Published online Dec 24, 2025. doi: 10.5306/wjco.v16.i12.115789
Revised: October 30, 2025
Accepted: November 20, 2025
Published online: December 24, 2025
Processing time: 59 Days and 6.4 Hours
Malignant diseases in both children and adults are a worldwide public health priority with a high socioeconomic burden. Ectonucleoside triphosphate diphosphohydrolase 6 (ENTPD6) molecule exhibits divergent expression patterns across different cancers. Its increased expression in some tumors may allow them to escape anti-tumor immune responses, potentially by inducing an immunosuppressive tumor microenvironment and favoring a poorer prognosis. Conversely, in vivo, a mutated ENTPD6 gene may induce effective cytotoxic T cell responses, thereby reducing liver tumor size. Additionally, low expression of ENTPD6 has been related to chemotherapy resistance, whereas specific ENTPD6-derived neo
Core Tip: Ectonucleoside triphosphate diphosphohydrolase 6 (ENTPD6) has dual effects on cancer development and progression, influencing immune surveillance and immune evasion through tumor microenvironment modulation. It serves as a promising novel molecular biomarker for prognosis and for predicting cancer therapy responses. Furth
- Citation: Bouayad A. Ectonucleoside triphosphate diphosphohydrolase 6: A double-edged sword in cancer prognosis and therapy. World J Clin Oncol 2025; 16(12): 115789
- URL: https://www.wjgnet.com/2218-4333/full/v16/i12/115789.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i12.115789
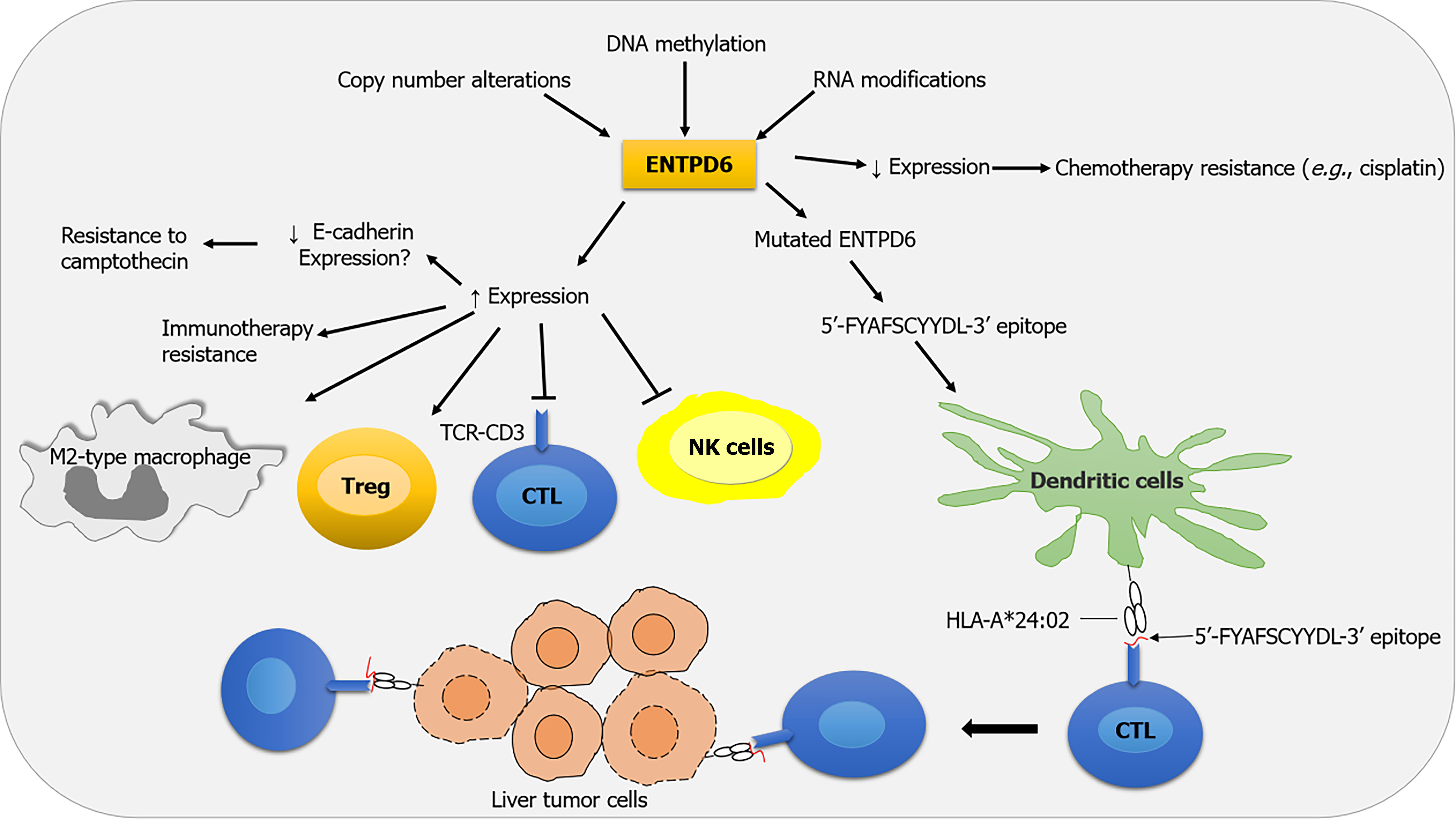
Malignant diseases in both children and adults are a worldwide public health priority with a high socioeconomic burden. Unfortunately, cancer cells often escape innate and adaptive immune responses, particularly T cell-mediated cytotoxicity. Indeed, cancer treatment may also lead to resistance and serious side effects, highly feared by physicians and patients, which need to be determined, effectively managed, and prevented. Even so, this is often not achieved, thus reducing survivorship and quality of life. Recently, Gang et al[1] found that overexpression of ectonucleoside triphosphate diphosphohydrolase 6 (ENTPD6) may contribute to tumor progression by modulating purine and pyrimidine metabolism, potentially promoting innate and adaptive immune evasion. This hydrolytic enzyme and its derived peptides are de
There is some evidence that ENTPD6 overexpression in certain tumors promotes an immune-resistant TME, thereby limiting inflammation and anticancer immune responses[1]. It is now well established that dysregulation of cellular processes (e.g., nucleotide, purine, and pyrimidine metabolism) can be reprogrammed by tumor cells in response to me
In contrast to the data discussed above, there is a body of experimental evidence that argues that ENTPD6 restrains cancer growth and proliferation. Notably, Chen et al[2] demonstrated that a mutated ENTPD6 gene can produce a specific peptide sequence (5’-FYAFSCYYDL-3’) that is presented by human leukocyte antigen (HLA)-A*24:02 molecules on tumor cells in vivo. Interestingly, cytotoxic cluster of differentiation 8+ T lymphocytes identify and eliminate liver tumor cells expressing this immunodominant epitope, thereby reducing the tumor size of HCC (Figure 1)[2].
Cancer cell resistance to chemotherapeutic agents can be due to several factors, such as low expression of ENTPD6, especially in testicular germ cell tumors and pancreatic cells[3,10]. While the mechanism underlying ENTPD6-associated drug resistance is not yet known, it has been suggested that the interaction between ENTPD6 and E-cadherin may contribute to cisplatin resistance[3]. Conversely, high ENTPD6 expression was significantly associated with resistance to camptothecin[1]. These findings suggest that ENTPD6 may serve as a promising molecular biomarker of chemotherapy resistance and a novel therapeutic target for modulating chemotherapy sensitivity in some solid tumors.
Cancer immunotherapies that activate the immune system have shown remarkable results for many patients. Resistance to immunotherapy can occur in 60%-80% of patients[11]. Several investigations indicate that both tumor cell-extrinsic and tumor cell-intrinsic factors contribute to the resistance mechanisms[11]. Tumor cell-intrinsic factors that contribute to immunotherapy resistance include expression or repression of certain genes and pathways in tumor cells that prevent immune cell infiltration or function within the TME. One reason why a tumor may not respond to immunotherapy is elevated ENTPD6 expression, possibly due to the induction of an immunosuppressive TME[1].
ENTPD6-derived neopeptide vaccines have shown promise, particularly in advanced HCC. Chen et al[2] demonstrated that among the 14 patients with HCC, only one patient expressed a specific peptide sequence 5’-FYAFSCYYDL-3’, identified as a dominant HCC neoantigen. This peptide is presented by HLA-A*24:02, highlighting the need for personalized vaccine design. Although peptide sequence 5’-FYAFSCYYDL-3’ presented by HLA-A*24:02 may trigger an eff
These findings indicate that there might have been a divergence in the function of ENTPD6 in tumorigenesis and antitumor immunity. Research on ENTPD6-derived peptides as a therapeutic target for cancer is still in its early stages, yet some epitopes previously identified in multi-omics platforms and validated in the coordinate human leukocyte antigen peptide affinity system have shown promise for HCC treatment.
| 1. | Gang W, Liu T, Zhang JX, Li YR, Zhu WJ, Wang JL, Dong WW, Zhang YY, Li YM, Yang LX, He LX, He WT. Systematic pan-cancer analysis reveals the prognostic and immunological roles of ectonucleoside triphosphate diphosphohydrolase 6. World J Clin Oncol. 2025;16:111627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Chen P, Chen D, Bu D, Gao J, Qin W, Deng K, Ren L, She S, Xu W, Yang Y, Xie X, Liao W, Chen H. Dominant neoantigen verification in hepatocellular carcinoma by a single-plasmid system coexpressing patient HLA and antigen. J Immunother Cancer. 2023;11:e006334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 3. | Tada Y, Yokomizo A, Shiota M, Song Y, Kashiwagi E, Kuroiwa K, Oda Y, Naito S. Ectonucleoside triphosphate diphosphohydrolase 6 expression in testis and testicular cancer and its implication in cisplatin resistance. Oncol Rep. 2011;26:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Yang L, Zhang Y, Yang L. Adenosine signaling in tumor-associated macrophages and targeting adenosine signaling for cancer therapy. Cancer Biol Med. 2024;21:995-1011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Luby A, Alves-Guerra MC. Targeting Metabolism to Control Immune Responses in Cancer and Improve Checkpoint Blockade Immunotherapy. Cancers (Basel). 2021;13:5912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Chen Y, Wen H, Zhou C, Su Q, Lin Y, Xie Y, Huang Y, Qiu Q, Lin J, Huang X, Tan W, Min C, Wang C. TNF-α derived from M2 tumor-associated macrophages promotes epithelial-mesenchymal transition and cancer stemness through the Wnt/β-catenin pathway in SMMC-7721 hepatocellular carcinoma cells. Exp Cell Res. 2019;378:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 7. | Huang P, Zhou X, Zheng M, Yu Y, Jin G, Zhang S. Regulatory T cells are associated with the tumor immune microenvironment and immunotherapy response in triple-negative breast cancer. Front Immunol. 2023;14:1263537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 8. | Joshi NS, Akama-Garren EH, Lu Y, Lee DY, Chang GP, Li A, DuPage M, Tammela T, Kerper NR, Farago AF, Robbins R, Crowley DM, Bronson RT, Jacks T. Regulatory T Cells in Tumor-Associated Tertiary Lymphoid Structures Suppress Anti-tumor T Cell Responses. Immunity. 2015;43:579-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 408] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 9. | Ye Z, Zheng M, Zeng Y, Wei S, Huang H, Wang Y, Liu Q, Lin Z, Chen S, Zheng Q, Chen L. A 13-Gene Metabolic Prognostic Signature Is Associated With Clinical and Immune Features in Stomach Adenocarcinoma. Front Oncol. 2021;11:612952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Fridley BL, Batzler A, Li L, Li F, Matimba A, Jenkins GD, Ji Y, Wang L, Weinshilboum RM. Gene set analysis of purine and pyrimidine antimetabolites cancer therapies. Pharmacogenet Genomics. 2011;21:701-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell. 2017;168:707-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2349] [Cited by in RCA: 4133] [Article Influence: 459.2] [Reference Citation Analysis (11)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/