Published online Feb 24, 2025. doi: 10.5306/wjco.v16.i2.97296
Revised: September 26, 2024
Accepted: November 19, 2024
Published online: February 24, 2025
Processing time: 197 Days and 18.8 Hours
Over the years, the numbers of treatment options for colorectal cancer (CRC) have increased, leading to notable improvements in the overall survival of CRC pa
To investigate the antitumor effects of SH-4-54, which are mediated by targeting CSCs relative to treatment outcomes.
CSCs were enriched by culturing CRC cells in serum-free medium. Hallmarks of stemness and IL-6/JAK2/STAT3 signaling were detected by Western blotting. Indicators of CSC malignancy, including proliferation, invasion, and tumor formation, were measured.
In this study, we employed SH-4-54, which exhibits anticancer activity in solid tumors through targeting the SH2 domain of both the signal transducer and activator of transcription (STAT)3 and the STAT5, and evaluated its effects on stemness and chemoresistance in colorectal CSCs. As expected, SH-4-54 treatment inhibited the phosp
Taken together, these results indicate that SH-4-54 is a promising molecule that exerts antitumor effects on colo
Core Tip: Targeting cancer stem cells (CSCs) has emerged as an appealing approach for combating colorectal cancer (CRC) and improving treatment outcomes. Specifically, the study employed a compound called SH-4-54, which targets the SH2 domain of both signal transducer and activator of transcription (STAT)3 and STAT5, thereby exhibiting anticancer activity in colorectal CSCs. Taken together, our study suggests that SH-4-54 is a promising molecule that can target and inhibit colorectal CSCs by modulating the STAT3 signaling pathway, thereby presenting a potential therapeutic approach for combating CRC.
- Citation: Zhang XF, Chen Q, Jiang Q, Hu QY. Targeting STAT3 with SH-4-54 suppresses stemness and chemoresistance in cancer stem-like cells derived from colorectal cancer. World J Clin Oncol 2025; 16(2): 97296
- URL: https://www.wjgnet.com/2218-4333/full/v16/i2/97296.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i2.97296
In 2007, Siddiquee et al[1] conducted virtual screening of the SH2 domain of the signal transducer and activator of transcription (STAT)3 protein and discovered small molecules containing salicylic acid structures that effectively inhibit STAT3 activity; among these, SH-4-54 is a novel small-molecule STAT3 inhibitor derived from the optimization of the BP-1-102 compound described by Haftchenary et al[2]. Surface plasmon resonance experiments revealed tight binding between SH-4-54 and STAT3, and fluorescence polarization assays indicated that SH-4-54 primarily binds to the SH2 domain of STAT3. The SH2 domain is one of the most conserved regions in STAT proteins and is a critical site for STAT3 phosphorylation[3]. Furthermore, subsequent Western blotting experiments confirmed that the inhibitory effect of SH-4-54 on p-STAT3 was dose-dependent and that SH-4-54 effectively suppressed the expression of signaling molecules downstream of STAT3. These results suggest that SH-4-54, a small-molecule STAT3 inhibitor, has potential therapeutic effects. Aberrant activation of STAT3 is associated with tumor proliferation, invasion, and metastasis in many cancer types. Therefore, blocking STAT3 activity can disrupt the growth and spread of these malignant tumors. The discovery of SH-4-54 provides new insights for the development of more effective anticancer drugs.
Colorectal cancer (CRC) is a significant type of cancer worldwide, accounting for 9% of all cancer cases. It is the second most common cancer in women and the third most common cancer in men[4,5]. Adenocarcinoma, which arises from epithelial cells of the colon and rectum, accounts for 90% of CRC cases[6]. Over 70% of CRC-related deaths are caused by liver metastasis[7]. Although surgery may offer curative potential, fewer than 25% of CRC patients are eligible for surgical treatment, and up to 70% of patients experience recurrence. Cancer stem cells (CSCs) play crucial roles in the occurrence, development, metastasis, recurrence, and resistance to treatment of CRC[8-10].
In BT73 xenograft mouse models, SH-4-54 inhibited the growth of glioblastoma and suppressed the phosphorylation of p-STAT3[2]. Encouragingly, SH-4-54 exerted even greater inhibitory effects against human brain CSCs (BCSCs), with no toxicity observed in normal fetal astrocytes[2]. These results suggest that SH-4-54 exhibits strong targeting specificity toward human tumor stem cells. Furthermore, SH-4-54 has been found to induce apoptosis in human multiple myeloma cell lines and inhibit constitutive phosphorylation of STAT3 (p-STAT3) without altering the expression level of the STAT3 protein. Moreover, SH-4-54 significantly reduces the transcriptional activity of STAT3 and consistently leads to decreases in levels of the proteins encoded by target genes of STAT3, such as c-Myc[11]. SH-4-54 has been shown to inhibit the growth of various tumor types and tumor stem cells by inhibiting the phosphorylation and transcriptional activity of STAT3 and reducing the levels of the proteins encoded by target genes of STAT3. These findings provide valuable information for the development of new anticancer drugs and treatment strategies and have important implications for overcoming challenges in cancer therapy. Future research will continue to explore the mechanisms of action of SH-4-54 and evaluate its potential in clinical applications.
STAT3 is a key marker in CRC stem cells (CRC-CSCs). When STAT3 is phosphorylated, p-STAT3 translocates into the cell nucleus where it becomes active, promoting the proliferation, angiogenesis, and invasion of CRC-CSCs[12,13]. IL-6 is a necessary factor for STAT3 phosphorylation. In CRC, IL-6 induces the proliferation of CRC-CSCs by mediating the p-STAT3[14]. Moreover, studies have shown that the expression of STAT3 is associated with chemoradiotherapy resistance in CRC. Inhibiting STAT3 can reverse resistance to chemical drugs such as 5-fluorouracil[15]. These findings suggest that STAT3 is a potential target for treating resistant CRC. Additionally, inhibiting the activity of p-STAT3 can induce apoptosis and cell cycle arrest in CRC cells[16]. The function of STAT3 allows CRC-CSCs to maintain stem cell characteristics, and CRC-CSCs contain higher levels of p-STAT3 than ordinary CRC cells do. Therefore, inhibiting STAT3 can effectively prevent the survival of CRC-CSCs[17].
These findings highlight the important role of STAT3 in CRC-CSCs. By inhibiting the phosphorylation and activity of STAT3, the proliferation, invasion, and angiogenesis of CRC-CSCs can be blocked, and drug resistance can be reversed. Therefore, STAT3 is considered a potential target for treating CRC and overcoming drug resistance. Further research will help us better understand the function and regulatory mechanisms of STAT3 in CRC-CSCs, providing a foundation for the development of new targeted treatment strategies. In summary, SH-4-54 is an effective inhibitor of STAT3 and strongly targets CSCs. This may be attributed to the fact that STAT3 is one of the key factors that maintains the stemness of CSCs. Additionally, targeting CRC-CSCs is a crucial strategy for the treatment of CRC, indicating that SH-4-54 may hold significant potential as a small-molecule drug that can target CRC-CSCs in the treatment of CRC.
The human CRC cells SW480 and LoVo were provided by the Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). All cells were guaranteed for no mycoplasma, chlamydia, or bacterial contamination. SW480 and LoVo cells were cultured in DMEM medium containing 10% fetal bovine serum, 100U/mL penicillin, and 100mg/mL streptomycin. The cells were maintained in a CO2 incubator at 37 °C with 5% CO2. The culture medium was replaced every three days.
To enrich tumor stem cells, SW480 and LoVo cell lines were cultured in tumor sphere culture medium composed of serum-free DMEM/F12 medium (Gibco, Waltham, MA, United States), 2% B27 supplement (Gibco Life Technologies, New York, NY, United States), 10 ng/mL human recombinant basic fibroblast growth factor (bFGF), and 10 ng/mL epidermal growth factor (EGF; R&D Systems, Minneapolis, MN, United States). The cells were seeded at a density of 1 × 104 viable cells per low-attachment six-well plate (Corning, New York, NY, United States). The culture medium was changed every other day until tumor spheres were observed within approximately two weeks.
The cells were seeded at a density of 1 × 104 cells per well in a 96-well plate containing 0.1% DMSO or SH-4-54 (cat. No. 1456632-40-8, Sigma-Aldrich) medium and incubated at 37 °C for 24 hours. Cell proliferation/viability was determined using the CCK-8 assay. The absorbance at 450 nm for the DMSO-treated group was set as 100%, and the data were presented as a percentage relative to the DMSO control.
The detection of stem cell markers was performed using flow cytometry (FACScan, BD Biosciences, United States). After being treated without SH-4-54 (mock group), with IC30 (IC30 group) or IC50 (IC50 group) of SH-4-54 for 72 hours, the spheres of SW480 and LoVo were trypsinized, followed by two washes with phosphate-buffered saline (PBS). Anti-ALDH1A1-PE antibody (eBioscience, United States) was added to the cell suspension. After incubating at 4 °C in the dark for 10 minutes, the fluorescence intensity was analyzed using flow cytometry.
The SW480 and LoVo cell spheres were lysed using RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China). The extracted proteins were then subjected to electrophoresis on a 10% SDS-PAGE gel and blocked in 5% skim milk for 1 hour. Subsequently, the membrane was incubated overnight at 4°C with the following primary antibodies (Beijing Boaosen Biotechnology Co., Ltd., Beijing, China): Rabbit anti-β-actin antibody (diluted 1:1000); anti-ALDH1A1 antibody (catalog No. Ab52492, Abcam), anti-CD44 antibody (catalog No. Ab254530, Abcam), anti-Nanog antibody (catalog No. Ab109250, Abcam), anti-GAPDH antibody (catalog No. Ab8245, Abcam), anti-IL-6 antibody (catalog No. Ab233706, Abcam), anti-JAK2 antibody (catalog No. Ab108596, Abcam), anti-phosphorylated JAK2 antibody (catalog No. Ab32101, Abcam), anti-STAT3 antibody (catalog No. Ab68153, Abcam), anti-phosphorylated STAT3 antibody (catalog No. Ab30647, Abcam). The membrane was subsequently incubated with a secondary antibody, goat anti-rabbit IgG-HRP (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.). The signal proteins were visualized using a chemiluminescence system (Millipore Corporation, Billerica, MA). β-actin was used as an internal reference for normalization, and semi-quantitative analysis was performed using ChemiDocTM XRS (Bio-Rad). All experiments were repeat for three times
To quantify DNA content and analyze cell cycle phases, cells were first collected and rinsed with ice-cold PBS. They were then fixed overnight at 4 °C in ice-cold 70% ethanol. After being washed with PBS three times, the cells were incubated with a final concentration of 100 μg/mL RNase A and 40 μg/mL propidium iodide (PI, Beyotime) in the dark for 15 minutes. Finally, the cells were analyzed using 3 Laser Navios flow cytometers from Beckman Coulter, located in Brea, CA, United States.
According to the manufacturer’s instructions (Beyotime Biotech, Shanghai, China), cell apoptosis was detected using the Annexin V-FITC/PI staining kit. After treating the SW480 and LoVo cell spheres with SH-4-54 for 24 hours, the cells were collected and washed twice with pre-chilled PBS. Then, the cells were stained with 5 μL of FITC Annexin V and 5 μL of PI at room temperature in the dark for 15 minutes. Apoptotic cells were analyzed using a FACScalibur flow cytometer and Cell Quest Pro software (BD Biosciences, Shanghai, China). The experiment was independently repeated three times.
Cell migration and invasion were evaluated using an 8 μm pore size Transwell system (Costar; Corning, Inc.). The Transwell inserts were used with or without Matrigel (BD Biosciences). Cells were dissociated into single cells and resuspended in DMEM medium at a density of 1 × 105 cells/mL. The upper chamber of the Transwell was filled with 200 μL of cell suspension, while 800 μL of DMEM medium supplemented with 10% FBS was added to the lower chamber. The cells were incubated at 37 °C in a cell culture incubator for 24 hours. After that, the cells were fixed with 4% paraformaldehyde for 10 minutes and stained with 0.25% crystal violet (MilliporeSigma) at room temperature for 10 minutes. The cells were then washed three times with PBS. Images of stained cells from five random views were captured using an X71 (U-RFL-T) fluorescence microscope (Olympus Corporation) at a magnification of × 20.
The SW480 and LoVo cell spheres were seeded at a density of 1 × 104 cells per well in a 6-well plate and cultured at 37 °C for 10 days to allow colony formation. After that, the cells were washed with PBS, fixed with 4% paraformaldehyde, and stained with 0.1% crystal violet (Sigma-Aldrich, St. Louis, MO). Images were captured using a fluorescence microscope, and the number of colonies (with each colony containing > 50 cells) was counted. The experiment was independently repeated three times.
This study adhered to the guidelines outlined in the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health. The Experimental Animal Ethics Committee of the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine approved the protocol (Protocol number: 2024074) at date of 03/12/2020. Strict humane endpoints were established, whereby animals experiencing > 30% body mass loss and labored breathing were euthanized. When both of these clinical signs were observed in rats, they were considered to have reached the experimental endpoint and were immediately euthanized following the protocol of the Experimental Animal Ethics Committee of the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine. Twelve nude mice aged 4 weeks and weighing between 18-22 g were used, and animal health and behavior were monitored every two days. After nude mice were bought, mice undergo an adaptation period of approximately one week before being subjected to tumor bearing experiment. All mice were kept under a regular light-dark schedule (6 a.m.-6 p.m.) and had unrestricted access to water and food. Individual housing was provided for all mice at either standard housing temperatures (22 ± 1 °C), as specified. The cells were suspended in DMEM/F12 medium, and 200 μL of CSCs (at concentration of 2.5 × 106 cells) were subcutaneously implanted under the skin on the back of the nude mice. All mice were separated into two groups, 6 for each group. In SH-5-45 treated group, after 12 days, the mice were treated with intraperitoneal injections of the indicated compound at a dosage of 10 mg/kg in 200 μL, twice a day. In mock group, same volume of PBS was injected intraperitoneally, twice a day. Tumor volume (V) was calculated using the formula length × width × width × 0.52 every 4 days. After 28 days after tumor bearing, isoflurane was used for euthanasia in nude mice. Anesthesia was induced with a low concentration (0.27%) and then a high concentration (5%) was given to rapidly induce unconsciousness until one minute after breathing stops. After the animals' hearts stopped, they were confirmed dead and solid tumors were removed and prepared for immunohistochemistry (IHC) analysis.
IHC was performed using the formalin-fixed and paraffin-embedded brain tissues. The primary antibodies used Ki67 (catlog No. Ab15580, Abcam) at dilution rate of 1:100. A universal DAB detection kit (Roche) was used to stain antibody bound tissues.
Data analysis was performed using SPSS 26.0 statistical software. Count data were presented as frequencies or percentages, and between-group comparisons were analyzed using the χ2 test to determine significant differences. A significance level of P < 0.05 was considered statistically significant, indicating the presence of a difference.
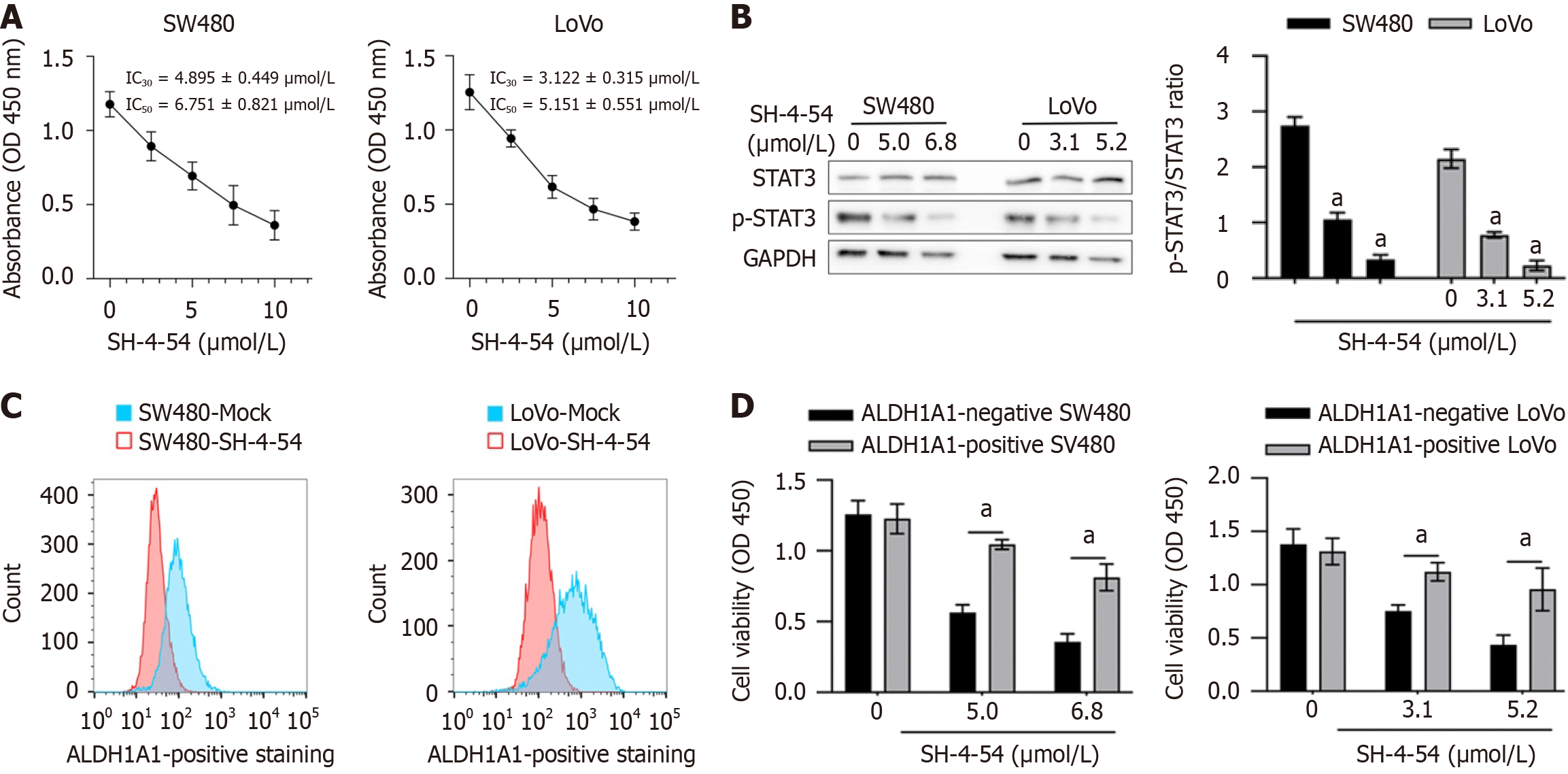
To evaluate the effect of SH-4-54 on proportion of ALDH1A1 in SW480 and LoVo cells, its cytotoxic effect was detected. 1-10 μmol/L SH-4-54 was employed for 24 hour-culture and then cell viability was measured by performing CCK-8 assay. As presented in Figure 1A, IC30 of SH-4-54 is 4.895 ± 0.449 μmol/L for SW480 and 3.122 ± 0.315 μmol/L for LoVo; IC50 of SH-4-54 is 6.751 ± 0.821 μmol/L for SW480 and 5.151 ± 0.551 μmol/L for LoVo. Meanwhile the significant inhibition of p-STAT3 was confirmed by performing western blot (Figure 1B). In CRC ALDH1A1, which marks CSCs and plays putative roles in tumor progression and drug resistance[18]. To evaluate the effect of SH-4-54 on the proportion of ALDH1A1 in SW480 and LoVo, cells were cultured with IC30 of SH-4-54 for 72 hours, respectively, and then ALDH1A1-positive proportion was detected by performing flow cytometry. As presented in Figure 1C, in both of these cell lines, SH-4-54 treatment significantly decreased ALDH1A1-positive proportion, indicating that SH-4-54 might be sensitive against ALDH1A1-positive proportion, but not ALDH1A1-negative proportion. To further confirm whether SH-4-54 is sensitive against ALDH1A1-positive proportion, ALDH1A1-positive or ALDH1A1-negative proportion was sorted respectively for sh-4-54 treatment. As presented in Figure 1D, compared to ALDH1A1-negative cells, SH-4-54 is more sensitive against ALDH1A1-positive cells.
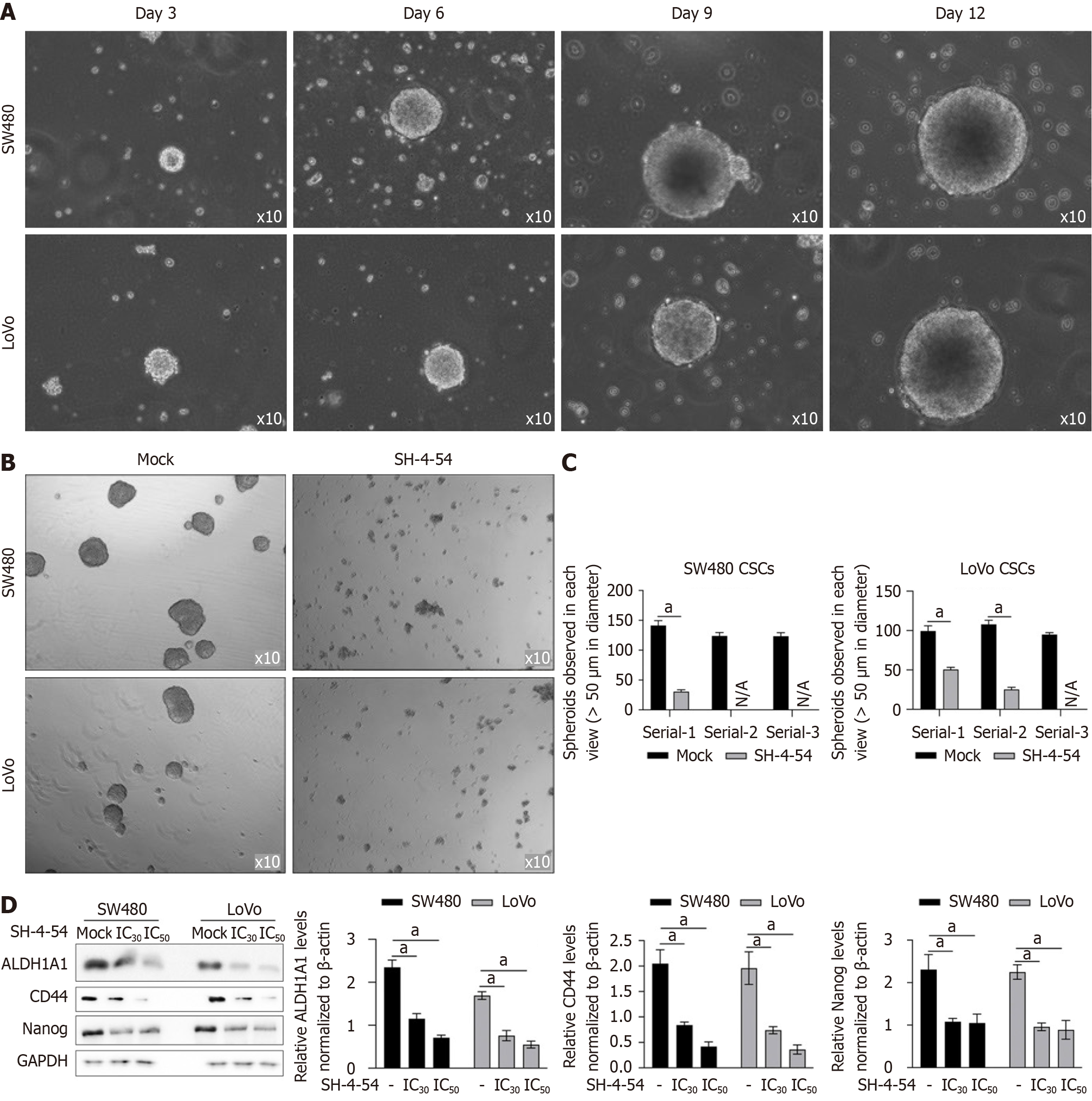
ALDH1A1-positive proportion is considered as a subpopulation presenting stemness feature and contributes to poor prognosis and outcome. By considering that SH-4-54 might affect ALDH1A1-positive proportion, it indicates that SH-4-54 may affects stemness in colorectal CSCs. We enriched CSCs by culturing SW480 or LoVo cells in serum-free medium for 12 days and formed spheres (Figure 2A) were collected for passaging. Compared to solvent control group (Mock), addition of SH-4-54 (IC30 of SH-4-54 is 4.895 ± 0.449 μmol/L for SW480 and 3.122 ± 0.315 μmol/L for LoVo) promoted dissociation of spheres after 72-hour incubation (Figure 2B), and significantly decreased ALDH1A1-positive proportion (Figure 2C). To further evaluate the effects of SH-4-54 on stemness, hallmarker genes, including CD44, Nanog and ALDH1A1 were detected. Expectedly, in CSCs, all these genes were upregulated compared to parental cells (PCs), which were significantly decreased by addition of SH-4-54 (Figure 2D).
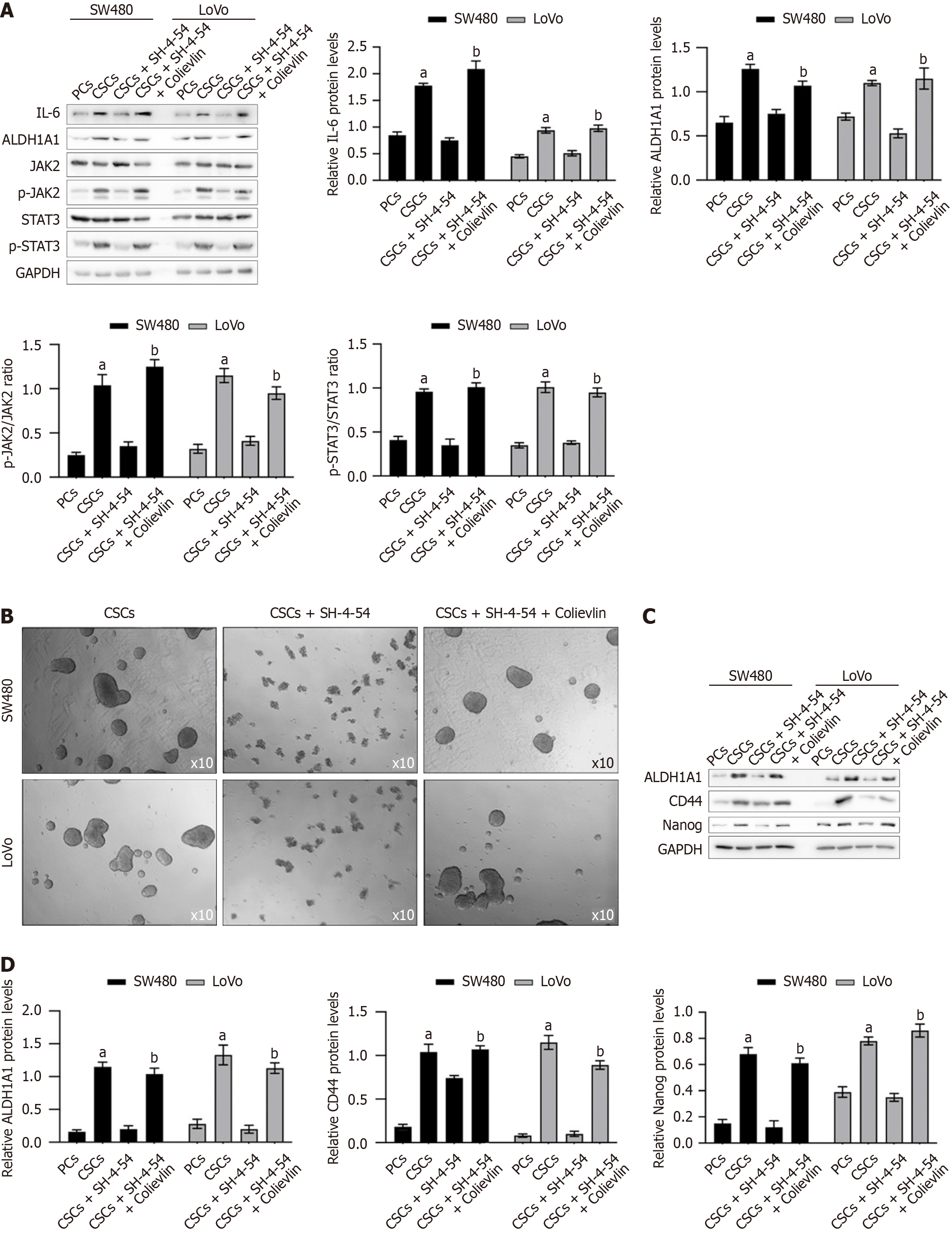
Previous results indicates that SH-4-54 inhibited STAT3 activity via inhibiting p-STAT3. STAT3 was reported to have an essential role in maintaining the expression of genes that are important for stem cell phenotype and are used as markers of CSCs[19]. To evaluate whether the effects of SH-4-54 on modifying stemness is via inhibiting STAT3 activity, we treated CSCs with SH-4-54, together with Colievlin, a brain penetrant neuroprotective peptide and a potent activator of STAT3 for 24h and analyzed stemness hallmarkers. As presented in Figure 3A, in CSCs compared to PCs, IL-6 was significantly stimulated by EGF, which is a supplement in serum-free medium, and then activates JAK2/STAT3 signaling. Expectedly, addition of SH-4-54 significantly decreased phosphorylated STAT3, without affecting IL-6 or JAK2. Addition of Colievlin significantly increased phosphorylated STAT3 compared to that without Colievlin, and thus upregulated stemness hallmarkers. Then, all these cells were further maintained for exert 12 days and then spheroid formation was observed. As presented in Figure 3B, it is clearly observed that, addition of SH-4-54 inhibited spheroid formation, which is reversed by addition of Colievlin, indicating that the inhibitory effect of SH-4-54 on stemness is potentially via inhibiting STAT3 activity. To further confirm the effect of SH-4-54 on stemness, after being cultured with SH-4-54, stemness hallmarkers including ALDH1A1, CD44 and Nanog were semi-quantitatively measured (Figure 3C and D). The results indicated that, SH-4-54 decreased all these stemness hallmarkers, and STAT3 activation by addition of Colievlin reversed the decease of all these hallmarkers, indicating that STAT3 activation is a major target of SH-4-54 to exert anti-stemness role.
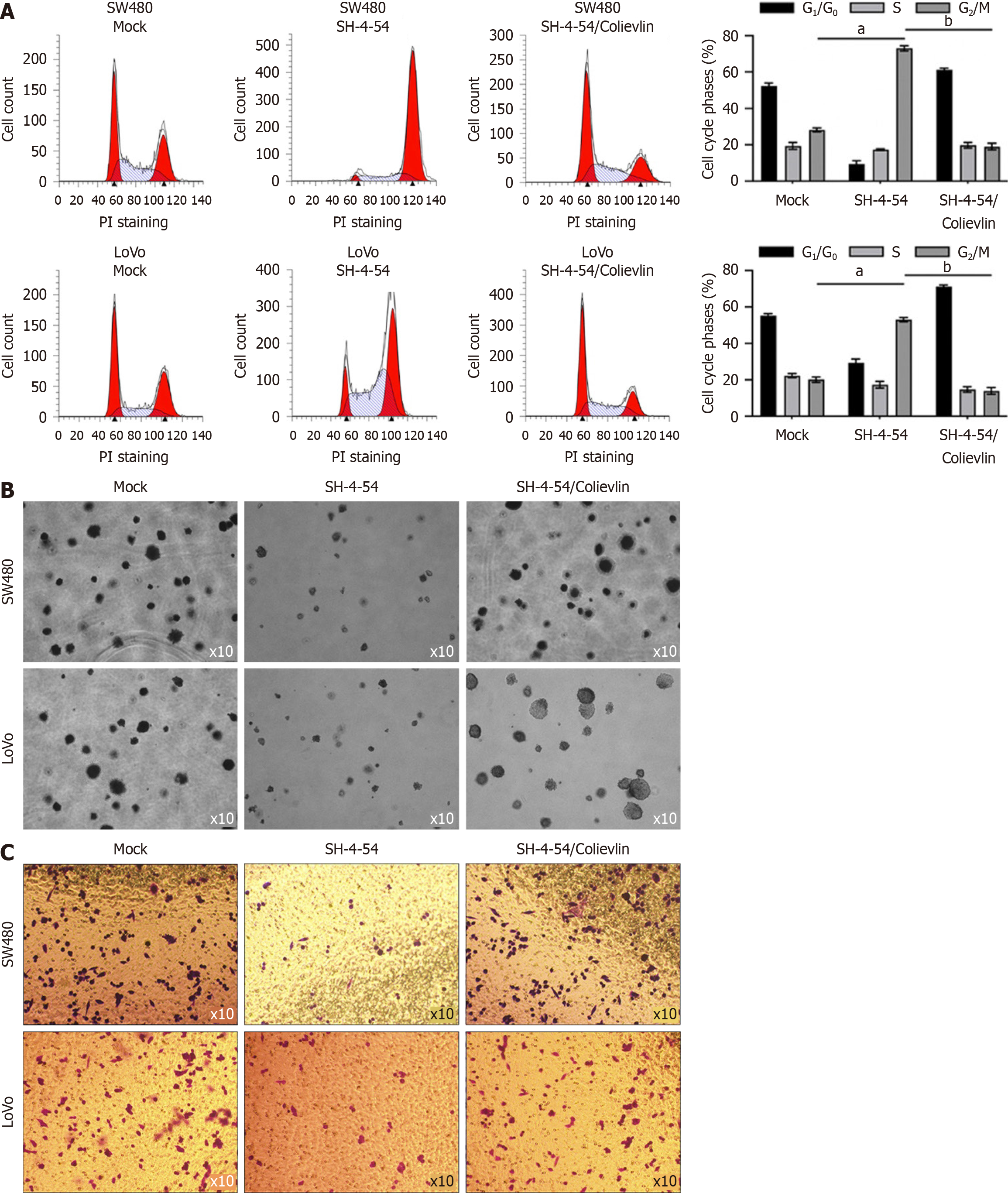
According to our results presenting that SH-4-54 regulates stemness in CSCs, which is critical for malignancies, we further evaluated the effects of SH-4-54 treatment on cell cycle distribution, tumor formation and invasion in vitro. As presented in Figure 4A, SH-4-54 treatment for 24 hours significantly increased proportion of G2/M phase, indicating its cell cycle arrest effect at G2 phase. In soft agar, addition of SH-4-54 for 10-day growth, decreased formed colonies, which is potentially due to decrease in proliferation and loss of stemness (Figure 4B). Then we also detect the effect of SH-4-54 treatment on invasion. Expectedly, SH-4-54 treatment for 24 hours significantly decreased invasion in CSCs (Figure 4C).
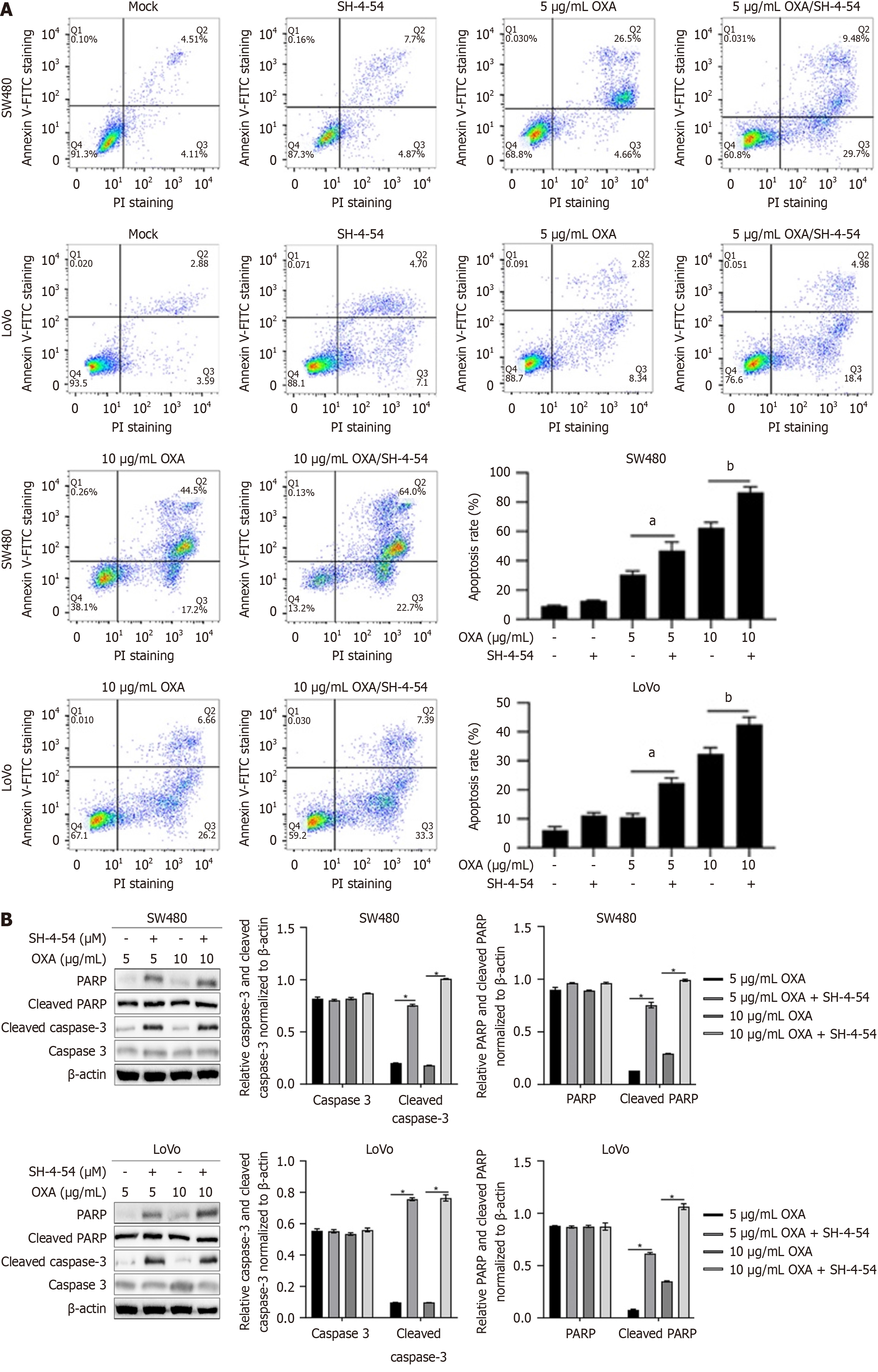
The presence of CSCs is a critical source of chemoresistance in CRCs. By considering the remarkable decreasing effects of SH-4-54 on stemness, we proposed that SH-4-54 treatment may affects chemosensitivity of CSCs. After being treated using oxaplatin (OXA) with or without SH-4-54 for 24 h, apoptosis was measured and results presented that, presence of SH-4-54 significantly increased OXA-induced apoptosis (Figure 5A). To further confirm the effects of SH-4-54 on OXA-induced apoptosis, cleaved-PARP and cleaved caspase-3 were semi-quantitatively measured by western blot. As presented in Figure 5B, presence of SH-4-54 significantly increased cleaved-PARP and cleaved caspase-3.
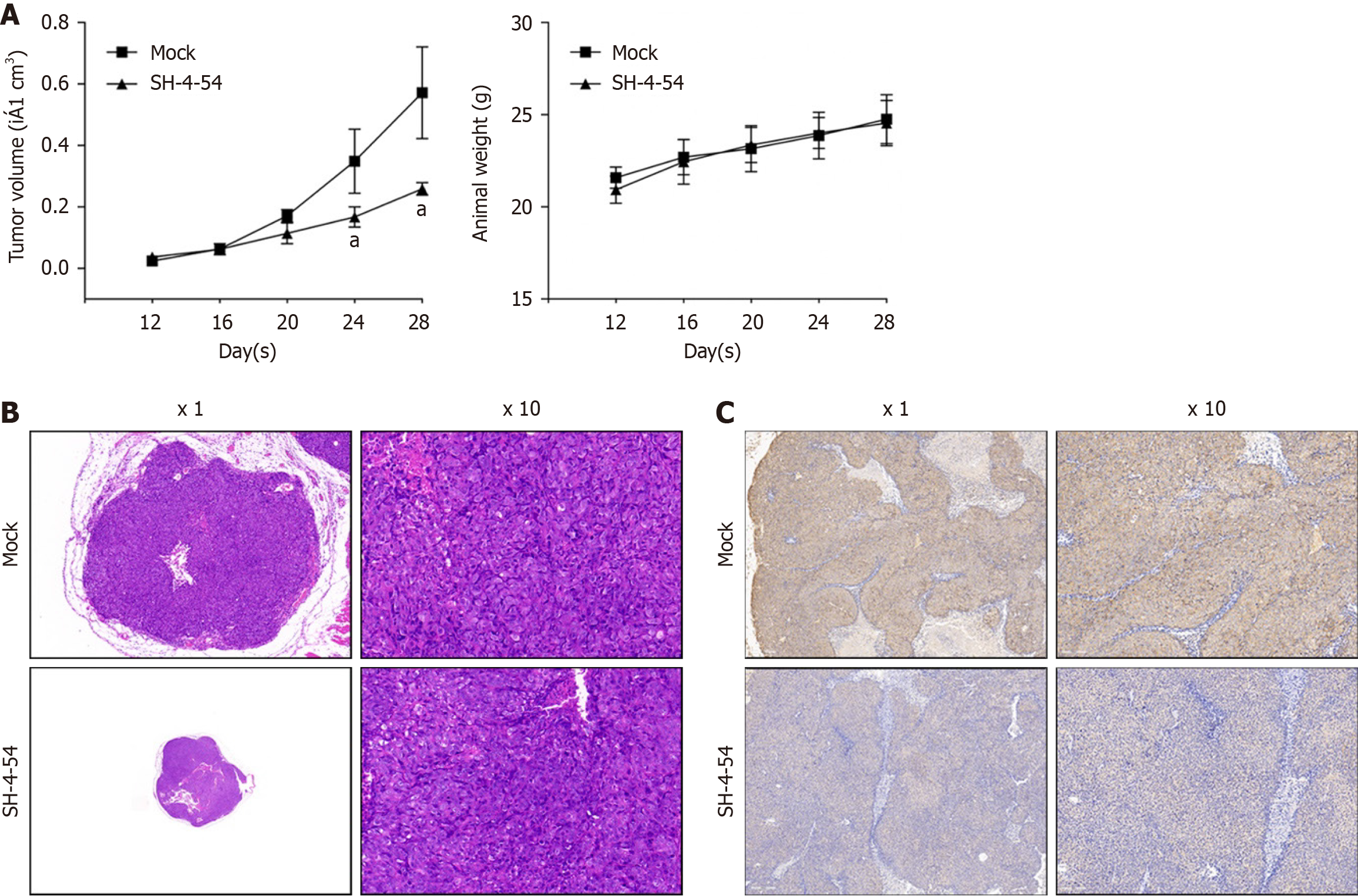
To further assess whether SH-4-54 inhibits the tumor-forming ability of CSCs in vivo, we subcutaneously injected 1 × 105 CSCs derived from SW480 into the backs of nude mice. Starting from day 10, mice were intraperitoneally injected with a dosage of 10mg/kg of SH-4-54 and continued to be treated for 20 days. The growth curve of subcutaneous tumors and animal weight were monitored throughout the experiment, at day 12, 16, 20, 24 and 28. As shown in Figure 6A, the largest tumor diameter in mock group is 1.25 cm, and SH-5-45 treated group is 0.43 cm, indicating that the subcutaneous tumor growth in mice treated with intraperitoneal injections of SH-4-54 was significantly slower than those of Mock group, without affecting animal weight obviously. Then, we evaluated the pathological structure, however, no obvious difference was observed between mock and SH-4-54 group (Figure 6B). Moreover, by performing Ki67 staining, there was no significant difference in the structure of the tumor tissues, indicating that the variation in tumor size may be due to differences in tumor growth rate (Figure 6C).
In the present study, SH-4-54 treatment significantly decreased the p-STAT3 in colorectal CSCs. The addition of Colivelin notably reversed the inhibitory effect of SH-4-54 on sphere formation, demonstrating that the inhibitory effect of SH-4-54 on stemness is mainly dependent on STAT3 activity.
Research on SH-4-54 also provides important clues for understanding the molecular mechanisms underlying STAT3 regulation, which is critical for CRC regulation[1,20,21]. By studying in depth, the mode of binding between SH-4-54 and STAT3 and its impact on downstream signaling, a better understanding of the role of STAT3 in processes such as cell proliferation, transcriptional regulation, and the immune response can be achieved. Further research will contribute to revealing the biological functions of STAT3 and the potential application of SH-4-54 as an antitumor drug. After the addition of SH-4-54, proliferation, tumor formation, invasion, and maintenance of stemness in CSCs were significantly reduced, indicating that the inhibitory effect of SH-4-54 on STAT3 is one of the main mechanisms involved in suppressing tumor stem cells. Notably, in this study, we measured IC30 and IC50 of SH-4-54 after 24-hour treatment due to the general doubling time of cells being close to 24 hours, without monitoring the inhibitory effect of SH-4-54 at other time points, including 48 h or 72 hours, which is a limitation of this inhibitory effect investigation. However, whether SH-4-54 directly inhibits STAT3 activity remains unclear, and this aspect is worth investigating with further studies.
CSCs are a small subset of tumor cells that can self-renew and differentiate and have the potential to drive tumor development and progression[8,9]. Compared to ordinary tumor cells, CSCs exhibit greater resistance to therapy and a greater ability to metastasize. By studying the biological characteristics and molecular mechanisms of CSCs, researchers can gain a better understanding of the pathogenesis of CRC and develop new treatment strategies targeting these distinct cell subsets. Therefore, targeting CSCs has become an effective approach for treating CRC. CSCs were enriched by culturing cells in a serum-free medium supplemented with EGF, bFGF and B27[10]. As expected, in enriched CSCs, the downstream target gene of EGF, IL-6, was significantly upregulated, which activated the JAK2/STAT3 signaling pathway[14,22]. The addition of SH-4-54 significantly decreased the IL-6 concentration, indicating that SH-4-54 acts as an anti-inflammatory agent in CSCs.
Currently, many studies are dedicated to discovering and developing therapeutic methods for targeting CSCs, including antibody therapies that target CSC surface markers, drugs that interfere with CSC signaling pathways, and the use of nanotechnology to deliver specific drugs to CSCs[16,17]. These strategies aim to suppress the self-renewal, differentiation, and metastatic capabilities of CSCs, thereby preventing the development and recurrence of CRC[23]. We found that SH-4-54 can inhibit the stemness of CSCs, suggesting that it may serve as a specific small-molecule inhibitor that targets tumor stem cells. SH-4-54 has significant inhibitory effects on glioblastoma and human BCSCs, but no toxicity has been observed in normal cells. Additionally, SH-4-54 induces apoptosis and suppresses STAT3 signaling pathway activity in human multiple myeloma cells. These findings provide further insight into the mechanism of action of SH-4-54 and its potential application in treating different types of cancer.
The presence of CSCs plays a crucial role in chemoresistance, mainly via their increased efflux activity. Inhibition of stemness by SH-4-54 sensitizes CSCs to OXA, suggesting promising potential for the use of SH-4-54 as an antitumor agent that targets the CSC subpopulation and induces chemosensitivity in CRC. Collectively, our results indicate that SH-4-54 acts as a specific CSC inhibitor by inhibiting STAT3 signaling, highlighting its potential for use as a promising chemotherapeutic agent that targets CSCs.
The authors would like to thank Dr. Yin Li (Chongqing University) for language editing.
| 1. | Siddiquee K, Zhang S, Guida WC, Blaskovich MA, Greedy B, Lawrence HR, Yip ML, Jove R, McLaughlin MM, Lawrence NJ, Sebti SM, Turkson J. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci U S A. 2007;104:7391-7396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 621] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 2. | Haftchenary S, Luchman HA, Jouk AO, Veloso AJ, Page BD, Cheng XR, Dawson SS, Grinshtein N, Shahani VM, Kerman K, Kaplan DR, Griffin C, Aman AM, Al-Awar R, Weiss S, Gunning PT. Potent Targeting of the STAT3 Protein in Brain Cancer Stem Cells: A Promising Route for Treating Glioblastoma. ACS Med Chem Lett. 2013;4:1102-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Lim CP, Cao X. Structure, function, and regulation of STAT proteins. Mol Biosyst. 2006;2:536-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 260] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 4. | Ghoncheh M, Mohammadian M, Mohammadian-Hafshejani A, Salehiniya H. The Incidence and Mortality of Colorectal Cancer and Its Relationship With the Human Development Index in Asia. Ann Glob Health. 2016;82:726-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Techner LM. On "pathogenesis and management of postoperative ileus" (clin colon rectal surg 2009;22:47-50). Clin Colon Rectal Surg. 2010;23:128. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Fleming M, Ravula S, Tatishchev SF, Wang HL. Colorectal carcinoma: Pathologic aspects. J Gastrointest Oncol. 2012;3:153-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 285] [Reference Citation Analysis (0)] |
| 7. | Neo JH, Ager EI, Angus PW, Zhu J, Herath CB, Christophi C. Changes in the renin angiotensin system during the development of colorectal cancer liver metastases. BMC Cancer. 2010;10:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Abdul Khalek FJ, Gallicano GI, Mishra L. Colon cancer stem cells. Gastrointest Cancer Res. 2010;S16-S23. [PubMed] |
| 9. | Vermeulen L, Todaro M, de Sousa Mello F, Sprick MR, Kemper K, Perez Alea M, Richel DJ, Stassi G, Medema JP. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc Natl Acad Sci U S A. 2008;105:13427-13432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 594] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 10. | Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, Clarke MF. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158-10163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1541] [Cited by in RCA: 1679] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 11. | Linher-Melville K, Nashed MG, Ungard RG, Haftchenary S, Rosa DA, Gunning PT, Singh G. Chronic Inhibition of STAT3/STAT5 in Treatment-Resistant Human Breast Cancer Cell Subtypes: Convergence on the ROS/SUMO Pathway and Its Effects on xCT Expression and System xc- Activity. PLoS One. 2016;11:e0161202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Corvinus FM, Orth C, Moriggl R, Tsareva SA, Wagner S, Pfitzner EB, Baus D, Kaufmann R, Huber LA, Zatloukal K, Beug H, Ohlschläger P, Schütz A, Halbhuber KJ, Friedrich K. Persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia. 2005;7:545-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 339] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 13. | Lassmann S, Schuster I, Walch A, Göbel H, Jütting U, Makowiec F, Hopt U, Werner M. STAT3 mRNA and protein expression in colorectal cancer: effects on STAT3-inducible targets linked to cell survival and proliferation. J Clin Pathol. 2007;60:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1747] [Cited by in RCA: 1806] [Article Influence: 106.2] [Reference Citation Analysis (0)] |
| 15. | Spitzner M, Roesler B, Bielfeld C, Emons G, Gaedcke J, Wolff HA, Rave-Fränk M, Kramer F, Beissbarth T, Kitz J, Wienands J, Ghadimi BM, Ebner R, Ried T, Grade M. STAT3 inhibition sensitizes colorectal cancer to chemoradiotherapy in vitro and in vivo. Int J Cancer. 2014;134:997-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 16. | Lin Q, Lai R, Chirieac LR, Li C, Thomazy VA, Grammatikakis I, Rassidakis GZ, Zhang W, Fujio Y, Kunisada K, Hamilton SR, Amin HM. Constitutive activation of JAK3/STAT3 in colon carcinoma tumors and cell lines: inhibition of JAK3/STAT3 signaling induces apoptosis and cell cycle arrest of colon carcinoma cells. Am J Pathol. 2005;167:969-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 184] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Lin L, Liu A, Peng Z, Lin HJ, Li PK, Li C, Lin J. STAT3 is necessary for proliferation and survival in colon cancer-initiating cells. Cancer Res. 2011;71:7226-7237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 252] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 18. | van der Waals LM, Borel Rinkes IHM, Kranenburg O. ALDH1A1 expression is associated with poor differentiation, 'right-sidedness' and poor survival in human colorectal cancer. PLoS One. 2018;13:e0205536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Galoczova M, Coates P, Vojtesek B. STAT3, stem cells, cancer stem cells and p63. Cell Mol Biol Lett. 2018;23:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 204] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 20. | Schulz-Heddergott R, Stark N, Edmunds SJ, Li J, Conradi LC, Bohnenberger H, Ceteci F, Greten FR, Dobbelstein M, Moll UM. Therapeutic Ablation of Gain-of-Function Mutant p53 in Colorectal Cancer Inhibits Stat3-Mediated Tumor Growth and Invasion. Cancer Cell. 2018;34:298-314.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 197] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 21. | Gargalionis AN, Papavassiliou KA, Papavassiliou AG. Targeting STAT3 Signaling Pathway in Colorectal Cancer. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (1)] |
| 22. | Yar Saglam AS, Alp E, Elmazoglu Z, Menevse S. Treatment with cucurbitacin B alone and in combination with gefitinib induces cell cycle inhibition and apoptosis via EGFR and JAK/STAT pathway in human colorectal cancer cell lines. Hum Exp Toxicol. 2016;35:526-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Agarwal S, Afaq F, Bajpai P, Kim HG, Elkholy A, Behring M, Chandrashekar DS, Diffalha SA, Khushman M, Sugandha SP, Varambally S, Manne U. DCZ0415, a small-molecule inhibitor targeting TRIP13, inhibits EMT and metastasis via inactivation of the FGFR4/STAT3 axis and the Wnt/β-catenin pathway in colorectal cancer. Mol Oncol. 2022;16:1728-1745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/