Published online Sep 24, 2024. doi: 10.5306/wjco.v15.i9.1198
Revised: August 5, 2024
Accepted: August 7, 2024
Published online: September 24, 2024
Processing time: 83 Days and 21.6 Hours
Lung cancer (LC) combined with chronic obstructive pulmonary disease (COPD) is a common combination of comorbidities. Anti-inflammation and modulation of oxidative/antioxidative imbalance may prevent COPD-induced LC, and are also crucial to the treatment of LC combined with COPD. Modern studies have shown that Tao Hong Si Wu Tang (THSW) has vasodilatory, anti-inflammatory, anti-fa
To observe the effects of THSW on COPD and LC in mice.
A total of 100 specific pathogen-free C57/BL6 mice were randomly divided into five groups: Blank control group (group A), model control group (group B), THSW group (group C), IL-6 group (group D), and THSW + IL-6 group (group E), with 20 mice in each group. A COPD mouse model was established using fumi
The blank control group exhibited a clear alveolar structure. The model control and IL-6 groups had thickened alveolar walls, with smaller alveolar lumens, interstitial edema, and several inflammatory infiltrating cells. Histopathological changes in the lungs of the THSW and THSW + IL-6 groups were less than those of the model control group. The serum IL-1β, IL-6, and TNF-α levels and IL-6R, JAK, p-JAK, STAT1/3, p-STAT1/3, FOXO, p-FOXO, and IL-7R expression levels in lung tissues of mice in the rest of the groups were significantly higher than those of the blank control group (P < 0.01). Compared with the model control group, the IL-6 group demonstrated significantly higher levels for the abovementioned proteins in the serum and lung tissues (P < 0.01), and the THSW group had significantly higher serum IL-1β, IL-6, and TNF-α levels and IL-7R expression levels in lung tissues (P < 0.01) but significantly decreased IL-6R, JAK, p-JAK, STAT1/3, p-STAT1/3, FOXO, p-FOXO, and IL-7R levels (P < 0.01).
THSW reduces the serum IL-1β, IL-6, and TNF-α levels in the mouse model with anti-inflammatory effects. Its anti-inflammatory mechanism lies in inhibiting the overactivation of the JAK/STAT1/3 signaling pathway.
Core Tip: The present study aimed to analyze the anti-inflammatory effects of Tao Hong Si Wu Tang (THSW) in a chronic obstructive pulmonary disease-lung cancer mouse model and elucidate its anti-inflammatory mechanism in inhibiting the development of positive-feedback inflammatory response by detecting the signaling pathway that may be induced by the inflammatory factors. The findings of this study will lay a theoretical foundation and provide a guide for the clinical app
- Citation: Wang GL, Xu YL, Zhao KM, Sui AF, Wang LN, Deng H, Wang G. Anti-inflammatory effects of Tao Hong Si Wu Tang in mice with lung cancer and chronic obstructive pulmonary disease. World J Clin Oncol 2024; 15(9): 1198-1206
- URL: https://www.wjgnet.com/2218-4333/full/v15/i9/1198.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i9.1198
Lung cancer (LC) combined with chronic obstructive pulmonary disease (COPD) is a common combination of comor
Tao Hong Si Wu Tang (THSW) has the core effect of removing blood stasis, and in combination with Chuan Xiong, it can help activate blood circulation and move qi. Modern studies have shown that THSW has vasodilatory, anti-inflammatory, anti-fatigue, anti-shock, immunoregulaory, lipid-reducing, micronutrient-supplementing, and anti-allergy effects[3-6]. THSW, when used in the treatment of non-small cell LC, can significantly improve lung function and other indi
The present study aimed to analyze the anti-inflammatory effects of THSW in a COPD-LC mouse model and elucidate its anti-inflammatory mechanism in inhibiting the development of positive-feedback inflammatory response by detecting the signaling pathway that may be induced by the inflammatory factors. The findings of this study will lay a theoretical foundation and provide a guide for the clinical application of THSW.
A total of 100 specific pathogen-free 5-8-week-old male C57/BL6 mice weighing 18-22 g, were purchased from Liaoning Changsheng Biotechnology Co. Prior to use, the mice were acclimatized and reared for 7 d.
The mice were randomly divided into five groups: Blank control (group A), model control (group B), THSW (group C), IL-6 (group D), and THSW + IL-6 (group E), with each group comprising 20 mice.
A COPD mouse model was first established for mice in groups B, C, D, and E using the method of smoking plus lipopolysaccharide (LPS) intra-airway drip. The mice were placed in a homemade plexiglass dyeing box (120 cm × 80 cm × 80 cm) to undergo passive smoking with 10 cigarettes 2 times a day for 30 min each time, with an interval of at least 4 h between the 2 times, for 30 consecutive days. Subsequently, the mice were placed in a homemade plexiglass dyeing box (120 cm × 80 cm × 80 cm) for passive smoking with 2 and 14 weather tubes dripped with 2 mL/kg LPS (50 μL), and smoking was suspended 2 times on the day of the LPS drip. Successful establishment of the COPD mouse model was confirmed 24 h after the final smoke exposure, and the in situ LC model was replicated in the COPD mouse model 1 wk later by obtain
The blank control group (group A) received no treatment, and experienced normal rearing until the end of the experi
At 2 h after the last oral gavage administration, mice in each group were intraperitoneally injected with sodium pento
Visual observation of the tumor and HE staining: The fixed lung tissues were obtained and HE-stained to observe histo
IL-1β, IL-6, and TNF-α contents in peripheral blood of mice: Enzyme-linked immunosorbent assay was used to detect the serum IL-1β, IL-6, and TNF-α levels of mice in each group.
Western blot analysis for detecting IL-6R, JAK, p-JAK, STAT1/3, p-STAT1/3, FOXO, p-FOXO, and IL-7R expression levels in lung tissues: Fresh lung tissues were obtained; 100 mg was weighed, and total protein was extracted by the lysis method. After protein quantification, the protein concentration was adjusted to 10 µg/µL, electrophoresis was conducted at a sample volume of 6 µL/well, the protein was transferred to the polyvinylidene difluoride membrane by the wet-transfer method, the primary and secondary antibodies were incubated, and then an electrochemiluminescence solution was used for visualization. A gel imaging analysis system was used to capture photos. The gray value of the bands was determined. The relative expression level of the target protein was calculated as the gray value of the target protein band/that of the internal reference protein band and statistically analyzed.
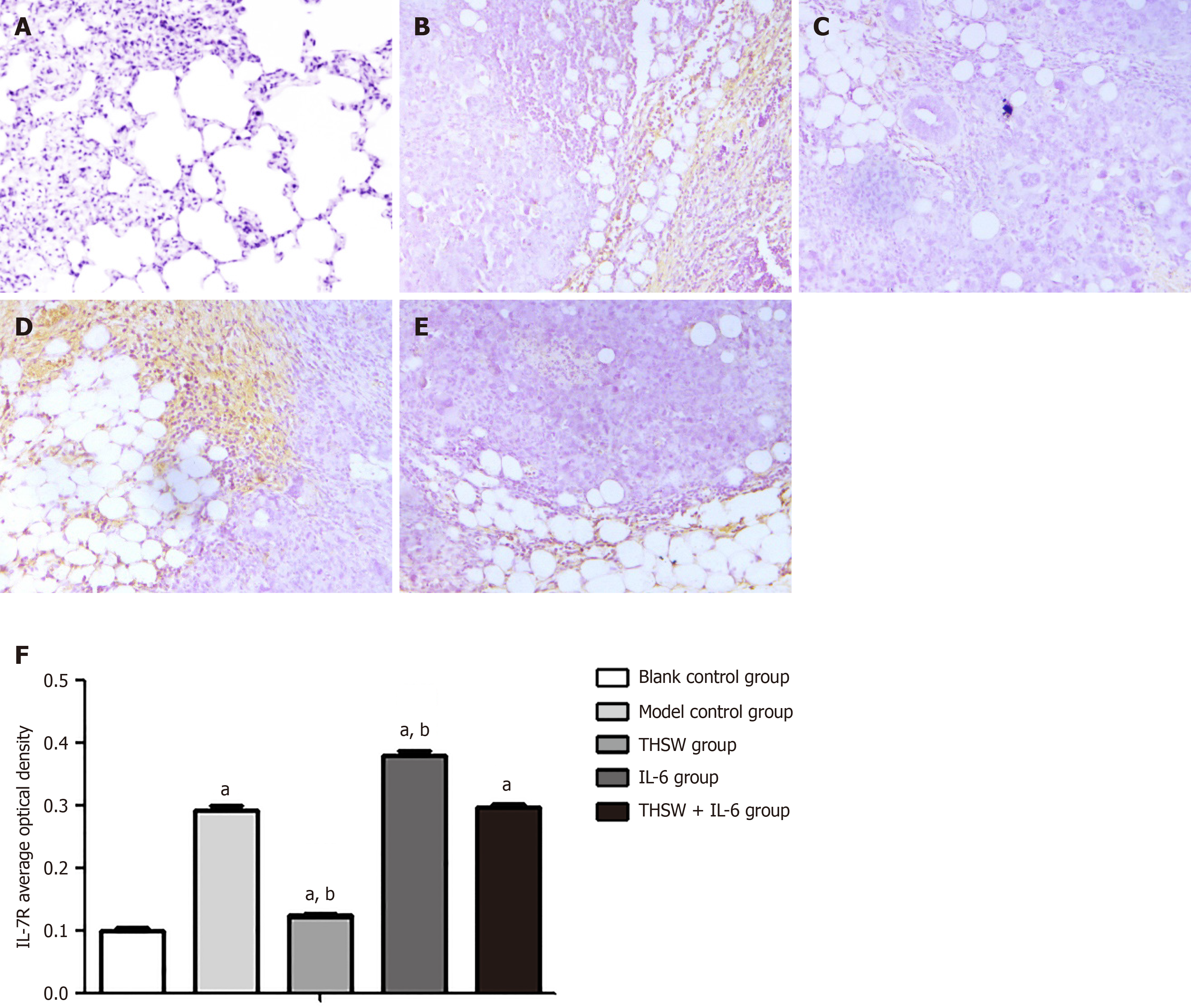
Detection of IL-6R and IL-7R protein expression levels by immunohistochemistry: Immunohistochemistry was used to detect IL-6R and IL-7R protein expression levels in the lung tissues of mice in each group.
The SPSS22.0 software package was used to statistically analyze the test results of each group. Measurement data are expressed as the mean ± SD, and the least significant difference method was used to for comparisons among groups. Significance was set at P < 0.05.
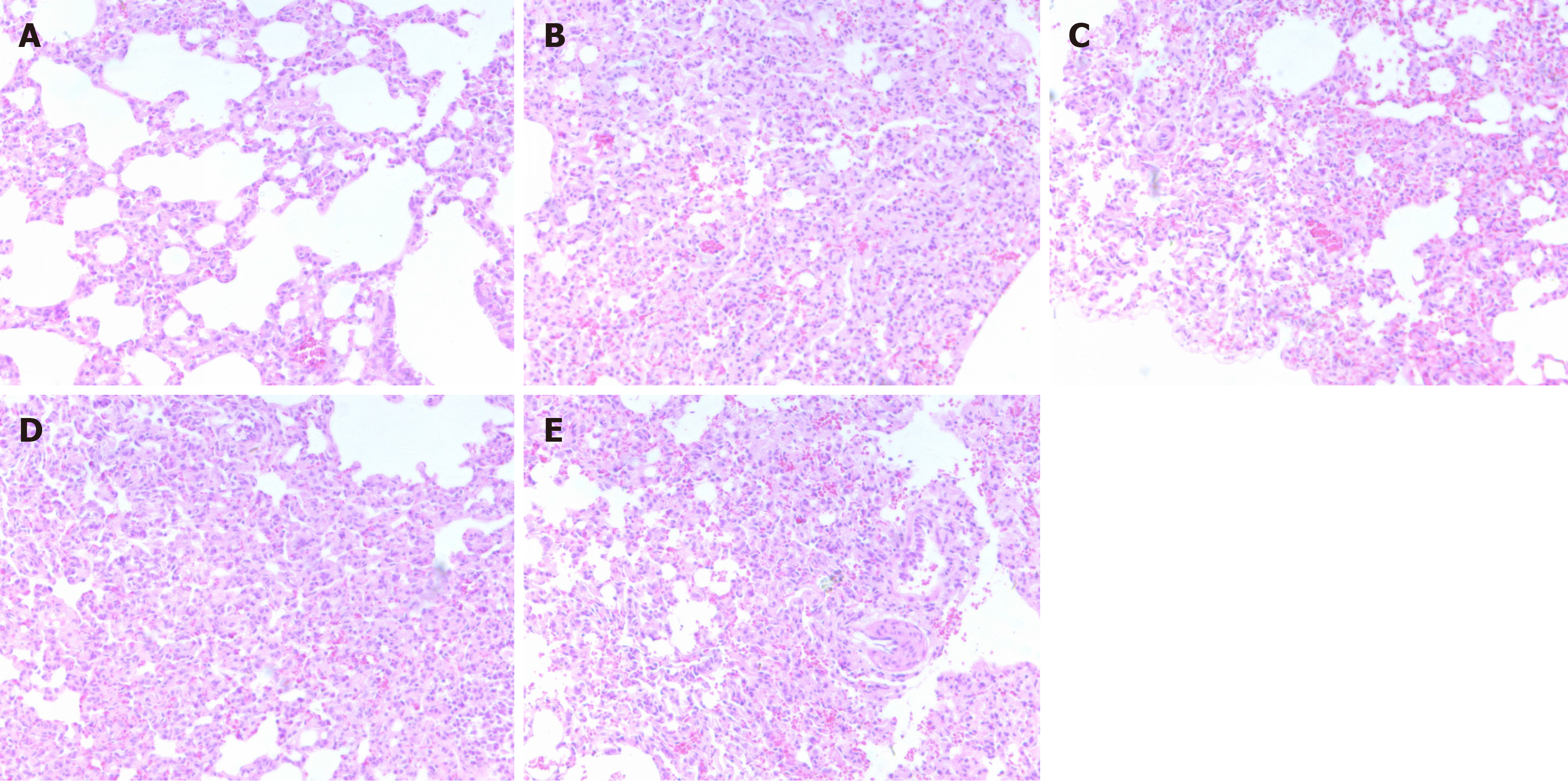
The HE staining of lung tissues of mice in each group revealed a clear alveolar structure, normal alveolar intervals, and no abnormal manifestations in mice in the blank control group; thickened alveolar walls, smaller alveolar cavities, interstitial lung edema, several inflammatory infiltrating cells, some deeply stained alveolar walls, and irregular cell nuclei in the model control and IL-6 groups; and significantly reduced pathologic changes in the lungs of the mice in the THSW and THSW + IL-6 groups compared with the model control group, especially in the THSW group, where the alveolar walls were significantly thinner, inflammatory cell infiltration was reduced, and the number of irregular nuclei was reduced (Figure 1).
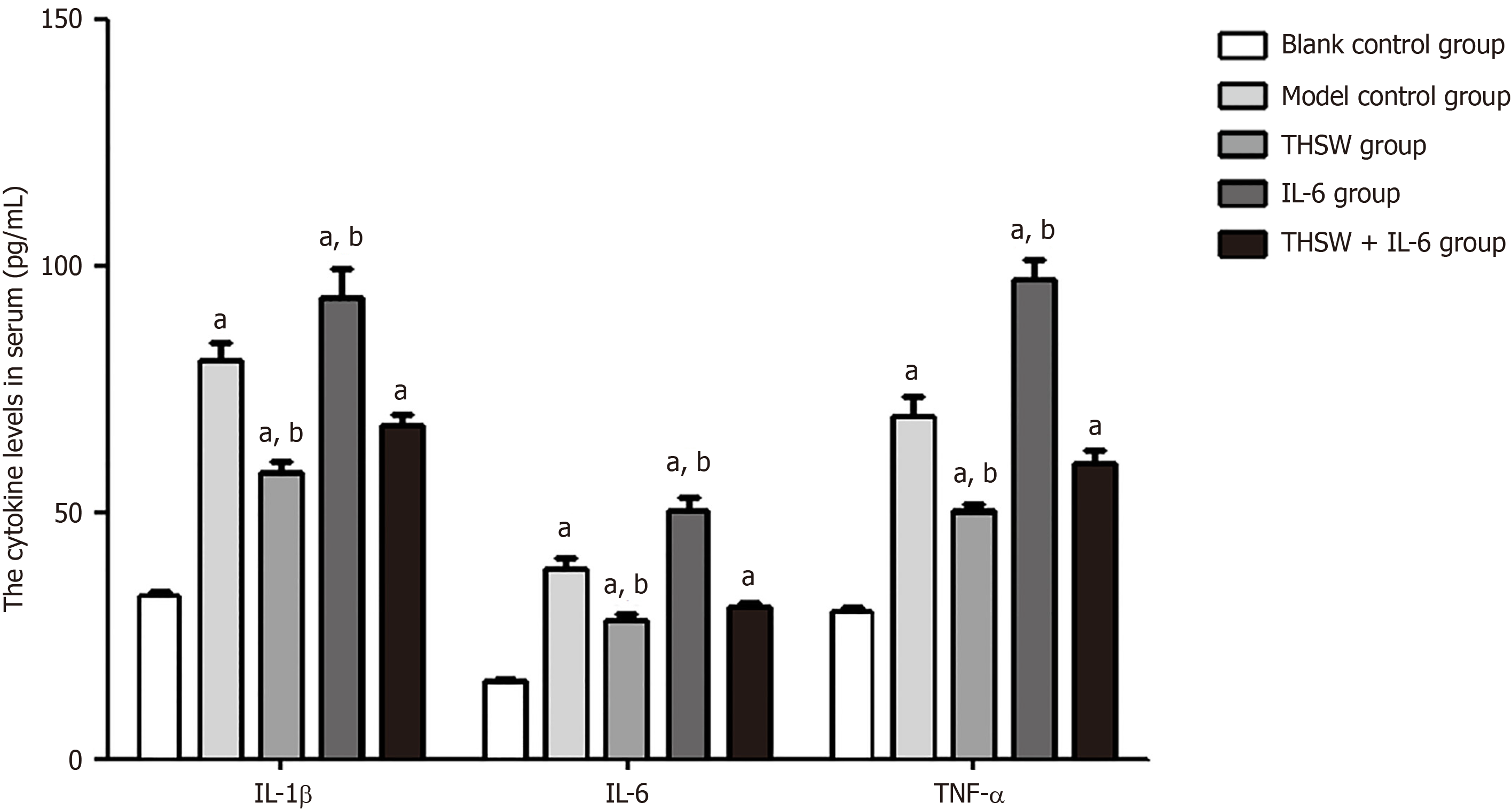
Compared with the blank control group, the other groups exhibited significantly higher serum IL-1β, IL-6, and TNF-α levels (P < 0.01). The serum IL-1β, IL-6, and TNF-α levels were significantly higher in the IL-6 group and significantly lower in the THSW group than in the model control group (P < 0.01; Figure 2).
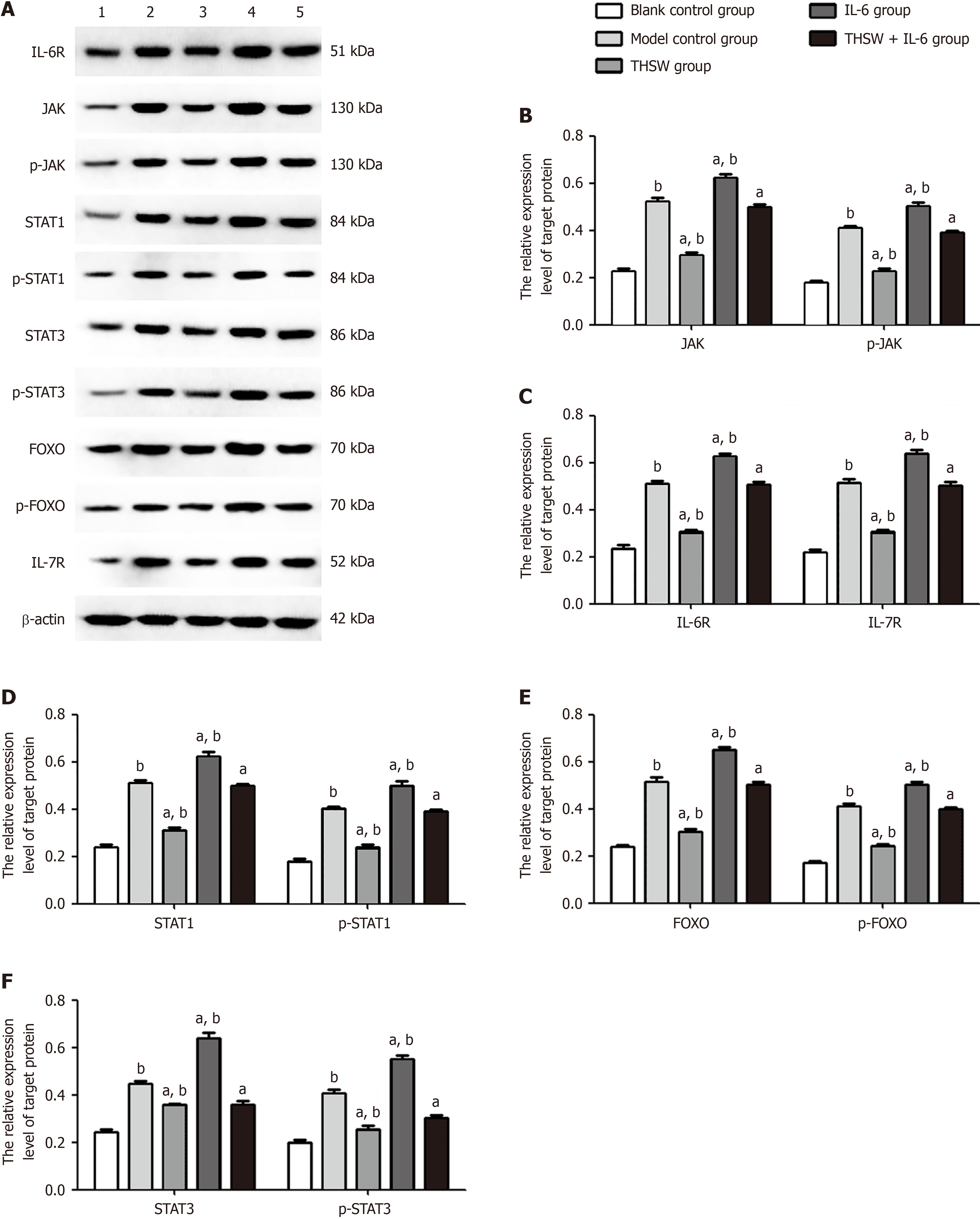
Compared with the blank control group, the other groups had significantly higher IL-6R, JAK, p-JAK, STAT1/3, p-STAT1/3, FOXO, p-FOXO, and IL-7R expression levels in the lung tissues (P < 0.01). Meanwhile, the same expression levels in the lung tissues were significantly higher in the IL-6 group and significantly lower in the THSW group than in the model control group (P < 0.01; Figure 3).
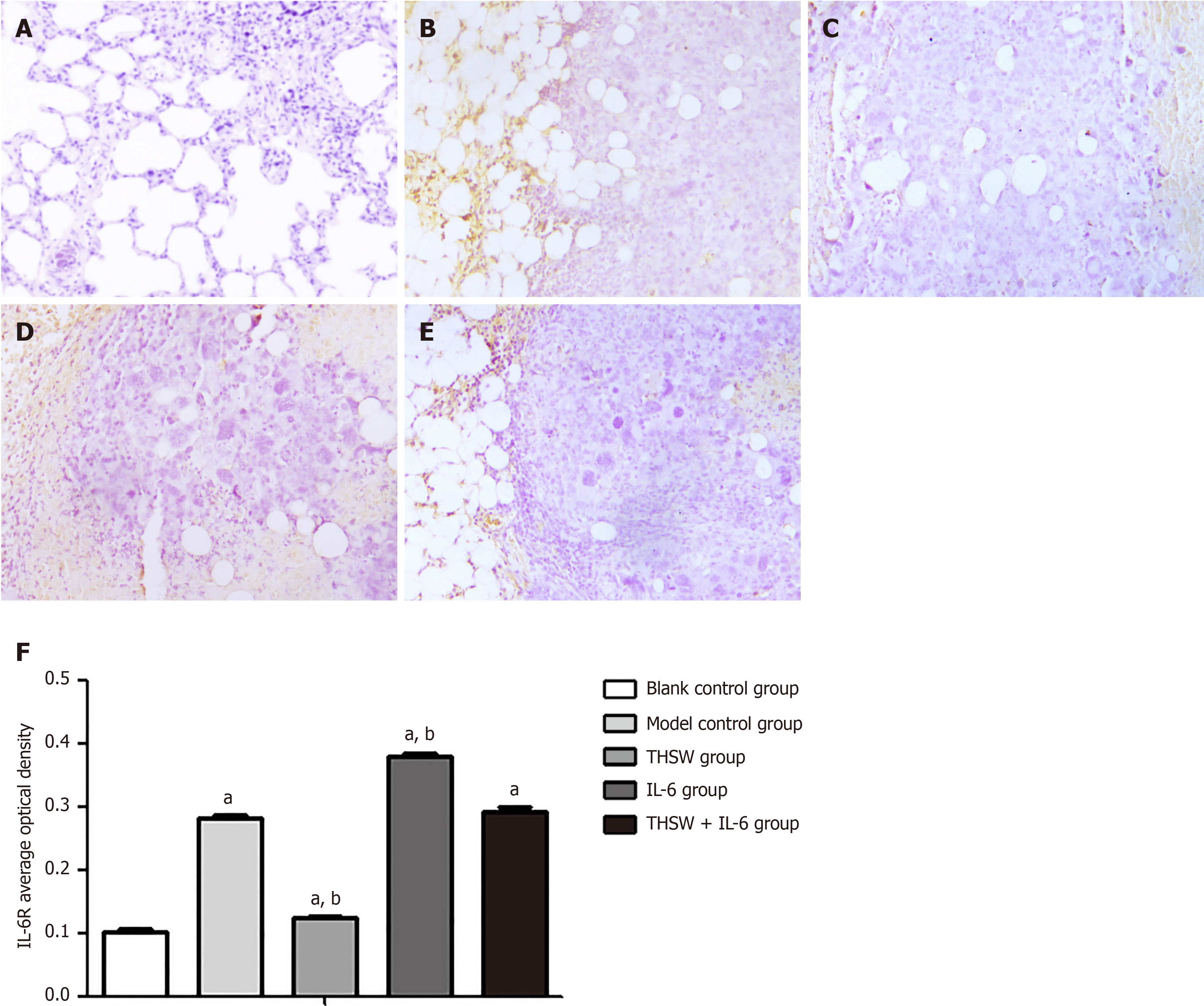
Compared with the blank control group, the other groups demonstrated significantly higher IL-6R and IL-7R expression levels in the lung tissues (P < 0.01). Meanwhile, the IL-6R and IL-7R expression levels in the lung tissues were significantly higher in the IL-6 group (P < 0.01) and significantly lower in the THSW group than in the model control group (P < 0.01; Figures 4 and 5).
THSW consists of six natural plant herbs, namely, peach kernel, safflower, Radix Rehmanniae Praeparata, Radix Angelicae Sinensis, Paeoniae Alba, and Rhizoma Ligustici Chuanxiong, which have clear vasodilatory[3], anti-inflammatory[4], anti-fatigue[5], and immune-regulating properties[6]. THSW regulates the activation of T and B lymphocytes by reducing the IL-6, IL-8, and TNF-α contents in the alveolar lavage fluid of patients with COPD[9-11]. THSW is a classic formula for activating blood circulation and removing blood stasis, and its anti-inflammatory effects are also evident in modern studies. In Niu et al's study[12], THSW reduced the serum IL-2, IL-17, IL-22, and IL-23 Levels in a psoriasis mouse model, and inhibited the IL-2, IL-17, IL-22, and IL-23 expression levels in the dorsal lesion tissues of the same model. Another study has demonstrated that polysaccharides are one of the main active ingredients of THSW, and that they exert mainly anti-inflammatory and antioxidant effects. The polysaccharide components of THSW significantly reduced the serum IL-1β, IL-6, and TNF-α levels in a rat model of ischemic stroke[13]. Our previous study has also indicated that THSW could reduce the inflammatory response in a COPD-LC mouse model, and that THSW exerted an anti-inflammatory effect in the same mouse model by inhibiting the secretion of the Th1- and Th17-type cytokines IFN-γ and IL-17, and promoting the secretion of the Th2- and Treg-type cytokines IL-4 and TGF-β[14].
The JAK/STAT signaling pathway is a series of chain reactions between intracellular proteins interacting with each other. It is involved in the processes of immunity, cell division, cell death, and tumor formation. In addition, the JAK/STAT1/3 signaling pathway is involved in the transcription of various inflammatory factors, and the activation of its classical pathway by IL-6 amplifies inflammatory responses[15,16]. Zhu et al's study has demonstrated that erythrulose polysaccharide could inhibit the inflammatory response in the gastric mucosa of rats with diabetic gastroparesis, and its mechanism was related to the modulation of the JAK/STAT3 signaling pathway, indicating the close relationship bet
THSW significantly reduced serum IL-1β, IL-6, and TNF-α levels in our COPD-LC mouse model, which confirmed its anti-inflammatory effects. The test results of key targets of the JAK/STAT1/3 signaling pathway proved that THSW could significantly downregulate the p-JAK, p-STAT1/3, and p-FOXO expression levels, and reduced phosphorylation levels of key targets, which indicated the inhibitory effect of THSW on this signaling pathway. To clarify its inhibitory effect on the JAK/STAT1/3 signaling pathway, we also included IL-6, which can activate the classical pathway of the JAK/STAT1/3 signaling pathway to mediate the inflammatory response. The inflammatory response of mice in the IL-6 group was significantly severe, whereas the expression levels of inflammatory factors and key targets of the JAK/STAT1/3 signaling pathway in mice in the IL-6 + THSW group were significantly reduced, which further proved that the anti-inflammatory mechanism of THSW in the COPD-LC mouse model was achieved by inhibiting the activation of the JAK/STAT1/3 signaling pathway.
| 1. | Ma H, Zhang Q, Zhao Y, Zhang Y, Zhang J, Chen G, Tan Y, Zhang Q, Duan Q, Sun T, Qi C, Li F. Molecular and Clinicopathological Characteristics of Lung Cancer Concomitant Chronic Obstructive Pulmonary Disease (COPD). Int J Chron Obstruct Pulmon Dis. 2022;17:1601-1612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 2. | Wang C, Xu J, Yang L, Xu Y, Zhang X, Bai C, Kang J, Ran P, Shen H, Wen F, Huang K, Yao W, Sun T, Shan G, Yang T, Lin Y, Wu S, Zhu J, Wang R, Shi Z, Zhao J, Ye X, Song Y, Wang Q, Zhou Y, Ding L, Yang T, Chen Y, Guo Y, Xiao F, Lu Y, Peng X, Zhang B, Xiao D, Chen CS, Wang Z, Zhang H, Bu X, Zhang X, An L, Zhang S, Cao Z, Zhan Q, Yang Y, Cao B, Dai H, Liang L, He J; China Pulmonary Health Study Group. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391:1706-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 1120] [Article Influence: 140.0] [Reference Citation Analysis (15)] |
| 3. | Tang LF, Chang H, Wang DD, Liu ZQ, Han L, Peng DY. [Pharmacology, molecular docking and experimental validation of target cell trapping association network to analyze the active ingredients of Tao Hong Si Wu Tang and the potential mechanism of action in regulating ischemic stroke]. Zhongguo Zhongyao Zazhi. 2023;17:4761-4773. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Wu CJ, Chen JT, Yen TL, Jayakumar T, Chou DS, Hsiao G, Sheu JR. Neuroprotection by the Traditional Chinese Medicine, Tao-Hong-Si-Wu-Tang, against Middle Cerebral Artery Occlusion-Induced Cerebral Ischemia in Rats. Evid Based Complement Alternat Med. 2011;2011:803015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Li SS, Chen ZC, Zhang CH. Effect of tao-hong-si-wu-tang, a traditional Chinese herbal medicine formula, on physical fatigue in mice. Afr J Tradit Complement Altern Med. 2012;10:60-65. [PubMed] |
| 6. | Jiao YB, Zhu LY, Liu ZH. [Study on the effect of Tao Hong Si Wu Tang on pain and immune function of postoperative patients with colon cancer]. Liaoning Zhongyi Zazhi. 2022;11:105-108. [DOI] [Full Text] |
| 7. | Zhang Y, Zhou KW, Wang BC. [Clinical study on the treatment of coughing and wheezing in advanced non-small cell lung cancer with qi stagnation and blood stasis by combining Tao Hong Si Wu Tang and San Zi Nuan Xiang Tang with additional subtractions]. Xinzhongyi. 2021;08:103-106. [DOI] [Full Text] |
| 8. | Li XC, Luo HY. [Therapeutic effects of artesunate modulating IL-6/STAT3 signaling pathway in a rat model of neuropathic pain]. Cuzhongyushenjing Jibing. 2024;02:187-191 + 205. |
| 9. | Ling XY, Han P, Cui XH. [Clinical analysis of the treatment of chronic obstructive pulmonary disease in the elderly with the addition and subtraction of Tao Hong Si Wu Tang combined with Er Chen Tang]. Shizhen Guoyi Guoyao. 2022;07:1678-1681. |
| 10. | Prudente R, Ferrari R, Mesquita C, Machado L, Franco E, Godoy I, Tanni S. Nine-Year Follow-Up of Interleukin 6 in Chronic Obstructive Pulmonary Disease - Complementary Results from Previous Studies. Int J Chron Obstruct Pulmon Dis. 2021;16:3019-3026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Liu F, Zhang X, Du W, Du J, Chi Y, Sun B, Song Z, Shi J. Diagnosis values of IL-6 and IL-8 levels in serum and bronchoalveolar lavage fluid for invasive pulmonary aspergillosis in chronic obstructive pulmonary disease. J Investig Med. 2021;69:1344-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Niu FQ, Li BN, Wang SN, Li YF, Zhou WL. [Exploring the anti-inflammatory effect of Tao Hong Si Wu Tang in the treatment of psoriasis and the mechanism of intervening effect on Caspase14 and EVPL]. Zhongguo Shiyan Fangjixue Zahi. 2024;. [DOI] [Full Text] |
| 13. | Zhang YN, Zhao MD, Tang LF, Hou XY, Zhang YZ, Han L, Peng DY. [Structural analysis of polysaccharides from Tao Hong Si Wu Tang and their anti-inflammatory effects on rats with ischemic stroke]. Anhui Zhongyiyao Daxue Xuebao. 2023;02:79-85. |
| 14. | Deng H, Wang GL, Xu YL, Wang G. [Experimental study on the anti-inflammatory effect of Tao Hong Si Wu Tang on COPD combined with lung cancer model mice]. Shijie Zhongxiyi Jiehe Zazhi. 2023;04:642-645 + 662. [DOI] [Full Text] |
| 15. | W u HH, Pan Z, Liu HY, Xu SW, Liu WM, Zhang YJ, Liu ZB. [Electroacupuncture modulates SOCS3/JAK1/STAT3 signaling pathway to improve lung inflammation in COPD]. Zhongguo Mianyixue Zazhi. 2024;. |
| 16. | Yew-Booth L, Birrell MA, Lau MS, Baker K, Jones V, Kilty I, Belvisi MG. JAK-STAT pathway activation in COPD. Eur Respir J. 2015;46:843-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Zhu XL, An H, Li RK, Zhang L, Wei CH, Miao LL, Wan SF. [Exploring the effect of rubragalin polysaccharide on inflammatory response in diabetic gastroparesis rats based on JAK2/STAT3 signaling pathway]. Zhongguo Yaolixue Tongbao. 2024;05:907-913. |
| 18. | Xi J, Zhang M, Zhang YY, Zhang C, Zhang YL, Wang R, Shen L, Li J, Song X. [Proteomic Identification of Nrf2-Mediated Phase II Enzymes Critical for Protection of Tao Hong Si Wu Decoction against Oxygen Glucose Deprivation Injury in PC12 Cells]. Nanfang Yike Daxue Xuebao. 2024;04:765-772. |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/