puncture needle under United States guidance in real-time, the needle was moved back and forth approximately ten times within the lesion, with three punctures in all. Pathological examination revealed red-stained cytoplasmic granular cells scattered with slightly deviated nuclei; no malignant tumor cells were found. (Figure 1D). Immunohistochemistry (IHC) showed that vimentin was (+), while CK (-), CD68 (-), S-100 (-), DPC4 (-), MyoD1 (-), Myogenin (-), and Desmin (-). These results indicated that mesenchymal-derived tumors are likely to be benign or intermediate tumors. Based on the EUS-FNA results, the possibility of malignancy was considered in the pancreatic occupancy. As the patient was a young woman in good health, aggressive surgery was recommended.
After considering the IHC results in conjunction with the Lin-Weiss-Bisceglia criteria, the final diagnosis was benign adrenocortical oncocytoma.
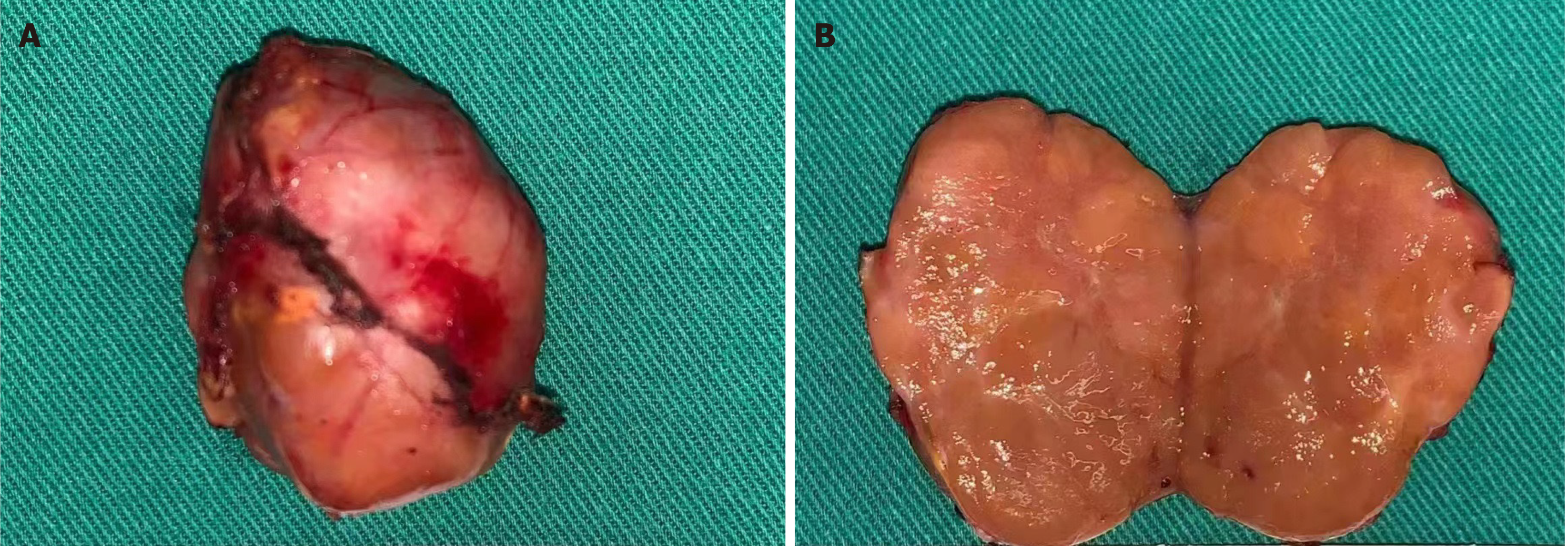
Following laparoscopic resection, the patient's tumor measured approximately 3 cm in diameter and had a tough and grayish-yellow appearance on the cut surface (Figure 2). Based on postoperative pathological analysis of the retroperitoneal mass, the tumor was identified as nodular, with a size of 4 cm × 3 cm × 3 cm, an intact envelope, mild to moderate cell heterogeneity, deviated and visible nucleoli with intranuclear pseudo-inclusions. The analysis further revealed rare (< 5 per 10 mm2) nuclear schizophrenia with no signs of necrosis or vascular or envelope invasion. In addition, a small amount of adrenal tissue was visible at the periphery. A panel of IHC markers were used revealing the following details: Synaptophysin (focally +), MelanA (+), SF-1 (weakly +), cgA (-), S-100 (-), CK (-), EMA (-), Desmin (-), and Ki-67 (+, 2%).
Following the surgical procedure, the patient’s postoperative recovery was smooth, with no reported complications, and no discomfort. After two years of follow-up, neither CT scans nor laboratory tests revealed any indications of tumor recurrence or metastasis.
Adrenocortical oncocytomas are rare tumors that primarily affect the adrenal glands. Extensive research has shown that females tend to be slightly more affected than males, with a female/male ratio of 1.5:1 to 2.1:1.4[7]. Moreover, most tumors are commonly located on the left side, accounting for approximately 64% of all cases[8]. Although adrenocortical oncocytomas are primarily considered benign, recent studies have indicated that some may exhibit borderline or malignant features. Furthermore, most oncocytomas are non-functional, accounting for approximately 66% of cases[8]. The case presented herein reflects these characteristics. To gather more information, we conducted a thorough review of the relevant literature using databases such as PubMed and MEDLINE, focusing on specific control vocabulary and keywords such as “adrenocortical oncocytoma”, “adrenal gland neoplasms”, and “adrenal glands”. We excluded cases involving symptomatic patients, functional oncocytomas, and those with inaccessible full texts and ultimately screened 60 instances. The summarized characteristics of the patients and tumors are summarized in Table 1, revealing that tumors are predominantly found in females (65%) and the left adrenal gland (65%), with the median age being 46 years and the median size being 8.5 cm. The proportion of benign, borderline, and malignant tumors was 45%, 29%, and 26%, respectively. Our results generally concurred with the existing literature on the topic. Adrenocortical oncocytoma does not display characteristic imaging performances, making it challenging to arrive at a diagnosis preoperatively. Moreover, imaging alone is often insufficient for predicting malignant behavior. In some cases, 18F-FDG was used for diagnosis, but due to multiple intracellular mitochondria in tumor cells, high uptake may show false positivity[2,3]. There is no single definitive method for decision-making regarding prognosis and treatment.
| Variable | n (%) or median (range) |
| Age | 46 (5-83) |
| Sex (female) | 39 (65) |
| Adrenal laterality | 60 |
| Left adrenal gland | 39 (65) |
| Right adrenal gland | 21 (35) |
| Size (cm)1 | 8.5 (1.7-19) |
| Nature | 158 |
| Benign | 26 (45) |
| Borderline | 17 (29) |
| Malignant | 15 (26) |
In this case, a preliminary abdominal CT scan revealed the presence of a pancreatic mass that was initially thought to be a solid pseudopapillary tumor, particularly in the patient's youth. However, the local healthcare facility only suspected a pancreatic tumor. Enhanced CT and enhanced MR scans conducted at our hospital suspected a retroperitoneal neurogenic tumor without clarifying the location or origin of the lesion. Considering the surgical risks and ambiguity surrounding postoperative care, we opted for EUS, which revealed that the tumor's location at the junction of the body and tail of the pancreas. The pathological results from EUS-FNA confirmed the lesion was likely to be intermediate in nature. We advocate a proactive surgical approach for this young patient with no contraindications. Conversely, for older patients with preexisting health issues, a strategy that emphasizes monitoring may be more appropriate, particularly if malignancy is not suspected. In conclusion, EUS-FNA is pivotal for tailoring treatment plans according to individual patient treatment.
In most cases analyzed, the tumors were swiftly excised following their detection, particularly when the tumor's characteristics were unclear. Even in cases where the cancer exhibited nonfunctional behavior and the patients were asymptomatic, surgical resection was preferred, except in three instances in which the masses demonstrated progressive growth during follow-up examinations[9,10]. CT-guided fine-needle aspiration was implemented twice, and EUS-FNA was utilized once to procure preoperative pathological findings for adrenocortical oncocytoma (Table 2). However, the authors of the referenced paper did not describe visual findings of EUS or the specific puncture procedure used. Our article elaborates on this procedure in detail, which may prove beneficial for the future preoperative diagnosis of this condition. The diagnosis of adrenocortical oncocytoma predominantly hinges on pathological assessment, which can aid in distinguishing oncocytoma from other tumors exhibiting granular, eosinophilic cytoplasm[11]. The Weiss system[12] was initially established as a guideline to differentiate benign and malignant neoplasms. Still, the modified Weiss system, proposed later by Bisceglia et al[13], added additional criteria for distinguishing between malignant, uncertain malignant potential (borderline), and benign tumors. Primary criteria (high mitotic rate (> 5 mitoses × 50 HPF), atypical mitoses, venous invasion) and minor criteria (large size and weight (> 10 cm and > 200 g), necrosis, capsular invasion, sinusoidal invasion) are discussed discerning various malignant tumors, as well as definitional criteria (predominantly cells with eosinophilic and granular cytoplasm, high nuclear grade, diffuse architectural pattern) standard to all types of oncocytic tumors.
Research has shown that EUS-FNA of the adrenal gland is reliable and safe, offering better results than the per
In conclusion, regardless of its functionality, adrenocortical oncocytoma is a tumor that is seldom encountered. It is generally considered as a benign neoplasm, although cases with borderline and malignant characteristics have been documented. For patients with nonfunctional adrenocortical oncocytomas, EUS-FNA may be performed to acquire preoperative pathology to help guide the subsequent steps toward establishing a precise prognosis and treatment plan. Given the limited research on this issue, long-term follow-up is recommended to foster a better understanding of prognosis.
We thank the patient and his family for providing signed informed consent.
| 1. | Tirkes T, Gokaslan T, McCrea J, Sandrasegaran K, Hollar MA, Akisik F, Lall C. Oncocytic neoplasms of the adrenal gland. AJR Am J Roentgenol. 2011;196:592-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 2. | Kim DJ, Chung JJ, Ryu YH, Hong SW, Yu JS, Kim JH. Adrenocortical oncocytoma displaying intense activity on 18F-FDG-PET: a case report and a literature review. Ann Nucl Med. 2008;22:821-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Sato N, Nakamura Y, Takanami K, Ono Y, Omata K, Morimoto R, Satoh F, Ise K, Yamada S, Kasajima A, Fujishima F, Watanabe M, Arai Y, Sasano H. Case report: adrenal oncocytoma associated with markedly increased FDG uptake and immunohistochemically positive for GLUT1. Endocr Pathol. 2014;25:410-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Obando JV. EUS-FNA and needle echogenicity in the age of personalized medicine. Gastrointest Endosc. 2016;84:434-435. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Patil R, Ona MA, Papafragkakis C, Duddempudi S, Anand S, Jamil LH. Endoscopic ultrasound-guided fine-needle aspiration in the diagnosis of adrenal lesions. Ann Gastroenterol. 2016;29:307-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Harada K, Yasuda M, Nakano Y, Yoshida K, Umeda Y, Yagi T, Yamazaki Y, Sasano H, Otsuka F. A rare case of oncocytic adrenocortical carcinoma clinically presented as an incidentaloma. Endocr J. 2020;67:883-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Audenet F, Méjean A, Chartier-Kastler E, Rouprêt M. Adrenal tumours are more predominant in females regardless of their histological subtype: a review. World J Urol. 2013;31:1037-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Kanitra JJ, Hardaway JC, Soleimani T, Koehler TJ, McLeod MK, Kavuturu S. Adrenocortical oncocytic neoplasm: A systematic review. Surgery. 2018;164:1351-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Saleh D, Gomaa W, Al-Maghrabi J. A Rare Concomitant Oncocytic Adrenocortical Neoplasm and Hepatocellular Carcinoma over a Four-year Duration: A Case Report and Review of Literature. Case Rep Pathol. 2019;2019:9137120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Hoang MP, Ayala AG, Albores-Saavedra J. Oncocytic adrenocortical carcinoma: a morphologic, immunohistochemical and ultrastructural study of four cases. Mod Pathol. 2002;15:973-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Xiao GQ, Pertsemlidis DS, Unger PD. Functioning adrenocortical oncocytoma: a case report and review of the literature. Ann Diagn Pathol. 2005;9:295-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Weiss LM. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am J Surg Pathol. 1984;8:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 582] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 13. | Bisceglia M, Ludovico O, Di Mattia A, Ben-Dor D, Sandbank J, Pasquinelli G, Lau SK, Weiss LM. Adrenocortical oncocytic tumors: report of 10 cases and review of the literature. Int J Surg Pathol. 2004;12:231-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 208] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 14. | Eloubeidi MA, Morgan DE, Cerfolio RJ, Eltoum IA. Transduodenal EUS-guided FNA of the right adrenal gland. Gastrointest Endosc. 2008;67:522-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Haseganu LE, Diehl DL. Left adrenal gland hemorrhage as a complication of EUS-FNA. Gastrointest Endosc. 2009;69:e51-e52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Kann PH. Endoscopic ultrasound imaging of the adrenals. Endoscopy. 2005;37:244-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Mizuide M, Ryozawa S, Fujita A, Ogawa T, Katsuda H, Suzuki M, Noguchi T, Tanisaka Y. Complications of Endoscopic Ultrasound-Guided Fine Needle Aspiration: A Narrative Review. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 18. | Dhooria S, Aggarwal AN, Gupta D, Behera D, Agarwal R. Utility and Safety of Endoscopic Ultrasound With Bronchoscope-Guided Fine-Needle Aspiration in Mediastinal Lymph Node Sampling: Systematic Review and Meta-Analysis. Respir Care. 2015;60:1040-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 19. | Krishnamurthy S, Ordóñez NG, Shelton TO, Ayala AG, Sneige N. Fine-needle aspiration cytology of a case of oncocytic adrenocortical carcinoma. Diagn Cytopathol. 2000;22:299-303. [PubMed] [DOI] [Full Text] |
| 20. | St-Amour P, Djafarrian R, Zingg T, La Rosa S, Demartines N, Matter M. Laparoscopic resection of an adrenal oncocytic neoplasm: Report of a case and review of the literature. Int J Surg Case Rep. 2020;76:305-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/