Published online Nov 24, 2025. doi: 10.5306/wjco.v16.i11.112514
Revised: August 12, 2025
Accepted: October 13, 2025
Published online: November 24, 2025
Processing time: 115 Days and 2.9 Hours
Breast cancer is a prevalent malignant tumor among women. Despite significant advancements in the development and implementation of various anti-breast cancer therapies, enhancing the efficacy of these drugs while minimizing their toxicity remains a challenge.
To explore the functional impact of the targeted long chain non-coding RNA (LncRNA) of Chidamide on the activity of natural killer (NK) cells via progra
This study screened the positive LncRNA molecule Linc01010 through high-throughput sequencing, which can counteract the pharmacological effects of Chidamide. Luciferase localization analysis revealed that the Linc01010 fragment was situated in the proximal exon 4-3 region, identified as its functionally active region. Electrophoretic mobility shift assays and RNA-protein pull-down experiments demonstrated the interaction between Chidamide-induced Linc01010 expression and the target protein mitogen-activated protein kinase kinase 6 (MKK6). Western blotting and quantitative polymerase chain reaction analyses indicated that Chidamide enhanced the expression of the downstream effector PD-L1 by activating the corresponding p38-mitogen-activated protein kinases pathway.
While investigating the effects of the Chidamide-Linc01010-MKK6-PD-L1 axis on the immune cell line NK-92, we observed that tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) secretion was significantly inhibited by this response axis. Furthermore, reducing TRAIL secretion within the tumor microenvironment diminished the death effects in breast cancer cells induced by Chidamide.
Our study provides a robust foundation for improving the effectiveness of current anti-breast cancer medications and for identifying new targets related to drug resistance.
Core Tip: Breast cancer is a prevalent malignant tumor among women. This study screened the positive long chain non-coding RNA molecule Linc01010 which can counteract the pharmacological effects of Chidamide. While investigating the effects of the Chidamide-Linc01010-mitogen-activated protein kinase kinase 6-programmed death-ligand 1 axis on the immune cell line natural killer-92, we observed that tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) secretion was significantly inhibited by this response axis. Furthermore, reducing TRAIL secretion within the tumor microenvironment diminished the death effects in breast cancer cells induced by Chidamide. Our study provides a robust foundation for improving the effectiveness of current anti-breast cancer medications and for identifying new targets related to drug resistance.
- Citation: Han H, Guo XY, Wen JX, Zhao XM, Zhou WQ. Immune regulation of Chidamide-induced Linc01010 accumulation in breast cancer cell death. World J Clin Oncol 2025; 16(11): 112514
- URL: https://www.wjgnet.com/2218-4333/full/v16/i11/112514.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i11.112514
Cancer, as a multifactorial-driven complex disease, involves multilevel mechanisms such as genetic mutations, epigenetic dysregulation, and microenvironmental interactions in its development. In recent years, cancer treatment strategies have gradually shifted from conventional chemotherapy and radiotherapy to targeted therapy and immunotherapy, but drug resistance and tumor heterogeneity remain major clinical challenges[1]. In particular, breast cancer is the most prevalent malignant tumor in women worldwide, accounting for 11.7% of new cancer cases worldwide in 2020 (2.3 million new cases per year)[2]. In recent years, the discovery of new targets [e.g., human epidermal growth factor receptor 2 (HER2)] and drugs (e.g., CDK4/6 inhibitors) for cancer therapy has significantly improved the prognosis of breast cancer patients[3,4]. However, despite the fact that endocrine therapy and HER2-targeted drugs have become the standard clinical regimen, patients with advanced disease still commonly face drug resistance, which ultimately leads to treatment failure[5].
Extensive research on the structure of the human genome has revealed that epigenetic modifications play a crucial role in regulating gene functions[6,7]. These modifications can disrupt the normal functioning of genes, leading to an increased risk of cancer development[8,9]. Histone acetylation regulation is a key epigenetic modification that governs the dynamic balance between histone acetyltransferases and histone deacetylase (HDAC). This balance determines the acetylation state of histones, which in turn affects gene regulation. Therefore, it plays a significant role in the occurrence and progression of breast cancer[10,11].
HDAC inhibitors (HDACi) have been demonstrated to activate the expression of anti-oncogenes and enhance the differentiation and apoptosis of tumor cells by inhibiting the activity of HDAC. This broad spectrum of anti-tumor capabilities has been substantiated by various studies[12]. Chidamide, a novel subtype-selective HDACi, has been shown to selectively inhibit the activity of class I HDAC1, HDAC2, HDAC3, and class II HDAC10[13,14]. Numerous studies have illustrated the efficacy of Chidamide in disrupting the tumor cell cycle, promoting tumor cell differentiation, inducing autophagy and apoptosis of tumor cells, and inhibiting neovascularization, thereby resulting in effective anti-tumor activity across various solid tumors[15,16]. However, the efficacy of therapeutic strategies that singularly target epigenetic modifications is often limited by the inherent immunosuppressive properties of the tumor microenvironment (TME); e.g., immune escape mechanisms mediated by the programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) axis.
The tumor and its internal environment are collectively referred to as the TME, characterized by interdependence and mutual reinforcement, as well as antagonism and competition. The TME significantly influences the occurrence, development, and metastasis of tumors, while also playing a critical role in their diagnosis, prevention, treatment, and prognosis[17,18]. During the study of tumors, researchers have observed a phenomenon that contradicts previous understanding: Certain immune cells often shift from being 'protectors' of the internal environment to becoming 'accomplices' of tumor cells, thereby promoting and sustaining tumor development. This observation challenges classical immunology, which posits that effective anti-tumor responses are mediated by immune active cells through immune surveillance mechanisms that combat harmful invaders. By exerting a pro-tumor effect, infiltrating tumor immune cells can significantly impact tumor progression and the efficacy of anti-cancer therapies. Consequently, quantifying tumor-infiltrating immune cells is anticipated to elucidate the complex role of the immune system in human cancer, particularly regarding tumor escape mechanisms and responses to treatment[19].
Currently, the molecular mechanism of HDACi interaction with the tumor immune microenvironment has not been fully elucidated. Notably, studies suggest that long chain non-coding RNAs (LncRNAs) may play a key bridging role in mediating this interaction.
LncRNA is an endogenous RNA molecule that does not code for proteins and has a transcript length exceeding 200 nucleotides. It is characterized by the absence of an open reading frame[20]. Despite its limited protein-coding capability, LncRNA serves as a promising biomarker for cancer prognosis, diagnosis, and development[21]. LncRNA interacts specifically with various proteins, including epigenetic modifiers, transcription factors, co-activators, and ribonucleoprotein complexes, to regulate gene expression[22]. This regulation can occur at the transcriptional, post-transcriptional, and epigenetic levels, influencing biological processes such as tumor cell proliferation, differentiation, migration, and apoptosis.
In previous research, Chidamide was used to treat breast cancer cell lines MCF-7 and MDA-MB-231, which were subsequently screened and analyzed using RNA high-throughput sequencing (RNA-seq)[23]. The results revealed that a LncRNA (Linc01010) showed significant differential expression after drug treatment. It is interesting to note that Linc01010 may be involved in the regulation of immune checkpoints (ICPs) through the mitogen-activated protein kinase kinase 6 (MKK6)/p38 signaling pathway. MKK6 kinase exhibits a dual function in solid tumors: Its oncogenic role has been demonstrated in metastatic studies of ovarian cancer, where the upstream regulator of p38 kinase, MKK4/MKK6, inhibited the metastatic colonization of tumor cells[24], whereas, its pro-carcinogenic activity has been reported in gastrointestinal tract tumors, as evidenced by the high expression status of MKK6 in esophageal and gastric cancer tissues[25].
Similarly, the p38-PD-L1 regulatory axis showed functional conservation in both muscle differentiation and immune escape. In muscle differentiation, the MKK6/p38/protein kinase B axis has been shown to have both anticancer and pro-myogenesis effects[26], suggesting that there may be a signaling pattern of this regulatory axis across cancer species.
The present study aimed to reveal the function of Chidamide in regulating natural killer (NK) cell activity by targeting the Linc01010-MKK6/p38-PD-1 axis, with a particular focus on the mechanism by which NK cells affect breast cancer cells. These studies will undoubtedly deepen our understanding of the role of LncRNAs and their molecular regulatory mechanisms, thus laying the foundation for identifying drug-resistant targets in breast cancer.
Human breast cancer cell line MCF-7, MDA-MB-231 and human NK-92 cell line were obtained from Pricella (Wuhan, China). RPMI-1640 medium, L-15 medium, fetal bovine serum (FBS) and Penicillin-streptomycin Cocktails were purchased from Life Technologies (Austin, TX, United States). Chidamide was supplied by Sigma-Aldrich (St. Louis, MO, United States). Luciferase Assay System and CellTiter 96® AQueous One Solution Cell Proliferation Assay were from Promega (Madison, MI, United States). High Pure RNA Isolation Kit was given from Roche Diagnostics GmbH (Mannheim, Germany). Transcriptor First Strand cDNA Synthesis Kit, Zero Blunt® TOPO® PCR Cloning Kit, MAXIscript® Kit, NorthernMaxTM Kit, ULYSIS® Nucleic Acid Labeling Kit, LightShift® Chemiluminescent RNA EMSA Kit, PierceTM Magnetic RNA-Protein Pull-Down Kit, Power SYBR Green PCR Master Mix, RIPA Cell Lysis Buffer, BCA Protein Assay Kit, Donkey Anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody Labeled with Alexa Fluor™ 790 and Donkey Anti-Goat IgG (H+L) Highly Cross-Adsorbed Secondary Antibody Labeled with Alexa Fluor™ 680 were from Life Technologies (Austin, TX, United States). Polyclonal anti-MKK6 antibody, polyclonal anti-MKK6 phospho (S207) antibody, polyclonal anti-STAT1 antibody, polyclonal anti-STAT1 phospho (S727) antibody, polyclonal anti-STAT3 antibody, polyclonal anti-STAT3 phospho (S727) antibody, polyclonal anti-p38 phospho (T180+Y182) antibody, polyclonal anti-PD-L1 antibody and polyclonal anti-β-actin antibody were obtained from Abcam Inc (Cambridge, MA, United States). LncRNA01010 small interfering RNA (siRNA) candidates were designed and synthesized by RiboBio (Guangzhou, China). The p38 inhibitor SB203580 was from GLPBIO (Montclair, CA, United States). Human TRAIL/TNFSF10 Quantikine® ELISA Kit and Human PD-1 Quantikine® ELISA Kit were obtained from R and D Systems Inc (Minneapolis, MN, United States). Human other chemicals were from Sangon Biotech (Shanghai, China).
The cells were authenticated using short tandem repeat and amelogenin gene locus to reconfirm the cell identity. Then the cells were incubated in medium with supplementation of 10% FBS, 100 U/mL penicillin and 100 g/mL streptomycin. All the cells were grown at 37 °C with 5% CO2 and 95% humidity. The cells were seeded at a density of 1.0 × 104 cells/mL in a 96-well plate, 5.0 × 105 cells/mL in a 6-well plate and 1.5 × 107 cells/mL in a 100 mm dish. The cells were grown to 70%-80% confluence and starved for 24 hours in basal medium [with dimethyl sulfoxide (DMSO)] without FBS and dealt with different compounds.
For the cultivation and collection of human NK cell line NK-92, take 1 × 105 cells/mL NK cells in a 25 mm flask and suspended them in complete culture medium (Pricella) containing 0.2 mmol/L inositol, 0.1 mmol/L β-mercaptothanol, 0.02 mmol/L folic acid, and 100 U/mL recombinant interleukin (IL)-2 for 48 hours.
The cells were plated in a 96-well plate (1.0 × 104 cells/mL). After 24 hours of starvation, cells were combined with 20 μM of Chidamide for 48 hours of incubation. The same concentrations of DMSO were added as a control. The CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTT) was performed and absorbance was measured at 490 nm on a microplate reader.
Cell viability was confirmed using the Muse Count and Viability reagent (Millipore) following the manufacturer’s protocols. Following trypsinization, 2 × 105 of harvested cells (50 uL cell suspension) were added with 450 uL Muse Count and Viability reagent (Millipore). The results were obtained with Muse Count and Viability software module, and the statistics showed the percentage of viable cells.
The 5.0 × 105 of breast cancer MCF-7 or MDA-MB-231 cells were seeded in each well of 6-wells plate with 4 mL medium. After incubation with Chidamide for 48 hours, 2.0 × 105 of NK-92 suspension cells were added and co-cultivated according to the same ratio of culture media for another 48 hours. The cell viability was assayed by MTT.
The 1.0 × 104/well of MCF-7 or MDA-MB-231 cells was added into E-plate 16 in duplicates and xCELLigence Real-time Cell Analyzer (RTCA) DP System was used to monitor cell kinetics across microelectronic sensors integrated into the bottom. The cells were starved without FBS and incubated with MCF-7 or MDA-MB-231 cells with different doses of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) (0-100 ng/mL) and 20 μM Chidamide. The same concentrations of DMSO were used as control. The assay was monitored every 60 minutes for 120 hours. For quantification, the cell index values at indicated time points were graphically represented at least three independent measurements.
We collected the supernatant or cell lysis after co-culturing the drug with the corresponding cells under the above experimental conditions, centrifuging at 12000 rpm for 30 minutes, and added the supernatant to a 96-well plate. The protein concentrations were measured using Quantikine® ELISA Assay Kit following the manufacturer’s instructions.
NK-92 cells were centrifuged at 1000 rpm for 5 minutes and added 500 μL of 4% paraformaldehyde solution to fix overnight at 4 °C. After washing with PBS, the cells were blocked with 1% bovine serum albumin at room temperature for 1 hour. The 100 μL of 1:250 diluted rabbit anti-human CD54 or rabbit anti-human CD16 primary antibody was added and incubated overnight at 4 °C. Goal anti-rabbit immunoglobulin G conjugated Fluro® 488 secondary antibody was incubated at room temperature for 1 hour. Finally, one portion of the fluorescently stained cells were added onto a glass slide and observed the distribution and intensity of NK-92 cell fluorescence using a fluorescence microscope at an excitation wavelength of 488 nm. The other part of cells was filtered through a 200-mesh filter and analyzed by flow cytometry for NK-92 cell content and CDs molecule expression.
The siRNAs were transfected into breast cancer cells maintained in a 6-well plate using Lipofectamine 3000 transfection reagent. The 6.6 μL siRNA was mixed with 125 μL medium without serum for 5 minutes, and the 3.75 μL Lipofectamine 3000 reagent was mixed with 125 μL medium without serum for 5 minutes. These two reagents were mixed and incubated at room temperature for 5 minutes. The cell culture medium was removed from each well of the 6-well plates, and the mixture of siRNA and Lipofectamine 3000 was added to each well. The 1 mL medium without serum was added with cells for 12 hours of incubation. Finally, the transfection mixture was removed and the cells were cultured with 2 mL of medium with serum for 24 hours.
The blunt-end polymerase chain reaction (PCR) products were subcloned into PCR-Blunt II-TOPO vector according to the manufacturer’s protocol. The reaction mixture was incubated for 5 minutes at room temperature. Then the reaction with competent DH5α bacteria was placed at 42 °C for 45 seconds and put on ice. Positive clones were selected by colony PCR and amplified in Luria-Bertani medium containing 50 μg/mL kanamycin. Plasmid DNA was isolated using a miniprep extraction kit.
The MAXIscript® Kit was used according to the manufacturer’s protocol. Firstly, conventional PCR was amplified of DNA template for synthesizing Northern blot probe. The primers were used: (1) Forward: 5’-TGAGGAGGTGGAGAACGTGG-3’; and (2) Reverse: 5’-GGACACAGATTGCTGACAAC-3’. SP6 RNA polymerases were added for the in vitro synthesis of RNA transcripts from Not I-linearized DNA templates. Then the reaction was mixed with ribonucleoside triphosphates and transcription buffer, and the mixture was incubated for 15 minutes at 37 °C. After terminated by Turbo DNase, the RNA probe was purified by spin column.
For the preparation of the labeling probe, ULYSIS® Nucleic Acid Labeling Kits were used. One μg of RNA was precipitated by adding 1/10 volume of 3 M sodium acetate (pH 5.2) and two volumes of absolute ethanol. The reaction was frozen at -70 °C for 30 minutes and then centrifuged for 15 minutes at 12000 rpm. The pellet was washed with 70% ethanol and resuspended in 20 μL of the labeling buffer. The DNA was denatured at 95 °C for 5 minutes and then snapped cool on ice. The tube was centrifuged briefly to redeposit the sample. Finally, 5 μL Alexa Fluor® 647 labeling reagent stock solution was added to the tube containing the denatured sample DNA to bring the final volume to 25 μL. The mixture was incubated at 90 °C for 10 minutes and the DNA was purified by a spin column according to the manufacturer’s protocol.
Total RNA was extracted from breast cancer cells using High Pure RNA Isolation Kit according to the manufacturer’s instructions. RNA quantitation was performed via quantitative PCR (qPCR). The total RNA was reverse-transcribed with Transcriptor First Strand cDNA Synthesis Kit and amplified by Power SYBR Green PCR Master Mix in an Applied Biosystems 7500 qPCR System. The primers were designed by primer 3 suit and libraries. The sequences of primers were shown in Table 1. Data normalization was based on correcting all Ct values for the average Ct values of GAPDH gene present in the reaction. Three independent biological replicates were performed.
| Gene name | Primer orientation | Sequence |
| Lin01010 | Forward | 5’-GCAAACTTGAGGAATGGGCA-3’ |
| Reverse | 5’-GCCTTGGCTTGCCTATTACC-3’ | |
| PD-1 | Forward | 5’-AGGATGGACACTGCTCTTGG-3’ |
| Reverse | 5’-CTTCTCCTGAGGAAATGCGC-3’ | |
| PD-L1 | Forward | 5’-GGAAATTCCGGCAGTGTACC-3’ |
| Reverse | 5’-GGCATTCAAGGGTTCAAGCA-3’ | |
| PD-L2 | Forward | 5’-ACAGCCAGTTTGCAAAAGGT-3’ |
| Reverse | 5’-TTGGTACTGTCCTTCGTCCC-3’ | |
| MKK6 | Forward | 5’-TTGGAGTCTGGGCATCACGA-3’ |
| Reverse | 5’-GTTGTGGCGATGGCTCCTCT-3’ | |
| STAT1 | Forward | 5’-CCAATGGAACTTGATGGCCC-3’ |
| Reverse | 5’-ACCTCGTCAAACTCCTCAGG-3’ | |
| STAT3 | Forward | 5’-AGTTCTCCTCCACCACCAAG-3’ |
| Reverse | 5’-CCTTGCCAGCCATGTTTTCT-3’ | |
| P38 | Forward | 5’-AGAATGTGCCAGGGGTGTG-3’ |
| Reverse | 5’-GTGGGGTTGCAGAAGTTCAG-3’ | |
| GAPDH | Forward | 5’-GAGTCAACGGATTTGGTCGT-3’ |
| Reverse | 5’-GACAAGCTTCCCGTTCTCAG-3’ |
MCF-7 cells were treated with Chidamide as described before. The growth medium was carefully removed, and the cells were rinsed with PBS. 300 μL 1 × lysis buffer was added to cover the cells. After rocking the dishes several times, attached cells were scraped from the dish. Cells were then transferred to a 1.5 mL tube and kept in ice for 1 minute. The luminometer was programmed to perform a 2-second measurement delay followed by a 10-second measurement read for luciferase activity. The 20 μL cell lysate was then mixed with 100 μL luciferase assay reagent. The samples were analyzed on Glomax® 96 luminometer and initiated reading.
The cell pellets collected from 6-well plates were incubated in RIPA buffer containing 0.1 mg/mL protease inhibitor. The cellular lysate was rotated for 2 hours at 4 °C followed by centrifugation for 30 minutes at 14000 g at 4 °C. Proteins were quantified using a BCA Protein Assay Kit. For immunoblotting, 20 μg proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. Western blot analyses were performed using the antibodies described above. The level of β-actin was used as the loading control. Protein bands were detected using LI-COR CLx Odyssey Scanning System at 700 nm or 800 nm channel.
Northern blot was performed according to the protocol described previously. In brief, a 15 μg aliquot of total RNA was collected in 5 μL of RNase-free H2O, supplemented with two volumes of RNA loading buffer. RNA samples were resolved on a 0.8% formaldehyde-agarose gel, capillary transferred onto a positively charged nylon transfer membrane, rinsed with H2O and ultraviolet crosslinked at 1200 mJ (0.1 mJ/cm2) for 60 seconds. North2South® hybridization buffer was added with the membrane at 55 °C for two hours to Alexa Fluor® 647-labeled RNA probe at a final concentration of 150 ng/mL. Odyssey Scanning System was performed to detect according to the manufacturer’s instructions.
Breast cancer tumor tissues were gathered and homogenated by the homogenizer Precellys® 24 machine. Approximately 20 μL reaction solution contained Alexa Fluor® 647-labeled sense RNA probe, unlabeled target RNA and protein extract was to demonstrate a functional Electrophoretic Mobility Shift Assays (EMSA). Reactions were electrophoresed, transferred, and crosslinked of binding reactions to the nylon membrane according to the steps in the protocol. The fluorescent-labeled RNA is detected using LI-COR CLx Odyssey Scanning System at 700 nm channel.
For RNA-Protein Pull-Down Assay, the synthesized sense RNA probe was labeled with streptavidin magnetic beads and incubated with protein lysis solution at 4 °C overnight. The RNA-protein complex was pulled down with magnetic beads for another 4 hours. After washing three times with PBS, the beads were eluted and the elution solution was detected by western blot with relative antibody using LI-COR CLx Odyssey Scanning System.
All data in the text and Figures were provided as mean ± SD. The results were analyzed by a one-way analysis of variance, followed by Tukey Post hoc comparisons. All analyses were performed using the Statistical Package for Social Science software v22.0 (IBM, Armonk, NY, United States). P < 0.05 was considered significant.
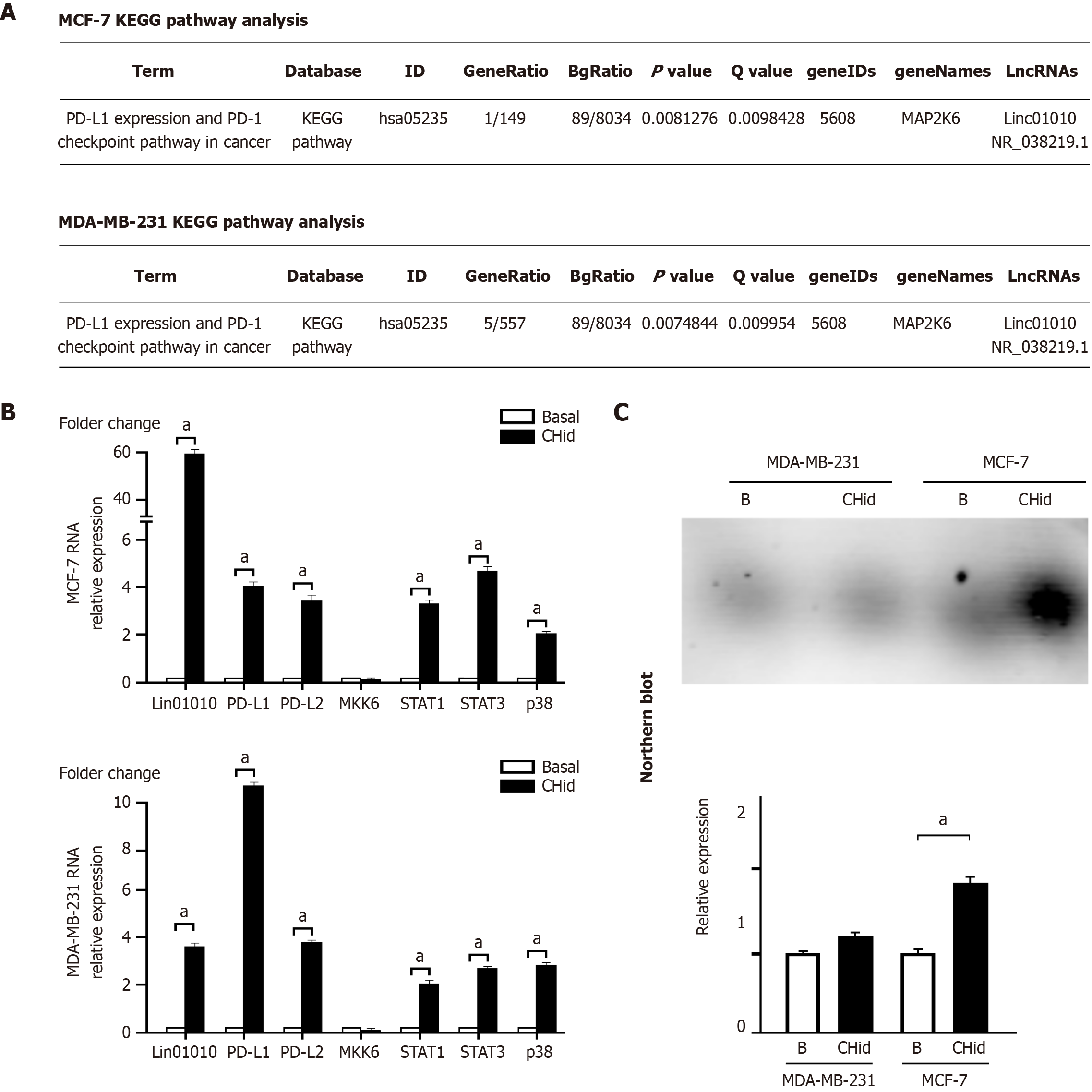
In our previous study, we analyzed the Gene Ontology enrichment function of LncRNA in Chidamide-treated breast cancer cells using the SRA database file PRJNA807845. Our findings revealed that among the 61 LncRNA molecules (33 exhibiting an increase and 28 showing a decrease) induced by Chidamide, the significant accumulation of Linc01010 was linked to enhanced activity in the downstream mitogen-activated protein kinases (MAPK) pathway, suggesting its potential role in signal transduction pathways[23]. When we conducted Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis to identify the relevant signaling pathways, we discovered that the direct target kinase was MAP2K6, also known as MKK6. Further evidence indicated that MKK6 was responsible for initiating the downstream PD-L1/PD-1 checkpoint (Figure 1A).
To investigate the mRNA changes of relevant factors in breast cancer cells, we conducted qPCR analysis. Our findings revealed that Chidamide-induced accumulation of Linc01010 significantly elevated the levels of key factors, including PD-1, PD-L1, and PD-L2, within the PD-1/PD-L1 checkpoint, particularly emphasizing PD-L1. The components of the STAT-MAPK pathway, such as p38, STAT1, and STAT3, exhibited notable changes, as illustrated in Figure 1B. Interestingly, no significant alteration in MKK6 was observed in the cells, suggesting that MKK6 may operate through protein-level phosphorylation rather than RNA-level transcriptional regulation.
To assess the actual RNA levels of Linc01010 in Chidamide-treated cells, we employed Northern blot analysis. Detailed information regarding probe cloning and labeling used in the Northern blot was provided in the attached Supplementary Figure 1. Figure 1C demonstrated that the Chidamide-induced expression of Linc01010 was more pronounced in MCF-7 cells compared to MDA-MB-231 cells, where this significant expression was not observed.
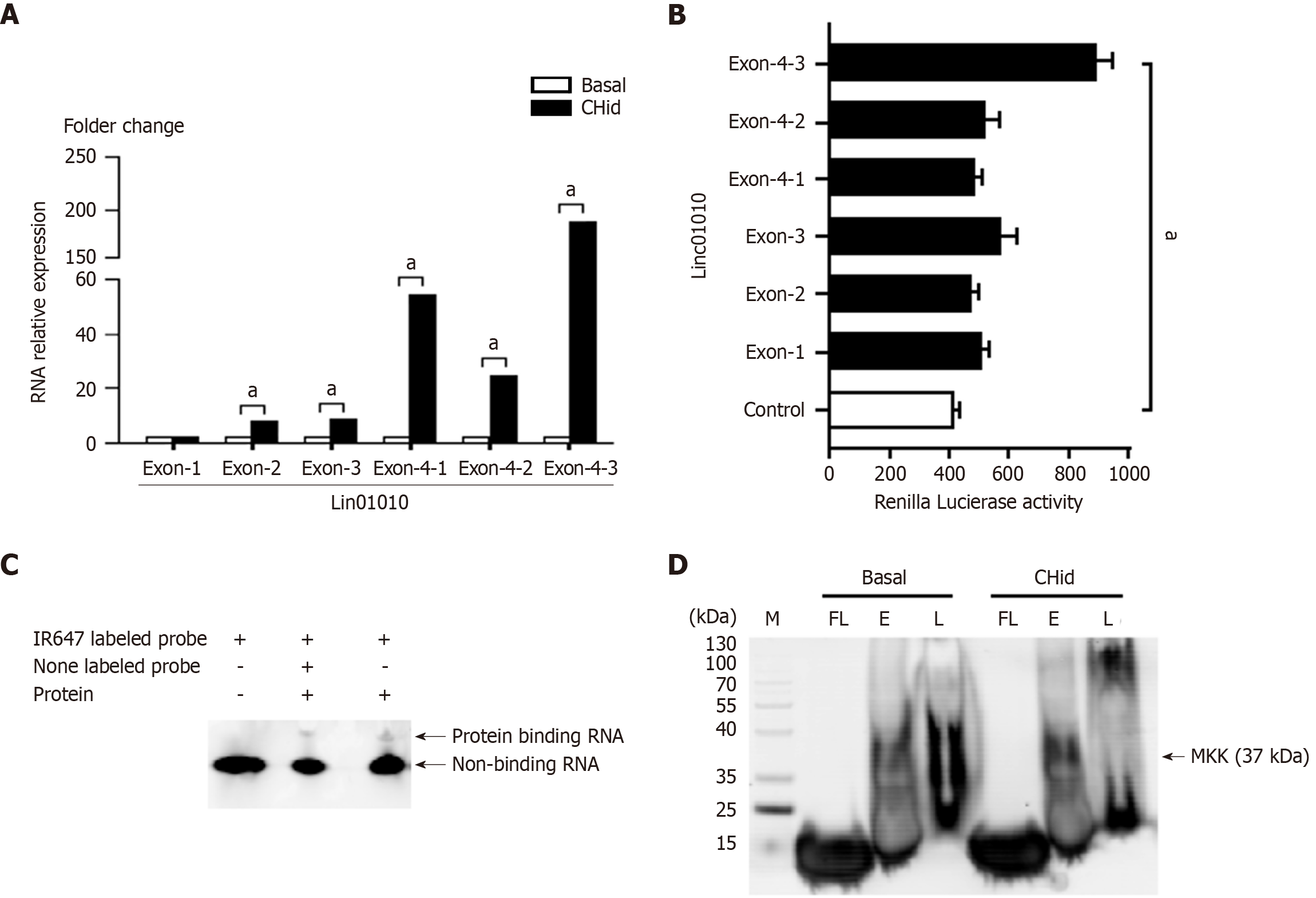
To elucidate the functional localization of Linc01010 components in breast cancer cells, we performed a sequence analysis of Linc01010 utilizing qPCR and a luciferase assay on MCF-7 cells. The primers were designed by primer 3 suit and libraries. The sequences of primers were shown in Table 2. The qPCR results demonstrated a significant increase in the levels of all exons, except exon 1. Notably, all sub-regions of exon 4 exhibited a marked elevation (Figure 2A). Furthermore, the luciferase assay indicated that the enzyme intensity in exon 4-3 reached a peak value, suggesting that this region may represent the functionally active area of the LncRNA in response to Chidamide treatment (Figure 2B).
| Gene name | Primer orientation | Sequence |
| Lin01010 E1 | Forward | 5’-GGATCTGTAGTTCTAGCCTCA-3’ |
| Reverse | 5’-AGTCCTCCTGATTTTCATTCCA-3’ | |
| Lin01010 E2 | Forward | 5’-GCTGGAGTGCAGTGGTGCAC-3’ |
| Reverse | 5’-CTTGGAAAGGTCAGACTTGA-3’ | |
| Lin01010 E3 | Forward | 5’-GGTCTCACTCTGTCACCCAG-3’ |
| Reverse | 5’-CTGTAGTCCCAGCTACTCAG-3’ | |
| Lin01010 E4 | Forward | 5’-GAATAGACTGGCTTCGGGGA-3’ |
| Reverse | 5’-CTGTTGCTGAAGAAGGCGTT-3’ | |
| Lin01010 E4-1 | Forward | 5’-GAATAGACTGGCTTCGGGGA-3’ |
| Reverse | 5’-TCTTTCCCCGATTCCTCAGG-3’ | |
| Lin01010 E4-2 | Forward | 5’-GGGAGGTCAGGGCAGTATTT-3’ |
| Reverse | 5’-ACCAGAGATGGCAGAACTTGA-3’ | |
| Lin01010 E4-3 | Forward | 5’-AGAATGTGCCAGGGGTGTG-3’ |
| Reverse | 5’-CTGTTGCTGAAGAAGGCGTT-3’ |
To investigate the binding of Linc01010 to target proteins in breast cancer cells, we employed EMSA and RNA-Protein Pull-Down Assay. Initially, we used the Basic Local Alignment Search Tool to obtain a 154 bp sequence of mouse origin as the primer, which exhibited high homology to the human exon 4-3 region. This fragment was subsequently synthesized as an RNA probe. The relevant sequence of RNA probes and the alignments of comparative genomics between human and mouse were be seen in Supplementary Figure 2. By analyzing samples from a previously established Chidamide-treated mouse breast cancer model, we investigated the interaction between Linc01010 and proteins using EMSA with an Alexa Fluor® 647 labeled fluorescent beacon. The results indicated that incubation with total protein extracts from drug-treated mouse tissue led to a reduction in the fluorescent intensity of the RNA probe compared to the fluorescent probe alone. Furthermore, new fluorescent bands corresponding to binding proteins were observed. The addition of 100 × non-fluorescent labeled RNA probes further diminished the fluorescent intensity of the RNA probe. Additionally, the fluorescent signal from the new protein binding complex also weakened, suggesting that a substantial number of unlabeled RNA probes reduced the fluorescent intensity by competitively binding to the target protein (Figure 2C). To identify the components of the binding proteins, we conducted an RNA-Protein Pull-Down Assay using magnetic beads labeled with the Linc01010 RNA probe. The presence of the MKK6 protein in the RNA-Protein mixture adsorbed by the magnetic beads was confirmed through western blot analysis (Figure 2D).
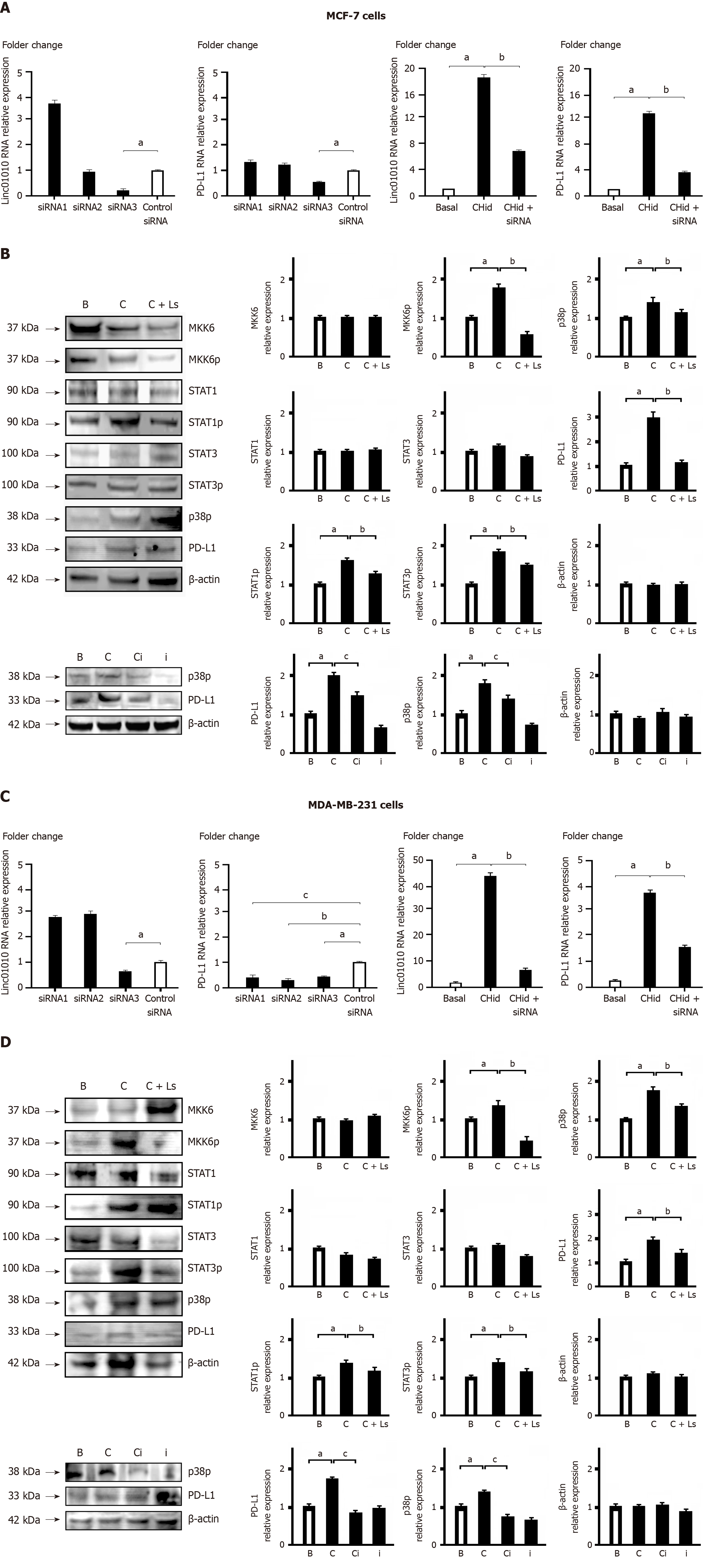
In our study, we aimed to elucidate the role of Linc01010 in the signal transduction cascade of breast cancer cells. To achieve this, we screened siRNA molecules to effectively silence Linc01010. We designed and synthesized three oligonucleotides based on the sequence of Linc01010 and transfected them into MCF-7 or MDA-MB-231 cells (Figure 3). Our results, as shown in Figure 3A and C, indicated that primer 3 exhibited the most significant inhibitory effect among the candidate siRNAs, while primers 1 and 2 showed no inhibitory effect. The results of qPCR demonstrated that the use of siRNA3 effectively reduced the levels of both Linc01010 and PD-L1 in breast cancer cells treated with Chidamide.
Subsequently, we examined the alterations in key factors of the signal transduction pathway during the interaction between Linc01010 and MKK6. In both MCF-7 and MDA-MB-231 cells, our results revealed a significant reduction in the phosphorylation levels of p38, STAT1, and STAT3 upon silencing Linc01010 using specific siRNA (siRNA3). Moreover, we observed that the activation of Linc01010-MKK6 by Chidamide resulted in enhanced downstream activity of PD-L1. Inhibition of p38 activity using the specific inhibitor SB203580 led to a significant repression of PD-L1 activity. This suggested that Linc01010 might facilitate the downstream accumulation of PD-L1 through the p38-MAPK pathway (Figure 3B and D).
We used immuno-fluorescent microscopy to observe and flow cytometry to detect the differential expression of CD54+ and CD16- molecules, and confirmed that the NK-92 cells used in this experiment belonged to the CD54+/CD16- subgroup and had good immune activity after IL-2 stimulation (Supplementary Figure 3).
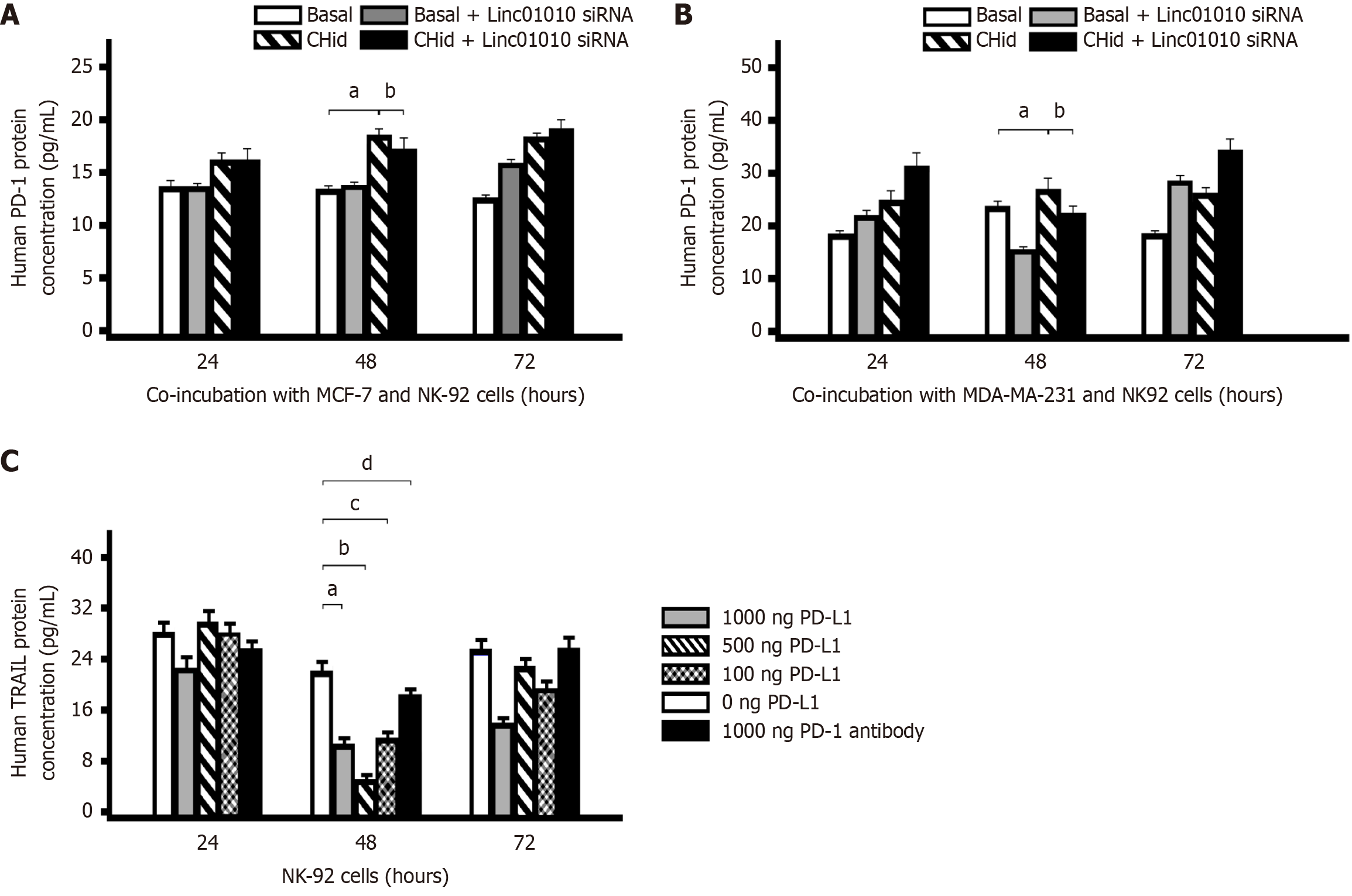
To determine the optimal time for Chidamide to induce PD-1 expressions in NK cells co-incubated, we set up a co-culture model with breast cancer cell and NK-92 cells. At first, MCF-7 or MDA-MB-231 breast cancer cells were transfected with Linc01010-specific siRNA. Following the suppression of Linc01010 function, two different cell lines of breast cancer and NK-92 were incubated with 20 μM Chidamide for 24 hours, 48 hours, and 72 hours, respectively. Enzyme-linked immunosorbent assay (ELISA) was employed to measure the PD-1 content in the cell lysate. Although breast cancer cells did not directly express PD-1 protein, it could activate NK-92 cells to express PD-1 through PD-L1 receptor in the co-cultured cell model. As illustrated in Figure 4A and B, the PD-1 level in the Chidamide-treated groups was significantly increased compared to the basal group after 24 hours and 72 hours of incubation. However, in Linc01010 siRNA group, PD-1 did not effectively inhibit the activation induced by Chidamide. Conversely, in Linc01010 siRNA group, PD-1 content was effectively reduced after 48 hours of Chidamide treatment, suggesting that 48 hours was the optimal time to maintain Linc01010 siRNA functionality.
Next, to investigate the impact of Chidamide-induced PD-L1 on the secretion of TRAIL in NK cells, we incubated NK-92 cells with different doses of human PD-L1 protein for 24 hours, 48 hours, and 72 hours, respectively. The ELISA assay was conducted to analyze TRAIL content. As illustrated in Figure 4C, an increase in PD-L1 dosage correlated with a decrease in TRAIL protein secretion. This finding indicated that the accumulation of PD-L1 in the TME significantly repressed TRAIL secretion by NK cells.
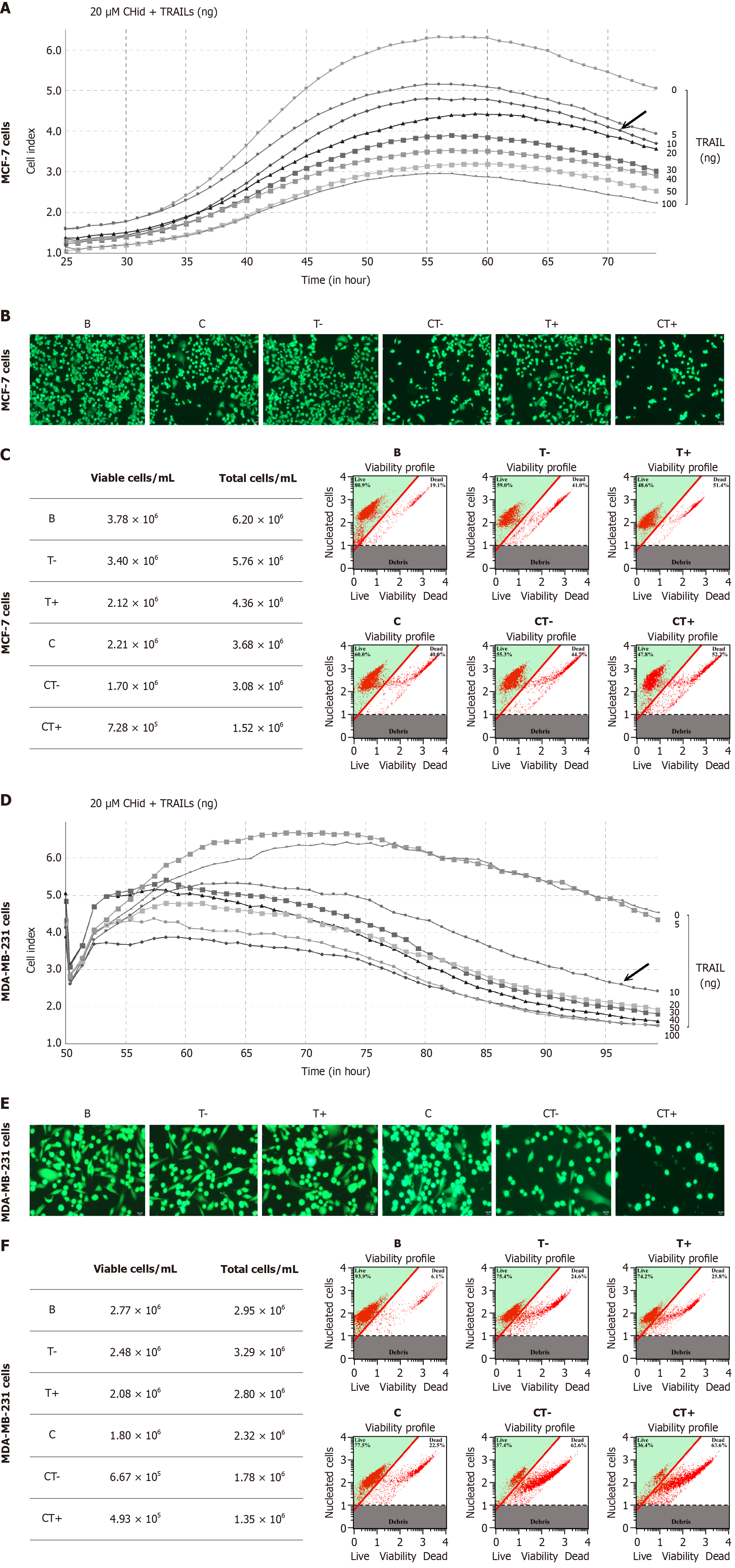
To investigate the impact of TRAIL secreted from NK cells on the pharmacological activity of breast cancer cells treated with Chidamide, we incubated MCF-7 or MDA-MB-231cells with varying doses of TRAIL protein (0-100 ng/mL) alongside 20 μM Chidamide, based on prior experimental results[27]. We then assessed the growth of breast cancer cells in this drug treatment environment using RTCA. The results indicated that different breast cancer cell lines exhibited slight variations in drug sensitivity to TRAIL (Figure 5). Notably, TRAIL demonstrated significant cytotoxicity against MCF-7 cells, with a marked effect observed after 48 hours of treatment with 10 ng/mL TRAIL. However, as low-dose TRAIL already displayed considerable toxicity, its combination with Chidamide did not yield enhanced pharmacological effects (Figure 5A and D). In contrast, the response of MDA-MB-231 cells to treatment was distinct. Administration of TRAIL or Chidamide alone resulted in only moderate toxic responses, while the combined pharmacological effects of the optimal dose (100 ng/mL) of TRAIL and Chidamide were pronounced, evidenced by a significant reduction in both the total number of MDA-MB-231 cells and cell viability. A decrease in TRAIL dosage correspondingly weakened its cytotoxic effects (Figure 5B and E).
Furthermore, fluorescent staining corroborated these experimental findings, revealing a reduction in the total number of breast cancer cells treated with TRAIL and Chidamide. Notable changes in cell morphology were observed, including roundness, swelling, increased fragmentation, and the presence of needle-like cells, along with elevated intracellular granular substances. These cytological changes provided evidence of the pharmacological effects of TRAIL and Chidamide on breast cancer cells (Figure 5C and F).
Breast cancer is a heterogeneous disease characterized by varying morphological and biological traits, which result in diverse clinical behaviors and treatment outcomes. Statistical research indicates that estrogen receptor (ER) positive breast cancer is the most prevalent subtype, accounting for 80%-85% of cases[28-31]. Triple-negative breast cancer (TNBC) is a distinct subtype defined by the absence of ERs, human epidermal growth factor receptor 2 (HER2), and progesterone receptors. TNBC is associated with early onset, high malignancy, aggressive invasion, a tendency for recurrence, distant metastasis, and a poor prognosis, representing approximately 15% of all breast cancer cases[32,33]. Currently, the clinical management of breast cancer faces challenges, including high rates of recurrence and metastasis, along with a lack of targeted therapies. Thus, investigating the molecular pathogenesis of breast cancer, as well as developing new technologies and drugs for its diagnosis and treatment, is both crucial and urgent[27,34].
In recent years, advancements in biotechnology have led to a significant focus on LncRNAs, which were previously regarded as mere ‘noise factors’ in tumors[35]. Certain LncRNAs exhibit abnormal expression patterns in cancer and are closely associated with the onset and progression of the disease[36]. Due to their high specificity to various tissues and cell types, some LncRNA molecules have the potential to serve as diagnostic biomarkers, prognostic indicators, and targeted therapeutic agents[37]. In our previous experiments, we observed an upregulation of LncRNA Linc01010 following Chidamide administration, as determined by RNA-seq high-throughput sequencing. KEGG enrichment analysis, utilizing the clustering analyzer package in R, indicated that the direct target kinase was MKK6 protein. MKK6 functions as an upstream activator of the MAPK signaling pathway, selectively activating the downstream p38 protein and contributing to various cellular processes induced by stress responses. These processes encompass cell cycle arrest, cell differentiation, transcriptional activation, and apoptosis[38]. Our bioinformatics results further revealed that the increase in Linc01010 induced by Chidamide was not only associated with the phosphorylation of key kinases in the p38 MAPK pathway but also regulated the downstream PD-L1/PD-1 checkpoint activity.
It is important to emphasize that we systematically evaluated the complementarities and limitations of bulk RNA-seq and single-cell RNA-seq technologies when using transcriptome profiling to resolve cancer mechanisms[39-42]. A suitable analytical strategy was adopted: Bulk RNA-seq was employed for differential expression screening and pathway enrichment analysis, but bulk sequencing may blur intratumor heterogeneity[43]. In order to ensure that the observed Chidamide-Linc01010-MKK6-p38-PD-L1 axis reflects more realistically the cytological basis of its action, we employed Luciferase Assays to analyze the expression of various exons within the complete sequence of the target LncRNA in MCF-7 cells under drug treatment. The findings demonstrated that, following Chidamide treatment, nearly all exon regions of Linc01010 displayed varying levels of activity, with exon 4 exhibiting the highest activity. Luciferase detection confirmed that the exon 4-3 region showed the greatest activity, suggesting that this region might serve as a functional domain for Linc01010 to interact with target proteins. Analysis of the exon 4-3 sequence indicated the presence of multiple transcription factor binding sites, including AP1, SP1, and HDAC1, highlighting its critical role in regulating the transcription and translation of key factors involved in cell proliferation, invasion, metastasis, and epithelial-mesenchymal transition. To investigate the binding of Linc01010 to its target protein and elucidate their functional mode of action, we coated the Linc01010 probe with magnetic beads and confirmed the presence of MKK6 protein in the elution mixture through EMSA and RNA-Protein Pull-Down Assay. This finding suggests an interaction between Linc01010 and MKK6, indicating that the biological function of Linc01010 is mediated through MKK6 in MCF-7 cells treated with Chidamide.
Next, we screened for potential channel protein expression during the action of Linc01010-MKK6. Western blot analysis revealed that Linc01010 could activate the p38-MAPK pathway, leading to the downstream accumulation of PD-L1 through the phosphorylation of STAT1 and STAT3. The p38-MAPK pathway is a well-established kinase activation pathway that transmits upstream signals to downstream reactive molecules via continuous kinase phosphorylation, thereby regulating various cellular biological processes. This pathway is closely associated with cell growth, development, differentiation, and programmed cell death. Jiang et al's research[44] indicates that 2α-hydroxyursolic acid can exert anti-tumor effects by modulating the p38-MAPK signaling pathway, which can induce cell apoptosis or inhibit cell proliferation. Hong et al's findings[45] confirmed that the knockout of p38-MAPK in cancer cells can enhance the invasion and migration activity of tumor cells. Additionally, it can promote the secretion of the tumor cytokine IL-6 in the TME by regulating miR365 activity.
PD-1, also known as CD279, is a membrane protein consisting of 288 amino acid residues. As a significant downstream effector of the p38-MAPK pathway, PD-1 functions as an ICP. PD-1 has two primary ligands, PD-L1 and PD-L2, which bind to receptors L1 or L2 to further suppress immune responses. Consequently, PD-1 plays a crucial role in regulating the immune system and promoting self-tolerance by inhibiting the inflammatory activity of immune cells[46,47].
Targeting PD-1 for immune regulation holds significant promise in the fight against tumors, infections, and autoimmune diseases[48]. Research has demonstrated that PD-L1 expression is typically elevated in various cancers and correlates with decreased survival rates in esophageal, pancreatic, and breast cancers[49]. PD-L1 inhibits anti-tumor activity by binding to immune effector cells, disrupting the interaction between PD-1 and PD-L1, and consequently diminishing the immune cell response in vitro. In this study, we investigated the relationship between Linc01010-MKK6 and PD-1/PD-L1 checkpoint dysfunction in breast cancer cells treated with Chidamide. Our findings indicate that the functional expression of PD-1/PD-L1 induced by Linc01010-MKK6 can be reversed through the overexpression of PD-L1 following the silencing of its key channel protein, p38. Therefore, we conclude that Linc01010 may elevate PD-L1 expression by activating the MKK6-p38-MAPK pathway. This functional alteration of PD-L1 can represent a stress-antagonistic response of breast cancer cells to drug stimulation.
The interplay between breast cancer and its surrounding microenvironment is intricately linked to the initiation and progression of the disease. The TME comprises tumor cell components, immune infiltrating cells, and other tumor-associated elements, such as secretory products and the extracellular matrix, all of which are vital to the proliferation, metastasis, invasion, and drug resistance of breast cancer cells.
Although current studies have mainly focused on T-lymphocyte immunosuppression mediated by the PD-1/PD-L1 axis, it is noteworthy that PD-1 is also widely expressed in other immune cells such as NK cells[50]. As a key member of natural immunity, NK cells are an important line of defense for early recognition and killing of tumor cells, and contain tumor progression through direct killing, release of antigens, recruitment and activation of other immune cells, and shaping of the anti-TME. However, when NK cells are exhausted or suppressed, they may instead promote the release of suppressor cytokines, exacerbating the immunosuppressive state of the TME and contributing to immune escape from the tumor.
In healthy individuals, NK cells usually express little PD-1 but in pathological states (e.g., cancer), their PD-1 expression is abnormally up-regulated. Binding of PD-L1 on the surface of tumor cells to PD-1 on NK cells significantly inhibits the cytotoxic function of NK cells (as evidenced by impaired CD107a expression, reduced perforin and granzyme B release, and diminished anti-tumor activity). Targeted blockade of the PD-1/PD-L1 axis reactivates the anti-tumor response of NK cells.
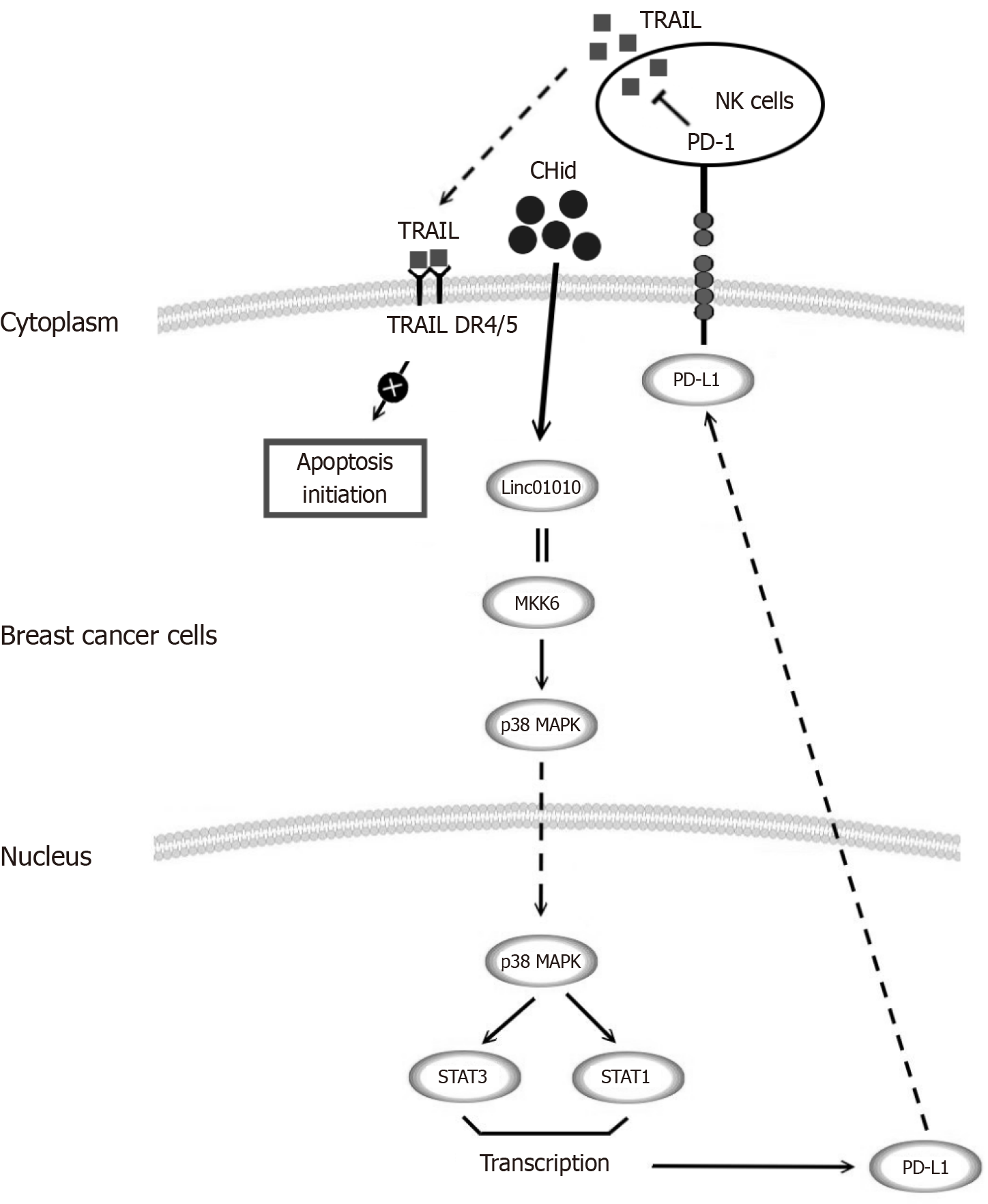
After activation, NK cells release toxic substances such as perforins and granzymes through cytotoxicity, leading to perforation of target cells and subsequent apoptosis, the FasCD95/FasL pathway, the tumor necrosis factor (TNF)-R1/TNF pathway and the TRAIL-R/TRAIL pathway to play a role in promoting the development of tumorigenic cells. The TRAIL-R/TRAIL pathway has attracted particular attention in recent years. Our previous studies had been shown that exogenous TRAIL enhanced the apoptostic and autophagic death of breast cancer cells by HDACi and that Chidamide-induced secretion of autophagic vesicles directly synergizes the pro-apoptotic effect of TRAIL, whereas the present study revealed that up-regulation of TRAIL expression was an important mechanism by which tumor cells were killed by NK cells[27,51]. In this study, Chidamide was found to induce high expression of the PD-L1 receptor on the surface of breast cancer cells. The elevated PD-L1 expression in vivo promoted the expression of PD-1 in NK-92 cells, which inhibited immune cell activity, as evidenced by a reduction in TRAIL secretion. This weakening of TRAIL activity not only created favorable conditions for tumor cells to escape the immune system but also represented a specific manifestation of the pharmacological action of breast cancer cells against Chidamide, which diminished the extent of Chidamide-induced apoptosis and excessive autophagy, among other pharmacological reactions (Figure 6). Of interest, recent studies revealed that the combination of chidamide with PD-1 blockers significantly enhanced anti-tumor immune effects in a sarcoma model[52]; meanwhile, local anesthetics were able to synergize with ICP inhibitors by modulating ion channels (e.g., Transient Receptor Potential Melastatin 7) in the TME[53,54]. These findings provide important translational medicine evidence for HDACi-based immune-combination therapeutic strategies, in particular therapeutic regimens targeting the TRAIL-NK axis.
Furthermore, from a biomarker perspective, Linc01010 is not the only non-coding RNA that regulates the p38-PD-L1 pathway. Pan-cancer analyses revealed that mitotic DNA integrity checkpoint kinases (e.g., BUB1B)[55,56], disulfide-bond-death-associated genes (e.g., SLC7A11)[57], and auto-ubiquitylating transcription factors (e.g., TRIM28)[58], etc., can affect the expression of ICPs through similar pathways, which highlights the complexity of tumor immune escape mechanisms and their conservation across different cancer types. Furthermore, the finding that natural products (e.g., chebulbul extract Coriolagin) can alleviate endoplasmic reticulum stress by modulating inflammatory vesicle signaling[59] suggests that they may be able to work synergistically with Chidamide to reverse the immunosuppressive state of the breast cancer microenvironment.
This study aimed to investigate the mechanism of Linc01010 in response to Chidamide, focusing on its interaction with MKK6 and its ability to activate the p38-MAPK pathway, which subsequently leads to an increase in downstream PD-L1 levels. The elevated PD-L1 expression was found to diminish the secretion of TRAIL by NK cells within the TME via PD-1 interaction, ultimately moderating the pharmacological effect of Chidamide on breast cancer cells. The findings from this study provide a robust foundation for enhancing the efficacy of current anti-breast cancer therapies and for identifying novel targets to combat drug resistance.
| 1. | Sonkin D, Thomas A, Teicher BA. Cancer treatments: Past, present, and future. Cancer Genet. 2024;286-287:18-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 378] [Article Influence: 189.0] [Reference Citation Analysis (0)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68881] [Article Influence: 13776.2] [Reference Citation Analysis (202)] |
| 3. | Moo TA, Sanford R, Dang C, Morrow M. Overview of Breast Cancer Therapy. PET Clin. 2018;13:339-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 337] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 4. | Spellman A, Tang SC. Immunotherapy for breast cancer: past, present, and future. Cancer Metastasis Rev. 2016;35:525-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Chiba A, Bawaneh A, Velazquez C, Clear KYJ, Wilson AS, Howard-McNatt M, Levine EA, Levi-Polyachenko N, Yates-Alston SA, Diggle SP, Soto-Pantoja DR, Cook KL. Neoadjuvant Chemotherapy Shifts Breast Tumor Microbiota Populations to Regulate Drug Responsiveness and the Development of Metastasis. Mol Cancer Res. 2020;18:130-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 6. | Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1920] [Cited by in RCA: 2371] [Article Influence: 169.4] [Reference Citation Analysis (0)] |
| 7. | Wu HJ, Chu PY. Epigenetic Regulation of Breast Cancer Stem Cells Contributing to Carcinogenesis and Therapeutic Implications. Int J Mol Sci. 2021;22:8113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Rahman MM, Brane AC, Tollefsbol TO. MicroRNAs and Epigenetics Strategies to Reverse Breast Cancer. Cells. 2019;8:1214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 9. | Que Y, Zhang XL, Liu ZX, Zhao JJ, Pan QZ, Wen XZ, Xiao W, Xu BS, Hong DC, Guo TH, Shen LJ, Fan WJ, Chen HY, Weng DS, Xu HR, Zhou PH, Zhang YZ, Niu XH, Zhang X. Frequent amplification of HDAC genes and efficacy of HDAC inhibitor chidamide and PD-1 blockade combination in soft tissue sarcoma. J Immunother Cancer. 2021;9:e001696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 10. | Ahmed S, Riegsecker S, Beamer M, Rahman A, Bellini JV, Bhansali P, Tillekeratne LM. Largazole, a class I histone deacetylase inhibitor, enhances TNF-α-induced ICAM-1 and VCAM-1 expression in rheumatoid arthritis synovial fibroblasts. Toxicol Appl Pharmacol. 2013;270:87-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Huang M, Zhang J, Yan C, Li X, Zhang J, Ling R. Small molecule HDAC inhibitors: Promising agents for breast cancer treatment. Bioorg Chem. 2019;91:103184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 12. | Benedetti R, Conte M, Altucci L. Targeting Histone Deacetylases in Diseases: Where Are We? Antioxid Redox Signal. 2015;23:99-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 13. | Xu Y, Zhang P, Liu Y. Chidamide tablets: HDAC inhibition to treat lymphoma. Drugs Today (Barc). 2017;53:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Cao L, Zhao S, Yang Q, Shi Z, Liu J, Pan T, Zhou D, Zhang J. Chidamide Combined With Doxorubicin Induced p53-Driven Cell Cycle Arrest and Cell Apoptosis Reverse Multidrug Resistance of Breast Cancer. Front Oncol. 2021;11:614458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Wang S, Ma Z, Shi Z, Huang Y, Chen T, Hou L, Jiang T, Yang F. Chidamide stacked in magnetic polypyrrole nano-composites counter thermotolerance and metastasis for visualized cancer photothermal therapy. Drug Deliv. 2022;29:1312-1325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Ning ZQ, Li ZB, Newman MJ, Shan S, Wang XH, Pan DS, Zhang J, Dong M, Du X, Lu XP. Chidamide (CS055/HBI-8000): a new histone deacetylase inhibitor of the benzamide class with antitumor activity and the ability to enhance immune cell-mediated tumor cell cytotoxicity. Cancer Chemother Pharmacol. 2012;69:901-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 228] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 17. | Lujan DA, Ochoa JL, Beswick EJ, Howard TA, Hathaway HJ, Perrone-Bizzozero NI, Hartley RS. Cold-Inducible RNA Binding Protein Impedes Breast Tumor Growth in the PyMT Murine Model for Breast Cancer. Biomedicines. 2024;12:340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | El-Ashmawy NE, Hussien FZ, El-Feky OA, Hamouda SM, Al-Ashmawy GM. Serum LncRNA-ATB and FAM83H-AS1 as diagnostic/prognostic non-invasive biomarkers for breast cancer. Life Sci. 2020;259:118193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Wang P, Ning S, Zhang Y, Li R, Ye J, Zhao Z, Zhi H, Wang T, Guo Z, Li X. Identification of lncRNA-associated competing triplets reveals global patterns and prognostic markers for cancer. Nucleic Acids Res. 2015;43:3478-3489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 181] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 20. | Kopp F, Mendell JT. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell. 2018;172:393-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1719] [Cited by in RCA: 2729] [Article Influence: 389.9] [Reference Citation Analysis (0)] |
| 21. | Zhou S, He Y, Yang S, Hu J, Zhang Q, Chen W, Xu H, Zhang H, Zhong S, Zhao J, Tang J. The regulatory roles of lncRNAs in the process of breast cancer invasion and metastasis. Biosci Rep. 2018;38:BSR20180772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | Li J, Liao Q, Guo Y, Zhang J, Zhang R, Liu Q, Liu H. Mechanism of crosstalk between DNA methylation and histone acetylation and related advances in diagnosis and treatment of premature ovarian failure. Epigenetics. 2025;20:2528563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 23. | Feng X, Han H, Guo Y, Feng X, Guo S, Zhou W. LncRNA ENST869 Targeting Nestin Transcriptional Region to Affect the Pharmacological Effects of Chidamide in Breast Cancer Cells. Front Oncol. 2022;12:874343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Hickson JA, Huo D, Vander Griend DJ, Lin A, Rinker-Schaeffer CW, Yamada SD. The p38 kinases MKK4 and MKK6 suppress metastatic colonization in human ovarian carcinoma. Cancer Res. 2006;66:2264-2270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Parray AA, Baba RA, Bhat HF, Wani L, Mokhdomi TA, Mushtaq U, Bhat SS, Kirmani D, Kuchay S, Wani MM, Khanday FA. MKK6 is upregulated in human esophageal, stomach, and colon cancers. Cancer Invest. 2014;32:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Di Rocco A, Camero S, Benedetti A, Lozanoska-Ochser B, Megiorni F, Marchese C, Stramucci L, Ciccarelli C, Bouché M, Bossi G, Marampon F, Zani BM. Antioncogenic and promyogenic action of the MKK6/p38/AKT axis induced by targeting MEK/ERK in embryonal rhabdomyosarcoma. Oncol Rep. 2022;48:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Zhou W, Han H, Xu J, Sun T, Feng X. Autophagic Vacuole Secretion Triggered by Chidamide Participates in TRAIL Apoptosis Effect in Breast Cancer Cells. Curr Pharm Des. 2021;27:2366-2380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Lumachi F, Brunello A, Maruzzo M, Basso U, Basso SM. Treatment of estrogen receptor-positive breast cancer. Curr Med Chem. 2013;20:596-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 204] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 29. | Alamri A, Rauf A, Khalil AA, Alghamdi A, Alafnan A, Alshammari A, Alshammari F, Malik JA, Anwar S. In Silico Screening of Marine Compounds as an Emerging and Promising Approach against Estrogen Receptor Alpha-Positive Breast Cancer. Biomed Res Int. 2021;2021:9734279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 30. | Ma T, Liang Y, Li Y, Song X, Zhang N, Li X, Chen B, Zhao W, Wang L, Yang Q. LncRNA LINP1 confers tamoxifen resistance and negatively regulated by ER signaling in breast cancer. Cell Signal. 2020;68:109536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 31. | Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1398] [Cited by in RCA: 1596] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 32. | Lv M, Xu P, Wu Y, Huang L, Li W, Lv S, Wu X, Zeng X, Shen R, Jia X, Yin Y, Gu Y, Yuan H, Xie H, Fu Z. LncRNAs as new biomarkers to differentiate triple negative breast cancer from non-triple negative breast cancer. Oncotarget. 2016;7:13047-13059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 33. | Rugo HS, Delord JP, Im SA, Ott PA, Piha-Paul SA, Bedard PL, Sachdev J, Le Tourneau C, van Brummelen EMJ, Varga A, Salgado R, Loi S, Saraf S, Pietrangelo D, Karantza V, Tan AR. Safety and Antitumor Activity of Pembrolizumab in Patients with Estrogen Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer. Clin Cancer Res. 2018;24:2804-2811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 294] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 34. | Roßwag S, Sleeman JP, Thaler S. RASSF1A-Mediated Suppression of Estrogen Receptor Alpha (ERα)-Driven Breast Cancer Cell Growth Depends on the Hippo-Kinases LATS1 and 2. Cells. 2021;10:2868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 35. | Qian W, Yang L, Ni Y, Yin F, Qin L, Yang Y. LncRNA LINC01857 reduces metastasis and angiogenesis in breast cancer cells via regulating miR-2052/CENPQ axis. Open Med (Wars). 2022;17:1357-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 36. | Gan L, Shangguan Q, Zhang F, Tong X, Qi D, Zhao Y, Ye X. HBV HBx-Downregulated lncRNA LINC01010 Attenuates Cell Proliferation by Interacting with Vimentin. Int J Mol Sci. 2021;22:12497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Li B, Liu D, Xu J, Lu Z, Liu Q, Zhao X, Wang X, Peng T, Xu J. Overexpression of lncRNA MAPT-AS1 exacerbates cell proliferation and metastasis in breast cancer. Transl Cancer Res. 2022;11:835-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 38. | Romero-Becerra R, Mora A, Manieri E, Nikolic I, Santamans AM, Montalvo-Romeral V, Cruz FM, Rodríguez E, León M, Leiva-Vega L, Sanz L, Bondía V, Filgueiras-Rama D, Jiménez-Borreguero LJ, Jalife J, Gonzalez-Teran B, Sabio G. MKK6 deficiency promotes cardiac dysfunction through MKK3-p38γ/δ-mTOR hyperactivation. Elife. 2022;11:e75250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Liu H, Karsidag M, Chhatwal K, Wang P, Tang T. Single-cell and bulk RNA sequencing analysis reveals CENPA as a potential biomarker and therapeutic target in cancers. PLoS One. 2025;20:e0314745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 32] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 40. | Liu H, Dong A, Rasteh AM, Wang P, Weng J. Identification of the novel exhausted T cell CD8 + markers in breast cancer. Sci Rep. 2024;14:19142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 98] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 41. | Li Y, Liu H. Clinical powers of Aminoacyl tRNA Synthetase Complex Interacting Multifunctional Protein 1 (AIMP1) for head-neck squamous cell carcinoma. Cancer Biomark. 2022;34:359-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 42. | Liu H, Li Y. Potential roles of Cornichon Family AMPA Receptor Auxiliary Protein 4 (CNIH4) in head and neck squamous cell carcinoma. Cancer Biomark. 2022;35:439-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 43. | Liu H, Li Y, Karsidag M, Tu T, Wang P. Technical and Biological Biases in Bulk Transcriptomic Data Mining for Cancer Research. J Cancer. 2025;16:34-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 83] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 44. | Jiang X, Li T, Liu RH. 2α-Hydroxyursolic Acid Inhibited Cell Proliferation and Induced Apoptosis in MDA-MB-231 Human Breast Cancer Cells through the p38/MAPK Signal Transduction Pathway. J Agric Food Chem. 2016;64:1806-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 45. | Hong B, Li H, Zhang M, Xu J, Lu Y, Zheng Y, Qian J, Chang JT, Yang J, Yi Q. p38 MAPK inhibits breast cancer metastasis through regulation of stromal expansion. Int J Cancer. 2015;136:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Gatalica Z, Snyder C, Maney T, Ghazalpour A, Holterman DA, Xiao N, Overberg P, Rose I, Basu GD, Vranic S, Lynch HT, Von Hoff DD, Hamid O. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev. 2014;23:2965-2970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 414] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 47. | Muenst S, Soysal SD, Gao F, Obermann EC, Oertli D, Gillanders WE. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2013;139:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 287] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 48. | Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2013;19:1021-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 436] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 49. | Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1835] [Cited by in RCA: 1845] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 50. | Li Z, Zhang H, Wang X, Wang Q, Xue J, Shi Y, Wang M, Wang G, Zhang J. Identification of cuproptosis-related subtypes, characterization of tumor microenvironment infiltration, and development of a prognosis model in breast cancer. Front Immunol. 2022;13:996836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 51. | Han H, Zhou H, Li J, Feng X, Zou D, Zhou W. TRAIL DR5-CTSB crosstalk participates in breast cancer autophagy initiated by SAHA. Cell Death Discov. 2017;3:17052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 52. | Jin Z, Zhang W, Liu H, Ding A, Lin Y, Wu SX, Lin J. Potential Therapeutic Application of Local Anesthetics in Cancer Treatment. Recent Pat Anticancer Drug Discov. 2022;17:326-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 53. | Liu H, Dilger JP, Lin J. The Role of Transient Receptor Potential Melastatin 7 (TRPM7) in Cell Viability: A Potential Target to Suppress Breast Cancer Cell Cycle. Cancers (Basel). 2020;12:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 54. | Liu H, Dilger JP, Lin J. Lidocaine Suppresses Viability and Migration of Human Breast Cancer Cells: TRPM7 as a Target for Some Breast Cancer Cell Lines. Cancers (Basel). 2021;13:234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 55. | Li R, Xiao C, Liu H, Huang Y, Dilger JP, Lin J. Effects of local anesthetics on breast cancer cell viability and migration. BMC Cancer. 2018;18:666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 56. | Rasteh AM, Liu H, Wang P. Pan-cancer genetic profiles of mitotic DNA integrity checkpoint protein kinases. Cancer Biomark. 2024;41:CBM240119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 57. | Liu H, Tang T. Pan-cancer genetic analysis of disulfidptosis-related gene set. Cancer Genet. 2023;278-279:91-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 105] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 58. | Dong A, Rasteh A, Wang P, Liu H. An in-silico pan-cancer bulk and single-cell profiling of transcription factors in protein autoubiquitination. Discov Oncol. 2025;16:1245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 59. | Ou L, Liu HR, Shi XY, Peng C, Zou YJ, Jia JW, Li H, Zhu ZX, Wang YH, Su BM, Lai YQ, Chen MY, Zhu WX, Feng Z, Zhang GM, Yao MC. Terminalia chebula Retz. aqueous extract inhibits the Helicobacter pylori-induced inflammatory response by regulating the inflammasome signaling and ER-stress pathway. J Ethnopharmacol. 2024;320:117428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 41] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/