Published online Sep 24, 2024. doi: 10.5306/wjco.v15.i9.1207
Revised: February 7, 2024
Accepted: June 3, 2024
Published online: September 24, 2024
Processing time: 309 Days and 20.1 Hours
Blastic plasmacytoid dendritic cell tumor (BPDCN) is a rare and highly invasive lymphohematopoietic tumor that originates from plasmacytoid dendritic cells. BPDCN has an extremely poor prognosis. Skin lesions are usually the first ma
In the present paper, the cases of 2 patients diagnosed with BPDCN are discussed. The immunohistochemistry analysis of these 2 patients revealed positivity for CD4, CD56, and CD123. Currently, no standard chemotherapy regimen is available for BPDCN. Therefore, intensive therapy for acute lymphoblastic leukemia was applied as the treatment method for these 2 cases.
Although allogeneic bone marrow transplantation could be further effective in prolonging the median survival the ultimate prognosis was unfavorable. Future treatment modalities tailored for elderly patients will help prolong survival.
Core Tip: This article reported on 2 cases of blastic plasmacytoid dendritic cell tumor (BPDCN) patients diagnosed and treated in our hospital, describing clinical manifestations, laboratory examination, and treatment process. A summarization of the current literature, combined with the experience of these 2 patients, allowed for our reasoned overall conclusions. Based on the 2-patient experience, BPDCN as a hematologic malignancy has a very poor prognosis. Treatment with acute lymphoblastic leukemia chemotherapy is effective in some way. Venetoclax, a BCL-2 inhibitor, can partially subside skin lesions. Unless stem cell transplantation is used, there is no significant improvement in overall survival time.
- Citation: Ma YQ, Sun Z, Li YM, Xu H. Blastic plasmacytoid dendritic cell neoplasm: Two case reports. World J Clin Oncol 2024; 15(9): 1207-1214
- URL: https://www.wjgnet.com/2218-4333/full/v15/i9/1207.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i9.1207
This article presents 2 cases of blastic plasmacytoid dendritic cell tumor (BPDCN) patients. Upon admission, the first patient exhibited widespread tumor cell dissemination throughout his body, with infiltrative plaques and bruise-like patches on the chest and back. The second patient initially presented with a limited plaque lesion on her upper arm without metastasis to other organs. Despite both patients receiving positive combined chemotherapy, and the first patient achieved temporary remission with the addition of venetoclax, while it did not alter the poor prognosis for either patient. Given that BPDCN is a highly malignant tumor and typical skin lesions may not be present, hematologists should remain vigilant for its possibility in order to facilitate early diagnosis and treatment; hematopoietic stem cell transplantation may offer a more effective means to prolong survival.
Case 1: The first patient was a 73-year-old man who had developed a dark red plucky mass on the lower back 6 months ago. The rash gradually increased in size, and 2 months ago, the patient had developed red or purplish papules on both chest and back. In addition, the patient experienced fatigue, night sweats, and weight loss of nearly 5 kg throughout this period. A family history of a similar disease or condition was denied.
Case 2: The second patient was a 57-year-old female who had developed a dark red cystic plaque on the right upper arm 10 months ago.
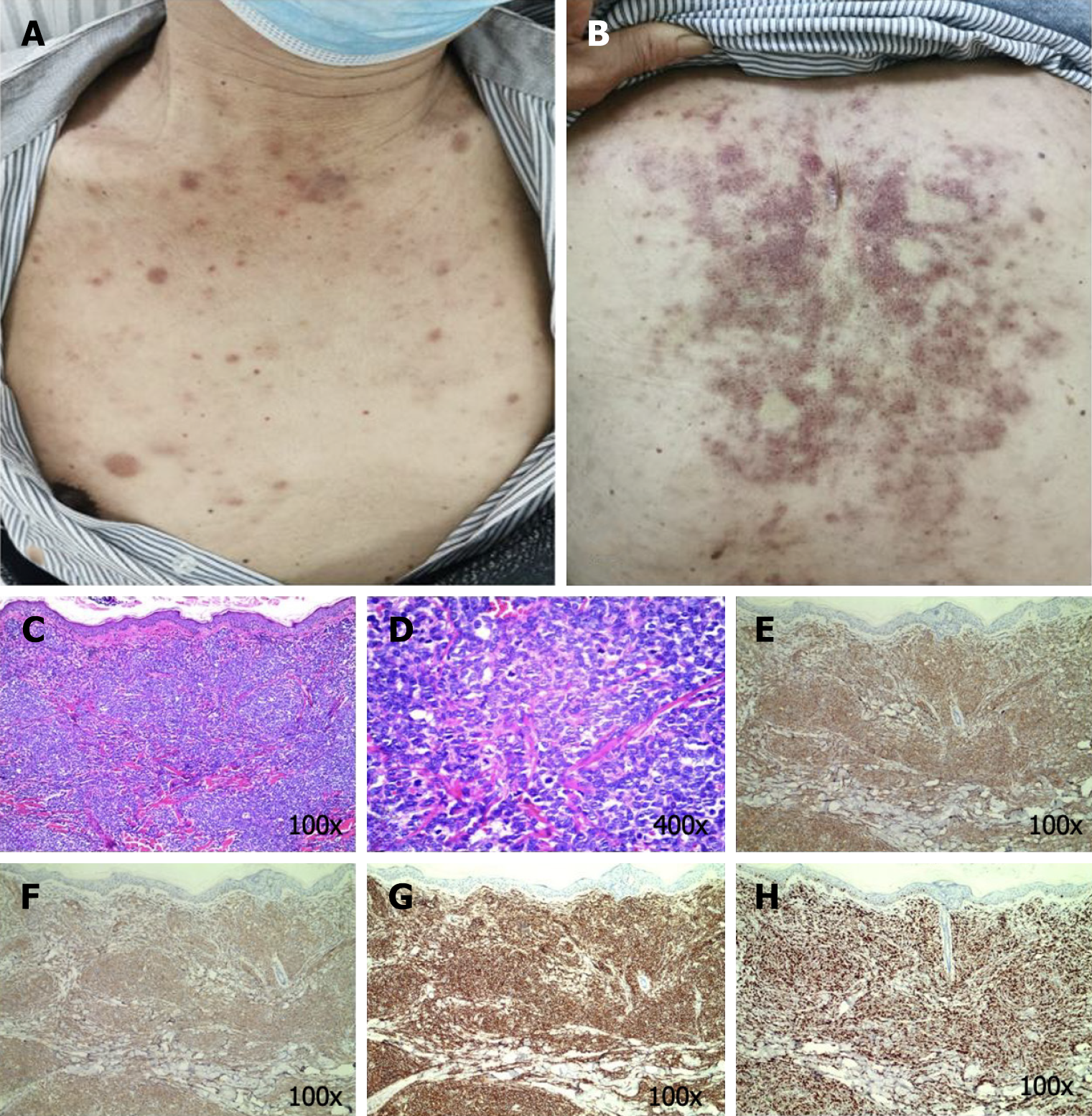
Case 1: The general condition of the patient could be described as follows: Fair and multiple bilateral cervical, axillary, and inguinal lymph nodes that were enlarged and partially fused. The skin examination revealed dark red or purplish red infiltrative papules, nodules, and plaques distributed widely on the chest and back. A few of these papules were fused into pieces. The nodules varied in size, with a maximum diameter of approximately 2 cm. In addition, the nodules were slightly elevated, leathery, and well-defined (Figure 1).
Case 2: Ten months prior, she presented with a dark red cystic plaque on her right arm, which was asymptomatic.
There was no relevant information for either patient, as determined by history taking.
A family history of a similar disease or condition was denied by both patients.
Case 1: The general condition of the patient could be described as follows: Fair and multiple bilateral cervical, axillary, and inguinal lymph nodes that were enlarged and partially fused. The skin examination revealed dark red or purplish red infiltrative papules, nodules, and plaques distributed widely on the chest and back. A few of these papules were fused into pieces. The nodules varied in size, with a maximum diameter of approximately 2 cm. In addition, the nodules were slightly elevated, leathery, and well-defined (Figure 1A and B).
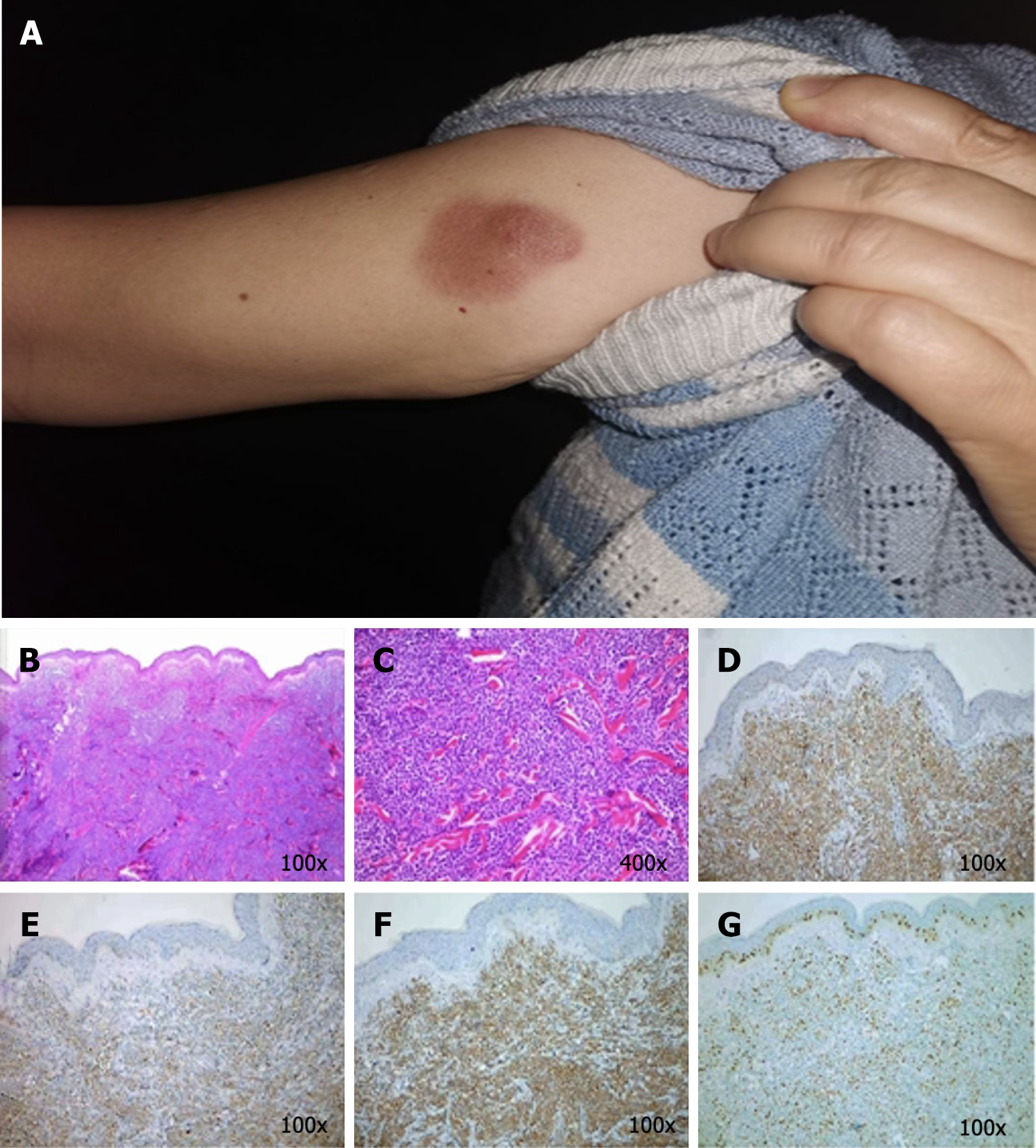
Case 2: The general condition of this patient could be described as follows: Fair and lymph nodes that were not palpable. The skin examination revealed an irregular dark red patch near the shoulder of her right upper arm, which was approximately 3 cm × 2.5 cm in size, with a distinct boundary. In the center, a cystic patch, the size of a broad bean, could be noted, and the patch was raised on the skin and was firm without ulceration (Figure 2A).
Case 1: The white blood cell count was increased significantly, while the platelet count was decreased. The bone marrow analysis revealed lymphocytic leukemia. The immunophenotype was consistent with that of BPDCN. The molecular genetics analysis was negative. The hematoxylin-eosin (HE) staining-based histopathological examination of the skin lesions revealed a marked zone of cell-free infiltration (Grenz zone) between the tumor cells and the epidermis, in addition to diffuse lymphocytic infiltration with atypia in the entire dermis, periadnices, and subcutaneous fat lobules (Figure 1C and 1D). Immunohistochemistry (IHC) revealed CD3 partial (++), CD4 (+++), CD8 focal (+), CD20 (–), CD30 (–), CD56 (+++), MPO (–), TdT (++), CD123 (+++), CD99(+), and Ki67 (75%+) (Figure 1E-H).
Case 2: The blood tests revealed no abnormalities. The bone marrow examination, and immunophenotyping were negative. The HE staining of the skin lesions revealed diffuse proliferation and infiltration of medium-sized lymphocytes in the entire dermis, with oval or irregular nuclei, fine chromatin, and frequent mitotic figures. The epidermis was not involved. (Figure 2B and C). The IHC analysis revealed the following: CD123 (+), CD4 (+), CD56 (+), CD79a (+), CD7 (+), CD99 weak (+), TdT (+), BCL-2 (+90%), CD10 (+/–), CD43 (+/abnormal expression), Ki67 (50% hot zone), C-myc (weak +), CD2 (–), CD3 (–), CD5 (–), CD8 (–), and CD20 (–) (Figure 2D-G).
Case 1: Computed tomography (CT) showed a small amount of pericardial effusion, a small amount of pleural effusion, multiple enlarged lymph nodes in both axilla, multiple liver cysts, and enlarged spleen.
Case 2: Positron emission tomography-CT were also negative.
Both patients were diagnosed as BPDCN.
Case 1: He was treated with two cycles of the harringtonine and cytarabine + vincristine and cisplatin regimen. Venetoclax was added to the regimen, although the patient discontinued the drug after 1 week and the rash developed again. Subsequently, the patient was treated with three cycles of the mini- hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD) plus venetoclax regimen, which caused a part of his rash to subside.
Case 2: She was treated with two cycles of hyper-CVAD/mitoxantrone and cytarabine chemotherapy. The disease was suitably controlled.
Case 1: On October 27, 2020, the patient developed a serious infection after the last chemotherapy and probably died of the disease after refusing further treatment and discharge from the hospital. Assuming the mortality, the overall survival in this case was approximately 13 months.
Case 2: On October 23, 2022, the patient presented with fatigue and a purulent infection of the middle finger of the right hand. The patient was then treated with platelet transfusion, anti-infection therapy, and leukocyte therapy. The patient was then discharged from the hospital, and after that, her prognosis remains unknown.
BPDCN is a highly invasive lymphohematopoietic tumor that originates from plasmacytoid dendritic cells (pDCs). BPDCN is rare, with an incidence of approximately 0.44% for hematological malignancies and 0.7% for primary cutaneous lymphomas[1-2]. BPDCN was first reported in 1994 by Adachi et al[3], who described it as a "CD4+/CD56 + lymphoma"[3]. In 2008, the World Health Organization (WHO) termed this disease BPDCN under the classification of acute myeloid leukemia (AML). Later in 2016, the WHO officially classified BPDCN independently from AML and the related precursor cell tumors[4].
The racial tendency of BPDCN remains unknown, and it is frequently detected in elderly people of age around 65 years, with a male-to-female ratio of nearly 3:1.9 for the occurrence of this disease[5]. The disease is highly invasive, and reports have stated a median survival time of just 12–14 months and a 5-year survival rate of just 0%–6%, suggesting the extremely poor prognosis of this disease. Cutaneous lesions are the first clinical manifestation in nearly 70%–85% of BPDCN cases, and lymph nodes, bone marrow, peripheral blood, central nervous system (CNS), testis, soft tissues, and other parts of the body might also be involved simultaneously. The mediastinum, pancreas, and mammary gland neoplasm invasiveness of BPDCN is also reported[6]. Julia et al[7] retrospectively analyzed 90 cases of BPDCN registered in the French Cutaneous Lymphoma Study Group database between November 1995 and January 2012 and identified the following three distinct features of lesions in this disease: (1) Nodular lesions; (2) "Bruise-like" plaques; and (3) disseminated lesions, with a few of the rashes capable of forming ulcers and a small percentage of patients having mucosal damages, similar to skin lesions with dark-purple spots, papules, or nodules[7]. In the present study conducted with 2 BPDCN patients, the first case of the elderly male presented mainly with a disseminated lesion characterized by multiple nodules, papules, and purplish spots, while the second case of the female presented with the more common solitary nodular lesion.
The pathogenesis of BPDCN remains to be elucidated to date. BPDCN originates from the precursors of pDCs. DCs are broadly divided into two subtypes: One is the classical CD11c+/CD123– cell type, which performs the function of antigen presentation; another is the CD123+/CD11c– cell type, also referred to as the pDCs, which produce type I interferons (interferon α mainly) that activate T cells for the antiviral action. These pDCs do not exist in healthy skin and accumulate only in the skin that requires wound healing, is infected, or has inflammation or neoplasms, playing an important mediatory role between the natural and acquired immune systems[8-9]. CD123 is one of the markers associated with pDCs. CD123 is the α-chain portion of the IL-3 receptor, and as the main low-affinity subunit, promotes the high-affinity binding of the IL-3 receptor to IL-3 when co-expressed with the β-subunit. Ceribelli et al[10] reported that the e-box transcription factor-4 (TCF4), which is also referred to as E2–2, plays a major regulatory role in BPDCN cells[10], and TCF4 knockout results in the downregulation of CD123 and CD56 in BPDCN cells[11].
The diagnosis of BPDCN relies mainly on biopsy and IHC examinations. Skin lesions are the first manifestation of BPDCN in most patients and, therefore, skin biopsy is particularly useful for an early diagnosis of this disease. Molecular genetics could also be used, as a reference, although no specific genetic change has been reported so far as a manifestation of this disease. The typical pathological manifestation of BPDCN is the non-epidermal infiltrating lesions, which occur frequently with an evident acellular infiltration zone (Grenz zone) between the regions of tumor cell infiltration and the epidermis. The dermis is diffused with single infiltrated medium-sized blast cells exhibiting an irregular karyotype, fine chromatin, one small nucleolus or a few in certain cases, and little cytoplasm that is grayish blue and has the characteristic micro-vacuoles and pseudopodia while the granules are lacking. Another prominent feature of this tumor is the absence of vascular infiltration or coagulative necrosis and growth around skin appendages[11]. In the present study, the pathological findings of the 2 patients revealed the above-stated typical features, such as Grenz zone, blast cells, and the lack of granules. The IHC features of BPDCN are also distinctive: The blast cells express CD123, CD4, CD45, CD56, CD303 (blood dendritic cell antigen 4), CD33, and human leukocyte antigen-DR, while no T cell, B cell, or myeloid cell-specific markers are expressed. Therefore, BPDCN is described as a CD45+/CD56+/CD4+/CD123+/CD33+/MPO– tumor in certain studies[12]. Each of the 2 cases discussed in the present paper expressed CD123, CD4, CD56, TDT, and MPO–. Molecular pathology of BPDCN suggests that monoclonal TCR gene rearrangement could occur in a few cases, while the Ig gene rearrangement is negative. Nearly 60% of the BPDCN patients could have chromosome karyotype abnormality. Lucioni et al[13] reported that deletion of the 9p21.3 locus is the most common chromosome abnormality observed in BPDCN, and the prognosis is often poor with the allelic deletion of the gene[13]. However, 12p is now considered the most common chromosomal abnormality in BPDCN after the report of Tang et al[14].
BPDCN is rare and, therefore, no consensus has been reached so far on the treatment guidelines for this disease. Most of the published therapeutic schedules are based on retrospective studies only. Patients who are being treated for the first time have a high probability of gaining complete remission (CR), although chances of a relapse also exist. Stem cell transplantation during the patient's first remission, particularly allogeneic stem cell transplantation, is considered, to date, the only treatment that could result in durable remissions[15]. The other treatment options reported in the literature include: (1) Intensive chemotherapy: Such treatments are based on the chemotherapy regimen for ALL, AML, and lymphoma. The commonly used schemes include the hyper-CVAD scheme (cyclophosphamide + vincristine + doxorubicin + dexamethasone sequential high-dose methotrexate + cytarabine), the daunorubicin + cytarabine + etoposide scheme, the cyclophosphamide + vincristine + doxorubicin + prednisone scheme, and the cyclophosphamide + vincristine + prednisone scheme; (2) hematopoietic stem cell transplantation: This includes autologous hematopoietic stem cell transplantation and allogeneic hematopoietic stem cell transplantation (allo-HSCT); (3) low-intensity chemotherapy: The commonly used regimens are azacytidine, pratrexate, lenalidomide plus bortezomib, bendamustine, and citaxel plus gemcitabine; (4) targeted therapy: The commonly used drugs include tagraxofusp (SL-401, targeting CD123), daratumumab (targeting CD38), the BCL-2 inhibitor venetoclax (the survival of blast cells relies highly on BCL-2), etc.; and (5) immunotherapy: The commonly used regimens include the use of PD-1/PD-L1 inhibitors, anti-CD123 chimeric antigen receptor T cell therapy, etc[11].
The first patient described in the present paper was initially treated with the harringtonine and cytarabine + vincristine and cisplatin chemotherapy regimen combined with venetoclax, and CR was achieved. After the relapse, the patient was then switched to hyper-CVAD chemotherapy combined with venetoclax. As mentioned above, venetoclax was used in his combination therapy as blast cells rely on the anti-apoptotic protein BCL-2 and are sensitive to this drug. The BCL-2 gene is expressed highly in BPDCN compared to its expression in normal pDCs[16]. Venetoclax was approved by the Food and Drug Administration in 2016 for the treatment of patients with chronic lymphocytic leukemia and 17p deletion[17]. Montero et al[18] reported that venetoclax reduced the burden of BPDCN cells in human peripheral blood, bone marrow, and spleen. Moreover, the histology and IHC analyses conducted in animal studies confirmed the decreased size of tumors in the tissues and the restoration of normal hematopoietic elements in the treated animals[18]. However, recently, Albiol et al[19] reported a case that presented with v relapse of BPDCN with the involvement of the CNS, and even though this patient used venetoclax as the salvage therapy after allo-HSCT, his disease continued to progress. Albiol et al[19], accordingly, reported the assumption that the effect of venetoclax was poor in advanced patients, particularly when the drug was used alone[19]. The first patient (male, 73) discussed in the present paper experienced myelosuppression after the last chemotherapy, and his prognosis was not very good considering that it was difficult for him to achieve the conditions for allogeneic stem cell transplantation and the myelosuppression caused by the chemotherapy regimen might not have been well tolerated in his case. It is expected that a treatment regimen with less toxicity and a higher response rate will be developed for elderly patients with BPDCN in the near future. The second patient (female, 57) discussed in the present paper also used hyper-HCVD as her chemotherapy regimen. This patient also reached CR, although myelosuppression complications appeared after the third cycle of mitoxantrone and cytarabine chemotherapy. This patient had presented with a solitary skin lesion and did not appear to be responding better to the chemotherapy compared to the first case of the elderly patient with systemic disseminated skin lesions. The female patient could be continuing with her other chemotherapy regimen, although overall, her prognosis is unfortunately expected to be poor in the long term because of the highly malignant tumor. The 2 patients were both elderly individuals. The male patient presented with a widespread rash, while the female patient exhibited a solitary skin lesion. Both patients underwent hyper-CVAD chemotherapy regimen, however, infection following disease recurrence ultimately resulted in an unfavorable prognosis for both individuals. These 2 patients alone are not consistent with the conclusion of relatively good prognosis of individual lesions reported in some literatures[20]. This could potentially be attributed to the high malignancy of BPDCN, leaving limited time for intervention and improvement of survival.
BPDCN is a rare and highly invasive lymphohematopoietic tumor, with skin lesions often as the initial symptom. BPDCN progresses rapidly and, therefore, has a short median survival time. Early diagnosis would provide the patients with a better chance of achieving effective treatment and prolonged survival. Therefore, it is recommended that when a patient presents with typical skin lesions, peripheral blood and bone marrow abnormalities, and enlarged lymph nodes, the possibility of BPDCN must be considered. Further skin biopsy and IHC examination are the gold standards for a definite diagnosis. However, no standard treatment scheme is currently available for this disease, and the pathogenesis of BPDCN remains to be elucidated to date, warranting studies with larger clinical samples for further investigations.
| 1. | Aoki T, Suzuki R, Kuwatsuka Y, Kako S, Fujimoto K, Taguchi J, Kondo T, Ohata K, Ito T, Kamoda Y, Fukuda T, Ichinohe T, Takeuchi K, Izutsu K, Suzumiya J. Long-term survival following autologous and allogeneic stem cell transplantation for blastic plasmacytoid dendritic cell neoplasm. Blood. 2015;125:3559-3562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 2. | Kerr D 2nd, Sokol L. The advances in therapy of blastic plasmacytoid dendritic cell neoplasm. Expert Opin Investig Drugs. 2018;27:733-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Adachi M, Maeda K, Takekawa M, Hinoda Y, Imai K, Sugiyama S, Yachi A. High expression of CD56 (N-CAM) in a patient with cutaneous CD4-positive lymphoma. Am J Hematol. 1994;47:278-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 121] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5219] [Cited by in RCA: 6991] [Article Influence: 699.1] [Reference Citation Analysis (0)] |
| 5. | Wang Y, Ma YJ, Xu WJ. [Advances in pathogenesis, diagnosis and treatment of blastocytic plasmacytoid dendritic cell tumors]. Xiandai Zhongliu Yixue. 2022;30:709-713. |
| 6. | Lee HJ, Park HM, Ki SY, Choi YD, Yun SJ, Lim HS. Blastic plasmacytoid dendritic cell neoplasm of the breast: A case report and review of the literature. Medicine (Baltimore). 2021;100:e25699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Julia F, Petrella T, Beylot-Barry M, Bagot M, Lipsker D, Machet L, Joly P, Dereure O, Wetterwald M, d'Incan M, Grange F, Cornillon J, Tertian G, Maubec E, Saiag P, Barete S, Templier I, Aubin F, Dalle S. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2013;169:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 8. | Kolerova A, Sergeeva I, Krinitsyna J, Pronkina N, Sizikova S, Filimonov P, Kryuchkova I. Blastic Plasmacytoid Dendritic Cell Neoplasm: Case Report and Literature Overview. Indian J Dermatol. 2020;65:217-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 9. | Villani AC, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, Griesbeck M, Butler A, Zheng S, Lazo S, Jardine L, Dixon D, Stephenson E, Nilsson E, Grundberg I, McDonald D, Filby A, Li W, De Jager PL, Rozenblatt-Rosen O, Lane AA, Haniffa M, Regev A, Hacohen N. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science. 2017;356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1636] [Cited by in RCA: 1790] [Article Influence: 198.9] [Reference Citation Analysis (0)] |
| 10. | Ceribelli M, Hou ZE, Kelly PN, Huang DW, Wright G, Ganapathi K, Evbuomwan MO, Pittaluga S, Shaffer AL, Marcucci G, Forman SJ, Xiao W, Guha R, Zhang X, Ferrer M, Chaperot L, Plumas J, Jaffe ES, Thomas CJ, Reizis B, Staudt LM. A Druggable TCF4- and BRD4-Dependent Transcriptional Network Sustains Malignancy in Blastic Plasmacytoid Dendritic Cell Neoplasm. Cancer Cell. 2016;30:764-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 11. | Khoury JD. Blastic Plasmacytoid Dendritic Cell Neoplasm. Curr Hematol Malig Rep. 2018;13:477-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Koerber RM, Held SAE, Vonnahme M, Feldmann G, Wenzel J, Gütgemann I, Brossart P, Heine A. Blastic plasmacytoid dendritic-cell neoplasia: a challenging case report. J Cancer Res Clin Oncol. 2022;148:743-748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Lucioni M, Novara F, Fiandrino G, Riboni R, Fanoni D, Arra M, Venegoni L, Nicola M, Dallera E, Arcaini L, Onida F, Vezzoli P, Travaglino E, Boveri E, Zuffardi O, Paulli M, Berti E. Twenty-one cases of blastic plasmacytoid dendritic cell neoplasm: focus on biallelic locus 9p21.3 deletion. Blood. 2011;118:4591-4594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Tang Z, Li Y, Wang W, Yin CC, Tang G, Aung PP, Hu S, Lu X, Toruner GA, Medeiros LJ, Khoury JD. Genomic aberrations involving 12p/ETV6 are highly prevalent in blastic plasmacytoid dendritic cell neoplasms and might represent early clonal events. Leuk Res. 2018;73:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Garnache-Ottou F, Vidal C, Biichlé S, Renosi F, Poret E, Pagadoy M, Desmarets M, Roggy A, Seilles E, Soret L, Schillinger F, Puyraimond S, Petrella T, Preudhomme C, Roumier C, MacIntyre EA, Harrivel V, Desbrosses Y, Gruson B, Geneviève F, Thepot S, Drebit Y, Leguay T, Gros FX, Lechevalier N, Saussoy P, Salaun V, Cornet E, Benseddik Z, Veyrat-Masson R, Wagner-Ballon O, Salanoubat C, Maynadié M, Guy J, Caillot D, Jacob MC, Cahn JY, Gressin R, Rose J, Quesnel B, Guerin E, Trimoreau F, Feuillard J, Gourin MP, Plesa A, Baseggio L, Arnoux I, Vey N, Blaise D, Lacroix R, Arnoulet C, Benet B, Dorvaux V, Bret C, Drenou B, Debliquis A, Latger-Cannard V, Bonmati C, Bene MC, Peterlin P, Ticchioni M, Rohrlich PS, Arnaud A, Wickenhauser S, Bardet V, Brechignac S, Papoular B, Raggueneau V, Vargaftig J, Letestu R, Lusina D, Braun T, Foissaud V, Tamburini J, Bennani H, Freynet N, Cordonnier C, Le Garff-Tavernier M, Jacques N, Maloum K, Roos-Weil D, Bouscary D, Asnafi V, Lhermitte L, Suarez F, Lengline E, Féger F, Battipaglia G, Mohty M, Bouyer S, Ghoual O, Dindinaud E, Basle C, Puyade M, Lafon C, Fest T, Roussel M, Cahu X, Bera E, Daliphard S, Jardin F, Campos L, Solly F, Guyotat D, Galoisy AC, Eischen A, Mayeur-Rousse C, Guffroy B, Recher C, Loosveld M, Garnier A, Barlogis V, Rosenthal MA, Brun S, Contentin N, Maury S, Callanan M, Lefebvre C, Maillard N, Okamba P, Ferrand C, Adotevi O, Saas P, Angelot-Delettre F, Binda D, Deconinck E. How should we diagnose and treat blastic plasmacytoid dendritic cell neoplasm patients? Blood Adv. 2019;3:4238-4251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 16. | DiNardo CD, Rausch CR, Benton C, Kadia T, Jain N, Pemmaraju N, Daver N, Covert W, Marx KR, Mace M, Jabbour E, Cortes J, Garcia-Manero G, Ravandi F, Bhalla KN, Kantarjian H, Konopleva M. Clinical experience with the BCL2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am J Hematol. 2018;93:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 357] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 17. | Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DC, Hymowitz SG, Jin S, Khaw SL, Kovar PJ, Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer PM, Oleksijew A, Park CH, Park CM, Phillips DC, Roberts AW, Sampath D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD, Xiao Y, Xue JC, Zhang H, Humerickhouse RA, Rosenberg SH, Elmore SW. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1953] [Cited by in RCA: 2415] [Article Influence: 185.8] [Reference Citation Analysis (0)] |
| 18. | Montero J, Stephansky J, Cai T, Griffin GK, Cabal-Hierro L, Togami K, Hogdal LJ, Galinsky I, Morgan EA, Aster JC, Davids MS, LeBoeuf NR, Stone RM, Konopleva M, Pemmaraju N, Letai A, Lane AA. Blastic Plasmacytoid Dendritic Cell Neoplasm Is Dependent on BCL2 and Sensitive to Venetoclax. Cancer Discov. 2017;7:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 178] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 19. | Albiol N, Novelli S, Mozos A, Pratcorona M, Martino R, Sierra J. Venetoclax in relapsed/refractory blastic plasmacytoid dendritic cell neoplasm with central nervous system involvement: a case report and review of the literature. J Med Case Rep. 2021;15:326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Pileri A, Delfino C, Grandi V, Agostinelli C, Pileri SA, Pimpinelli N. Blastic plasmacytoid dendritic cell neoplasm (BPDCN): the cutaneous sanctuary. G Ital Dermatol Venereol. 2012;147:603-608. [PubMed] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/