Published online Nov 24, 2024. doi: 10.5306/wjco.v15.i11.1428
Revised: September 11, 2024
Accepted: September 25, 2024
Published online: November 24, 2024
Processing time: 177 Days and 22.9 Hours
Intracranial epidermoid cyst (IEC) transformation to malignant squamous cell carcinoma (SCC) is extremely rare, and its etiology is yet unknown. Currently, SCC is treated by performing surgery, followed by a combination of radiotherapy and chemotherapy. It is crucial to identify efficient and trustworthy therapeutic targets for SCC to improve its diagnosis, prognosis, and treatment.
In this study, we report the case of a 47-year-old female patient with SCC, which progressed from IEC in the left internal capsule region. The patient was sought treatment at our hospital for severe diplopic vision, accompanied with speech disorder and memory loss. Based on the clinical and postoperative pathology, this patient was finally diagnosed with SCC. To identify disease-causing variants, whole exome sequencing (WES) was performed on the proband. WES revealed two pathogenic missense mutations on Gap junction protein beta 2 (GJB2) (c.257C>T) and Toll-like receptor 2 (TLR2) (c.1039A>G), respectively.
This study provided the first clinical evidence for demonstrating the role of GJB2 and TLR2 in IEC development and treatment. We further confirmed WES as a robust and reliable technique for underlying rare and complex disease-related genetic factor identification.
Core Tip: Intracranial epidermoid cyst (IEC) malignant transformation is an uncommon lesion, accompanied by the spread of tumor cells, resulting in a poor prognosis. In this report, we present the case of a 47-year-old woman who was diagnosed with a malignant transition from IEC to squamous cell carcinoma. After surgical resection and chemoradiotherapy, the patient's condition was effectively controlled. It's noteworthy that whole exome sequencing revealed the significant role of Gap junction protein beta 2 and Toll-like receptor 2 in IEC development.
- Citation: Song ZN, Cheng Y, Wang DD, Li MJ, Zhao XR, Li FW, Liu Z, Zhu XR, Jia XD, Wang YF, Liang FF. Whole exome sequencing identifies risk variants associated with intracranial epidermoid cyst deterioration: A case report. World J Clin Oncol 2024; 15(11): 1428-1434
- URL: https://www.wjgnet.com/2218-4333/full/v15/i11/1428.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i11.1428
Intracranial epidermoid cyst (IEC) is a benign tumor involving the embryonic remnant tissue[1], and it constitutes approximately 0.2%-1.8%[2] of all brain tumors. It is most prevalent within the cerebellopontine angle region, and it is associated with a slow progression and long disease course[3,4]. Generally, surgical intervention can achieve complete resection and lead to a favorable prognosis. The occurrence of IEC malignant transformation is infrequent[4], and it does not exhibit any specific clinical signs or imaging characteristics. Following undetected deterioration, tumor cells typically spread through the cerebrospinal fluid to cause extensive intracranial dissemination and poor clinical results.
Whole exome sequencing (WES) is a robust and reliable tool for rare and complex disease-related genetic variant screening[5]. The exon region constitutes less than 2% of the human genome, but it harbors about 85% of disease-associated variants. Due to its ability to process a large amount of data quickly and at a lower cost, WES is a highly efficient substitute for whole genome sequencing. As a result, it is increasingly applied to enhance scientific and clinical research[6,7].
Here, we report a rare case of IEC malignant transformation to squamous cell carcinoma (SCC). Using WES, potential disease-causing variations were identified in the patient. This investigation was carried out with full consent from the patient.
A 47-year-old female patient sought treatment at our hospital for severe diplopia, along with speech disorder, memory loss, slow reaction, irritability, deterioration in the strength of her right limb muscles, nausea, and vomiting which spanned over a one-month period.
The patient complained of falling twice, but denied any symptoms of visual impairment, loss of consciousness, limb convulsions, choking while drinking, and urinary incontinence.
The patient reported no history of surgery, trauma, significant infections, or other notable medical conditions.
The patient had no personal or family history.
The patient had severe diplopia and decline in the strength of her right limb muscles.
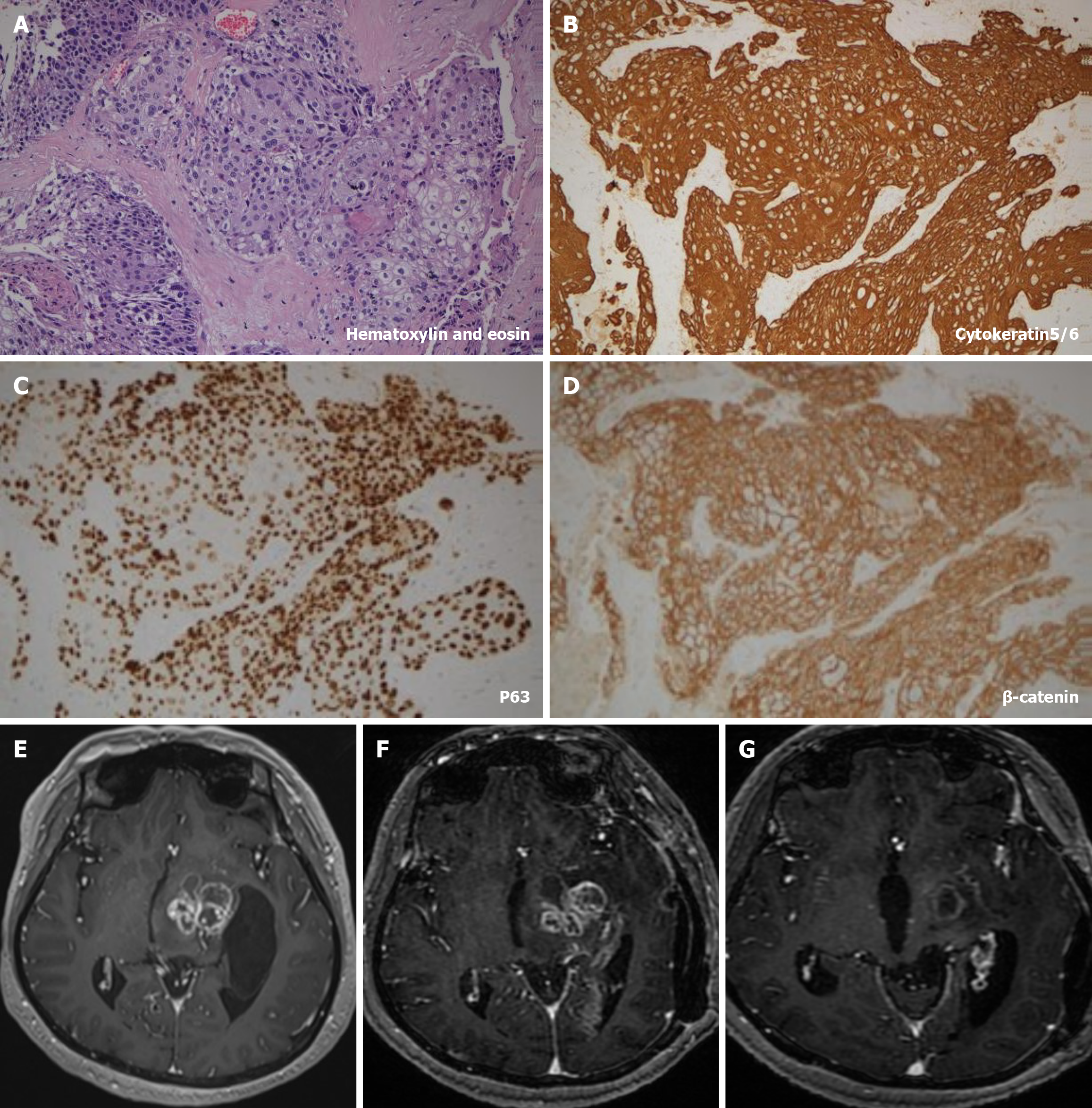
The postoperative pathology (Figure 1A-D), which included both hematoxylin and eosin and immunohistochemistry staining [cytokeratin5/6 (+), P63 (+), β-catenin (+), Ki-67: 40%], confirmed the presence of the tumor.
The contrast-enhanced magnetic resonance imaging (MRI) of brain (Figure 1E) revealed solid masses in the left basal ganglia and thalamus, and showed multiple circular intensification, with significant invasion surrounding normal brain tissue.
To identify potential pathogenic variants, we performed paired-end WES on a Nextseq CN500 instrument (Illumina) using genomic DNA from tumor tissue and peripheral blood leukocytes. After multiple purification and amplification rounds, the final library fragment size was approximately between 250-350 bps. The mean sequencing depth was 86.3X, and the average read number was 9.5 × 107 bps.
Following the completion of quality control and sequence alignment of the raw reads, we employed GATK-Haplotype Caller (v4.1.5.0) to detect single nucleotide polymorphisms and insertions/deletions, and utilized ANNOVAR to annotate variants. Variants were filtered based on the following criteria: (1) Any variants with a minor allele frequency ≥ 0.01 within the 1000 Genomes, ESP, or ExAC databases were eliminated from analysis; (2) Variants annotated as synonymous single nucleotide variants in the refGene database were eliminate; (3) Only variants found within the exonic, splicing, 5'-untranslated region (UTR), UTR3, and splicing regions were considered; (4) Prediction regarding the variated-mediated effect on protein coding utilized Sorting Intolerant from Tolerant, LRT, Mutation Assessor, Mutation Taster, FATHMM, and PROVEAN, and retained variants estimated to be deleterious by ≥ 2 software tools; and (5) Computed the combined annotation dependent depletion (CADD) score for individual variants, and filtered out variants with CADD_PHRED < 2. Using the aforementioned strict criteria, we identified heterozygous variants within the Gap junction protein beta 2 (GJB2) (c.257C>T) (p.Thr86Met) and Toll-like receptor 2 (TLR2) (c.1039A>G) (p.Lys347Glu) genes as potential disease-causing mutations (Table 1).
| Chr | Position | Gene | Transcript | cDNA | Amino acid |
| Chr4 | 153703946 | Toll-like receptor 2 | NM_001318789: exon3 | 1039A>G | 347K>E |
| Chr13 | 20189325 | Gap junction protein beta 2 | NM_004004: exon2 | 257C>T | 86T>M |
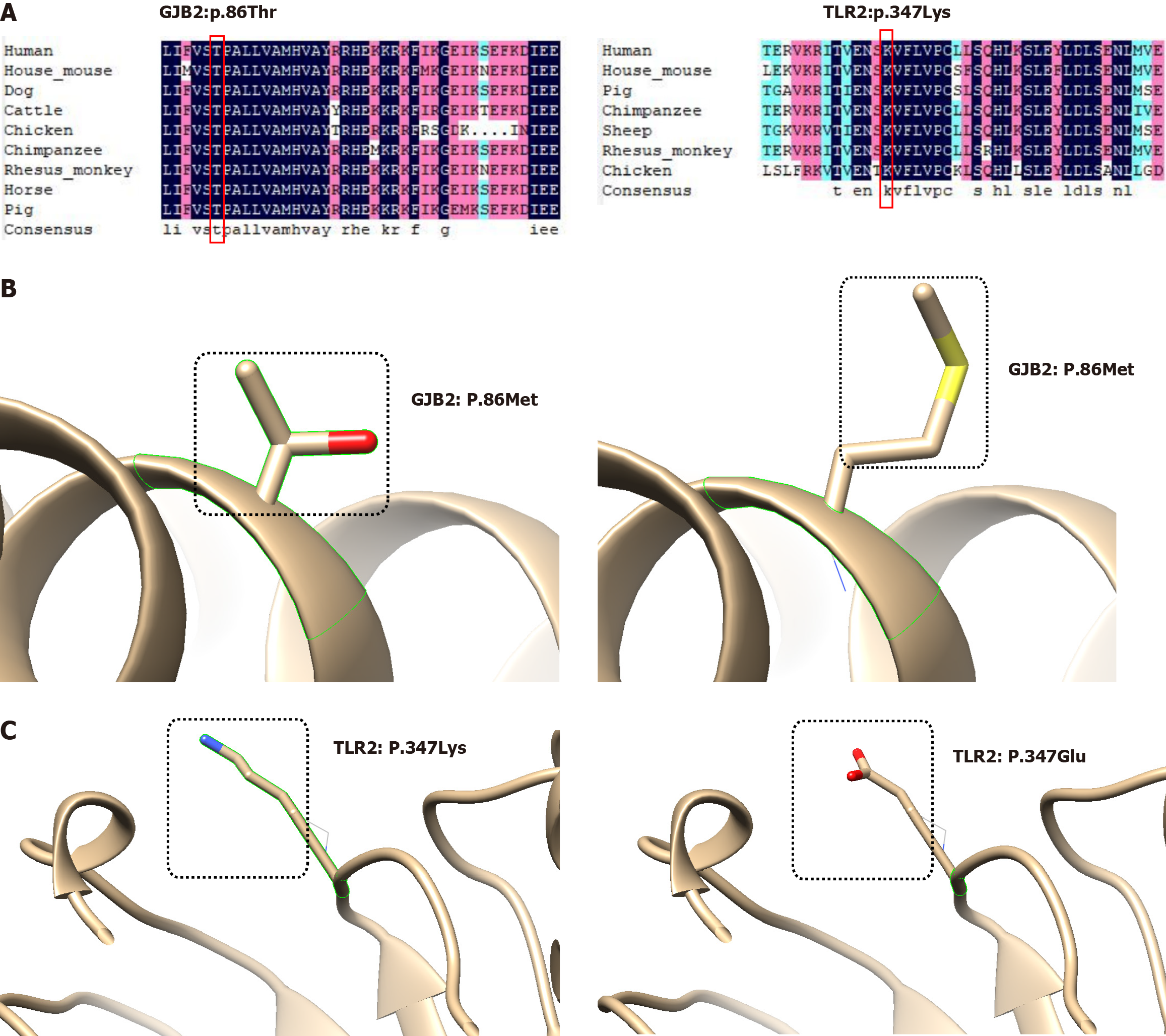
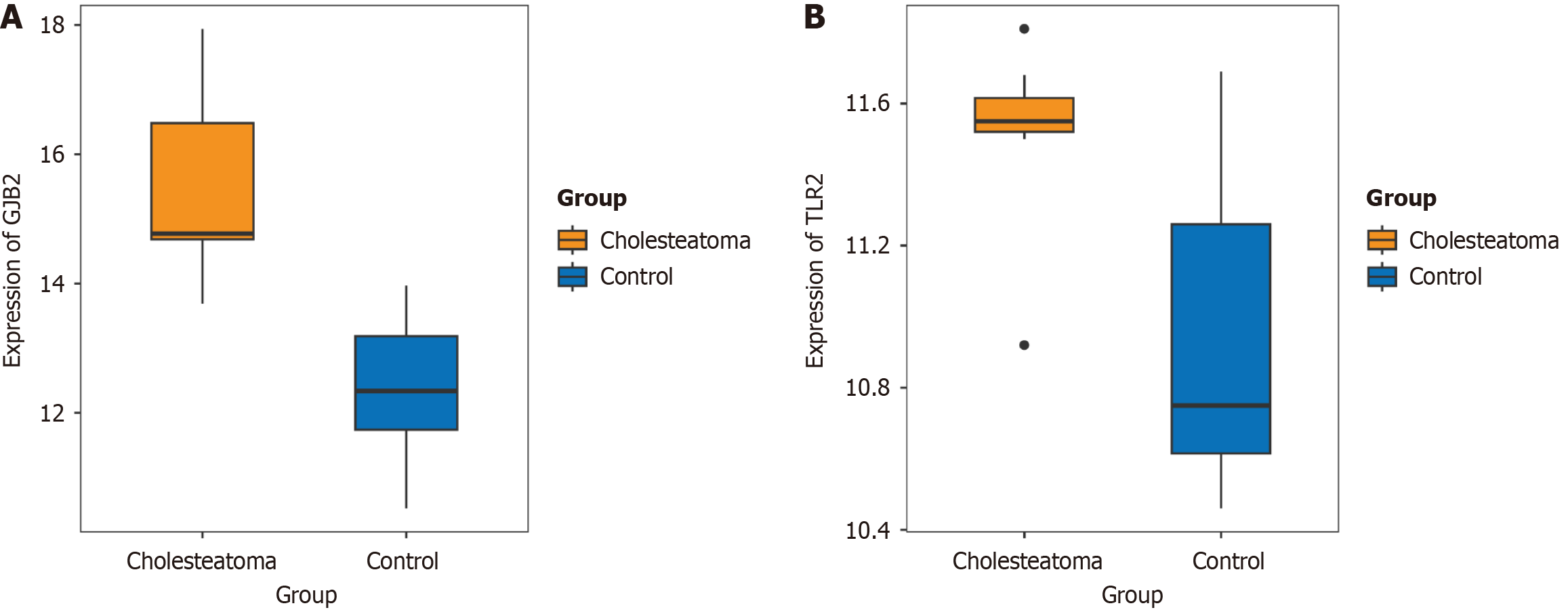
DNAMAN was used for amino acid sequence conservation analysis, both GJB2: T86 and TLR2: K347 were confirmed to be highly conserved across multiple species. Generally, mutations occurring in highly conserved amino acids significantly impact the protein. Finally, we employed Chimera (v1.17.3) to visualize protein structural alterations that resulted from the aforementioned amino acid mutations (Figure 2). Furthermore, the mRNA expression profile dataset GSE42556[8] of paired samples from patients with middle ear cholesteatoma was available in the Gene Expression Omnibus database. Using the Wilcoxon test, a significant over-expression of GJB2 and TLR2 has been observed in cholesteatoma patients (Figure 3), which is consistent with previously published studies[8,9].
The patient was ultimately diagnosed with a malignant transformation of an IEC into SCC.
At our institution, the patient underwent endoscopic microscopy combined with supra-tentorial and infra-tentorial approach for intracranial multiple mass resection under combined intravenous anesthesia. Postoperative symptomatic support treatments including epilepsy prevention, infection prevention, fluid infusion, nutritional support, and phlegm reduction were administered. The patient continued to experience fever and received multiple lumbar puncture fluid drainage treatments. The cerebrospinal fluid culture results showed Staphylococcus warneri, and anti-infective treatment was given. One week post discharge, the patient suffered a relapse caused by surgical residue, which was managed through radiotherapy. Following radiotherapy, the patient also received chemotherapy and Bevacizumab treatment.
Following the combined treatment, the patient's clinical symptoms were monitored, and routine MRI examinations were conducted. As previously indicated, the patient's clinical symptoms and contrast-enhanced MRI scan at the most recent visit (November 20, 2023) revealed remarkable improvement compared to the initial visit (Figure 1E-G), indicating effective control of the aforementioned SCC. Furthermore, telephone follow-up calls have been conducted continuously up to the present time.
IEC transformation to SCC is an extremely rare and asymptomatic event[10]. Although several studies have reported on this event, none have addressed the underlying mechanism at molecular level. WES of the genomic DNA extracted from a patient with IEC, who developed SCC was performed to identify potential mutations responsible for SCC transformation. The analysis revealed two risk variants: GJB2 c.257C>T (p.Thr86Met) and TLR2 c.1039A>G (p.Lys347Glu).
GJB2 encodes the gap junction protein connexin 26, which is heavily involved in hearing loss. In some studies, GJB2 has been demonstrated to accelerate development of various cancer types, including lung adenocarcinoma, colorectal cancer, and cholesteatoma[8,11,12]. Additionally, the GJB2 gene mutation is also known to cause skin disorders, and GJB2 variants are more common in patients with cholesteatoma than in normal populations[13]. Gap junctions provide strong cell-to-cell connections for direct intracellular communication, namely, cellular signaling and exchange of smaller molecules. This activity strictly modulates embryogenesis, homeostasis, and normal organ function[14]. Connexin upregulation strongly inhibits tumor development in several cancers[11]. In addition, GJB2 was found to be involved in biological processes, such as, identical protein binding, response to lipopolysaccharide, and cell body, according to gene ontology functional enrichment analysis. Hence, mutations in GJB2 may alter the structure of the gap junction protein connexin 26, thereby modifying cell-to-cell communication and accelerating the development of IEC.
The TLR2-encoding protein belongs to the TLR family, and it critically regulates pathogen recognition and innate immune activation. TLR2 is highly conserved and ubiquitously expressed within neurons, astrocytes, and microglia[15,16]. Pathogen-associated molecular patterns activate TLR2 to promote downstream signaling pathways that regulate host inflammatory responses[9,17]. There is some indication that this gene also regulates multiple autoimmune disease pathologies. According to an article by Hirai et al[18], TLR2 expression is strongly elevated in chronic otitis media and middle ear cholesteatoma. Emerging evidence suggests a substantial link between microglial TLR2 and innate and adaptive immunity against brain tumors, including glioblastoma[19,20]. Chronic endothelial inflammation of epidermoid cysts is considered as a major contributor to the malignant epidermoid cyst. Mutations in TLR2 can impede the immune response, and promote the development of inflammation. To further assess the effect of these identified mutations on gene expression, protein structure and function, we intend to conduct experimental validation in future research. Furthermore, the pathogenicity of these mutations will be investigated in a broader population.
Malignant transformation of IEC to SCC is a rare but noteworthy condition that is associated with poor clinical outcomes. In this study, we presented a female patient who suffered the malignant transition from IEC to SCC. Using WES, two risk variants on GJB2 (c.257C>T) and TLR2 (c.1039A>G) were identified for the malignant transformation of the patient. GJB2 may affect cell-to-cell communication by altering the structure of connexins, leading to the development of IEC. TLR2, on the other hand, affects the development of immune response and inflammation. To sum up, both GJB2 and TLR2 may be actively implicated in SCC pathogenesis, and are reliable targets for SCC prevention and therapy.
| 1. | Okada T, Fujitsu K, Ichikawa T, Miyahara K, Tanino S, Uriu Y, Hataoka S, Tanaka Y, Suzuki K, Niino H, Yagishita S, Kato I. Intracranial epidermoid cyst with proliferative folliculosebaceous epithelium: Report of a rare case and discussion on pathogenesis. Neuropathology. 2018;38:510-515. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Roh TH, Park YS, Park YG, Kim SH, Chang JH. Intracranial squamous cell carcinoma arising in a cerebellopontine angle epidermoid cyst: A case report and literature review. Medicine (Baltimore). 2017;96:e9423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Gerges MM, Godil SS, Rumalla K, Liechty B, Pisapia DJ, Magge RS, Schwartz TH. Genomic profile of a primary squamous cell carcinoma arising from malignant transformation of a pineal epidermoid cyst. Acta Neurochir (Wien). 2019;161:1829-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Vellutini EA, de Oliveira MF, Ribeiro AP, Rotta JM. Malignant transformation of intracranial epidermoid cyst. Br J Neurosurg. 2014;28:507-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Wang X, Shen X, Fang F, Ding CH, Zhang H, Cao ZH, An DY. Phenotype-Driven Virtual Panel Is an Effective Method to Analyze WES Data of Neurological Disease. Front Pharmacol. 2018;9:1529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Menzel M, Ossowski S, Kral S, Metzger P, Horak P, Marienfeld R, Boerries M, Wolter S, Ball M, Neumann O, Armeanu-Ebinger S, Schroeder C, Matysiak U, Goldschmid H, Schipperges V, Fürstberger A, Allgäuer M, Eberhardt T, Niewöhner J, Blaumeiser A, Ploeger C, Haack TB, Tay TKY, Kelemen O, Pauli T, Kirchner M, Kluck K, Ott A, Renner M, Admard J, Gschwind A, Lassmann S, Kestler H, Fend F, Illert AL, Werner M, Möller P, Seufferlein TTW, Malek N, Schirmacher P, Fröhling S, Kazdal D, Budczies J, Stenzinger A. Multicentric pilot study to standardize clinical whole exome sequencing (WES) for cancer patients. NPJ Precis Oncol. 2023;7:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 7. | Leung GKC, Mak CCY, Fung JLF, Wong WHS, Tsang MHY, Yu MHC, Pei SLC, Yeung KS, Mok GTK, Lee CP, Hui APW, Tang MHY, Chan KYK, Liu APY, Yang W, Sham PC, Kan ASY, Chung BHY. Identifying the genetic causes for prenatally diagnosed structural congenital anomalies (SCAs) by whole-exome sequencing (WES). BMC Med Genomics. 2018;11:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Klenke C, Janowski S, Borck D, Widera D, Ebmeyer J, Kalinowski J, Leichtle A, Hofestädt R, Upile T, Kaltschmidt C, Kaltschmidt B, Sudhoff H. Identification of novel cholesteatoma-related gene expression signatures using full-genome microarrays. PLoS One. 2012;7:e52718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Lee HY, Park MS, Byun JY, Kim YI, Yeo SG. Expression of pattern recognition receptors in cholesteatoma. Eur Arch Otorhinolaryngol. 2014;271:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Narasimhaiah D, Nair P, Kesavadas C, Poyuran R. Rapid malignant transformation of an intracranial epidermoid cyst: Report of a case. Neuropathology. 2023;43:268-272. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Sirnes S, Lind GE, Bruun J, Fykerud TA, Mesnil M, Lothe RA, Rivedal E, Kolberg M, Leithe E. Connexins in colorectal cancer pathogenesis. Int J Cancer. 2015;137:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Meng F, Sun X, Guo W, Shi Y, Cheng W, Zhao L. Recognition and combination of multiple cell-death features showed good predictive value in lung adenocarcinoma. Heliyon. 2023;9:e22434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | James AL, Chadha NK, Papsin BC, Stockley TL. Pediatric cholesteatoma and variants in the gene encoding connexin 26. Laryngoscope. 2010;120:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Choung YH, Park K, Kang SO, Markov Raynov A, Ho Kim C, Choung PH. Expression of the gap junction proteins connexin 26 and connexin 43 in human middle ear cholesteatoma. Acta Otolaryngol. 2006;126:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Li Y, Tong Q, Wang Y, Cheng Y, Geng Y, Tian T, Yuan Y, Fan Y, Lu M, Zhang K. Phosphorylated α-synuclein deposited in Schwann cells interacting with TLR2 mediates cell damage and induces Parkinson's disease autonomic dysfunction. Cell Death Discov. 2024;10:52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Szczepański M, Szyfter W, Jenek R, Wróbel M, Lisewska IM, Zeromski J. Toll-like receptors 2, 3 and 4 (TLR-2, TLR-3 and TLR-4) are expressed in the microenvironment of human acquired cholesteatoma. Eur Arch Otorhinolaryngol. 2006;263:603-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Jani C, Solomon SL, Peters JM, Pringle SC, Hinman AE, Boucau J, Bryson BD, Barczak AK. TLR2 is non-redundant in the population and subpopulation responses to Mycobacterium tuberculosis in macrophages and in vivo. mSystems. 2023;8:e0005223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Hirai H, Kariya S, Okano M, Fukushima K, Kataoka Y, Maeda Y, Nishizaki K. Expression of toll-like receptors in chronic otitis media and cholesteatoma. Int J Pediatr Otorhinolaryngol. 2013;77:674-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Chang CY, Jeon SB, Yoon HJ, Choi BK, Kim SS, Oshima M, Park EJ. Glial TLR2-driven innate immune responses and CD8(+) T cell activation against brain tumor. Glia. 2019;67:1179-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz K, Edwards MR, Michelsen KS, Kroeger KM, Liu C, Muhammad AK, Clark MC, Arditi M, Comin-Anduix B, Ribas A, Lowenstein PR, Castro MG. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009;6:e10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 295] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/