Published online Feb 24, 2024. doi: 10.5306/wjco.v15.i2.271
Peer-review started: October 23, 2023
First decision: November 29, 2023
Revised: December 11, 2023
Accepted: January 10, 2024
Article in press: January 10, 2024
Published online: February 24, 2024
Processing time: 119 Days and 10.9 Hours
X-ray gastric cancer (GC) screening has been shown to decrease mortality. Population-based X-ray GC screening has been performed in Hiroshima Prefe
To evaluate time trends and efficacy of population-based X-ray GC screening and identify challenges and suggested solutions for the future.
This was a population-based retrospective study. The data were derived from aggregated data of the Hiroshima Regional Health Medical Promotion Organization, including the number and rate of participants and those requiring esophagogastroduodenoscopies (EGDs), the number and rate of participants diagnosed as having GC, and the positive predictive value of the abnormal findings detected by X-ray and confirmed by EGDs. The number and rate of esophageal cancers were also collected. Further, the cost of detecting one GC was evaluated.
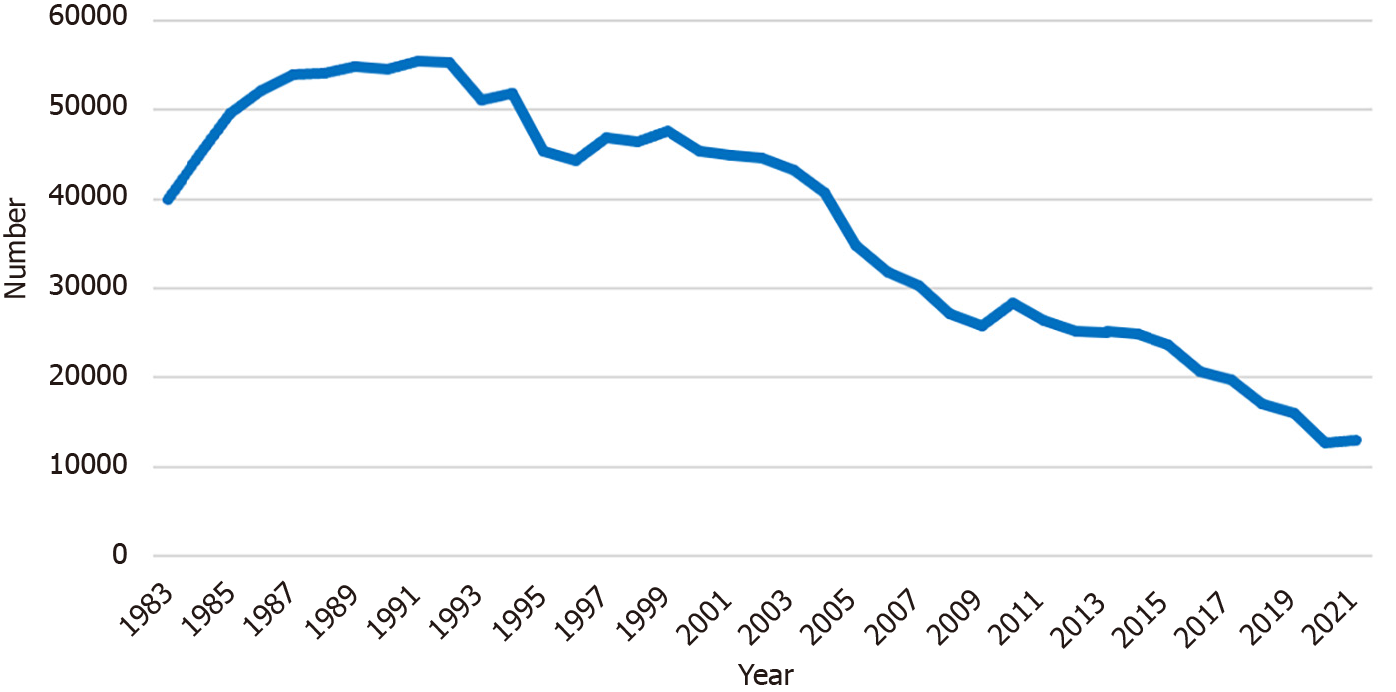
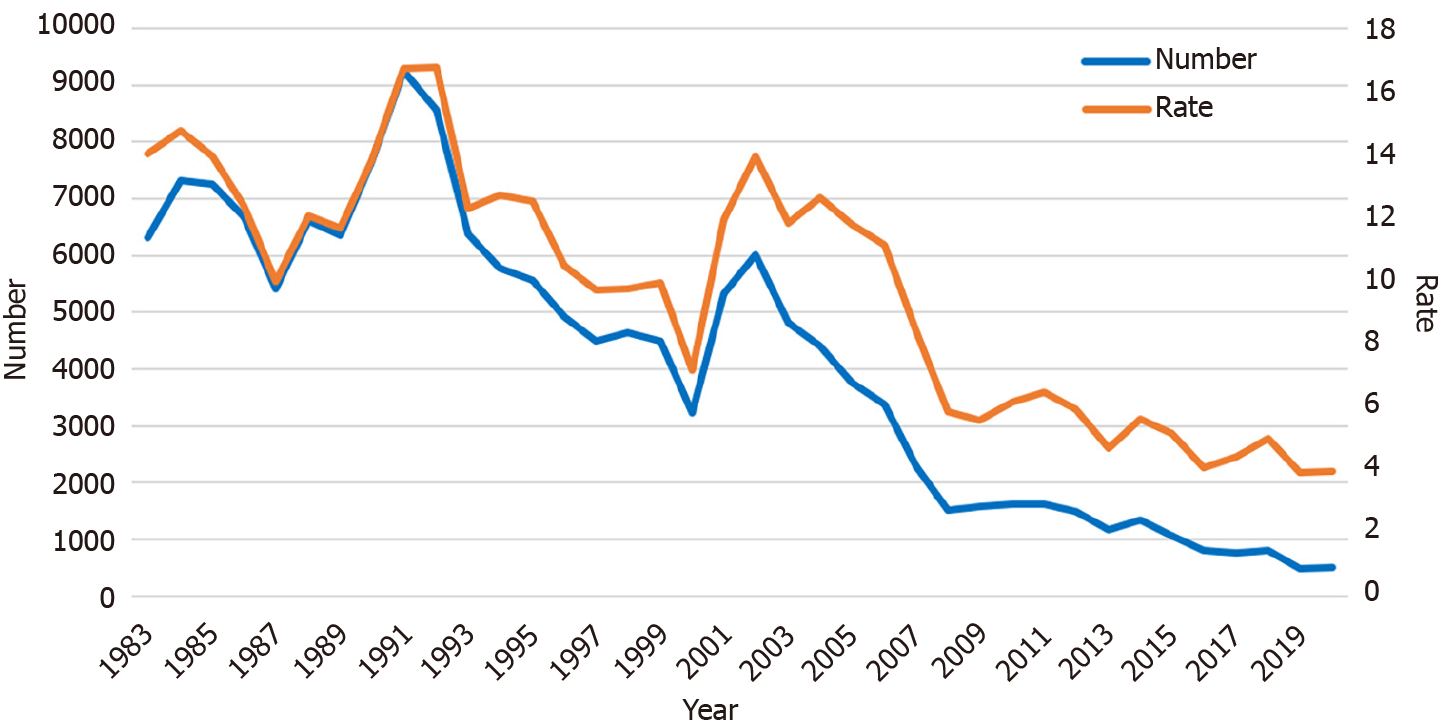
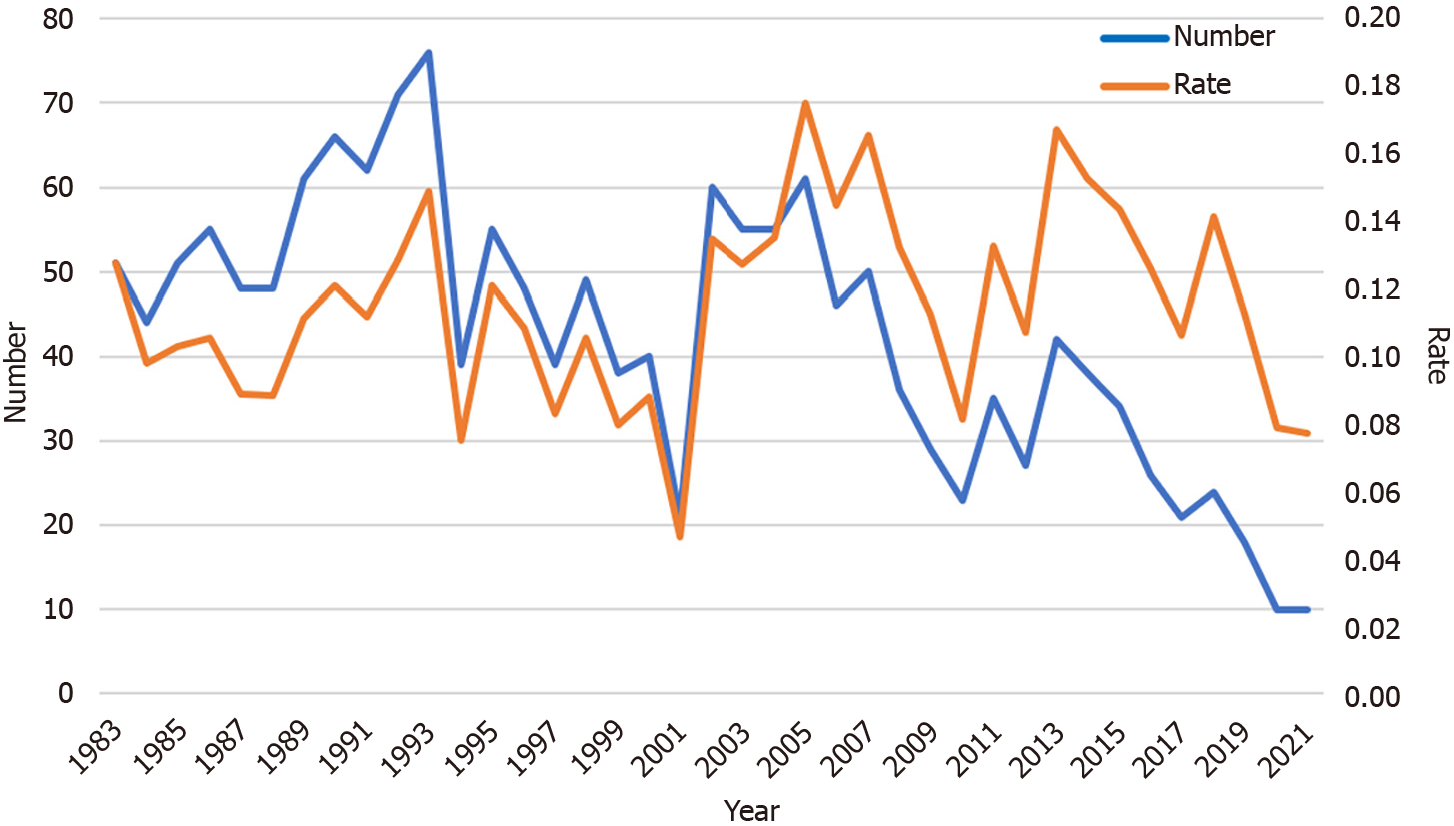
The number of participants has decreased during the last four decades, from 39925 in 1983 to 12923 in 2021. The rate of those requiring EGDs decreased significantly in recent years (P < 0.001). The number of participants diagnosed as having GC has also declined, from 76 to 10 cases. However, the rate of cases diagnosed as GC among the participants remained around 0.1%. The positive predictive value increased significantly in recent years except during 1983-1991. The number and rate of accidentally detected esophageal cancers have risen recently, from 0% in 2008 to 0.02% in 2021, one-fifth of the diagnosis rate of GC. One GC diagnosis costs approximately 4200000 Japanese Yen (30000 United States Dollars) for the X-ray screenings and EGDs.
X-ray GC screening in Hiroshima has been efficient, but one challenge is the cost. Esophageal cancers may also need to be considered because they have gradually increased in recent years.
Core Tip: This was a population-based retrospective study to evaluate the time trends and efficacy of population-based X-ray gastric cancer screening in Hiroshima over the last four decades. The number of participants and those requiring esophagogastroduodenoscopies has decreased significantly. The number of participants diagnosed as having gastric cancer has also declined. However, the rate of cases diagnosed as gastric cancer among the participants remained around 0.1%. The positive predictive value also increased significantly. The number and rate of accidentally detected esophageal cancers have risen recently. One gastric cancer diagnosis costs approximately 4200000 Japanese Yen (30000 United States Dollars) for the X-ray screenings and esophagogastroduodenoscopies.
- Citation: Vu NTH, Urabe Y, Quach DT, Oka S, Hiyama T. Population-based X-ray gastric cancer screening in Hiroshima prefecture, Japan. World J Clin Oncol 2024; 15(2): 271-281
- URL: https://www.wjgnet.com/2218-4333/full/v15/i2/271.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i2.271
Gastric cancer (GC) is the fifth most common cancer worldwide and has the fourth highest mortality rate[1]. Notably, the survival rate for GC has improved globally over the years due to advancements in both diagnosis and treatment[2,3]. In Japan, there has also been a decline in the adjusted incidence and mortality rates of GC over the last few decades[4]. Helicobacter pylori (H. pylori) infection is considered the leading cause of GC[5]. Therefore, this decrease in GC is mainly attributed to reducing H. pylori infection rates due to improvements in hygiene and the efficacy of H. pylori eradication therapy[6,7]. Despite this reduction, the incidence and mortality rates of GC are still the third-highest in Japan[8]. Consequently, the burden of GC remains substantial, making it a critical public health issue.
In addition to the H. pylori eradication regimen, it is crucial to implement a GC screening program to lower GC mortality. In the East Asian countries, only Japan and Korea have a national program for GC screening[9], and it has extensively contributed to the declining mortality from GC in both countries[10,11].
GC screening in Japan has been conducted in local areas for a long time-since the 1960s. Not until 1983, however, was it expanded nationwide in accordance with the Health Law for the aged[12]. Cancer screening in Japan can be categorized into population-based screening, which attempts to reduce overall mortality rates in target populations, and opportunistic screening, which aims to minimize individual risk. Effective population-based screening is a cornerstone of cancer control in Japan.
Although there are complications such as intestinal obstruction due to barium, aspiration pneumonia, and anaphylactic shock, radiographic screening has been the main approach for GC screening in Japan. Currently, the government of Japan has suggested either radiographic or endoscopic examination for GC screening[12]. The updated version of the Japanese Guidelines for GC Screening in 2018 recommended radiographic screening for population-based and opportunistic screenings as its advantages outweigh its risks. Likewise, endoscopic screening is also recommended for population-based and opportunistic screenings because its benefits outweigh its harms. Although more cases of GC have been detected with endoscopic rather than radiographic screening, it was reported that the reduction in GC mortality was not significantly different between the two screening methods[13]. GC screening using radiographic examination has been shown to be safe, cost-effective, accurate, and has a remarkable capacity for mass processing[14]. In addition, the GC risk stratification method named the ABC method, which uses H. pylori antibody and pepsinogen I and II, has been applied in some areas in Japan[15].
Hiroshima Prefecture is located in the southwestern part of Japan’s main island of Honshu and is the centre of the Chugoku region, with a total land area of 8480 km2 and a population of 2.8 million people. The Hiroshima Regional Health Medical Promotion Organization is in charge of GC screening in Hiroshima Prefecture, where radiographic screening has been chosen for population-based GC screening. The ABC method has not been applied in the prefecture. It is thus essential to assess the trends of X-ray GC screening from the past and investigate future perspectives. Hence, this study aimed to evaluate the trends and efficacy of population-based X-ray GC screening in Hiroshima Prefecture for the last 39 years, from 1983 to 2021, to identify the challenges and develop future solutions.
This was a population-based retrospective study. Hiyama T and Vu NTH contributed to the study’s conception, design, and execution of the research. The data were derived from the aggregated data of the Hiroshima Regional Health Medical Promotion Organization, Hiroshima, Japan. Vu NTH, Hiyama T, and Urabe Y conducted data collection and curation with the staff of this institution. Data obtained from this organization may represent the majority of residents living in Hiroshima Prefecture, where residents aged 40 years and older qualify for participation in an annual population-based X-ray GC screening. Each year, coupons were issued to eligible residents by the health center of each local city and town in Hiroshima Prefecture. Even residents under 40 could take the screening if they desired.
High-resolution double-contrast agents for the upper gastrointestinal tract were utilized for the X-ray examination in Hiroshima Prefecture. Consequently, the capability of gastric radiography examination to detect lesions was greatly enhanced. The citizens were ineligible for barium X-ray screening if they had one or more of the following conditions: Past medical history of total gastrectomy, hypersensitive to barium sulfate products, or pregnant (or possibly pregnant) women. Radiographic examination was performed using the standard 8-image method. The screening report was double-read (double-checked) by two radiologists on two separate occasions.
We collected the number of participants who underwent X-ray GC screening during the last 39 years, from 1983 to 2021, and examined the age distribution of participants from 2002 to 2021. Participants with a suspicious X-ray abnormality (cancers or other gastric lesions such as ulcers) were recommended to undergo detailed examination with esophagogastroduodenoscopy (EGD). We also gathered the number of participants and the rate of those requiring EGD during the study period. Participants who were ineligible to undergo X-ray gastric screening were not included among those for whom EGDs were required. Moreover, the number and rate of participants diagnosed as having GC, and the invasion depth of each cancer, were recorded. Invasion depth was divided into early stage, i.e., invasion depth is the lamina propria or the submucosa, and advanced stage, i.e., invasion depth is the muscularis propria or deeper. We derived the positive predictive value for GC detected by X-ray and confirmed by EGD. We also obtained data on the number, clinical stage (early or advanced), and rate of esophageal cancer detected in the screening from 2008 to 2021. Additionally, reported complications of X-ray GC screenings from 2007 to 2021 were evaluated.
To examine the time trend of each rate, the 39 years were divided into four periods: First nine years (1983–1991), second 10 years (1992–2001), third 10 years (2002–2011), and fourth 10 years (2012–2021). Each rate was compared among the four periods.
One X-ray GC screening in Hiroshima Prefecture costs approximately 3000 Japanese Yen (JPY) [21 United States Dollars (USD)]. The patient pays 30% of the total cost, with the remaining covered by the government. One EGD costs approximately 15000 JPY (110 USD). The cost of detecting one GC was calculated based on the detection rate of GC.
Hiyama T, Vu NTH, Oka S, Quach DT, and Urabe Y performed data analysis. The collected data were organized in an Excel spreadsheet (Microsoft, Redmond, WA, United States). All statistical analyses were performed using MedCalc® Statistical Software version 19.6.1 (MedCalc Software Ltd., Ostend, Belgium). Categorical variables are presented as numbers and percentages. The difference between two proportions was compared with the chi-squared test. A P-value of less than 0.05 was considered statistically significant. A Bonferroni correction (P-value of less than 0.0083) was used to compare each rate among the four periods. During the study period, Oka S, Hiyama T, and Quach DT supervised and ensured the overall integrity of the research. Vu NTH drafted the initial manuscript; Hiyama T, Quach DT, Urabe Y, and Oka S reviewed and edited the manuscript.
Ethical approval for this study was obtained from the Ethical Committee of Hiroshima University (approval No. E2023-0018).
Figure 1 shows annual trends in the number of Hiroshima Prefecture residents participating in population-based X-ray GC screening. In 1983, 39925 participants underwent the screening. This number gradually increased to 55406 people in 1991. After that, the number of participants began to decrease. There were 12923 participants remaining until 2021. During the period between 2015 and 2020, the rate of the eligible population in Hiroshima Prefecture taking part in GC screening ranged from 5.3% to 7.3%, which was average compared to other prefectures in Japan[16,17].
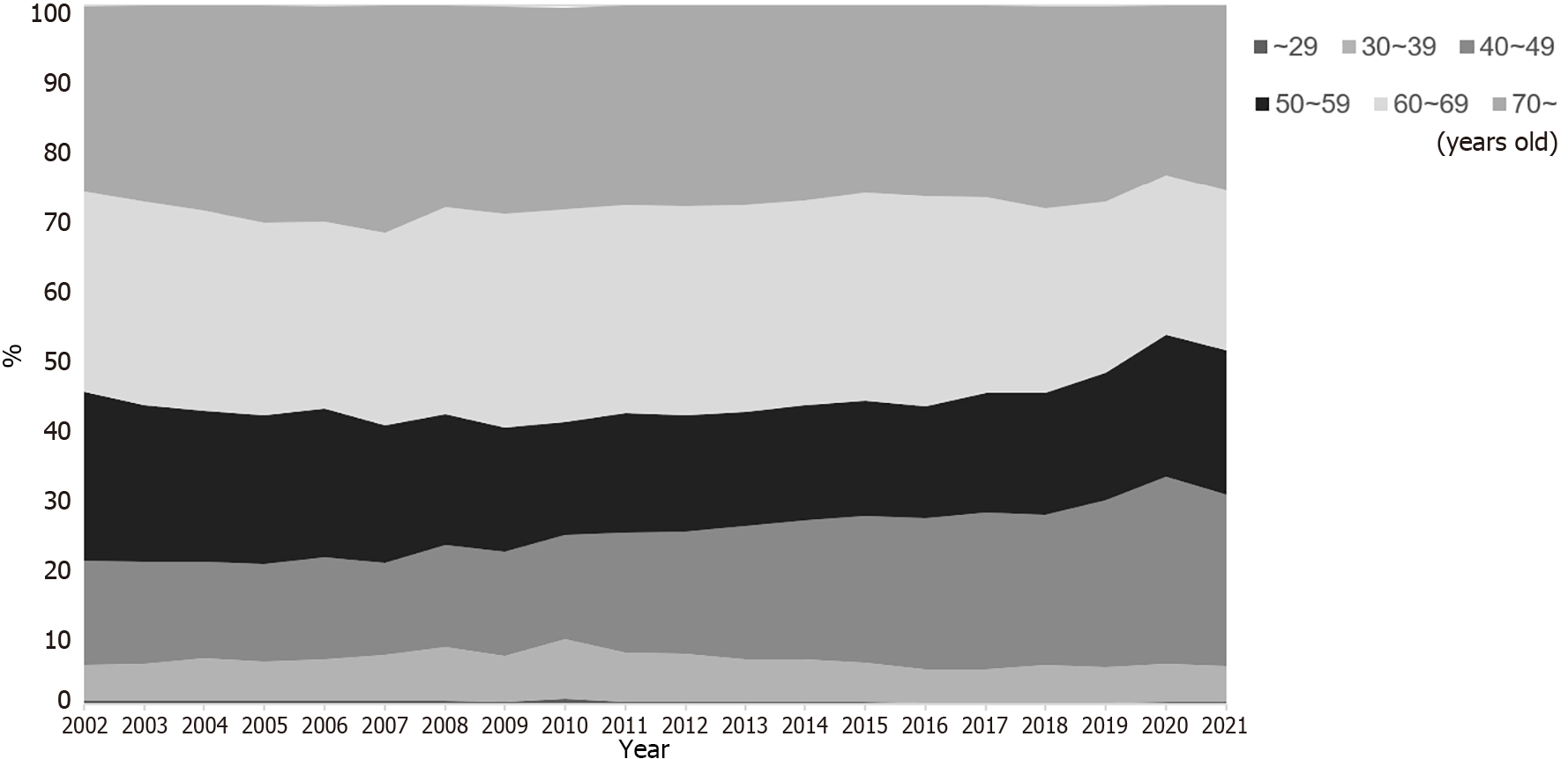
Figure 2 indicates the age distribution of the participants from 2002 and 2021. The rate of participants less than 50 years of age among all participants was 20.6% in 2002. The rate gradually increased over the years to 30.0% in 2021.
During the 10 years between 1983 and 1992, the number of screening participants who required subsequent detailed examination by EGD ranged from 6000 to 9000. After 1992, this number also declined annually. From 2012 to 2021, only 500 to 1500 participants needed an EGD (Figure 3). The rate of participants who required EGDs also decreased significantly in recent years (P < 0.001, first period: 13.0%, second: 12.0%, third: 10.4%, and fourth: 5.1%).
The number of participants with a confirmed diagnosis of GC also dropped over the last 39 years. The 76 cases of GC in 1993 were the most ever recorded. Until 2020 and 2021, the number of participants diagnosed as having GC was only 10 (Figure 4). However, the rate of cases diagnosed as GC remained around 0.1% during the study period, with no significant differences among the four periods.
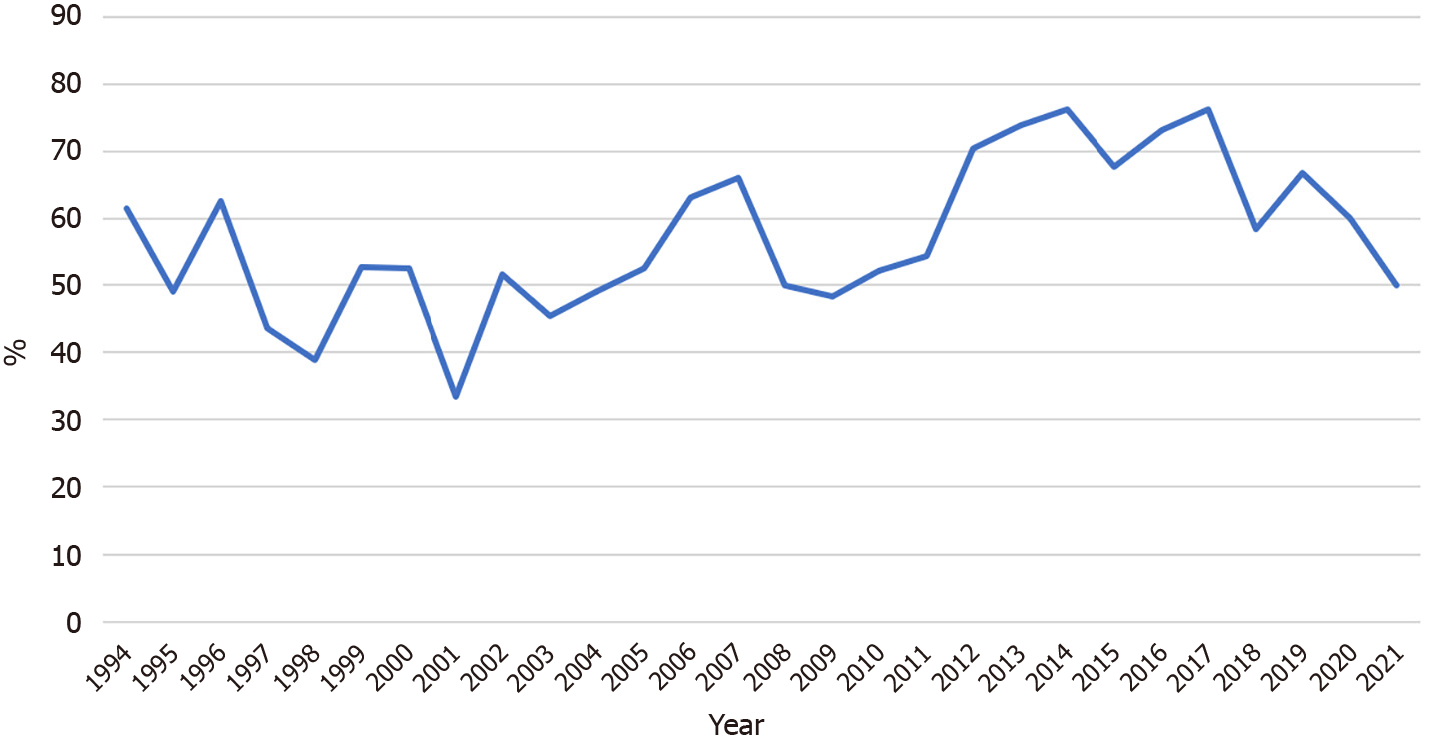
The rates of early-stage GC among all the GC detected in the screening from 1994 to 2021 are shown in Figure 5. This rate remained around 55% during the research period.
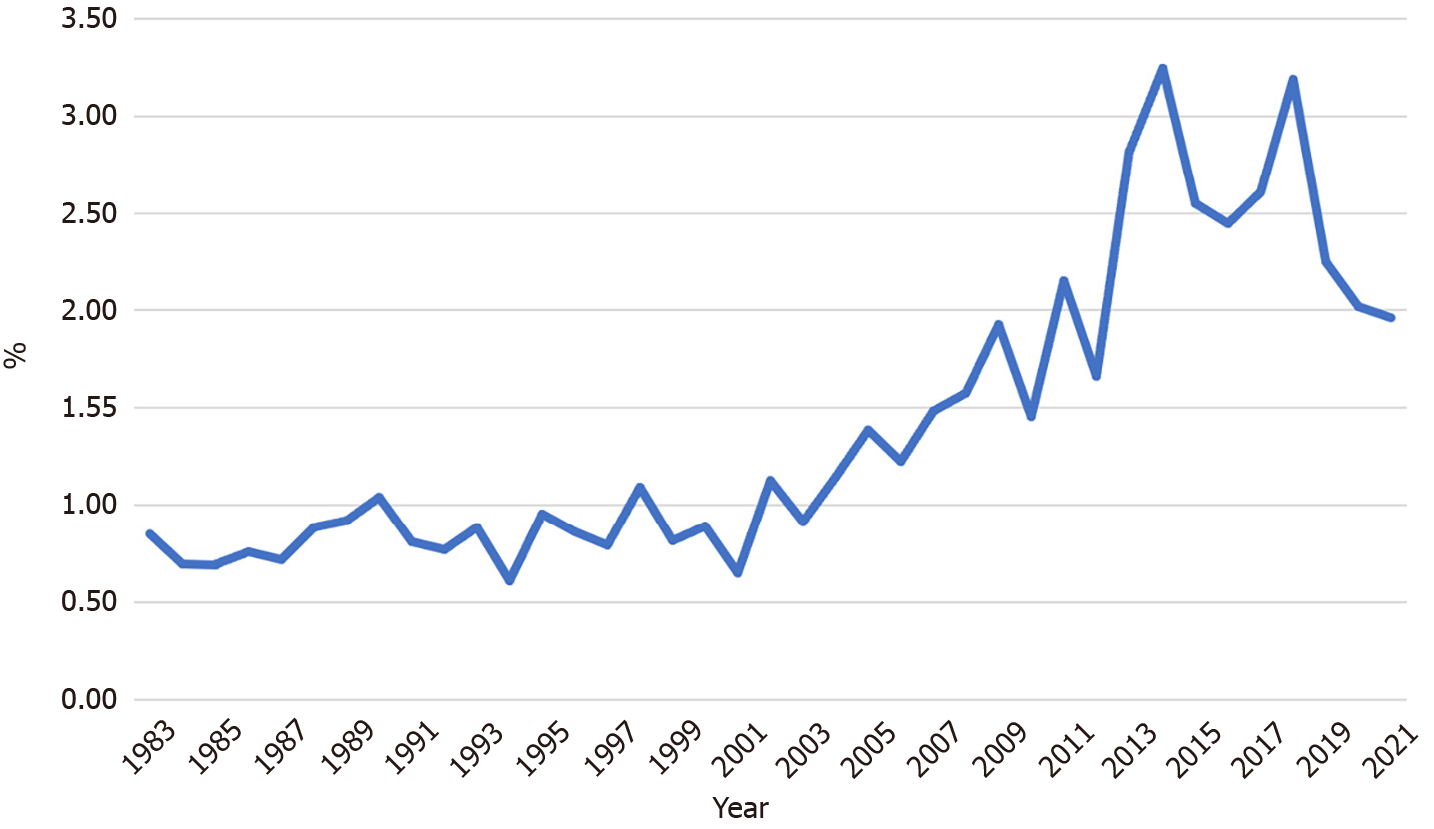
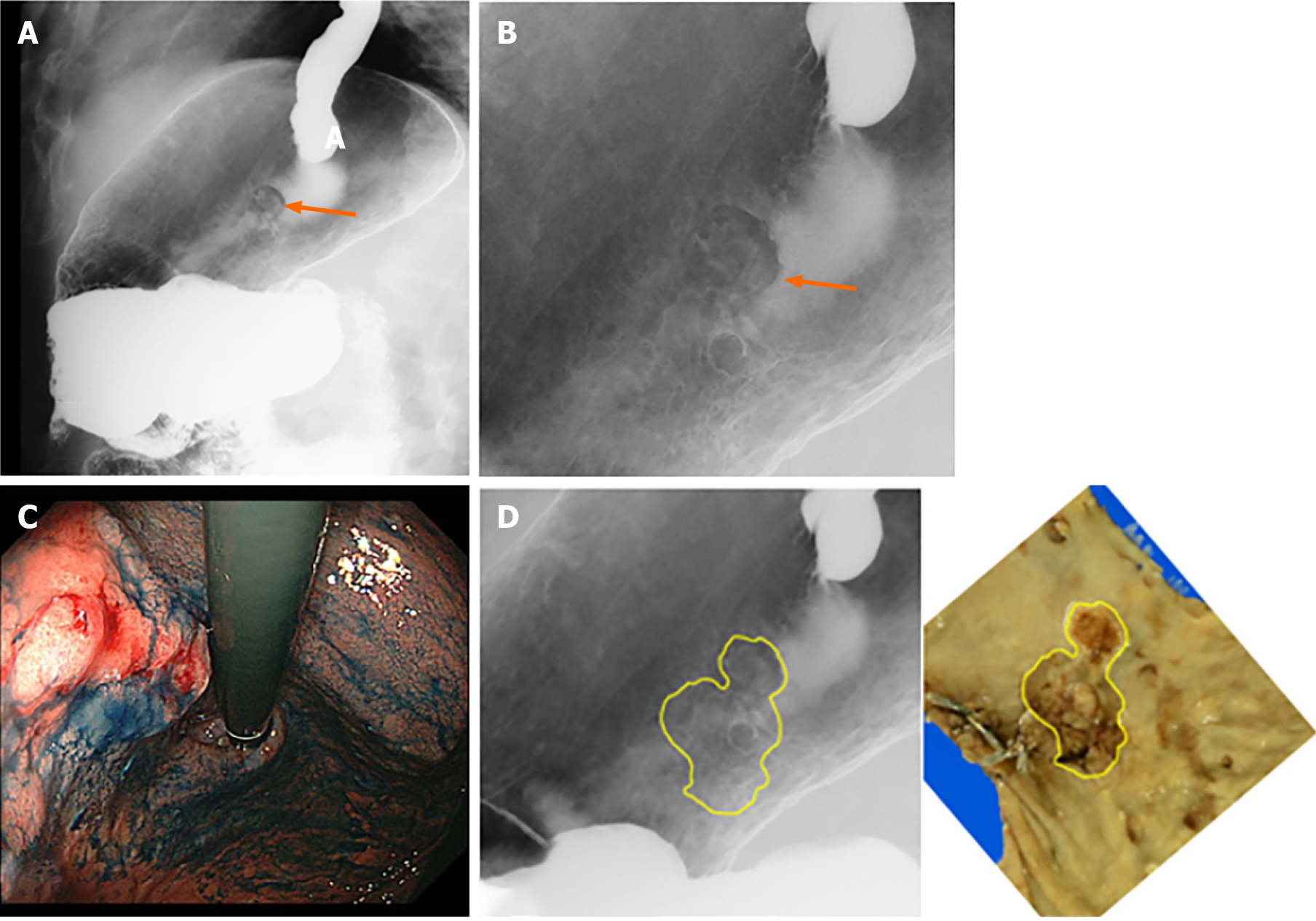
Figure 6 depicts the gradual increase in the positive predictive value of the GC screening program, from 0.8% in 1983 to 2%–3% over the last five years. The positive predictive value increased significantly in recent years except for the first period (P < 0.001, first period: 0.8%, second: 0.8%, third: 1.3%, and fourth 2.5%). Figure 7 illustrates an example of GC detected during the screening program.
The number and rate of esophageal cancers among the participants in GC screening also rose steadily from 2008 to 2021. In 2008, the rate of diagnosis was 0% (0 cases), but it grew to 0.02%, one-fifth of the diagnosis rate of GC, between 2017 and 2021 (3–4 cases/year). During the observation period, 28 esophageal cancers were detected in total. Among them, 9 (32%) were in the early stages, 17 (61%) were in advanced stages, and the remaining 2 (7%) were in an unknown clinical stage. Among all esophageal cancers, the rate of early-stage esophageal cancers fluctuated, and no consistent trend could be observed during the period due to the limited number of patients per year (data not shown).
As for complications of X-ray GC screenings from 2007 to 2021, 9 cases were reported: 4 cases of intestinal obstruction due to barium, 2 cases of barium aspiration, 2 cases of rib fracture due to compression, and 1 case of barium reflux into the common bile duct. The complication rate was 0.003% among the 335873 residents who underwent X-ray GC screening during the period.
The detection rate of GC was approximately 0.1%. In other words, detection of one GC required the performance of approximately 1000 gastric X-ray screenings. Regarding the cost aspect, detecting one GC cost approximately 3000000 JPY (21400 USD) for the gastric X-ray screenings [approximately 3000 JPY (21.4 USD) per X-ray screening]. The cost of performing EGDs should also be considered. Approximately 8% of the applicants required EGDs; thus, 80 EGDs were needed to detect one GC, the cost of which was approximately 1200000 JPY (8600 USD) [approximately 15000 JPY (107 USD) per EGD]. Thus, the total cost to diagnose one incidence of GC using X-ray screenings and EGDs was approximately 4200000 JPY (30000 USD).
GC development is an interactive, multistep, and multifactorial process[18,19] with a variety of factors influencing its development. Previous research has shown that more than 90% of GC cases in Japan were associated with H. pylori infection[20]. Other factors, including family history, diet, lifestyle choices, genetics, socioeconomic status, and other environmental factors, also contribute to the formation of GC[21]. The development of GC is a gradual process, including the formation of precancerous lesions. This involves sequential histopathological changes in the gastric mucosa, beginning with atrophic gastritis and the loss of the parietal cell mass and followed by intestinal metaplasia, dysplasia, and, ultimately, carcinoma[22]. The primary prevention strategies for GC include intervention in GC etiology by improving dietary habits and reducing the incidence of H. pylori infection. Consuming more fresh fruit and vegetables and limiting salt and salt-preserved foods may reduce the risk of GC. Further, lifestyle modifications such as increasing physical activity and restricting smoking may also decrease the risk of GC[21]. Secondary prevention efforts should focus on early detection and treatment of GC.
There is considerable regional variation in GC mortality and incidence, with Eastern Asia and Eastern Europe having the highest age-standardized incidence rates[23]. GC screening has been initiated in countries with a high prevalence of GC, such as Japan and Korea[24]. The implementation of GC screening in Asia subsequently increased the diagnosis of early GC, reduced mortality, and improved 5-year survival[11,25]. Previous studies have also indicated a reduced risk of GC mortality in patients undergoing X-ray GC screening[14,26]. In a population-based X-ray GC screening study with a 13-year follow-up in Japan, Lee et al[27] concluded that the screened participants had a 48% reduced risk of mortality from GC compared to the unscreened participants[27]. Additionally, X-ray GC screening has been shown to be as equally effective as endoscopic screening in reducing GC mortality[13,28].
The present study indicated that although the number of participants in population-based X-ray GC screening in Hiroshima Prefecture had fallen during the last 39 years, the screening program has still been efficient. Our findings were consistent with the fiscal 2015–2018 nationwide aggregate surveys of the Japanese Society of Gastrointestinal Cancer Screening. The rate of GC in these surveys ranged from 0.13% to 0.16%[16].
The downtrend in participants may be due to the fact that the residents have had the option to participate in GC screening programs at private facilities rather than those offered by the Hiroshima Regional Health Medical Promotion Organization. Additionally, endoscopic GC screening has become widespread recently. Another reason is that the incidence and mortality from GC in Japan has also decreased annually. As a result, residents’ awareness of GC also decreased, leading them to believe that attending a screening may be unnecessary. This downtrend may also be attributable to the annual decline of Hiroshima Prefecture’s population. The population peaked at 2.89 million in 1998 and gradually declined to 2.77 million in 2022[15].
During the study period, the positive predictive value of GC screening also grew gradually. Annual radiographic screening for all adults over 40 was established in the 1960s as a secondary preventive measure for GC in Japan[12]. The use of high-concentration, low-viscosity barium preparations, double-contrast radiography, and the introduction of digital X-ray devices have substantially enhanced the visibility of lesions on gastric radiographic examinations. The utilization of high-concentration thin barium sulfate has become prevalent in Japan since the 2000s. The Japanese Society of Gastrointestinal Cancer Screening published new guidelines on imaging methods to recommend this use for GC screening in 2005[29]. Furthermore, the transition from traditional film to digital images occurred during the 2000s and the 2010s[30]. These modifications have the potential to enhance the detection rate of minor lesions. This Japanese radiographic screening technology has been highly regarded internationally[31] and has been shown in two case-control studies to reduce GC mortality[14,26]. It was also mentioned in the Japanese Guidelines for GC Screening in 2005 as a modality associated with reduced GC mortality[11].
In our study, the number of participants with a confirmed diagnosis of GC decreased, whereas the rate of cases diagnosed as GC remained at around 0.1% among all participants. The prevalence of H. pylori infection has decreased in Japanese populations year by year. H. pylori infection is a definite carcinogen; therefore, thinking simply, the number of GC patients is expected to decrease year by year as well. However, our data did not indicate a decrease; rather, the rate remained stable at around 0.1% among all participants. There are two possible reasons for this. The first reason may be improvements in X-ray imaging and diagnostic skills, and the second may be changes in the participants. It is conceivable that the number of participants infected with H. pylori and/or gastrointestinal symptoms, who are at high risk of GC, might have increased.
One of the challenges for the future is whether X-ray screening should be replaced by endoscopic screening. The revised 2018 Japanese Guidelines have also approved endoscopic examinations for GC screening[32]. Even though endoscopic screening detected more cases of GC than radiographic screening, there was no significant difference in the reduction of GC mortality between the two screening modalities[13]. Another study reported that radiographic screening is as effective as endoscopic screening in reducing GC mortality[28]. Further, it is impossible to replace all conventional radiography with endoscopic examinations because of issues with endoscopy capacity, budget, and the high human resource of endoscopists[12,33,34]. In the Japanese medical system, upper endoscopic screening is five-fold more expensive than gastric X-ray screening [approximately 15000 JPY (110 USD) vs 3000 JPY (21 USD) per applicant]. There is also a need to establish a certification system for screening endoscopists as well as physician education on endoscopic screening and image interpretation. However, endoscopic screening also has some limitations, including complications and overdiagnosis. Serious complications may even lead to death. Infection control is also essential, so the endoscope needs to be appropriately cleaned[32]. X-ray GC screening has recently utilized imaging and artificial intelligence (AI) to detect H. pylori-infected gastritis and gastric mucosal atrophy[35]. This will enhance the diagnostic efficiency of X-ray GC screening. Therefore, it may be necessary to continue using radiographic examinations with a high processing capacity for population-based GC screening.
The risk of developing GC depends on the background condition, particularly that of H. pylori infection and gastric mucosal atrophy[36]. Because of the sharp drop in H. pylori infection rates, the background of GC risk has changed compared to the past[6,7,37-39]. Recently, the prevalence of H. pylori infection in Japan has been shown to vary by birth year, with those born in the 1970s or later having a low prevalence[37]. According to recent Japanese research, the risk of the cumulative incidence of GC in the H. pylori-infected population was 17.0% in men, 7.7% in women, but < 1% in the non-infected population[40]. As a result, identifying patients as H. pylori-infected should be required for effective GC screening[38,41,42]. GC screening targeting all populations of a certain age every year may become inefficient, and there may be a cost-effectiveness issue.
Currently, one case of X-ray GC screening in Japan costs approximately 3000 JPY (21 USD). Compared to the other cancer screening programs in Hiroshima Prefecture, the cost of GC screening is equivalent to that of cervical cancer screening. Nevertheless, the expenses of GC screening are higher than those for lung and colon cancer screening and lower than those for breast cancer screening. Lung and colon cancer screenings cost around 1100 JPY (8 USD), whereas breast cancer screening costs nearly 4300 JPY (31 USD).
Another finding in our study is that the number and rate of esophageal cancers among the participants in GC screening have increased recently. The incidence of esophageal cancer varies across regions and populations. The regions of Eastern Asia, Southern Africa, and Eastern Africa have exhibited the highest incidence rates. Specific risk factors, including tobacco use, alcohol consumption, and hot beverage consumption, are probable contributors to the high incidence rates in these regions[43]. In Japan, an estimated 26600 individuals were newly diagnosed as having esophageal cancer, and 11100 deaths were attributed to this cancer in 2021[8]. The best outcomes for esophageal cancer are associated with early diagnosis, commonly known as “early stages”[44]. Therefore, screening and early detection are critical for esophageal cancer control in high-risk populations[43]. Unfortunately, there are currently no established guidelines for esophageal cancer screening in Japan. Hence, individuals with esophageal cancer risk factors, such as high consumption of tobacco, alcohol, or hot beverages, may be encouraged to have additional esophageal X-rays during GC screening.
Our results showed that the detection rate of GC was approximately 0.1%, and, as a result, the cost for one case of GC diagnosed was approximately 4200000 JPY (30000 USD), including X-rays and EGDs, not a small amount. According to the National Cancer Center Japan report, the estimated 5-year survival rate of all GC patients in Japan, including those with early and advanced stages, is 65%[8]. If this percentage were to hold in the present study, 65% of diagnosed GC patients would survive for five years after the screening. In that case, the cost would rise to about 6500000 JPY (46400 USD) to diagnose one GC patient who remained alive for five years after the screening. Therefore, in the future, it may be necessary to stratify individuals based on their GC risk by identifying risk factors, such as a history of H. pylori infection and gastric mucosal atrophy, and determining the screening interval. For individuals with a low risk of GC, such as those never infected with H. pylori and who have no gastric mucosal atrophy, their screening interval could be lengthened or perhaps they could be eliminated from population-based GC screening. This may lead to major cost savings for the government and the participants. The ABC method of GC risk stratification has been applied in some areas in Japan. However, H. pylori-eradicated cases are classified as having low risk of GC by the ABC method[45]. The number of H. pylori-eradicated cases has increased, and even after eradication, the risk of GC remains relatively high if the grade of atrophy of the gastric mucosa is high[46]. Development of a simple, low-cost method for determining GC risk is desired.
Several future challenges lie ahead for X-ray GC screening in Japan. Improving the sensitivity and specificity of the imaging technology to detect early-stage GC is essential to enhancing the quality of screening. Improvements to the quality of machine learning algorithms and AI can potentially increase the accuracy of X-ray interpretation for GC detection. In addition, reducing the radiation exposure associated with X-ray screening is an important consideration for patient safety. Furthermore, one of the upcoming challenges is to enhance the engagement of citizens in the screening programs. Moreover, it is critical to prioritize the resolution of financial obstacles to establish an effective population-based screening program.
There are several limitations in our study. First, the ultimate purpose of GC screening is to reduce the mortality rate of GCs in the target populations. However, the mortality rate of GCs was not assessed in this study. To examine the mortality rate, different approaches, such as examining cancer registries, are needed. This issue will be the next research topic. Second, in a few applicants, it was unknown whether they had undergone EGDs and the results thereof. Because this number was small, it may be considered to be a very small bias. Third, we still need to obtain detailed data, such as the location of the cancers, for this study. However, the primary purpose of this study is to examine the major trends over the last four decades. The details are for future studies. Fourth, the cost to detect one GC should include not only the cost of X-ray gastric screenings and EGDs but also the cost of treatments for complications. We had no data on this cost. However, the cost may be ignored because rate of complications for X-ray GC screening shown by our study was quite low at 0.003%.
In conclusion, GC screening in Hiroshima Prefecture continues to be efficient. However, one of the challenges is the cost. Therefore, risk stratification may be needed, such as eliminating from screening participants never infected with H. pylori and those without gastric mucosal atrophy. Esophageal cancers may also need to be considered because there has been a gradual increase in their incidence in recent years.
Gastric cancer (GC) is the fifth most common cancer worldwide and has the fourth highest mortality rate. In Japan, population X-ray GC screening has been shown to decrease mortality. This screening has been performed in Hiroshima Prefecture since 1983; however, the time trends and the efficacy of the method over 39 years have not been assessed.
The updated version of the Japanese Guidelines for GC Screening recommended both radiographic screening and endoscopic screening for population-based GC screening. Although more cases of GC have been detected with endoscopic rather than radiographic screening, some studies reported that the reduction in GC mortality was not significantly different between the two screening methods. Endoscopic screening also has some limitations, including complications and overdiagnosis. Therefore, it may be necessary to continue using radiographic examinations with a high processing capacity for population-based GC screening.
This study aimed to evaluate the trends and efficacy of population-based X-ray GC screening in Hiroshima Prefecture for the last 39 years, from 1983 to 2021, to identify the challenges and develop future solutions. These findings may provide valuable insights for early detection, improving treatment outcomes, evaluating and improving screening programs, and enhancing public health awareness and education about GC.
This was a population-based retrospective study. The data were derived from the aggregated data of the Hiroshima Regional Health Medical Promotion Organization, Hiroshima, Japan. High-resolution double-contrast agents for the upper gastrointestinal tract were utilized for the X-ray examination in Hiroshima Prefecture. Participants with a suspicious X-ray abnormality (cancers or other gastric lesions such as ulcers) were recommended to undergo detailed examination with esophagogastroduodenoscopy (EGD).
The number of participants has decreased during the last four decades. The rate of those requiring EGDs decreased significantly in recent years. The number of participants diagnosed as having GC has also declined. However, the rate of cases diagnosed as GC among the participants remained around 0.1%, and the positive predictive value increased significantly in recent years. The number and rate of accidentally detected esophageal cancers have risen recently.
GC screening in Hiroshima Prefecture continues to be efficient. However, one of the challenges is the cost. Therefore, risk stratification may be needed, such as eliminating from screening participants never infected with Helicobacter pylori and those without gastric mucosal atrophy. Esophageal cancers may also need to be considered because they have gradually increased in recent years.
It is crucial to conduct multicenter, prospective, and follow-up studies to determine the efficacy of population-based X-ray GC screening and propose suitable solutions to improve GC screening in Japan.
The authors thank Shinji Tanaka, Honorary Professor, Hiroshima University, and President of the Japan Gastroenterological Endoscopy Society; Masaharu Yoshihara, Honorary Professor, Hiroshima University; and Kenji Nakagaki, Takayuki Harakawa, and Hiroya Matsuoka, Hiroshima Regional Health Medical Promotion Organization, for their support.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68525] [Article Influence: 13705.0] [Reference Citation Analysis (201)] |
| 2. | Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14:26-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 773] [Article Influence: 96.6] [Reference Citation Analysis (1)] |
| 3. | Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, Ogunbiyi OJ, Azevedo E Silva G, Chen WQ, Eser S, Engholm G, Stiller CA, Monnereau A, Woods RR, Visser O, Lim GH, Aitken J, Weir HK, Coleman MP; CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2711] [Cited by in RCA: 3686] [Article Influence: 460.8] [Reference Citation Analysis (1)] |
| 4. | Katanoda K, Hori M, Saito E, Shibata A, Ito Y, Minami T, Ikeda S, Suzuki T, Matsuda T. Updated Trends in Cancer in Japan: Incidence in 1985-2015 and Mortality in 1958-2018-A Sign of Decrease in Cancer Incidence. J Epidemiol. 2021;31:426-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 5. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3246] [Article Influence: 129.8] [Reference Citation Analysis (1)] |
| 6. | Kamada T, Haruma K, Ito M, Inoue K, Manabe N, Matsumoto H, Kusunoki H, Hata J, Yoshihara M, Sumii K, Akiyama T, Tanaka S, Shiotani A, Graham DY. Time Trends in Helicobacter pylori Infection and Atrophic Gastritis Over 40 Years in Japan. Helicobacter. 2015;20:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Lin Y, Kawai S, Sasakabe T, Nagata C, Naito M, Tanaka K, Sugawara Y, Mizoue T, Sawada N, Matsuo K, Kitamura T, Utada M, Ito H, Shimazu T, Kikuchi S, Inoue M; Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan. Effects of Helicobacter pylori eradication on gastric cancer incidence in the Japanese population: a systematic evidence review. Jpn J Clin Oncol. 2021;51:1158-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | National Cancer Center. Center for Cancer Control and Information Services (in Japanese). 2022. [cited 1 Jan 2024]. Available from: https://ganjoho.jp/reg_stat/statistics/stat/cancer/5_stomach.html#anchor1. |
| 9. | Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh KG, Goh KL, Wu KC, Wu DC, Sollano J, Kachintorn U, Gotoda T, Lin JT, You WC, Ng EK, Sung JJ; Asia Pacific Working Group on Gastric Cancer. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 660] [Article Influence: 36.7] [Reference Citation Analysis (1)] |
| 10. | Kim Y, Jun JK, Choi KS, Lee HY, Park EC. Overview of the National Cancer screening programme and the cancer screening status in Korea. Asian Pac J Cancer Prev. 2011;12:725-730. [PubMed] |
| 11. | Hamashima C, Shibuya D, Yamazaki H, Inoue K, Fukao A, Saito H, Sobue T. The Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol. 2008;38:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 281] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 12. | Hamashima C. Cancer screening guidelines and policy making: 15 years of experience in cancer screening guideline development in Japan. Jpn J Clin Oncol. 2018;48:278-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 13. | Hagiwara H, Moki F, Yamashita Y, Saji K, Iesaki K, Suda H. Gastric cancer mortality related to direct radiographic and endoscopic screening: A retrospective study. World J Gastroenterol. 2021;27:5595-5609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Fukao A, Tsubono Y, Tsuji I, HIsamichi S, Sugahara N, Takano A. The evaluation of screening for gastric cancer in Miyagi Prefecture, Japan: a population-based case-control study. Int J Cancer. 1995;60:45-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Nagasaki N, Ito M, Boda T, Kotachi T, Takigawa H, Oka S, Tanaka S. Identification of Helicobacter pylori-related gastric cancer risk using serological gastritis markers and endoscopic findings: a large-scale retrospective cohort study. BMC Gastroenterol. 2022;22:299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | The Japanese Society of Gastrointestinal Cancer Screening. Results of the Gastric Cancer Screening Survey (in Japanese). [cited 2 Jan 2023]. Available from: https://www.jsgcs.or.jp/files/uploads/2019zenkoku_igan.pdf. |
| 17. | Hirohima Cancer Net. Gastric Cancer Screening (in Japanese). Aug 6, 2021. [cited 2 Jan 2023]. Available from: https://www.pref.hiroshima.lg.jp/site/gan-net/yoboukenshin-jushinritsu-jushinritsu.html#i. |
| 18. | Zhang P, Yang M, Zhang Y, Xiao S, Lai X, Tan A, Du S, Li S. Dissecting the Single-Cell Transcriptome Network Underlying Gastric Premalignant Lesions and Early Gastric Cancer. Cell Rep. 2019;27:1934-1947.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 304] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 19. | Venerito M, Vasapolli R, Rokkas T, Malfertheiner P. Gastric cancer: epidemiology, prevention, and therapy. Helicobacter. 2018;23:e12518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 20. | Shirani M, Pakzad R, Haddadi MH, Akrami S, Asadi A, Kazemian H, Moradi M, Kaviar VH, Zomorodi AR, Khoshnood S, Shafieian M, Tavasolian R, Heidary M, Saki M. The global prevalence of gastric cancer in Helicobacter pylori-infected individuals: a systematic review and meta-analysis. BMC Infect Dis. 2023;23:543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 21. | Iwu CD, Iwu-Jaja CJ. Gastric cancer epidemiology: Current trend and future direction. Hygiene. 2023;3:256-268. [DOI] [Full Text] |
| 22. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 3294] [Article Influence: 549.0] [Reference Citation Analysis (6)] |
| 23. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56651] [Article Influence: 7081.4] [Reference Citation Analysis (134)] |
| 24. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20718] [Article Influence: 1883.5] [Reference Citation Analysis (23)] |
| 25. | Russo AE, Strong VE. Gastric Cancer Etiology and Management in Asia and the West. Annu Rev Med. 2019;70:353-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 26. | Oshima A, Hirata N, Ubukata T, Umeda K, Fujimoto I. Evaluation of a mass screening program for stomach cancer with a case-control study design. Int J Cancer. 1986;38:829-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 107] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Lee KJ, Inoue M, Otani T, Iwasaki M, Sasazuki S, Tsugane S; JPHC Study Group. Gastric cancer screening and subsequent risk of gastric cancer: a large-scale population-based cohort study, with a 13-year follow-up in Japan. Int J Cancer. 2006;118:2315-2321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 149] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 28. | Matsumoto S, Ishikawa S, Yoshida Y. Reduction of gastric cancer mortality by endoscopic and radiographic screening in an isolated island: A retrospective cohort study. Aust J Rural Health. 2013;21:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Imamura K, Ohashi S, Kitagawa S, Gotou H, Shibuya D, Sugino Y. A new stomach radiography guideline, Stomach Radiography Standardization Committee. The Japan Society of Gastroenterological Cancer Screening. Tokyo: Medical Review Company, 2005. |
| 30. | The Japanese Society of Gastrointestinal Cancer Screening. New Guidelines of Radiography for Gastric Cancer Screening. 2011. [cited 1 Jan 2024]. Available from: https://www.jsgcs.or.jp/about/notation/index. |
| 31. | Chamberlain J, Day NE, Hakama M, Miller AB, Prorok PC. UICC workshop of the Project on Evaluation of Screening Programmes for Gastrointestinal Cancer. Int J Cancer. 1986;37:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Hamashima C; Systematic Review Group and Guideline Development Group for Gastric Cancer Screening Guidelines. Update version of the Japanese Guidelines for Gastric Cancer Screening. Jpn J Clin Oncol. 2018;48:673-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 296] [Article Influence: 37.0] [Reference Citation Analysis (3)] |
| 33. | Hamashima C, Goto R. Potential capacity of endoscopic screening for gastric cancer in Japan. Cancer Sci. 2017;108:101-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 34. | Mabe K, Inoue K, Kamada T, Kato K, Kato M, Haruma K. Endoscopic screening for gastric cancer in Japan: Current status and future perspectives. Dig Endosc. 2022;34:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 35. | Togo R, Yamamichi N, Mabe K, Takahashi Y, Takeuchi C, Kato M, Sakamoto N, Ishihara K, Ogawa T, Haseyama M. Detection of gastritis by a deep convolutional neural network from double-contrast upper gastrointestinal barium X-ray radiography. J Gastroenterol. 2019;54:321-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 36. | Terasawa T, Nishida H, Kato K, Miyashiro I, Yoshikawa T, Takaku R, Hamashima C. Prediction of gastric cancer development by serum pepsinogen test and Helicobacter pylori seropositivity in Eastern Asians: a systematic review and meta-analysis. PLoS One. 2014;9:e109783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 37. | Ueda J, Gosho M, Inui Y, Matsuda T, Sakakibara M, Mabe K, Nakajima S, Shimoyama T, Yasuda M, Kawai T, Murakami K, Kamada T, Mizuno M, Kikuchi S, Lin Y, Kato M. Prevalence of Helicobacter pylori infection by birth year and geographic area in Japan. Helicobacter. 2014;19:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 38. | Sugano K. Screening of gastric cancer in Asia. Best Pract Res Clin Gastroenterol. 2015;29:895-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 39. | Wang C, Nishiyama T, Kikuchi S, Inoue M, Sawada N, Tsugane S, Lin Y. Changing trends in the prevalence of H. pylori infection in Japan (1908-2003): a systematic review and meta-regression analysis of 170,752 individuals. Sci Rep. 2017;7:15491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 40. | Kawai S, Wang C, Lin Y, Sasakabe T, Okuda M, Kikuchi S. Lifetime incidence risk for gastric cancer in the Helicobacter pylori-infected and uninfected population in Japan: A Monte Carlo simulation study. Int J Cancer. 2022;150:18-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (2)] |
| 41. | Mizota Y, Yamamoto S. How long should we continue gastric cancer screening? From an epidemiological point of view. Gastric Cancer. 2019;22:456-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Kishikawa H. The Clinical Benefits, Limitations, and Perspectives of the ABC Method. Intern Med. 2020;59:1471-1472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Morgan E, Soerjomataram I, Rumgay H, Coleman HG, Thrift AP, Vignat J, Laversanne M, Ferlay J, Arnold M. The Global Landscape of Esophageal Squamous Cell Carcinoma and Esophageal Adenocarcinoma Incidence and Mortality in 2020 and Projections to 2040: New Estimates From GLOBOCAN 2020. Gastroenterology. 2022;163:649-658.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 796] [Cited by in RCA: 739] [Article Influence: 184.8] [Reference Citation Analysis (1)] |
| 44. | Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1956] [Cited by in RCA: 2001] [Article Influence: 153.9] [Reference Citation Analysis (5)] |
| 45. | Kwon H, Lee SY, Kim JH, Lee SP, Sung IK, Park HS, Shim CS. ABC Classification Is Less Useful for Older Koreans Born before 1960. Gut Liver. 2019;13:522-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Ford AC, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta-analysis. Gut. 2020;69:2113-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 324] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang B, China S-Editor: Li L L-Editor: A P-Editor: Yu HG