Published online Oct 24, 2025. doi: 10.5306/wjco.v16.i10.105117
Revised: May 26, 2025
Accepted: September 3, 2025
Published online: October 24, 2025
Processing time: 285 Days and 17.5 Hours
Melanoma is an aggressive type of skin cancer notorious for its resistance to chemotherapy, radiotherapy and immunotherapy, which greatly impacts its le
Core Tip: Oncogenic B-Raf proto-oncogene, serine/threonine kinase activates the hedgehog signaling pathway, triggering glioma-associated oncogene homolog (GLI) 1/2 transcription factors that promote melanoma cell invasion and sustain cancer stem cell self-renewal, contributing to the malignancy’s therapeutic resistance. Histone deacetylases 1/2 (HDAC1/2) suppress the acetylation of GLI1/2, facilitating their activation. Inhibiting HDAC1/2 may stabilize GLI proteins in their inactive, acetylated form, offering a novel approach to inhibit melanoma progression. Hedgehog signaling induces HDAC1/2 expression, creating a feedback loop that amplifies GLI-mediated transcription, promoting cancer stem cell renewal. Disrupting this loop could unveil new therapeutic avenues for overcoming melanoma’s resistance to treatment.
- Citation: Rather RA. Oncogenic B-Raf proto-oncogene, serine/threonine kinase-mediated hedgehog signalling in the pathogenesis and targeted therapy of melanoma. World J Clin Oncol 2025; 16(10): 105117
- URL: https://www.wjgnet.com/2218-4333/full/v16/i10/105117.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i10.105117
Melanoma is a significant global public health concern and ranks among the leading causes of cancer-related mortality in Western countries[1]. According to Cancer Statistics 2025, it is estimated that there will be approximately 8430 deaths and 104960 new cases of melanoma in situ in the United States in 2025[2]. This highly lethal disease arises as a consequence of malignant transformation of melanin-producing cells (melanocytes) in human skin through intricate mechanisms[3], making it imperative for the researchers to understand the mechanisms behind such transformation. These melanin-producing cells exhibit phenotypic prominence, yet their histological characteristics remain highly obscure[4]. In human skin, melanocytes are located within the ultraviolet (UV)-permeable range, specifically in the basal layer of the epidermis, where they are exposed to UV light, including UVA and UVB rays[5]. Melanocytes are derived from multipotent neural crest (NC) cells at the dorsal borders of the neural tube, and migrate beneath the epidermis to populate various skin tissues, forming a heterogeneous group of cells responsible for pigmentation and determining skin phototype[6,7]. Tissue-resident melanocyte stem cells (MSCs) and melanocyte precursors play integral roles in the renewal and maintenance of melanocytes[8]. It is believed that the developmental processes governing melanocyte formation have a tendency to reactivate during melanoma formation. For example, embryonic NC stem cells gene expression signature is recapitulated during tumor initiation in zebrafish melanoma model, suggesting the role of NC stem cells in development of melanoma[9]. SOX10 and RAC1, two crucial genes involved in NC development, demonstrate how their disruption can hinder melanoma formation[10]. Likewise, MSX1, MITF, PAX3 and FOXD3 are pivotal in initiating NC development, facilitating NC cell migration and contributing to melanocyte formation[11]. Their activation during the onset of melanoma enhances cellular plasticity and fosters drug resistance[11]. Melanomas have been found to exhibit additional indicators characteristic of embryonic and regenerative melanocyte lineage, such as endothelin and KIT proto-oncogene, receptor tyrosine kinase[12]. Several groups of proteins, transcription factors, extracellular secreted ligands, trans
Besides transmitting signals for embryonic development and tumorigenesis, inappropriate activation of HH pathway imparts radio- and chemo-resistant phenotype to many solid tumors, including melanoma. As a result, prognosis of HH-driven tumors is often poor[17]. A better understanding of HH signaling is likely to open new therapeutic avenues for targeted therapy of melanoma and other solid tumors. In fact, to develop an effective anti-melanoma therapy, it is crucial to understand the mechanisms by which melanoma cells acquire chemo-resistance or how resistant cells are formed within the tumor. Consequently, there is a growing focus on conducting studies aimed at discovering novel inhibitors of the HH pathway that could be safely utilized in human intervention trials. In this review, we attempt to critically summarize the important aspects of HH pathway and its involvement in development and progression of melanoma. We also explore the role of the HH pathway in imparting a treatment-resistant phenotype to melanoma, an emerging new area of research. The hope is that understanding the precise role of HH signaling in melanoma pathogenesis may provide a rationale for improving existing cancer therapies and identifying novel targets for therapeutic intervention of me
The structural architecture of HH signaling cascade is simple. In the absence of HH ligands, PTCH TM receptor inhibits the function of SMO receptor[18]. PTCH and SMO are conserved whereas the mammalian HH proteins are diversified into, Sonic-HH (SHH), Indian-HH (IHH), and Desert-HH (DHH)[19]. These HH proteins encompasses a small family of secreted signaling proteins that jointly regulate multiple aspects of animal development, tissue homeostasis and regeneration[20]. HH signaling is initiated by binding of HH ligands to PTCH (which resides in the non-motile primary cilium) and is mediated by GLI transcription effector family, whose activity is finely tuned by a number of molecular interactions, trafficking and post-translation modifications[21,22]. The HH signaling is strongly influenced by HH in
HH signaling pathway plays a crucial role in intracellular communication during embryonic development, organ formation, tissue maintenance, and regeneration[25]. However, it is often dysregulated in various forms of cancer[22]. Major components of HH signal reception and transduction are as follows.
HH ligands comprise a small family of secreted signaling proteins that are present in majority of metazoans[19]. As morphogens, these secreted proteins are essential during embryogenesis and throughout adulthood, impacting both health and disease. HH proteins achieve their biological functions by inducing gene expression changes in target cells in a concentration-dependent manner, regulating their identity, proliferation, death, or metabolism depending on the specific tissue or organ[26]. The loss of these proteins in Drosophila embryos results in the loss of normal segmented patterns and the formation of a uniform coat of bristles, a feature reminiscent of HH coats. Consequently, Drosophila embryos lacking the Hh gene appear to resemble hedgehogs[27]. In higher animals, HH proteins are involved in tissue formation, wound repair, regeneration, stem cell maintenance and tumorigenesis[28,29]. The processes of wound healing and tumorigenesis share common molecular mechanisms, with both being influenced by HH signaling[29]. HH proteins mediate essential normal shaping (patterning) during many stages of animal development, and abnormal HH function is associated with birth defects and cancer in adult animals[29,30]. HH ligands participate in tissue pattering and growth by acting as classical morphogens during the formation of range of tissues and organs[31,32]. Morphogens are signaling molecules that, based on their concentration, shape cell patterns by triggering gene expression changes in target cells. HH ligands form a gradient of varying concentration from sites of secretion, inducing concentration-dependent differentiation of different cell types[33]. HH proteins consist of two domains: the amino-terminal domain (HhN), responsible for bio
SMO is a type of membrane-localized frizzled (class F) G protein-coupled receptor (GPCR) encoded by the SMO gene in humans[42]. This 7-pass integral membrane protein is highly conserved across species, spanning from flies to humans. SMO plays a pivotal role in embryonic development and tissue homeostasis[43]. Recent structural information revealed SMO is regulated by binding of various ligands and signals through G protein-dependent and -independent mechanisms. Structurally, SMO is a GPCR, consisting of N-terminal cysteine-rich domain (CRD), seven-pass transmembrane helices domain (TMD) and an intracellular C-terminal domain (hinge domain)[44]. SMO protein contains two ligand biding sites; one in heptahelical transmembrane domain (TMD) and one in the extracellular CRD[45]. The CRD is stacked atop the TMD, separated by an intervening wedge-like linker domain. Typically, SMO signaling is regulated by its trafficking within the primary cilium[46]. Signal output from SMO is influenced by its functional domains, post-translational modifications, subcellular distribution and trafficking[47]. Oncogenic forms of SMO have been identified in various types of human malignancies[48]. Although, SMO shows similarities in ligand binding with other GPCRs, the molecular basis of SMO activity and its regulation is imprecisely determined and deserves further examination. SMO expression shows positive correlation with tumor size, invasiveness, metastasis and recurrence[49]. Therefore, inhibition of SMO regresses tumor growth in melanoma models. SMO inhibitors have received huge significance in the treatment of skin and brain cancers and many such inhibitors have entered into clinical trials[50,51]. However, SMO mutations induce drug-resistance against SMO antagonists[52].
PTCH is encoded by a gene that belongs to the PTCH gene family. Multiple isoforms of PTCH mRNA have been identified and characterized in humans and mice, which are generated by the complex alternative use of several distinct exons[53]. The most important diseases associated with PTCH1 are holoprosencephaly, basal cell nevus syndrome and melanoma[54]. There are two PTCH homolog genes in vertebrates[55,56]. PTCH1 and PTCH2 exhibit overlapping functions in the formation of embryonic structures and in tissue homeostasis[57]. And there is 73% homology between the two subtypes. Both TM proteins function as receptors for SHH, IHH and DHH ligands, and are capable of binding all HH family members with similar affinity. Both PTCH1 and PTCH2 can form a complex with SMO[55]. Physiologically, PTCH functions to repress the activity of SMO[58]. Although PTCH1 and PTCH2 functionally overlap in the repression of SMO, the spatiotemporal expression of BOC (PTCH1 co-receptor) and GAS1 (PTCH2 co-receptor) are believed to de
In cells, the GLI proteins exist as three distinct zinc-finger transcription factors-GLI1, GLI2 and GLI3-which mediate HH signaling at the distal end of the pathway[65]. While GLI1 and GLI2 operate as transcriptional activators, GLI3 typically serves as a transcriptional repressor[66]. GLI2 has dual functions of activating and inhibiting transcription but mainly functions as a transcriptional activator[67]. The expression of GLI1 is often used as readout of HH pathway activation[68]. In the absence of HH ligands, the activity of SMO protein is negatively regulated by PTCH and the GLI proteins are cleaved proteolytically to form the repressor GLI, majorly obtained for GLI3, which inhibits HH signaling and down
SUFU is a highly conserved protein that negatively regulates HH signaling by binding to GLI zinc-finger transcription factors, thereby inhibiting the activation of target gene expression[74]. This protein is encoded by the SUFU gene in humans[75]. In mammals, the mutations in this gene have deleterious effects on embryo development and are associated with cancer-predisposing syndromes and congenital anomalies[76]. Additionally, SUFU can act as a tumor suppressor, as its depletion promotes tumorigenesis in TP53-/- mice[77]. Several vertebrate homologues of SUFU are found in a wide variety of organisms[78]. Structurally, SUFU protein has two domains. However, in eukaryotic SUFU, an additional domain exists at the C-terminus of the protein which interacts with the C-terminal domain of GLI transcription factors, inhibiting their activity[79]. In the human SUFU-GLI complex, SUFU has been observed to switch between “open” and “closed” conformations. The “closed” form of SUFU is stabilized upon binding to GLI, and is inhibited by HH treatment. Conversely, the “open” form of SUFU is promoted by the dissociation of GLI and the activation of HH signaling[80]. In addition to with GLI transcription factors, SUFU has been shown to interact with peroxisomal biogenesis factor 26 (PEX26) which is required for protein import into peroxisomes[81]. The interaction between SUFU and PEX26 suggests a potential link between HH signaling and peroxisomal function. Silencing PEX26, as an unconventional mechanism, has been shown to kill drug-resistant cancer cells and prevent drug resistance in melanoma cells[82].
In addition to the canonical PTCH receptors, HH ligands signal through three co-receptors: GAS1, CDON and BOC. Together, these co-receptors are needed during embryogenesis to mediate accurate HH signaling[83]. BOC and GAS1 form unique heterogeneous complexes through their interactions with PTCH1 and PTCH2 receptors, respectively, and mediate different kinetic SMO derepression programs through distinct ligand reception patterns[59]. CDON is a TM glycoprotein that binds directly to HH ligand and is required in conjunction with PTCH, BOC and GAS1 to promote HH signaling[84]. Binding of HH ligands to co-receptors GAS1, CDON and BOC leads to SMO derepression and phos
HH signaling is regulated by major phosphorylation events through protein kinases, which act as either positive or negative regulators (Table 1)[87-116].
| Protein kinases | Effect on HH signaling | Ref. |
| Protein kinase A | Inhibits GLI1, GLI2, GLI3 | [87] |
| Activates SMO | ||
| Stabilizes SUFU | ||
| Casein kinase 1 | Activates/inhibits GLI | [88] |
| Activates SMO | ||
| Casein kinase 2 | Activates GLI1, GLI2 | [89,90] |
| Glycogen synthase kinase 3β | Inhibits GLI2, GLI3 | [15] |
| Stabilizes SUFU | ||
| G protein-coupled receptor kinase 2 | Stabilizes and activates SMO | [91] |
| Dual-specificity tyrosine phosphorylation-regulated kinase 1A and Dual-specificity tyrosine phosphorylation-regulated kinase 1B | Activates/inhibits GLI | [92] |
| Dual-specificity tyrosine phosphorylation-regulated kinase 2 | Inhibits GLI2 | [93] |
| Extracellular signal-regulated kinases 1/2 | Activates HH signaling | [94,95] |
| Protein kinase B | Activates HH signaling | [96] |
| Ribosomal protein S6 kinase 1 | Activates GLI1 | [97,98] |
| Protein kinase C α, δ | Activates/inhibits GLI1 | [99,100] |
| Atypical protein kinase Cι/λ | Activates GLI1 | [101] |
| 5’ adenosine monophosphate-activated protein kinase | Inhibits/activates HH signaling | [102,103] |
| Unc-51 Like kinase 3 | Activates/inhibits GLI | [104] |
| Serine/threonine-protein kinase | Activates HH signaling | [105] |
| Integrin-linked kinase | Activates HH signaling | [106] |
| RIO kinase 3 | Activates HH signaling | [107] |
| Never in mitosis gene A-related kinase 2 | Increases SUFU stability repressing GLI2 | [108,109] |
| Liver kinase B1 | Inhibits HH signaling | [110] |
| Polo-like kinase 1 | Inhibits GLI1 | [111] |
| Mitogen-activated protein kinase kinase kinase 1 | Inhibits GLI1 | [112] |
| Mitogen-activated protein kinase kinase kinase 2/3 | Enhances GLI1-SUFU association | [113] |
| Mitogen-activated protein kinase kinase kinase 10 | Activates GLI1 and GLI2 | [114] |
| C-Jun N-terminal kinase | Activates GLI2 and GLI3 | [115,116] |
Although there is no direct physical interaction between PTCH and SMO, steroidal metabolites (such as cholesterol) are believed to mediate communication between the two receptors and act as SMO agonists[117]. The domain architecture of vertebrate PTCH (a 12-pass TM protein) and SMO (a 7-pass TM protein) show intimate links between cholesterol and HH signaling[118]. There are indications that sterol depletion inhibits the accumulation of SMO in the primary cilium[119]. Further, SMO activation is impaired through a cholesterol deficiency either by depleting cholesterol by using methyl-β-cyclodextrin in culture cells or by inhibiting the function of 7-dehydrocholesterol reductase that converts 7-dehydrocholesterol to cholesterol[120]. PTCH1 belongs to a family of resistance-nodulation-cell division pump proteins whose role in bacteria is to transport diverse molecular cargos such as antibiotics and sterols across lipid membranes[58]. It is believed that in the absence of HH ligands, PTCH1 transports the endogeneous modulators (endogenous agonists) to cell outside. PTCH1 also contains a putative sterol-sensing domain that regulates sterol metabolism[121].
An important class of endogenous SMO activators comprises of oxysterols, of which the most potent in SMO activation is 20(S)-hydroxycholesterol [20(S)-OHC][122]. Several clues make it unlikely that 20(S)-OHC is the endogenous SMO modulator. First, 20(S)-OHC could not be detected in cultured cells that are responsive to HH ligands[123]. 20(S)-OHC shows no synergism with SHH ligand in pathway activation[124]. Further, SMO mutations that impair the binding to 20(S)-OHC do not inhibit SMO activity. Recent crystal structure models of SMO action have shown that a cholesterol molecule binds to the cysteine rich domain (CRD) of the SMO and SMO lacking CRD or bearing mutations in key amino acids required for cholesterol binding, does not respond to cholesterol[125]. Cholesterol binding to SMO is competitively inhibited by both 20(S)-OHC and cyclopamine, consistent with a common binding site in the CRD[126]. Both these molecules allosterically regulate SMO protein. The cytoplasmic tail of SMO also participates in endogenous modulation of SMO. Phosphatidylinositol 4-photosphate promotes ciliary accumulation and pathway activation by binding to SMO’s cytoplasmic tail[127].
Cancer development and progression entail a multitude of biological processes, such as the malfunctioning of pivotal signaling pathways, increased drug efflux via adenosine triphosphate-binding cassette transporters, acquisition of mutations, evasion of apoptosis, activation of DNA damage response mechanisms, epithelial-mesenchymal transition, and adaptation of cancer stem cells (CSCs)[128]. The HH/PTCH/SMO/GLI signaling pathway significantly contributes to both the development of melanoma and its resistance to therapy[129]. A multitude of modulators have been identified as HH pathway antagonists. Fundamentally, these modulators target three key sites in HH signaling: HH ligands (SHH neutralizing antibodies, robotnikinin), SMO protein (cyclopamine and its derivatives IPI-926, Cyc-T and synthetic compounds GDC-0449, Cur61414, XL-139 and LDE-225); and GLI transcription factors (HPI-1, HPI-2, GANT-56 and GANT-61).
Cyclopamine, a sterol alkaloid obtained from the corn lily plant (Veratrum californicum), acts as an antagonist of the SMO receptor and has been utilized for treating and preventing basal cell carcinoma[130]. It directly binds to the TM helices of the SMO protein, inhibiting HH signaling. At low concentrations (< 10 μmol/L), cyclopamine specifically blocks HH signaling, but at high concentrations, it induces cell death without affecting HH signaling[131]. Several semi-synthetic derivatives, such as KAAD-cyclopamine, IPI-609, IPI-926, and Cyc-T, have been developed[132]. These derivatives exhibit improved stability in both acidic and aqueous environments. IPI-926 has progressed to phase II human intervention trials[133].
Due to dearth of natural products that cause selective and specific inhibition of HH pathway, efforts have been made to identify synthetic HH antagonists with higher potency than that of cyclopamine[133]. SMO agonists such as vismodegib (also known as erivedge or GDC-0449) have been found to be effective in regressing basal cell carcinoma in PTCH+/- mice[134]. This molecule is orally active and has entered into phase I and phase II human intervention trials[135]. Several synthetic compounds have been developed that bind to SMO but show no structural similarity to cyclopamine[136]. Recently, a small molecule inhibitor robotnikinin has been reported to bind SHH protein and inhibits the HH signalling in cultured skins cells. The IC50 of robotnikinin is approximately 3 μmol/L in the Shh-LIGHT2 cells[137].
Due to its pivotal role in melanoma pathogenesis, the HH signaling pathway has attracted considerable attention as a promising therapeutic target. Numerous small-molecule modulators have been identified and studied for their ability to inhibit HH signaling (Table 2)[138-168].
| Compound | EC50 | Mechanism of action | Ref. |
| Cyclopamine | 300 nM | Directly blocks HH signaling by binding to and inhibiting SMO receptors | [138,139] |
| KAAD-cyclopamine | 20 nM | Cyclopamine-KAAD is a potent cell-permeable analog of cyclopamine that specifically inhibits HH signaling (with similar or lower toxicity) by binding to SMOA1 and promoting its exit from the endoplasmic reticulum | [140] |
| SAG | 3 nM | SAG is a chlorobenzothiophene-containing HH pathway agonist that regulates the SMO activity by directly binding to SMO’s heptahelical bundle | [141] |
| Jervine | 500-700 nM | Jervine is a steroidal alkaloid that blocks HH signaling by binding to SMO receptors | [142] |
| Cyclopamine tartrate | 20 nM | CycT is an improved analogue of cyclopamine that inhibits the SMO receptor by binding to it | [139] |
| Cur-61414 | 100-200 nM | CUR-61414 (aminoproline, mol. wt. 513Da) is a specific and highly potent antagonist of SMO that can block elevated HH signaling activity arising from oncogenic mutations in PTCH-1 | [143] |
| SANT-1 | 200 nM | SANT-1 is an SMI of SMO and functions as a potent SHH pathway antagonist | [144] |
| SANT-2 | 20 nM | SANT-2, belonging to the benzimidazole class of compounds, is an SMI and a cell-permeable antagonist of the SMO receptor | [145] |
| SANT-3 | 30 nM | An SMI of SMO receptor | [127] |
| SANT-4 | 100 nM | An SMI of SMO receptor | [127] |
| Compound 5 | 200 nM | SMI targeting the SMO receptor | [146] |
| Compound Z | < 1 nM | Compound Z has an efficacy of less than 1 nM and is classified as a second-generation SMO inhibitor | [147] |
| Vismodegib | < 20 nM | Vismodegib (GDC-0449) is a first-in-class, SMI of SMO | [135] |
| Saridegib | - | Saridegib (IPI-926), a semisynthetic analogue of cyclopamine with a seven-membered D-ring, functions as an SMI of SMO with improved potency and improved plasma half life However, saridegib is a substrate of the P-glycoprotein transporter, therefore, its extended use causes drug resistance | [133] |
| Sonidegib (LDE225) | < 20 nM | Orally bioavailable cyclopamine-derived SMO inhibitor that causes cell cycle arrest and apoptosis in variety of cell lines | [148] |
| TAK-441 | 4.6 nM | TK-441 is an SMI of SMO that blocks castration-resistant progression of LNCaP xenografts by obstructing paracrine HH signaling | [149] |
| 2-amino-thiazoles | 30 nM | 2-Aminothiazole (JK184) is inhibitor of Shh-N-mediated Gli-transcription and retards the growth of cancer cell lines with aberrantly activated HH signaling | [150] |
| Gant-58 | 5 μM | SMI that inhibits GLI1-mediated GLI luciferase | [150] |
| Gant-61 | 5 μM | SMI that inhibits GLI1-mediated GLI luciferase | [150] |
| IPI-296 | < 20 nM | IPI-926 is a novel, natural product-based SMI of SMO | [151] |
| BMS-833923 (XL139) | < 20 nM | BMS-833923 is an orally bioavailable SMI of the SMO receptor that dose-dependently affects HH signature gene transcription in vitro | [152] |
| L4 | 2.33 nM | L-4 is a potent, orally bioavailable antagonist of the SMO receptor that exhibits potent anti-tumor effects against medulloblastoma, BCC and other cancers | [153] |
| Robotnikinin | < 10 μM | SMI that interferes with SHH protein function | [154] |
| Vitamin D3 | 100 μM | Vitamin D3 is an SMI that binds to SMO and blocks GLI reporter activity in cultured fibroblasts in vitro | [155] |
| RU-SKI 41 | 0.66 μM | 5-acyl-6,7-dihydrothieno(3,2-c) pyridine 41 (termed RU-SKI43) is an SMI that blocks HHAT | 103] |
| RU-SKI 43 | 2.38 μM | SMI that inhibits HH acyltransferase | [156] |
| RU-SKI 101 | - | SMI that inhibits HH acyltransferase | [156] |
| RUSKI-201 | 0.20 μM | SMI that blocks HH acyltransferase | [157] |
| CA1 | - | SMI of SMO that affects ciliary localization and ciliogenesis | [158] |
| CA2 | - | SMI of SMO that affects ciliary localization and ciliogenesis | [158] |
| NVP-LEQ506 | 2-4 nM | Orally bioavailable SMI of SMO with potential antineopastic activity | [159] |
| PF-04449913 | 191 nM | PF-04449913 is a novel oral SMI that selectively binds to SMO | [160] |
| LY2940680 (taladegib) | - | SMI that inhibits SMO | [161] |
| BRD-6851 | 0.4 μM | SMI that inhibits SMO | [132] |
| SEN450 | - | SMI that inhibits SMO | [162] |
| MRT-83 | 10 nM | A novel, potent SMO antagonist from the acylguanidine family of SMIs | [163] |
| Arcyriaflavin C | 11.3 μM | Attenuate GLI1 activity through PKC/MAPK pathway blockade | [164] |
| Physalins F | 0.66 μM | Attenuate GLI1 activity through PKC/MAPK pathway blockade | [164] |
| HPI1 | 1.5 μM | SMI influences the processing, activation, and/or trafficking of GLI1 | [165] |
| HPI2 | 2 μM | SMI influences the processing, activation, and/or trafficking of GLI1 | [165] |
| HPI3 | 3 μM | SMI influences the processing, activation, and/or trafficking of GLI1 | [165] |
| HPI4 (Ciliobrevin A) | 7 μM | SMI influences the processing, activation, and/or trafficking of GLI1 | [165] |
| Arsenic trioxide | 2.7 μM | SMI inhibits GLI transcription factors. ATO directly bound to GLI1 protein | [166] |
| Glabrescione B | - | Glabrescione B is an SMI that interferes with GLI1/DNA interaction in HH-dependent tumor cells well as the self-renewal of tumor-derived stem cells | [167] |
| Pyrazolo-imidazole smoothib | 157 ± 118 nM | Smoothib functions as an antagonist of SMO by binding to its heptahelical bundle, preventing its localization to the cilia. This subsequently reduces the expression of HH target genes | [168] |
Inhibitors of the HH signaling cascade often show limited efficacy as monotherapy in melanoma due to pathway redundancy and compensatory signaling mechanisms. This limited efficacy is further attributed to several intrinsic (such as genetic mutations and epigenetic alterations within tumor cells) and extrinsic factors (including stromal interactions, immune cell infiltration, and extracellular matrix composition) that undermine their therapeutic potential[169,170]. The primary challenges lies in the redundancy and compensatory nature of cellular signaling transduction pathways. Melanoma cells often circumvent HH pathway inhibition by activating parallel signaling pathways such as PI3K, mitogen-activated protein kinase (MAPK), or transforming growth factor β pathways[170,171]. In particular, the BRAF/MAPK pathway can directly stimulate GLI1, independently of SMO, thereby diminishing the effectiveness of SMO-targeted therapies[101,170]. Resistance also emerges from mutations within SMO or downstream effectors. For instance, specific point mutations like D473H in SMO can hinder drug binding, rendering inhibitors such as vismodegib less effective[172]. Additionally, non-canonical activation of GLI transcription factors - mediated by oncogenes like RAS or signaling pathways such as AKT and transforming growth factor β - further complicates treatment, as this bypasses the conventional SMO-dependent axis of HH signaling. Pharmacokinetic limitations represent another obstacle. Some HH inhibitors have poor tumor penetration due to poor bioavailability, rapid metabolism, or active efflux by membrane transporters like P-glycoprotein[173,174]. Saridegib, for example, is a known substrate of P-glycoprotein, which can lead to diminished drug levels at the tumor site and subsequent resistance[175]. Not all tumors rely on HH signaling for growth and survival; in many cases, only specific subpopulations of cancer cells are HH-dependent[176]. Moreover, interactions within the tumor microenvironment, especially those involving stromal cells, can support paracrine activation of the pathway, which is not always suppressed by conventional HH inhibitors[177]. Lastly, the physiological importance of the HH pathway in normal tissue homeostasis poses a therapeutic challenge. Long-term inhibition may result in toxicities, particularly in tissues where HH signaling is crucial, such as in the maintenance of hair follicles and taste buds[178,179]. These cumulative factors underscore the complexity of HH-targeted therapies and highlight the need for combination strategies or more selective inhibitors to overcome these limitations.
Furthermore, melanoma is notoriously resistant to conventional treatments, primarily due to its high genetic heterogeneity, rapid mutation rate, immune evasion, genomic amplifications, activation of aberrant signaling pathways (such as MAPK and PI3K-AKT), presence of cancer stem-like cells, erroneous DNA repair mechanisms, and enhanced survival strategies including resistance to apoptosis[180,181]. Although immunotherapy offers a promising strategy by harnessing the patient’s immune system to recognize and eliminate tumor cells, its effectiveness in melanoma is limited[182]. This is partly because melanoma has developed sophisticated mechanisms to evade immune detection, including alterations in the tumor microenvironment and upregulation of immune checkpoints[183]. Recent studies have identified the HH signaling pathway as a contributor to tumor immune evasion and poor responses to immunotherapy, further compli
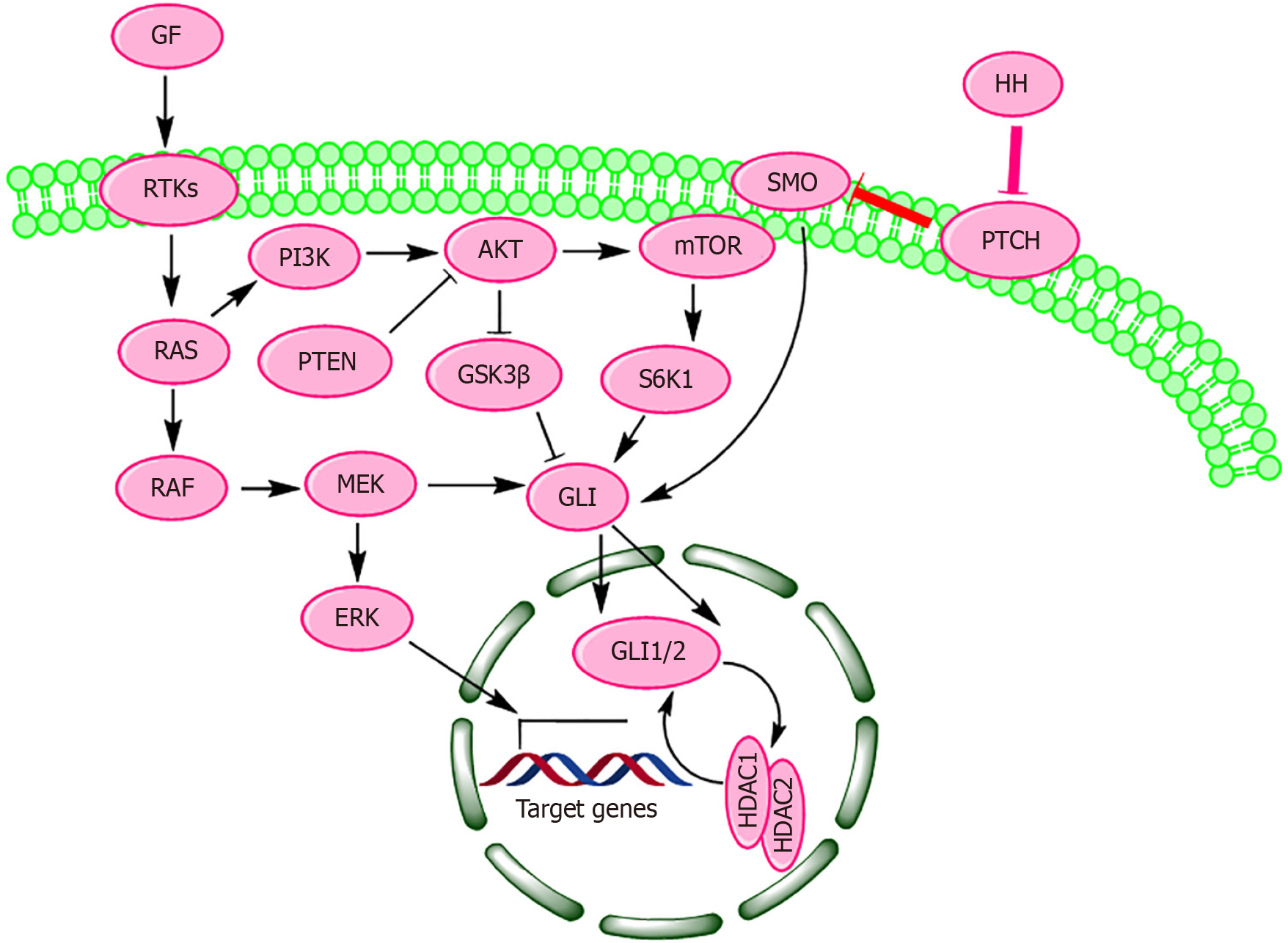
The HH signaling is activated in multitude of cancer including melanoma[22]. The mutations in HH pathway genes cause ligand-independent activation of this pathway. However, ligand-dependent activation of HH pathway is potentiated through crosstalk with other signaling pathways such as RAS/Raf proto-oncogene, serine/threonine kinase (RAF)/mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) pathway[188]. The way HH interacts with this pathway is likely to lead development of new combination therapies with improved antitumor efficacy and survival in animal models. The first connecting link between oncogenic BRAF and HH signaling emerged from re
HH pathway has been reported to be involved in the pathogenesis of melanoma and other skin cancers[193]. This pathway regulates various biological processes including cell differentiation, proliferation, tissue polarity, stem cell maintenance, embryonic patterning, embryonic development and tumor formation[194,195]. In human skin, the SHH pathway participates in the maintenance of MSCs, and in the regulation of hair follicle and sebaceous gland development[196]. Constitutively active RAS-RAF-MEK-ERK pathway has been found to induce HH signaling through translocation of GLI1 and GLI2 into the nucleus (Figure 1)[96]. There is a strong possibility that oncogenic BRAF promotes HH path
The regulation of HH signaling by BRAF occurs primarily through indirect mechanisms. Studies have demonstrated that activation of the MAPK/ERK pathway can upregulate GLI1 expression and activity in various cancer types[199]. However, the interaction between MAPK/ERK and HH signaling can vary depending on the cellular context. In some instances, activation of MAPK/ERK signaling has been observed following HH pathway inhibition, indicating a complex and context-dependent relationship between these pathways[200]. Oncogenic BRAF typically activates HH signaling via MAPK/ERK pathway through two distinct mechanisms: direct phosphorylation, where BRAF-activated ERKs move into the nucleus to phosphorylate GLIs, and indirect phosphorylation, where ERKs phosphorylate intermediate proteins that subsequently modify GLI transcription factors[94]. Given that most malignant melanomas contain activating mutations in BRAF, many potent inhibitors of oncogenic BRAF such as vemurafenib, dabrafenib and encorafenib were developed over the last two decades[201]. Many of these BRAF inhibitors (for example, vemurafenib) show significantly high clinical response rate in patients with BRAF V600E-mutant melanomas, yet the majority of melanoma patients ultimately develop drug resistance[202]. Although multiple resistance mechanisms are known for melanoma, many of BRAF inhibitor-resistant melanomas are driven by unknown mechanisms[203,204]. Recently, HH signaling has received tremendous attention as a central regulatory pathway that imparts drug resistant phenotype to melanoma tumors. Mechanistically, HH signaling is simple and comprises of HH ligands (such as SHH, IHH, and DHH), TM receptors, and transcription factors[205]. In canonical HH signaling, binding of secreted HH ligands to the TM receptor PTCH activates the GPCR SMO, which triggers an intracellular signaling cascade leading to the formation of transcription factors GLI 1 and 2 (GLI1/GLI2) and their translocation into the nucleus (Figure 1)[101,206]. GLI1/GLI2 transcription factors have been found to play a causal role in both the resistance to BRAF V600E-targeted therapy and the proinvasive behavior of melanoma cells[189]. Consequently, melanoma cell lines with acquired drug-resistance and melanoma tissue samples show increased levels of GLI1 and GLI2 compared to naive cells and normal tissues[189]. This raises the possibility of using GLI1/GLI2 inhibitors to reverse drug-resistance in BRAF driven melanoma cells. Furthermore, aberrant HH signaling has been associated with the maintenance of melanoma stem cells (MSCs)[207]. MSCs, also known as me
The self-renewal capacity and differentiation potential of CSCs contribute significantly to the heterogeneity, metastasis, recurrence, and chemo-radioresistance observed in melanoma tumors[217]. CSCs typically originate from normal stem cells through a series of gene mutations, epigenetic modifications, and dysregulated signaling pathways, in conjunction with persistent alterations in the tumor microenvironment[218]. However, the precise origin of MSCs remains unde
The most important biomarkers of MSCs are CD20 (a cell surface marker normally associated with B cells), adenosine triphosphate-binding cassette sub-family B member 5, C-X-C chemokine receptor type 6, CD44, SOX10, CD133, CD271 and aldehyde dehydrogenase (ALDH)[223,224]. Among these MSC biomarkers, the identification and functional validation of CD271 and ALDH have been particularly pivotal in understanding tumor heterogeneity and progression[225,226]. CD271, also known as p75 neurotrophin receptor, has been recognized as a marker of MSCs[227]. Studies have demonstrated that CD271-positive melanoma cells possess self-renewal capabilities and can recapitulate the heterogeneity of the original tumor upon serial transplantation in immunedeficient mice[225]. These cells maintain their tu
In melanoma, intratumoral heterogeneity, progression and drug resistance result from the unique characteristics of MSCs[232]. These MSCs harbor distinct protein signatures and tumor growth-driving pathways, whose activation is driven by driver mutation-dependent signals[232]. CSCs are usually identified on their ability to generate tumorspheres in suspension cultures, to possess high invasive behavior, to give rise to the heterogeneous original tumor when ino
HDACs have recently received attention for their role in the drug-resistant phenotype of melanoma, thereby positioning HDAC inhibitors as promising tools for combating this therapy resistance[238,239]. Expression of the class 1 histone deacetylases HDAC8 and HDAC3 are associated with improved survival of patients with metastatic melanoma[240]. HDAC8, in particular, regulates stress response pathways in melanoma to mediate escape from BRAF inhibitor therapy. For example, introduction of HDAC8 into drug-naïve melanoma cells conveyed resistance both in vitro and in vivo[241]. HDAC8-mediated BRAF inhibitor resistance is mediated via activation of receptor tyrosine kinases and MAPK signaling. Inhibitors targeting HDAC8 specifically curb the ability of melanoma cells to adapt to various stressors, including inhibition of BRAF-MEK signaling[241]. Additionally, HDAC inhibitors have been observed to restore sensitivity to BRAF inhibitors by modifying PI3K and survival signaling pathways in a specific subgroup of melanoma cases[242]. Although HDACs function at the histone level, they also regulate nonhistone substrates, and introduction of HDAC8 decreased the acetylation of c-JUN, increasing its transcriptional activity and enriching for an AP-1 gene signature. Using patient- and in vivo-derived melanoma cell lines with acquired BRAF inhibitor resistance, it has been reported that combined treatment with the BRAF inhibitor encorafenib and HDAC inhibitor panobinostat in 2D and 3D culture systems synergistically induced caspase-dependent apoptotic cell death[242]. Similarly, HDAC inhibition overcomes acute resistance to MEK inhibition in BRAF-mutant colorectal cancer by downregulation of cellular FLICE-like inhibitory protein, long isoform. Further, combined HDAC inhibitor/MEK inhibitor treatment resulted in dramatically attenuated tumor growth in BRAF MT xenografts[243].
The failure of current therapeutic regimens to fully eradicate MSCs poses a significant challenge to the healthcare system. It is now clear that HH signaling plays a crucial role in maintaining MSCs, and inducing apoptosis in these cells by targeting key signaling pathways represents a promising approach in cancer treatment. Oncogenic BRAF has been shown to activate HH signaling in BRAF-mutant melanoma tumors, drawing considerable attention to the potential of targeting the HH signaling cascade alongside BRAF signaling as a viable option to retard tumor growth and prevent recurrence. Several agents have been developed to specifically target these pathways, and since aberrant activation of HH is associated with enhanced proliferation and cancer development in the skin and other tissues, creating potent small molecule inhibitors for this pathway may provide successful therapeutic interventions for HH pathway-dependent cancers. Developing HH pathway inhibitors for melanoma is crucial due to their potential to target CSCs, overcome therapeutic resistance, and enhance combination therapies, thereby altering the tumor microenvironment and preventing metastasis. Ultimately, these inhibitors offer new avenues for more effective and personalized therapies, improving patient outcomes. However, there is a need to develop clinically relevant models to understand the crosstalk between oncogenic BRAF and HH signaling in sustaining melanoma tumors and to uncover new therapeutic targets.
| 1. | Schadendorf D, Fisher DE, Garbe C, Gershenwald JE, Grob JJ, Halpern A, Herlyn M, Marchetti MA, McArthur G, Ribas A, Roesch A, Hauschild A. Melanoma. Nat Rev Dis Primers. 2015;1:15003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 464] [Article Influence: 42.2] [Reference Citation Analysis (2)] |
| 2. | Siegel RL, Kratzer TB, Giaquinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA Cancer J Clin. 2025;75:10-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 1521] [Article Influence: 1521.0] [Reference Citation Analysis (3)] |
| 3. | Naik PP. Cutaneous Malignant Melanoma: A Review of Early Diagnosis and Management. World J Oncol. 2021;12:7-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 4. | Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445:843-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 888] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 5. | Sun X, Zhang N, Yin C, Zhu B, Li X. Ultraviolet Radiation and Melanomagenesis: From Mechanism to Immunotherapy. Front Oncol. 2020;10:951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Sommer L. Generation of melanocytes from neural crest cells. Pigment Cell Melanoma Res. 2011;24:411-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Dupin E, Le Douarin NM. Development of melanocyte precursors from the vertebrate neural crest. Oncogene. 2003;22:3016-3023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 130] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Castro-Pérez E, Singh M, Sadangi S, Mela-Sánchez C, Setaluri V. Connecting the dots: Melanoma cell of origin, tumor cell plasticity, trans-differentiation, and drug resistance. Pigment Cell Melanoma Res. 2023;36:330-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Kaufman CK, Mosimann C, Fan ZP, Yang S, Thomas AJ, Ablain J, Tan JL, Fogley RD, van Rooijen E, Hagedorn EJ, Ciarlo C, White RM, Matos DA, Puller AC, Santoriello C, Liao EC, Young RA, Zon LI. A zebrafish melanoma model reveals emergence of neural crest identity during melanoma initiation. Science. 2016;351:aad2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 315] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 10. | Shakhova O. Neural crest stem cells in melanoma development. Curr Opin Oncol. 2014;26:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Wessely A, Steeb T, Berking C, Heppt MV. How Neural Crest Transcription Factors Contribute to Melanoma Heterogeneity, Cellular Plasticity, and Treatment Resistance. Int J Mol Sci. 2021;22:5761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Mort RL, Jackson IJ, Patton EE. The melanocyte lineage in development and disease. Development. 2015;142:620-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 246] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 13. | Salaritabar A, Berindan-Neagoe I, Darvish B, Hadjiakhoondi F, Manayi A, Devi KP, Barreca D, Orhan IE, Süntar I, Farooqi AA, Gulei D, Nabavi SF, Sureda A, Daglia M, Dehpour AR, Nabavi SM, Shirooie S. Targeting Hedgehog signaling pathway: Paving the road for cancer therapy. Pharmacol Res. 2019;141:466-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 14. | Robbins DJ, Fei DL, Riobo NA. The Hedgehog signal transduction network. Sci Signal. 2012;5:re6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 331] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 15. | Li C, Chi S, Xie J. Hedgehog signaling in skin cancers. Cell Signal. 2011;23:1235-1243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Petrova R, Joyner AL. Roles for Hedgehog signaling in adult organ homeostasis and repair. Development. 2014;141:3445-3457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 319] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 17. | McMillan R, Matsui W. Molecular pathways: the hedgehog signaling pathway in cancer. Clin Cancer Res. 2012;18:4883-4888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Jiang J. Hedgehog signaling mechanism and role in cancer. Semin Cancer Biol. 2022;85:107-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 160] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 19. | Bürglin TR. The Hedgehog protein family. Genome Biol. 2008;9:241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Ingham PW. Hedgehog signaling. Curr Top Dev Biol. 2022;149:1-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 21. | Niewiadomski P, Niedziółka SM, Markiewicz Ł, Uśpieński T, Baran B, Chojnowska K. Gli Proteins: Regulation in Development and Cancer. Cells. 2019;8:147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 22. | Jing J, Wu Z, Wang J, Luo G, Lin H, Fan Y, Zhou C. Hedgehog signaling in tissue homeostasis, cancers, and targeted therapies. Signal Transduct Target Ther. 2023;8:315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 139] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 23. | Xavier GM, Seppala M, Barrell W, Birjandi AA, Geoghegan F, Cobourne MT. Hedgehog receptor function during craniofacial development. Dev Biol. 2016;415:198-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 24. | Nozawa YI, Lin C, Chuang PT. Hedgehog signaling from the primary cilium to the nucleus: an emerging picture of ciliary localization, trafficking and transduction. Curr Opin Genet Dev. 2013;23:429-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 25. | Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059-3087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2300] [Cited by in RCA: 2357] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 26. | Míguez DG, Iannini A, García-Morales D, Casares F. The effects of Hh morphogen source movement on signaling dynamics. Development. 2022;149:dev199842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 27. | Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2958] [Cited by in RCA: 2936] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 28. | Hosio M, Jaks V, Lagus H, Vuola J, Ogawa R, Kankuri E. Primary Ciliary Signaling in the Skin-Contribution to Wound Healing and Scarring. Front Cell Dev Biol. 2020;8:578384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Arwert EN, Hoste E, Watt FM. Epithelial stem cells, wound healing and cancer. Nat Rev Cancer. 2012;12:170-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 366] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 30. | Wijgerde M, McMahon JA, Rule M, McMahon AP. A direct requirement for Hedgehog signaling for normal specification of all ventral progenitor domains in the presumptive mammalian spinal cord. Genes Dev. 2002;16:2849-2864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 211] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 31. | Camacho-Aguilar E, Warmflash A. Insights into mammalian morphogen dynamics from embryonic stem cell systems. Curr Top Dev Biol. 2020;137:279-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Hooper JE, Scott MP. Communicating with Hedgehogs. Nat Rev Mol Cell Biol. 2005;6:306-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 610] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 33. | Prince E, Marcetteau J, Thérond PP. Circulating Hedgehog: a fresh view of a classic morphogen. Development. 2020;147:dev186395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Jeong J, McMahon AP. Cholesterol modification of Hedgehog family proteins. J Clin Invest. 2002;110:591-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Buglino JA, Resh MD. Palmitoylation of Hedgehog proteins. Vitam Horm. 2012;88:229-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Blassberg R, Jacob J. Lipid metabolism fattens up hedgehog signaling. BMC Biol. 2017;15:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 37. | Tukachinsky H, Kuzmickas RP, Jao CY, Liu J, Salic A. Dispatched and scube mediate the efficient secretion of the cholesterol-modified hedgehog ligand. Cell Rep. 2012;2:308-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 38. | Dierker T, Dreier R, Petersen A, Bordych C, Grobe K. Heparan sulfate-modulated, metalloprotease-mediated sonic hedgehog release from producing cells. J Biol Chem. 2009;284:8013-8022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Hall TM, Porter JA, Beachy PA, Leahy DJ. A potential catalytic site revealed by the 1.7-A crystal structure of the amino-terminal signalling domain of Sonic hedgehog. Nature. 1995;378:212-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 152] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 40. | Jägers C, Roelink H. Association of Sonic Hedgehog with the extracellular matrix requires its zinc-coordination center. BMC Mol Cell Biol. 2021;22:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Bonn-Breach R, Gu Y, Jenkins J, Fasan R, Wedekind J. Structure of Sonic Hedgehog protein in complex with zinc(II) and magnesium(II) reveals ion-coordination plasticity relevant to peptide drug design. Acta Crystallogr D Struct Biol. 2019;75:969-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 42. | Wright SC, Kozielewicz P, Kowalski-Jahn M, Petersen J, Bowin CF, Slodkowicz G, Marti-Solano M, Rodríguez D, Hot B, Okashah N, Strakova K, Valnohova J, Babu MM, Lambert NA, Carlsson J, Schulte G. A conserved molecular switch in Class F receptors regulates receptor activation and pathway selection. Nat Commun. 2019;10:667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 43. | McCabe JM, Leahy DJ. Smoothened goes molecular: new pieces in the hedgehog signaling puzzle. J Biol Chem. 2015;290:3500-3507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Zhang X, Zhao F, Wu Y, Yang J, Han GW, Zhao S, Ishchenko A, Ye L, Lin X, Ding K, Dharmarajan V, Griffin PR, Gati C, Nelson G, Hunter MS, Hanson MA, Cherezov V, Stevens RC, Tan W, Tao H, Xu F. Crystal structure of a multi-domain human smoothened receptor in complex with a super stabilizing ligand. Nat Commun. 2017;8:15383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 45. | Byrne EFX, Sircar R, Miller PS, Hedger G, Luchetti G, Nachtergaele S, Tully MD, Mydock-McGrane L, Covey DF, Rambo RP, Sansom MSP, Newstead S, Rohatgi R, Siebold C. Structural basis of Smoothened regulation by its extracellular domains. Nature. 2016;535:517-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 291] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 46. | Mukhopadhyay S, Badgandi HB, Hwang SH, Somatilaka B, Shimada IS, Pal K. Trafficking to the primary cilium membrane. Mol Biol Cell. 2017;28:233-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 47. | Ruiz-Gómez A, Molnar C, Holguín H, Mayor F Jr, de Celis JF. The cell biology of Smo signalling and its relationships with GPCRs. Biochim Biophys Acta. 2007;1768:901-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Carballo GB, Honorato JR, de Lopes GPF, Spohr TCLSE. A highlight on Sonic hedgehog pathway. Cell Commun Signal. 2018;16:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 347] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 49. | Jeng KS, Sheen IS, Leu CM, Tseng PH, Chang CF. The Role of Smoothened in Cancer. Int J Mol Sci. 2020;21:6863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 50. | Peer E, Tesanovic S, Aberger F. Next-Generation Hedgehog/GLI Pathway Inhibitors for Cancer Therapy. Cancers (Basel). 2019;11:538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 51. | Skoda AM, Simovic D, Karin V, Kardum V, Vranic S, Serman L. The role of the Hedgehog signaling pathway in cancer: A comprehensive review. Bosn J Basic Med Sci. 2018;18:8-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 541] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 52. | Nicheperovich A, Townsend-Nicholson A. Towards Precision Oncology: The Role of Smoothened and Its Variants in Cancer. J Pers Med. 2022;12:1648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 53. | Nagao K, Toyoda M, Takeuchi-Inoue K, Fujii K, Yamada M, Miyashita T. Identification and characterization of multiple isoforms of a murine and human tumor suppressor, patched, having distinct first exons. Genomics. 2005;85:462-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Reinders MG, van Hout AF, Cosgun B, Paulussen AD, Leter EM, Steijlen PM, Mosterd K, van Geel M, Gille JJ. New mutations and an updated database for the patched-1 (PTCH1) gene. Mol Genet Genomic Med. 2018;6:409-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 55. | Carpenter D, Stone DM, Brush J, Ryan A, Armanini M, Frantz G, Rosenthal A, de Sauvage FJ. Characterization of two patched receptors for the vertebrate hedgehog protein family. Proc Natl Acad Sci U S A. 1998;95:13630-13634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 199] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 56. | Cretnik M, Poje G, Musani V, Kruslin B, Ozretic P, Tomas D, Situm M, Levanat S. Involvement of p16 and PTCH in pathogenesis of melanoma and basal cell carcinoma. Int J Oncol. 2009;34:1045-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 57. | Veenstra VL, Dingjan I, Waasdorp C, Damhofer H, van der Wal AC, van Laarhoven HW, Medema JP, Bijlsma MF. Patched-2 functions to limit Patched-1 deficient skin cancer growth. Cell Oncol (Dordr). 2018;41:427-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 58. | Roberts B, Casillas C, Alfaro AC, Jägers C, Roelink H. Patched1 and Patched2 inhibit Smoothened non-cell autonomously. Elife. 2016;5:e17634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 59. | Izzi L, Lévesque M, Morin S, Laniel D, Wilkes BC, Mille F, Krauss RS, McMahon AP, Allen BL, Charron F. Boc and Gas1 each form distinct Shh receptor complexes with Ptch1 and are required for Shh-mediated cell proliferation. Dev Cell. 2011;20:788-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 60. | Adolphe C, Hetherington R, Ellis T, Wainwright B. Patched1 functions as a gatekeeper by promoting cell cycle progression. Cancer Res. 2006;66:2081-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 61. | AACR Project GENIE Consortium. AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 2017;7:818-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1205] [Cited by in RCA: 1487] [Article Influence: 165.2] [Reference Citation Analysis (0)] |
| 62. | Signetti L, Elizarov N, Simsir M, Paquet A, Douguet D, Labbal F, Debayle D, Di Giorgio A, Biou V, Girard C, Duca M, Bretillon L, Bertolotto C, Verrier B, Azoulay S, Mus-Veteau I. Inhibition of Patched Drug Efflux Increases Vemurafenib Effectiveness against Resistant Braf(V600E) Melanoma. Cancers (Basel). 2020;12:1500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 63. | Rahnama F, Shimokawa T, Lauth M, Finta C, Kogerman P, Teglund S, Toftgård R, Zaphiropoulos PG. Inhibition of GLI1 gene activation by Patched1. Biochem J. 2006;394:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 64. | Avery JT, Zhang R, Boohaker RJ. GLI1: A Therapeutic Target for Cancer. Front Oncol. 2021;11:673154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 65. | Kasper M, Regl G, Frischauf AM, Aberger F. GLI transcription factors: mediators of oncogenic Hedgehog signalling. Eur J Cancer. 2006;42:437-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 310] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 66. | Ruiz i Altaba A. Gli proteins encode context-dependent positive and negative functions: implications for development and disease. Development. 1999;126:3205-3216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 279] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 67. | Pan Y, Bai CB, Joyner AL, Wang B. Sonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation. Mol Cell Biol. 2006;26:3365-3377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 418] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 68. | Gupta S, Takebe N, Lorusso P. Targeting the Hedgehog pathway in cancer. Ther Adv Med Oncol. 2010;2:237-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 221] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 69. | Matissek SJ, Elsawa SF. GLI3: a mediator of genetic diseases, development and cancer. Cell Commun Signal. 2020;18:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 70. | Larsen LJ, Møller LB. Crosstalk of Hedgehog and mTORC1 Pathways. Cells. 2020;9:2316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 71. | Jeng KS, Chang CF, Lin SS. Sonic Hedgehog Signaling in Organogenesis, Tumors, and Tumor Microenvironments. Int J Mol Sci. 2020;21:758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 156] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 72. | Kurtović M, Piteša N, Bartoniček N, Ozretić P, Musani V, Čonkaš J, Petrić T, King C, Sabol M. RNA-seq and ChIP-seq Identification of Unique and Overlapping Targets of GLI Transcription Factors in Melanoma Cell Lines. Cancers (Basel). 2022;14:4540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 73. | Yao K, Zhou E, Cheng C. A B-Raf V600E gene signature for melanoma predicts prognosis and reveals sensitivity to targeted therapies. Cancer Med. 2022;11:1232-1243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 74. | Huang D, Wang Y, Tang J, Luo S. Molecular mechanisms of suppressor of fused in regulating the hedgehog signalling pathway. Oncol Lett. 2018;15:6077-6086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 75. | Stone DM, Murone M, Luoh S, Ye W, Armanini MP, Gurney A, Phillips H, Brush J, Goddard A, de Sauvage FJ, Rosenthal A. Characterization of the human suppressor of fused, a negative regulator of the zinc-finger transcription factor Gli. J Cell Sci. 1999;112 (Pt 23):4437-4448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 138] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 76. | Huq AJ, Walsh M, Rajagopalan B, Finlay M, Trainer AH, Bonnet F, Sevenet N, Winship IM. Mutations in SUFU and PTCH1 genes may cause different cutaneous cancer predisposition syndromes: similar, but not the same. Fam Cancer. 2018;17:601-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 77. | Cheng SY, Yue S. Role and regulation of human tumor suppressor SUFU in Hedgehog signaling. Adv Cancer Res. 2008;101:29-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 78. | Pearse RV 2nd, Collier LS, Scott MP, Tabin CJ. Vertebrate homologs of Drosophila suppressor of fused interact with the gli family of transcriptional regulators. Dev Biol. 1999;212:323-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 79. | Cherry AL, Finta C, Karlström M, Jin Q, Schwend T, Astorga-Wells J, Zubarev RA, Del Campo M, Criswell AR, de Sanctis D, Jovine L, Toftgård R. Structural basis of SUFU-GLI interaction in human Hedgehog signalling regulation. Acta Crystallogr D Biol Crystallogr. 2013;69:2563-2579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 80. | Zhang Y, Fu L, Qi X, Zhang Z, Xia Y, Jia J, Jiang J, Zhao Y, Wu G. Structural insight into the mutual recognition and regulation between Suppressor of Fused and Gli/Ci. Nat Commun. 2013;4:2608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 81. | Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD, Dreze M, Ayivi-Guedehoussou N, Klitgord N, Simon C, Boxem M, Milstein S, Rosenberg J, Goldberg DS, Zhang LV, Wong SL, Franklin G, Li S, Albala JS, Lim J, Fraughton C, Llamosas E, Cevik S, Bex C, Lamesch P, Sikorski RS, Vandenhaute J, Zoghbi HY, Smolyar A, Bosak S, Sequerra R, Doucette-Stamm L, Cusick ME, Hill DE, Roth FP, Vidal M. Towards a proteome-scale map of the human protein-protein interaction network. Nature. 2005;437:1173-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2192] [Cited by in RCA: 2091] [Article Influence: 99.6] [Reference Citation Analysis (0)] |
| 82. | Dahabieh MS, Huang F, Goncalves C, Flores González RE, Prabhu S, Bolt A, Di Pietro E, Khoury E, Heath J, Xu ZY, Rémy-Sarrazin J, Mann KK, Orthwein A, Boisvert FM, Braverman N, Miller WH, Del Rincón SV. Silencing PEX26 as an unconventional mode to kill drug-resistant cancer cells and forestall drug resistance. Autophagy. 2022;18:540-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 83. | Echevarría-Andino ML, Allen BL. The hedgehog co-receptor BOC differentially regulates SHH signaling during craniofacial development. Development. 2020;147:dev189076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 84. | Siebold C, Rohatgi R. The Inseparable Relationship Between Cholesterol and Hedgehog Signaling. Annu Rev Biochem. 2023;92:273-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 85. | Zhulyn O, Hui CC. Sufu and Kif7 in limb patterning and development. Dev Dyn. 2015;244:468-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 86. | Allen BL, Song JY, Izzi L, Althaus IW, Kang JS, Charron F, Krauss RS, McMahon AP. Overlapping roles and collective requirement for the coreceptors GAS1, CDO, and BOC in SHH pathway function. Dev Cell. 2011;20:775-787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 228] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 87. | Kotani T. Protein kinase A activity and Hedgehog signaling pathway. Vitam Horm. 2012;88:273-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 88. | Jiang J. CK1 in Developmental Signaling: Hedgehog and Wnt. Curr Top Dev Biol. 2017;123:303-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 89. | Jia H, Liu Y, Xia R, Tong C, Yue T, Jiang J, Jia J. Casein kinase 2 promotes Hedgehog signaling by regulating both smoothened and Cubitus interruptus. J Biol Chem. 2010;285:37218-37226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 90. | Firnau MB, Brieger A. CK2 and the Hallmarks of Cancer. Biomedicines. 2022;10:1987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 91. | Chen Y, Li S, Tong C, Zhao Y, Wang B, Liu Y, Jia J, Jiang J. G protein-coupled receptor kinase 2 promotes high-level Hedgehog signaling by regulating the active state of Smo through kinase-dependent and kinase-independent mechanisms in Drosophila. Genes Dev. 2010;24:2054-2067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 92. | Mao J, Maye P, Kogerman P, Tejedor FJ, Toftgard R, Xie W, Wu G, Wu D. Regulation of Gli1 transcriptional activity in the nucleus by Dyrk1. J Biol Chem. 2002;277:35156-35161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 149] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 93. | Stecca B, Pandolfi S. Hedgehog-Gli signaling in basal cell carcinoma and other skin cancers: prospects for therapy. Res Rep Biol. 2015;6:55-71. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 94. | Bardwell AJ, Wu B, Sarin KY, Waterman ML, Atwood SX, Bardwell L. ERK2 MAP kinase regulates SUFU binding by multisite phosphorylation of GLI1. Life Sci Alliance. 2022;5:e202101353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 95. | Kasper M, Schnidar H, Neill GW, Hanneder M, Klingler S, Blaas L, Schmid C, Hauser-Kronberger C, Regl G, Philpott MP, Aberger F. Selective modulation of Hedgehog/GLI target gene expression by epidermal growth factor signaling in human keratinocytes. Mol Cell Biol. 2006;26:6283-6298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 176] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 96. | Montagnani V, Stecca B. Role of Protein Kinases in Hedgehog Pathway Control and Implications for Cancer Therapy. Cancers (Basel). 2019;11:449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 97. | Pandolfi S, Stecca B. Cooperative integration between HEDGEHOG-GLI signalling and other oncogenic pathways: implications for cancer therapy. Expert Rev Mol Med. 2015;17:e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 98. | Gu D, Xie J. Non-Canonical Hh Signaling in Cancer-Current Understanding and Future Directions. Cancers (Basel). 2015;7:1684-1698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 99. | Riobo NA, Haines GM, Emerson CP Jr. Protein kinase C-delta and mitogen-activated protein/extracellular signal-regulated kinase-1 control GLI activation in hedgehog signaling. Cancer Res. 2006;66:839-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 100. | Cai Q, Li J, Gao T, Xie J, Evers BM. Protein kinase Cdelta negatively regulates hedgehog signaling by inhibition of Gli1 activity. J Biol Chem. 2009;284:2150-2158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 101. | Pietrobono S, Gagliardi S, Stecca B. Non-canonical Hedgehog Signaling Pathway in Cancer: Activation of GLI Transcription Factors Beyond Smoothened. Front Genet. 2019;10:556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 237] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 102. | Xu Q, Liu X, Zheng X, Yao Y, Wang M, Liu Q. The transcriptional activity of Gli1 is negatively regulated by AMPK through Hedgehog partial agonism in hepatocellular carcinoma. Int J Mol Med. 2014;34:733-741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 103. | Li YH, Luo J, Mosley YY, Hedrick VE, Paul LN, Chang J, Zhang G, Wang YK, Banko MR, Brunet A, Kuang S, Wu JL, Chang CJ, Scott MP, Yang JY. AMP-Activated Protein Kinase Directly Phosphorylates and Destabilizes Hedgehog Pathway Transcription Factor GLI1 in Medulloblastoma. Cell Rep. 2015;12:599-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 104. | Maloverjan A, Piirsoo M, Kasak L, Peil L, Østerlund T, Kogerman P. Dual function of UNC-51-like kinase 3 (Ulk3) in the Sonic hedgehog signaling pathway. J Biol Chem. 2010;285:30079-30090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 105. | Zhu J, Liu C, Liu F, Wang Y, Zhu M. Knockdown of PFTAIRE Protein Kinase 1 (PFTK1) Inhibits Proliferation, Invasion, and EMT in Colon Cancer Cells. Oncol Res. 2016;24:137-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 106. | Barakat B, Yu L, Lo C, Vu D, De Luca E, Cain JE, Martelotto LG, Dedhar S, Sadler AJ, Wang D, Watkins DN, Hannigan GE. Interaction of smoothened with integrin-linked kinase in primary cilia mediates Hedgehog signalling. EMBO Rep. 2013;14:837-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 107. | Tariki M, Wieczorek SA, Schneider P, Bänfer S, Veitinger S, Jacob R, Fendrich V, Lauth M. RIO kinase 3 acts as a SUFU-dependent positive regulator of Hedgehog signaling. Cell Signal. 2013;25:2668-2675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 108. | Wang Y, Li Y, Hu G, Huang X, Rao H, Xiong X, Luo Z, Lu Q, Luo S. Nek2A phosphorylates and stabilizes SuFu: A new strategy of Gli2/Hedgehog signaling regulatory mechanism. Cell Signal. 2016;28:1304-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 109. | Zhou F, Huang D, Li Y, Hu G, Rao H, Lu Q, Luo S, Wang Y. Nek2A/SuFu feedback loop regulates Gli-mediated Hedgehog signaling pathway. Int J Oncol. 2017;50:373-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 110. | Zhuang Z, Wang K, Cheng X, Qu X, Jiang B, Li Z, Luo J, Shao Z, Duan T. LKB1 inhibits breast cancer partially through repressing the Hedgehog signaling pathway. PLoS One. 2013;8:e67431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 111. | Zhang T, Xin G, Jia M, Zhuang T, Zhu S, Zhang B, Wang G, Jiang Q, Zhang C. The Plk1 kinase negatively regulates the Hedgehog signaling pathway by phosphorylating Gli1. J Cell Sci. 2019;132:jcs220384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 112. | Antonucci L, Di Magno L, D'Amico D, Manni S, Serrao SM, Di Pastena F, Bordone R, Yurtsever ZN, Caimano M, Petroni M, Giorgi A, Schininà ME, Yates Iii JR, Di Marcotullio L, De Smaele E, Checquolo S, Capalbo C, Agostinelli E, Maroder M, Coni S, Canettieri G. Mitogen-activated kinase kinase kinase 1 inhibits hedgehog signaling and medulloblastoma growth through GLI1 phosphorylation. Int J Oncol. 2019;54:505-514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 113. | Lu J, Liu L, Zheng M, Li X, Wu A, Wu Q, Liao C, Zou J, Song H. MEKK2 and MEKK3 suppress Hedgehog pathway-dependent medulloblastoma by inhibiting GLI1 function. Oncogene. 2018;37:3864-3878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 114. | An Y, Cai B, Chen J, Lv N, Yao J, Xue X, Tu M, Tang D, Wei J, Jiang K, Wu J, Li Q, Gao W, Miao Y. MAP3K10 promotes the proliferation and decreases the sensitivity of pancreatic cancer cells to gemcitabine by upregulating Gli-1 and Gli-2. Cancer Lett. 2013;329:228-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 115. | Yang J, Wang J, Liu Y, Zhang Y, Huang W, Zou Y, Qiu Y, Cai W, Gao J, Zhou H, Wu Y, Liu W, Ding Q, Zhang Y, Yin PH, Tan W. PGE2-JNK signaling axis non-canonically promotes Gli activation by protecting Gli2 from ubiquitin-proteasomal degradation. Cell Death Dis. 2021;12:707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 116. | Yang J, Wang J, Zhang Y, Huang W, Zhang S, Yin P, Tan W. c-Jun phosphorylated by JNK is required for protecting Gli2 from proteasomal-ubiquitin degradation by PGE2-JNK signaling axis. Biochim Biophys Acta Mol Cell Res. 2023;1870:119418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 117. | Hu A, Song BL. The interplay of Patched, Smoothened and cholesterol in Hedgehog signaling. Curr Opin Cell Biol. 2019;61:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 118. | Kowatsch C, Woolley RE, Kinnebrew M, Rohatgi R, Siebold C. Structures of vertebrate Patched and Smoothened reveal intimate links between cholesterol and Hedgehog signalling. Curr Opin Struct Biol. 2019;57:204-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 119. | Weiss LE, Milenkovic L, Yoon J, Stearns T, Moerner WE. Motional dynamics of single Patched1 molecules in cilia are controlled by Hedgehog and cholesterol. Proc Natl Acad Sci U S A. 2019;116:5550-5557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 120. | Luchetti G, Sircar R, Kong JH, Nachtergaele S, Sagner A, Byrne EF, Covey DF, Siebold C, Rohatgi R. Cholesterol activates the G-protein coupled receptor Smoothened to promote Hedgehog signaling. Elife. 2016;5:e20304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 195] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 121. | Radhakrishnan A, Rohatgi R, Siebold C. Cholesterol access in cellular membranes controls Hedgehog signaling. Nat Chem Biol. 2020;16:1303-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 122. | Raleigh DR, Sever N, Choksi PK, Sigg MA, Hines KM, Thompson BM, Elnatan D, Jaishankar P, Bisignano P, Garcia-Gonzalo FR, Krup AL, Eberl M, Byrne EFX, Siebold C, Wong SY, Renslo AR, Grabe M, McDonald JG, Xu L, Beachy PA, Reiter JF. Cilia-Associated Oxysterols Activate Smoothened. Mol Cell. 2018;72:316-327.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 123. | Myers BR, Sever N, Chong YC, Kim J, Belani JD, Rychnovsky S, Bazan JF, Beachy PA. Hedgehog pathway modulation by multiple lipid binding sites on the smoothened effector of signal response. Dev Cell. 2013;26:346-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 124. | Huang P, Nedelcu D, Watanabe M, Jao C, Kim Y, Liu J, Salic A. Cellular Cholesterol Directly Activates Smoothened in Hedgehog Signaling. Cell. 2016;166:1176-1187.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 294] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 125. | Hedger G, Koldsø H, Chavent M, Siebold C, Rohatgi R, Sansom MSP. Cholesterol Interaction Sites on the Transmembrane Domain of the Hedgehog Signal Transducer and Class F G Protein-Coupled Receptor Smoothened. Structure. 2019;27:549-559.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 126. | Nachtergaele S, Mydock LK, Krishnan K, Rammohan J, Schlesinger PH, Covey DF, Rohatgi R. Oxysterols are allosteric activators of the oncoprotein Smoothened. Nat Chem Biol. 2012;8:211-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 223] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 127. | Wu F, Zhang Y, Sun B, McMahon AP, Wang Y. Hedgehog Signaling: From Basic Biology to Cancer Therapy. Cell Chem Biol. 2017;24:252-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 247] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 128. | Ayob AZ, Ramasamy TS. Cancer stem cells as key drivers of tumour progression. J Biomed Sci. 2018;25:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 561] [Cited by in RCA: 649] [Article Influence: 81.1] [Reference Citation Analysis (0)] |
| 129. | Kumar V, Vashishta M, Kong L, Wu X, Lu JJ, Guha C, Dwarakanath BS. The Role of Notch, Hedgehog, and Wnt Signaling Pathways in the Resistance of Tumors to Anticancer Therapies. Front Cell Dev Biol. 2021;9:650772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 130. | Augustin MM, Ruzicka DR, Shukla AK, Augustin JM, Starks CM, O'Neil-Johnson M, McKain MR, Evans BS, Barrett MD, Smithson A, Wong GK, Deyholos MK, Edger PP, Pires JC, Leebens-Mack JH, Mann DA, Kutchan TM. Elucidating steroid alkaloid biosynthesis in Veratrum californicum: production of verazine in Sf9 cells. Plant J. 2015;82:991-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 131. | Ng JM, Curran T. The Hedgehog's tale: developing strategies for targeting cancer. Nat Rev Cancer. 2011;11:493-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 321] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 132. | Dockendorff C, Nagiec MM, Weïwer M, Buhrlage S, Ting A, Nag PP, Germain A, Kim HJ, Youngsaye W, Scherer C, Bennion M, Xue L, Stanton BZ, Lewis TA, Macpherson L, Palmer M, Foley MA, Perez JR, Schreiber SL. Macrocyclic Hedgehog Pathway Inhibitors: Optimization of Cellular Activity and Mode of Action Studies. ACS Med Chem Lett. 2012;3:808-813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 133. | Tremblay MR, Lescarbeau A, Grogan MJ, Tan E, Lin G, Austad BC, Yu LC, Behnke ML, Nair SJ, Hagel M, White K, Conley J, Manna JD, Alvarez-Diez TM, Hoyt J, Woodward CN, Sydor JR, Pink M, MacDougall J, Campbell MJ, Cushing J, Ferguson J, Curtis MS, McGovern K, Read MA, Palombella VJ, Adams J, Castro AC. Discovery of a potent and orally active hedgehog pathway antagonist (IPI-926). J Med Chem. 2009;52:4400-4418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 134. | Nitzki F, Becker M, Frommhold A, Schulz-Schaeffer W, Hahn H. Patched knockout mouse models of Basal cell carcinoma. J Skin Cancer. 2012;2012:907543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 135. | Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD, Solomon JA, Yoo S, Arron ST, Friedlander PA, Marmur E, Rudin CM, Chang AL, Low JA, Mackey HM, Yauch RL, Graham RA, Reddy JC, Hauschild A. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 947] [Cited by in RCA: 1074] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 136. | Schaefer GI, Perez JR, Duvall JR, Stanton BZ, Shamji AF, Schreiber SL. Discovery of small-molecule modulators of the Sonic Hedgehog pathway. J Am Chem Soc. 2013;135:9675-9680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 137. | Stanton BZ, Peng LF, Maloof N, Nakai K, Wang X, Duffner JL, Taveras KM, Hyman JM, Lee SW, Koehler AN, Chen JK, Fox JL, Mandinova A, Schreiber SL. A small molecule that binds Hedgehog and blocks its signaling in human cells. Nat Chem Biol. 2009;5:154-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 241] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 138. | Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16:2743-2748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1184] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 139. | Girardi D, Barrichello A, Fernandes G, Pereira A. Targeting the Hedgehog Pathway in Cancer: Current Evidence and Future Perspectives. Cells. 2019;8:153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 140. | Chen JK. I only have eye for ewe: the discovery of cyclopamine and development of Hedgehog pathway-targeting drugs. Nat Prod Rep. 2016;33:595-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 141. | Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci U S A. 2002;99:14071-14076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 815] [Cited by in RCA: 826] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 142. | Khanfar MA, El Sayed KA. The Veratrum alkaloids jervine, veratramine, and their analogues as prostate cancer migration and proliferation inhibitors: biological evaluation and pharmacophore modeling. Med Chem Res. 2013;22:4775-4786. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 143. | Williams JA, Guicherit OM, Zaharian BI, Xu Y, Chai L, Wichterle H, Kon C, Gatchalian C, Porter JA, Rubin LL, Wang FY. Identification of a small molecule inhibitor of the hedgehog signaling pathway: effects on basal cell carcinoma-like lesions. Proc Natl Acad Sci U S A. 2003;100:4616-4621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 235] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 144. | Wickström M, Dyberg C, Shimokawa T, Milosevic J, Baryawno N, Fuskevåg OM, Larsson R, Kogner P, Zaphiropoulos PG, Johnsen JI. Targeting the hedgehog signal transduction pathway at the level of GLI inhibits neuroblastoma cell growth in vitro and in vivo. Int J Cancer. 2013;132:1516-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 145. | Büttner A, Seifert K, Cottin T, Sarli V, Tzagkaroulaki L, Scholz S, Giannis A. Synthesis and biological evaluation of SANT-2 and analogues as inhibitors of the hedgehog signaling pathway. Bioorg Med Chem. 2009;17:4943-4954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 146. | Dijkgraaf GJ, Alicke B, Weinmann L, Januario T, West K, Modrusan Z, Burdick D, Goldsmith R, Robarge K, Sutherlin D, Scales SJ, Gould SE, Yauch RL, de Sauvage FJ. Small molecule inhibition of GDC-0449 refractory smoothened mutants and downstream mechanisms of drug resistance. Cancer Res. 2011;71:435-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 304] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 147. | Nguyen NM, Cho J. Hedgehog Pathway Inhibitors as Targeted Cancer Therapy and Strategies to Overcome Drug Resistance. Int J Mol Sci. 2022;23:1733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 148. | Rimkus TK, Carpenter RL, Qasem S, Chan M, Lo HW. Targeting the Sonic Hedgehog Signaling Pathway: Review of Smoothened and GLI Inhibitors. Cancers (Basel). 2016;8:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 376] [Cited by in RCA: 454] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 149. | Ibuki N, Ghaffari M, Pandey M, Iu I, Fazli L, Kashiwagi M, Tojo H, Nakanishi O, Gleave ME, Cox ME. TAK-441, a novel investigational smoothened antagonist, delays castration-resistant progression in prostate cancer by disrupting paracrine hedgehog signaling. Int J Cancer. 2013;133:1955-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 150. | Mahindroo N, Punchihewa C, Fujii N. Hedgehog-Gli signaling pathway inhibitors as anticancer agents. J Med Chem. 2009;52:3829-3845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 151. | Richards DA, Stephenson J, Wolpin BM, Becerra C, Hamm JT, Messersmith WA, Devens S, Cushing J, Schmalbach T, Fuchs CS. A phase Ib trial of IPI-926, a hedgehog pathway inhibitor, plus gemcitabine in patients with metastatic pancreatic cancer. J Clin Oncol. 2012;30:213-213. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 152. | Pietrobono S, Stecca B. Targeting the Oncoprotein Smoothened by Small Molecules: Focus on Novel Acylguanidine Derivatives as Potent Smoothened Inhibitors. Cells. 2018;7:272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 153. | Zhu M, Wang H, Wang C, Fang Y, Zhu T, Zhao W, Dong X, Zhang X. L-4, a Well-Tolerated and Orally Active Inhibitor of Hedgehog Pathway, Exhibited Potent Anti-tumor Effects Against Medulloblastoma in vitro and in vivo. Front Pharmacol. 2019;10:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 154. | Hitzenberger M, Schuster D, Hofer TS. The Binding Mode of the Sonic Hedgehog Inhibitor Robotnikinin, a Combined Docking and QM/MM MD Study. Front Chem. 2017;5:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 155. | Tang JY, Xiao TZ, Oda Y, Chang KS, Shpall E, Wu A, So PL, Hebert J, Bikle D, Epstein EH Jr. Vitamin D3 inhibits hedgehog signaling and proliferation in murine Basal cell carcinomas. Cancer Prev Res (Phila). 2011;4:744-751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 156. | Lanyon-Hogg T, Masumoto N, Bodakh G, Konitsiotis AD, Thinon E, Rodgers UR, Owens RJ, Magee AI, Tate EW. Synthesis and characterisation of 5-acyl-6,7-dihydrothieno[3,2-c]pyridine inhibitors of Hedgehog acyltransferase. Data Brief. 2016;7:257-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 157. | Rodgers UR, Lanyon-Hogg T, Masumoto N, Ritzefeld M, Burke R, Blagg J, Magee AI, Tate EW. Characterization of Hedgehog Acyltransferase Inhibitors Identifies a Small Molecule Probe for Hedgehog Signaling by Cancer Cells. ACS Chem Biol. 2016;11:3256-3262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 158. | Wu VM, Chen SC, Arkin MR, Reiter JF. Small molecule inhibitors of Smoothened ciliary localization and ciliogenesis. Proc Natl Acad Sci U S A. 2012;109:13644-13649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 159. | Peukert S, He F, Dai M, Zhang R, Sun Y, Miller-Moslin K, McEwan M, Lagu B, Wang K, Yusuff N, Bourret A, Ramamurthy A, Maniara W, Amaral A, Vattay A, Wang A, Guo R, Yuan J, Green J, Williams J, Buonamici S, Kelleher JF 3rd, Dorsch M. Discovery of NVP-LEQ506, a second-generation inhibitor of smoothened. ChemMedChem. 2013;8:1261-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 160. | Munchhof MJ, Li Q, Shavnya A, Borzillo GV, Boyden TL, Jones CS, LaGreca SD, Martinez-Alsina L, Patel N, Pelletier K, Reiter LA, Robbins MD, Tkalcevic GT. Discovery of PF-04449913, a Potent and Orally Bioavailable Inhibitor of Smoothened. ACS Med Chem Lett. 2012;3:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 161. | Bendell J, Andre V, Ho A, Kudchadkar R, Migden M, Infante J, Tiu RV, Pitou C, Tucker T, Brail L, Von Hoff D. Phase I Study of LY2940680, a Smo Antagonist, in Patients with Advanced Cancer Including Treatment-Naïve and Previously Treated Basal Cell Carcinoma. Clin Cancer Res. 2018;24:2082-2091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 162. | Ferruzzi P, Mennillo F, De Rosa A, Giordano C, Rossi M, Benedetti G, Magrini R, Pericot Mohr Gl, Miragliotta V, Magnoni L, Mori E, Thomas R, Tunici P, Bakker A. In vitro and in vivo characterization of a novel Hedgehog signaling antagonist in human glioblastoma cell lines. Int J Cancer. 2012;131:E33-E44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 163. | Roudaut H, Traiffort E, Gorojankina T, Vincent L, Faure H, Schoenfelder A, Mann A, Manetti F, Solinas A, Taddei M, Ruat M. Identification and mechanism of action of the acylguanidine MRT-83, a novel potent Smoothened antagonist. Mol Pharmacol. 2011;79:453-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 164. | Hosoya T, Arai MA, Koyano T, Kowithayakorn T, Ishibashi M. Naturally occurring small-molecule inhibitors of hedgehog/GLI-mediated transcription. Chembiochem. 2008;9:1082-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 165. | Hyman JM, Firestone AJ, Heine VM, Zhao Y, Ocasio CA, Han K, Sun M, Rack PG, Sinha S, Wu JJ, Solow-Cordero DE, Jiang J, Rowitch DH, Chen JK. Small-molecule inhibitors reveal multiple strategies for Hedgehog pathway blockade. Proc Natl Acad Sci U S A. 2009;106:14132-14137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 253] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 166. | Beauchamp EM, Ringer L, Bulut G, Sajwan KP, Hall MD, Lee YC, Peaceman D, Ozdemirli M, Rodriguez O, Macdonald TJ, Albanese C, Toretsky JA, Uren A. Arsenic trioxide inhibits human cancer cell growth and tumor development in mice by blocking Hedgehog/GLI pathway. J Clin Invest. 2011;121:148-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 285] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 167. | Infante P, Mori M, Alfonsi R, Ghirga F, Aiello F, Toscano S, Ingallina C, Siler M, Cucchi D, Po A, Miele E, D'Amico D, Canettieri G, De Smaele E, Ferretti E, Screpanti I, Uccello Barretta G, Botta M, Botta B, Gulino A, Di Marcotullio L. Gli1/DNA interaction is a druggable target for Hedgehog-dependent tumors. EMBO J. 2015;34:200-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 168. | Kremer L, Schultz-Fademrecht C, Baumann M, Habenberger P, Choidas A, Klebl B, Kordes S, Schöler HR, Sterneckert J, Ziegler S, Schneider G, Waldmann H. Discovery of a Novel Inhibitor of the Hedgehog Signaling Pathway through Cell-based Compound Discovery and Target Prediction. Angew Chem Int Ed Engl. 2017;56:13021-13025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 169. | Atwood SX, Chang AL, Oro AE. Hedgehog pathway inhibition and the race against tumor evolution. J Cell Biol. 2012;199:193-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 170. | Piteša N, Kurtović M, Bartoniček N, Gkotsi DS, Čonkaš J, Petrić T, Musani V, Ozretić P, Riobo-Del Galdo NA, Sabol M. Signaling Switching from Hedgehog-GLI to MAPK Signaling Potentially Serves as a Compensatory Mechanism in Melanoma Cell Lines Resistant to GANT-61. Biomedicines. 2023;11:1353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 171. | Corrales E, Levit-Zerdoun E, Metzger P, Mertes R, Lehmann A, Münch J, Lemke S, Kowar S, Boerries M. PI3K/AKT signaling allows for MAPK/ERK pathway independency mediating dedifferentiation-driven treatment resistance in melanoma. Cell Commun Signal. 2022;20:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 172. | Jamieson C, Martinelli G, Papayannidis C, Cortes JE. Hedgehog Pathway Inhibitors: A New Therapeutic Class for the Treatment of Acute Myeloid Leukemia. Blood Cancer Discov. 2020;1:134-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 173. | Lei ZN, Tian Q, Teng QX, Wurpel JND, Zeng L, Pan Y, Chen ZS. Understanding and targeting resistance mechanisms in cancer. MedComm (2020). 2023;4:e265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 172] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 174. | Nguyen TT, Duong VA, Maeng HJ. Pharmaceutical Formulations with P-Glycoprotein Inhibitory Effect as Promising Approaches for Enhancing Oral Drug Absorption and Bioavailability. Pharmaceutics. 2021;13:1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 175. | Lee MJ, Hatton BA, Villavicencio EH, Khanna PC, Friedman SD, Ditzler S, Pullar B, Robison K, White KF, Tunkey C, LeBlanc M, Randolph-Habecker J, Knoblaugh SE, Hansen S, Richards A, Wainwright BJ, McGovern K, Olson JM. Hedgehog pathway inhibitor saridegib (IPI-926) increases lifespan in a mouse medulloblastoma model. Proc Natl Acad Sci U S A. 2012;109:7859-7864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 176. | Peacock CD, Wang Q, Gesell GS, Corcoran-Schwartz IM, Jones E, Kim J, Devereux WL, Rhodes JT, Huff CA, Beachy PA, Watkins DN, Matsui W. Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc Natl Acad Sci U S A. 2007;104:4048-4053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 380] [Cited by in RCA: 360] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 177. | Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D, Nannini-Pepe M, Kotkow K, Marsters JC, Rubin LL, de Sauvage FJ. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 748] [Cited by in RCA: 802] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 178. | Kumari A, Ermilov AN, Allen BL, Bradley RM, Dlugosz AA, Mistretta CM. Hedgehog pathway blockade with the cancer drug LDE225 disrupts taste organs and taste sensation. J Neurophysiol. 2015;113:1034-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 179. | Kumari A, Ermilov AN, Grachtchouk M, Dlugosz AA, Allen BL, Bradley RM, Mistretta CM. Recovery of taste organs and sensory function after severe loss from Hedgehog/Smoothened inhibition with cancer drug sonidegib. Proc Natl Acad Sci U S A. 2017;114:E10369-E10378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 180. | Al Hmada Y, Brodell RT, Kharouf N, Flanagan TW, Alamodi AA, Hassan SY, Shalaby H, Hassan SL, Haikel Y, Megahed M, Santourlidis S, Hassan M. Mechanisms of Melanoma Progression and Treatment Resistance: Role of Cancer Stem-like Cells. Cancers (Basel). 2024;16:470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 181. | Patel M, Eckburg A, Gantiwala S, Hart Z, Dein J, Lam K, Puri N. Resistance to Molecularly Targeted Therapies in Melanoma. Cancers (Basel). 2021;13:1115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 182. | Knight A, Karapetyan L, Kirkwood JM. Immunotherapy in Melanoma: Recent Advances and Future Directions. Cancers (Basel). 2023;15:1106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 167] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 183. | Fruntealată RF, Marius M, Boboc IKS, Mitran SI, Ciurea ME, Stoica GA. Mechanisms of Altered Immune Response in Skin Melanoma. Curr Health Sci J. 2023;49:297-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 184. | Giammona A, Crivaro E, Stecca B. Emerging Roles of Hedgehog Signaling in Cancer Immunity. Int J Mol Sci. 2023;24:1321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 185. | Huang AC, Zappasodi R. A decade of checkpoint blockade immunotherapy in melanoma: understanding the molecular basis for immune sensitivity and resistance. Nat Immunol. 2022;23:660-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 396] [Article Influence: 99.0] [Reference Citation Analysis (0)] |
| 186. | Wagstaff W, Mwamba RN, Grullon K, Armstrong M, Zhao P, Hendren-Santiago B, Qin KH, Li AJ, Hu DA, Youssef A, Reid RR, Luu HH, Shen L, He TC, Haydon RC. Melanoma: Molecular genetics, metastasis, targeted therapies, immunotherapies, and therapeutic resistance. Genes Dis. 2022;9:1608-1623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 187. | Namikawa K, Yamazaki N. Targeted Therapy and Immunotherapy for Melanoma in Japan. Curr Treat Options Oncol. 2019;20:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (2)] |
| 188. | Sigafoos AN, Paradise BD, Fernandez-Zapico ME. Hedgehog/GLI Signaling Pathway: Transduction, Regulation, and Implications for Disease. Cancers (Basel). 2021;13:3410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 189. | Faião-Flores F, Alves-Fernandes DK, Pennacchi PC, Sandri S, Vicente AL, Scapulatempo-Neto C, Vazquez VL, Reis RM, Chauhan J, Goding CR, Smalley KS, Maria-Engler SS. Targeting the hedgehog transcription factors GLI1 and GLI2 restores sensitivity to vemurafenib-resistant human melanoma cells. Oncogene. 2017;36:1849-1861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 190. | Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A, Rosenthal A, Epstein EH Jr, de Sauvage FJ. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1005] [Cited by in RCA: 997] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 191. | Wang J, Cui B, Li X, Zhao X, Huang T, Ding X. The emerging roles of Hedgehog signaling in tumor immune microenvironment. Front Oncol. 2023;13:1171418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 192. | Stecca B, Mas C, Clement V, Zbinden M, Correa R, Piguet V, Beermann F, Ruiz i Altaba A. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc Natl Acad Sci U S A. 2007;104:5895-5900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 429] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 193. | Horák P, Kreisingerová K, Réda J, Ondrušová L, Balko J, Vachtenheim J Jr, Žáková P, Vachtenheim J. The Hedgehog/GLI signaling pathway activates transcription of Slug (Snail2) in melanoma cells. Oncol Rep. 2023;49:75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 194. | Jia Y, Wang Y, Xie J. The Hedgehog pathway: role in cell differentiation, polarity and proliferation. Arch Toxicol. 2015;89:179-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 195. | Armas-López L, Zúñiga J, Arrieta O, Ávila-Moreno F. The Hedgehog-GLI pathway in embryonic development and cancer: implications for pulmonary oncology therapy. Oncotarget. 2017;8:60684-60703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 196. | Gola M, Czajkowski R, Bajek A, Dura A, Drewa T. Melanocyte stem cells: biology and current aspects. Med Sci Monit. 2012;18:RA155-RA159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 197. | Sithanandam G, Kolch W, Duh FM, Rapp UR. Complete coding sequence of a human B-raf cDNA and detection of B-raf protein kinase with isozyme specific antibodies. Oncogene. 1990;5:1775-1780. [PubMed] |
| 198. | Dankner M, Rose AAN, Rajkumar S, Siegel PM, Watson IR. Classifying BRAF alterations in cancer: new rational therapeutic strategies for actionable mutations. Oncogene. 2018;37:3183-3199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 376] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 199. | Xu X, Lu Y, Li Y, Prinz RA. Sonic Hedgehog Signaling in Thyroid Cancer. Front Endocrinol (Lausanne). 2017;8:284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 200. | Scheidt T, Alka O, Gonczarowska-Jorge H, Gruber W, Rathje F, Dell'Aica M, Rurik M, Kohlbacher O, Zahedi RP, Aberger F, Huber CG. Phosphoproteomics of short-term hedgehog signaling in human medulloblastoma cells. Cell Commun Signal. 2020;18:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 201. | Proietti I, Skroza N, Michelini S, Mambrin A, Balduzzi V, Bernardini N, Marchesiello A, Tolino E, Volpe S, Maddalena P, Di Fraia M, Mangino G, Romeo G, Potenza C. BRAF Inhibitors: Molecular Targeting and Immunomodulatory Actions. Cancers (Basel). 2020;12:1823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 202. | Proietti I, Skroza N, Bernardini N, Tolino E, Balduzzi V, Marchesiello A, Michelini S, Volpe S, Mambrin A, Mangino G, Romeo G, Maddalena P, Rees C, Potenza C. Mechanisms of Acquired BRAF Inhibitor Resistance in Melanoma: A Systematic Review. Cancers (Basel). 2020;12:2801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 203. | Zhong J, Yan W, Wang C, Liu W, Lin X, Zou Z, Sun W, Chen Y. BRAF Inhibitor Resistance in Melanoma: Mechanisms and Alternative Therapeutic Strategies. Curr Treat Options Oncol. 2022;23:1503-1521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 204. | Luebker SA, Koepsell SA. Diverse Mechanisms of BRAF Inhibitor Resistance in Melanoma Identified in Clinical and Preclinical Studies. Front Oncol. 2019;9:268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 156] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 205. | Briscoe J, Thérond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1167] [Cited by in RCA: 1409] [Article Influence: 108.4] [Reference Citation Analysis (0)] |
| 206. | Villavicencio EH, Walterhouse DO, Iannaccone PM. The sonic hedgehog-patched-gli pathway in human development and disease. Am J Hum Genet. 2000;67:1047-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 290] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 207. | Sari IN, Phi LTH, Jun N, Wijaya YT, Lee S, Kwon HY. Hedgehog Signaling in Cancer: A Prospective Therapeutic Target for Eradicating Cancer Stem Cells. Cells. 2018;7:208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 157] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 208. | Girouard SD, Murphy GF. Melanoma stem cells: not rare, but well done. Lab Invest. 2011;91:647-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 209. | Santini R, Vinci MC, Pandolfi S, Penachioni JY, Montagnani V, Olivito B, Gattai R, Pimpinelli N, Gerlini G, Borgognoni L, Stecca B. Hedgehog-GLI signaling drives self-renewal and tumorigenicity of human melanoma-initiating cells. Stem Cells. 2012;30:1808-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 210. | Kozovska Z, Gabrisova V, Kucerova L. Malignant melanoma: diagnosis, treatment and cancer stem cells. Neoplasma. 2016;63:510-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 211. | Marzagalli M, Raimondi M, Fontana F, Montagnani Marelli M, Moretti RM, Limonta P. Cellular and molecular biology of cancer stem cells in melanoma: Possible therapeutic implications. Semin Cancer Biol. 2019;59:221-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 212. | Coni S, Mancuso AB, Di Magno L, Sdruscia G, Manni S, Serrao SM, Rotili D, Spiombi E, Bufalieri F, Petroni M, Kusio-Kobialka M, De Smaele E, Ferretti E, Capalbo C, Mai A, Niewiadomski P, Screpanti I, Di Marcotullio L, Canettieri G. Selective targeting of HDAC1/2 elicits anticancer effects through Gli1 acetylation in preclinical models of SHH Medulloblastoma. Sci Rep. 2017;7:44079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 213. | Coni S, Di Magno L, Canettieri G. Determination of Acetylation of the Gli Transcription Factors. Methods Mol Biol. 2015;1322:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 214. | Canettieri G, Di Marcotullio L, Greco A, Coni S, Antonucci L, Infante P, Pietrosanti L, De Smaele E, Ferretti E, Miele E, Pelloni M, De Simone G, Pedone EM, Gallinari P, Giorgi A, Steinkühler C, Vitagliano L, Pedone C, Schinin ME, Screpanti I, Gulino A. Histone deacetylase and Cullin3-REN(KCTD11) ubiquitin ligase interplay regulates Hedgehog signalling through Gli acetylation. Nat Cell Biol. 2010;12:132-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 279] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 215. | Seto E, Yoshida M. Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb Perspect Biol. 2014;6:a018713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 916] [Cited by in RCA: 1529] [Article Influence: 127.4] [Reference Citation Analysis (0)] |
| 216. | Lai F, Guo ST, Jin L, Jiang CC, Wang CY, Croft A, Chi MN, Tseng HY, Farrelly M, Atmadibrata B, Norman J, Liu T, Hersey P, Zhang XD. Cotargeting histone deacetylases and oncogenic BRAF synergistically kills human melanoma cells by necrosis independently of RIPK1 and RIPK3. Cell Death Dis. 2013;4:e655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 217. | Cochrane CR, Szczepny A, Watkins DN, Cain JE. Hedgehog Signaling in the Maintenance of Cancer Stem Cells. Cancers (Basel). 2015;7:1554-1585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 200] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 218. | Shackleton M. Normal stem cells and cancer stem cells: similar and different. Semin Cancer Biol. 2010;20:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 219. | Murphy GF, Wilson BJ, Girouard SD, Frank NY, Frank MH. Stem cells and targeted approaches to melanoma cure. Mol Aspects Med. 2014;39:33-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 220. | Parmiani G. Melanoma Cancer Stem Cells: Markers and Functions. Cancers (Basel). 2016;8:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 221. | van Schaijik B, Davis PF, Wickremesekera AC, Tan ST, Itinteang T. Subcellular localisation of the stem cell markers OCT4, SOX2, NANOG, KLF4 and c-MYC in cancer: a review. J Clin Pathol. 2018;71:88-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 148] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 222. | Santini R, Pandolfi S, Montagnani V, Pietrobono S, Pimpinelli N, Borgognoni L, Stecca B. Regulation of melanoma initiating cells by Hedgehog signaling and SOX2. J Transl Med. 2014;12:O4. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 223. | Zabierowski SE, Herlyn M. Melanoma stem cells: the dark seed of melanoma. J Clin Oncol. 2008;26:2890-2894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 224. | Yin Q, Shi X, Lan S, Jin H, Wu D. Effect of melanoma stem cells on melanoma metastasis. Oncol Lett. 2021;22:566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 225. | Civenni G, Walter A, Kobert N, Mihic-Probst D, Zipser M, Belloni B, Seifert B, Moch H, Dummer R, van den Broek M, Sommer L. Human CD271-positive melanoma stem cells associated with metastasis establish tumor heterogeneity and long-term growth. Cancer Res. 2011;71:3098-3109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 251] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 226. | Luo Y, Dallaglio K, Chen Y, Robinson WA, Robinson SE, McCarter MD, Wang J, Gonzalez R, Thompson DC, Norris DA, Roop DR, Vasiliou V, Fujita M. ALDH1A isozymes are markers of human melanoma stem cells and potential therapeutic targets. Stem Cells. 2012;30:2100-2113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 233] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 227. | Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP, Butler PD, Yang GP, Joshua B, Kaplan MJ, Longaker MT, Weissman IL. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466:133-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 601] [Cited by in RCA: 566] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 228. | Yue L, Huang ZM, Fong S, Leong S, Jakowatz JG, Charruyer-Reinwald A, Wei M, Ghadially R. Targeting ALDH1 to decrease tumorigenicity, growth and metastasis of human melanoma. Melanoma Res. 2015;25:138-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 229. | Ciccone V, Simonis V, Del Gaudio C, Cucini C, Ziche M, Morbidelli L, Donnini S. ALDH1A1 confers resistance to RAF/MEK inhibitors in melanoma cells by maintaining stemness phenotype and activating PI3K/AKT signaling. Biochem Pharmacol. 2024;224:116252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 230. | Sun Q, Lee W, Mohri Y, Takeo M, Lim CH, Xu X, Myung P, Atit RP, Taketo MM, Moubarak RS, Schober M, Osman I, Gay DL, Saur D, Nishimura EK, Ito M. A novel mouse model demonstrates that oncogenic melanocyte stem cells engender melanoma resembling human disease. Nat Commun. 2019;10:5023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 231. | Moon H, Donahue LR, Choi E, Scumpia PO, Lowry WE, Grenier JK, Zhu J, White AC. Melanocyte Stem Cell Activation and Translocation Initiate Cutaneous Melanoma in Response to UV Exposure. Cell Stem Cell. 2017;21:665-678.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 232. | Alamodi AA, Eshaq AM, Hassan SY, Al Hmada Y, El Jamal SM, Fothan AM, Arain OM, Hassan SL, Haikel Y, Megahed M, Hassan M. Cancer stem cell as therapeutic target for melanoma treatment. Histol Histopathol. 2016;31:1291-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 233. | Marzagalli M, Moretti RM, Messi E, Marelli MM, Fontana F, Anastasia A, Bani MR, Beretta G, Limonta P. Targeting melanoma stem cells with the Vitamin E derivative δ-tocotrienol. Sci Rep. 2018;8:587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 234. | Wang ML, Chiou SH, Wu CW. Targeting cancer stem cells: emerging role of Nanog transcription factor. Onco Targets Ther. 2013;6:1207-1220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 235. | Pietrobono S, Santini R, Gagliardi S, Dapporto F, Colecchia D, Chiariello M, Leone C, Valoti M, Manetti F, Petricci E, Taddei M, Stecca B. Targeted inhibition of Hedgehog-GLI signaling by novel acylguanidine derivatives inhibits melanoma cell growth by inducing replication stress and mitotic catastrophe. Cell Death Dis. 2018;9:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 236. | Santini R, Pietrobono S, Pandolfi S, Montagnani V, D'Amico M, Penachioni JY, Vinci MC, Borgognoni L, Stecca B. SOX2 regulates self-renewal and tumorigenicity of human melanoma-initiating cells. Oncogene. 2014;33:4697-4708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 175] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 237. | Pandolfi S, Montagnani V, Penachioni JY, Vinci MC, Olivito B, Borgognoni L, Stecca B. WIP1 phosphatase modulates the Hedgehog signaling by enhancing GLI1 function. Oncogene. 2013;32:4737-4747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 238. | Yeon M, Kim Y, Jung HS, Jeoung D. Histone Deacetylase Inhibitors to Overcome Resistance to Targeted and Immuno Therapy in Metastatic Melanoma. Front Cell Dev Biol. 2020;8:486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 239. | Palamaris K, Moutafi M, Gakiopoulou H, Theocharis S. Histone Deacetylase (HDAC) Inhibitors: A Promising Weapon to Tackle Therapy Resistance in Melanoma. Int J Mol Sci. 2022;23:3660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 240. | Wilmott JS, Colebatch AJ, Kakavand H, Shang P, Carlino MS, Thompson JF, Long GV, Scolyer RA, Hersey P. Expression of the class 1 histone deacetylases HDAC8 and 3 are associated with improved survival of patients with metastatic melanoma. Mod Pathol. 2015;28:884-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 241. | Emmons MF, Faião-Flores F, Sharma R, Thapa R, Messina JL, Becker JC, Schadendorf D, Seto E, Sondak VK, Koomen JM, Chen YA, Lau EK, Wan L, Licht JD, Smalley KSM. HDAC8 Regulates a Stress Response Pathway in Melanoma to Mediate Escape from BRAF Inhibitor Therapy. Cancer Res. 2019;79:2947-2961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 242. | Gallagher SJ, Gunatilake D, Beaumont KA, Sharp DM, Tiffen JC, Heinemann A, Weninger W, Haass NK, Wilmott JS, Madore J, Ferguson PM, Rizos H, Hersey P. HDAC inhibitors restore BRAF-inhibitor sensitivity by altering PI3K and survival signalling in a subset of melanoma. Int J Cancer. 2018;142:1926-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 243. | Carson R, Celtikci B, Fenning C, Javadi A, Crawford N, Carbonell LP, Lawler M, Longley DB, Johnston PG, Van Schaeybroeck S. HDAC Inhibition Overcomes Acute Resistance to MEK Inhibition in BRAF-Mutant Colorectal Cancer by Downregulation of c-FLIPL. Clin Cancer Res. 2015;21:3230-3240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/