Published online May 24, 2023. doi: 10.5306/wjco.v14.i5.190
Peer-review started: September 18, 2022
First decision: November 11, 2022
Revised: November 23, 2022
Accepted: April 25, 2023
Article in press: April 25, 2023
Published online: May 24, 2023
Processing time: 242 Days and 9.1 Hours
The therapy of left-sided malignant colonic obstruction continues to be one of the largest problems in clinical practice. Numerous studies on colonic stenting for neoplastic colonic obstruction have been reported in the last decades. Thereby the role of self-expandable metal stents (SEMS) in the treatment of malignant colonic obstruction has become better defined. However, numerous prospective and retrospective investigations have highlighted serious concerns about a possible worse outcome after endoscopic colorectal stenting as a bridge to surgery, particularly in case of perforation. This review analyzes the most recent evidence in order to highlight pros and cons of SEMS placement in left-sided malignant colonic obstruction.
Core Tip: Self-expandable metal stents (SEMS) should be considered as a primary option in palliative treatment of malignant left-sided colonic obstruction. In patients with conceivably curable left-sided colon cancer, SEMS placement as a bridge to surgery should be carefully discussed, specifically focusing on lower risk and lower permanent stoma rates, but potentially higher recurrence rates when compared to surgery. In this scenario the endoscopic expertise has a significant impact on the complication rate.
- Citation: Russo S, Conigliaro R, Coppini F, Dell'Aquila E, Grande G, Pigò F, Mangiafico S, Lupo M, Marocchi M, Bertani H, Cocca S. Acute left-sided malignant colonic obstruction: Is there a role for endoscopic stenting? World J Clin Oncol 2023; 14(5): 190-197
- URL: https://www.wjgnet.com/2218-4333/full/v14/i5/190.htm
- DOI: https://dx.doi.org/10.5306/wjco.v14.i5.190
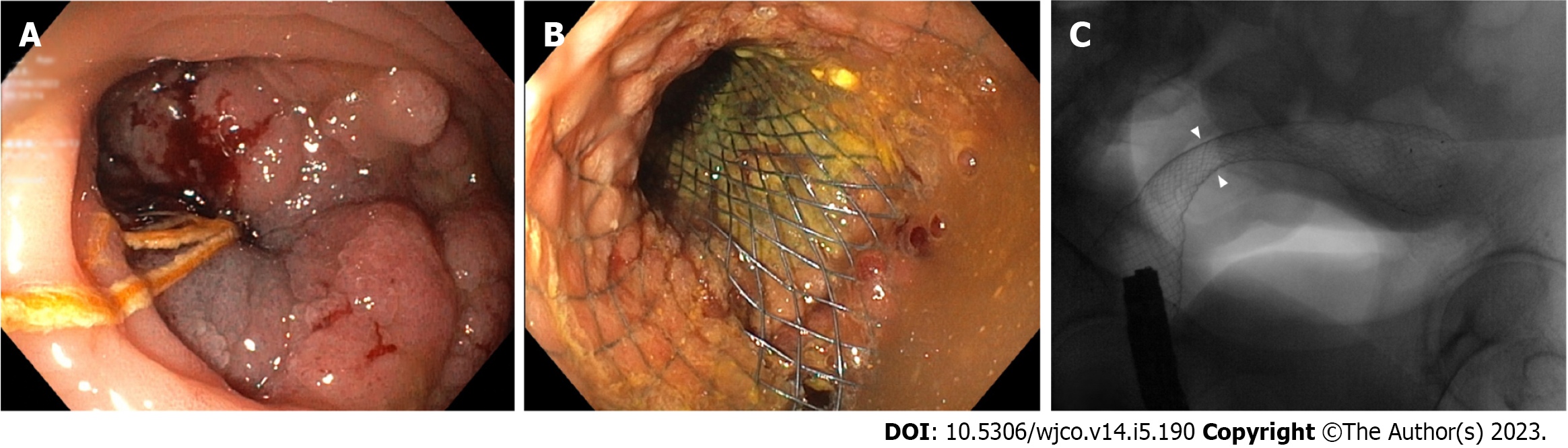
Colorectal cancer (CRC) is the third most frequently diagnosed malignancy in the world and the second cause of cancer-related mortality[1]. CRC is still among the most common reason for large bowel obstruction in adults and about 20% of patients with CRC are admitted with emergency[2-4]. Obstructive CRC most frequently develops in the sigmoid colon, with 75% of tumors located distal to the splenic flexure[5]. Emergency surgery (ES) is the standard approach for obstructive right-sided colon cancer, along with primary resection and ileocolic anastomosis[6]. However, it is debatable whether emergency or radical surgery following stenting as a bridge to surgery (BTS) should be considered for obstructive left-sided colorectal cancer[7]. Self- expandable metal stents (SEMS) for BTS (Figure 1) have shown excellent short-term results, but related complications such as perforations may be disastrous and long-term outcomes are still a matter of debate[8-11].
Over the last decades, many papers have been published on colonic stenting for neoplastic obstruction, including randomized controlled trials (RCT), post-hoc analysis and systematic reviews. Moreover, in 2020 the European Society of Gastrointestinal Endoscopy (ESGE) released updated guidelines on this topic[7]. Even though the role of SEMSs in the management of malignant colonic obstruction has been better defined, several issues still remain. Although screening programs are widespread in developed countries, large bowel obstruction is one of the most common causes of ES in patients with CRC[7,12]. For example, in the United Kingdom, the rate of colorectal cancer presenting as an emergency remains at 20%[13]. Colonic SEMS placement is mainly suggested for patients who have obstructive symptoms and CT-results compatible with obstructing CRC. Acute colorectal obstruction (ACRO) is a medical emergency related to CRC that occurs more frequently in patients with advanced disease, in whom ES is responsible of significant morbidity and mortality than elective surgery, particularly in aged patients[14,15]. These patients usually present to the emergency department with nausea, vomiting, constipation and/or abdominal distention, often combined with poor intake of food from the previous days[16].
In ACRO, the main therapeutic aim is to decrease colonic distension and to prevent complications (i.e. necrosis, perforation), generally associated with pneumoperitoneum and systemic inflammatory response syndrome. Therefore, colonic stenting is an interesting option to obtain this goal in ACRO, as a BTS and for palliative purposes in patients with advanced and/or unfit for surgery CRC[7,15].
Effective stent placement makes it feasible to perform non-surgical intestinal decompression and prepare the colon for a forthcoming elective oncologic resection. Furthermore, in CRC obstruction, the proximal colon is frequently dilated with vascular insufficiency, with an increased risk of colostomy/ileostomy in case of ES. As shown in many studies, in this situation SEMSs may decompress the dilated proximal colon, thus obviating the requirement of ES with colostomy/ileostomy[17].
To evaluate the severity of obstruction, in Japan a modified point score system called ColoRectal Obstruction Scoring System (CROSS) (Table 1) is widely used. CROSS 0 patients need ES or SEMS placement. CROSS 1 or 2 patients are candidates for elective surgery. In CROSS 3 and 4 patients SEMS placement is not required because they can receive food. A post hoc analysis of two prospective, observational, single-arm multicenter clinical trials demonstrated the short-term high efficacy and safety of SEMS placement as a BTS for patients with obstructive CRC classified as CROSS 0, 1, and 2[18].
In a large cohort prospective study, the clinical success rate of SEMS placement was 95.5% and the technical success rate 97.9%. Major adverse events included perforation (2.1%), stent migration (1.0%), and stent occlusion (0.8%)[19]. The primary cause of perforation was the procedure itself (0.8%) followed by comorbidities (impending perforation, obstructive colitis) not manifest prior to SEMS insertion (0.6%). In a retrospective study, the technical success rate for stent placement for left-sided malignant colonic obstruction (LS-MCO) and rectal obstruction did not differ, but the clinical success rate was lower in patients with rectal obstruction (85.4% vs 92.1%; P = 0.02). In addition, the latter group of patients had a higher complication rate (37.4% vs 25.1%; P = 0.01), due to an increased risk of extra-intestinal cancer[20]. Furthermore, it is well established from the literature that expertise, method, lesion characteristics, and the location of the obstruction or architecture of the colon, such as tortuosity, have a significant impact on the technical and clinical failure rates for colonic stenting[7,21]. Since there have been growing concerns about protracted and technically challenging stent placement in complex patients, the Colonic Stent Safe Procedure Research Group, in collaboration with the Japan Gastroenterological Endoscopy Society, has developed mini-guidelines to ensure the procedural safety and efficacy for colonic stent placement. A post-hoc analysis[22] of a large multicenter clinical trial identified the risk factors for difficult colonic stenting cases such as a CROSS score of 0 before SEMS placement, evidence of peritoneal carcinomatosis, tumor site in the right colon, stricture length ≥5 cm and placement of multiple SEMSs[22]. In light of this evidence, Kuwai et al[22] concluded that before attempting SEMS placement for obstructive CRC clinicians must anticipate technical challenges.
Various SEMS have been developed, but they can be classified as covered and uncovered. A recent meta-analysis examined the effectiveness of uncovered vs covered stents in treating colonic obstruction either as a curative BTS or palliative option. Uncovered SEMSs presented less complications (e.g. tumor overgrowth and displacement), longer SEMS patency (mean duration 18 mo), while the risk of tumor ingrowth was higher, as expected. Rates of technical success, clinical success, perforation, stool impaction and stent obstruction were similar in both groups[21].
It is difficult to make recommendations regarding the SEMS length or diameter, as few studies have shown conflicting results. When selecting a stent after fluoroscopic measurement of colonic stricture length, it is widely accepted in clinical practice to follow a simple rule: to prepare for stent fores
Emergency surgery is burdened by high anastomotic leakage rates, up to 33%[12]. Furthermore a recent study suggests that emergency presentation remains an independent poor prognostic indicator after curative colorectal resection[24]. The optimal management of left-sided malignant large bowel obstruction is less clear than the right-sided cancer where the surgical approach is highly recommended[25].
Several surgical options exist for left-sided bowel obstruction including primary resection (with or without anastomosis), subtotal colectomy (with or without anastomosis) or unfunctioning ileostomy/colostomy with interval resection[24,25].
For the first time in 1994 Tejero et al[26] described the technique of SEMS placement in 2 patients with ACRO as a BTS. Nearly twenty years after this initial description, the debate is still open regarding the role of SEMSs as a BTS for symptomatic LS-MCO because interpretation of the literature on this subject is still challenging.
The fundamental hypotheses driving the growing interest in SEMS placement are that it can turn ES into elective surgery, reducing preoperative morbidity. Webster et al[25] analyzed 19 international guidelines for the treatment of LS-MCO from 2010 to 2018 and asked whether ES or stent placement as a bridge to surgery was the best procedure in terms of morbidity, mortality and long-term oncological outcomes. They concluded that there was a lack of high-quality evidence[25]. The more recent guidelines of the European Society of Gastrointestinal Endoscopy recommend to reserve colonic stenting in case of clinical symptoms and radiological signs of obstructing CRC, without evidence of perforation (strong recommendation, low quality evidence)[7].
In 2011, one of the first multicenter randomized trials comparing ES with colonic stenting as a BTS for left-sided CRC showed that colonic stenting had no decisive clinical advantages for global health status, mortality, morbidity and stoma rates. Moreover their results raised concerns about overt and silent perforations responsible for tumor spread[27].
A systematic review and meta-analysis of RCTs on colonic stenting as a BTS vs ES for acute symptomatic malignant left sided colonic obstruction[12] showed that patients treated with SEMS as a BTS had less short-term overall morbidity and reduced rates of both permanent and transient stoma. Albeit influenced by local expertise, level of obstruction and patient’s clinical status, stenting as a BTS for LS-MCO showed lower risk than ES in the short-term morbidity (60 d after surgery). However, recurrence rate data between the two groups showed a clear trend in favour of ES over stenting as a BTS (26% vs 40%), although this was not statistically significant.
In a subsequent multicenter randomized controlled trial (ESCO trial) comparing stenting as a BTS to ES for malignant colonic obstruction, Arezzo et al[28] reported a similar short term complications rate between the two groups but a higher stoma rate in the ES group (P = 0.031). Looking at the long term oncologic results of the ESCO trial, no difference was observed between the two groups in terms of overall survival, time to progression and disease free survival[29]. These results have also been confirmed in a more recent meta-analysis by Cirocchi et al[30].
While the majority of studies tried to understand if SEMS placement is more convenient than ES[12,31,32], there are few studies comparing the bridge to elective surgery approach such as decompressive stoma (DS) vs SEMS placement. Creation of a DS is a quite simple procedure with a near 100% success rate and can be performed in almost all patients while, as mentioned above, colonic stenting is an intervention requiring specific technical skills and expertise (in both colonoscopy and fluoroscopic techniques), including the ability to select correctly the patient based on stricture’s length and location, and carries risks of adverse events. A population-based cohort study[33] comparing the two bridge to elective surgery approaches showed that SEMS appears to be a safest procedure, with a shorter hospital admission, as well as in palliative care. In a recent meta-analysis of seven studies (1 prospective, 6 retrospective), involving 646 and 712 patients who underwent SEMS and DS approaches respectively, Zhang et al found a significantly lower complication rate in the SEMS group than in the DS group (8.68 vs 16.85%; P = 0.004), without differences in short-term mortality and permanent stoma rates. In line with the previously cited study[33], the authors concluded that SEMSs may be a better alternative to DS for obstructive CRC, but highlighted the lack of high-quality RCTs[34].
Finally, a newly published randomized trial with a longer follow-up (3 y) and larger population compared to prior studies, randomized patients with left-sided obstructive colon cancer to colonic stenting or surgical decompression. The authors showed that among patients undergoing potentially curative treatment, there were no significant differences in 30-d postoperative mortality or duration of hospital stay between stenting followed by delayed elective surgery and emergency surgery group. Moreover the use of a stoma resulted more frequent in patients treated with immediate surgery than in patients treated with SEMS (67.9% vs 47.5%; P = 0.003), without substantial differences in peri-operative morbidity, intensive care use, quality of life and 3-y recurrence or mortality[35].
The proper timing of surgery subsequent to SEMS placement as a BTS is not clear yet. Adequate radial stent expansion, ischemia reversibility of the colon proximal to the stricture and colon cleansing require sufficient time after SEMS deployment. In order to reduce the risk of stoma and postoperative complications, such as anastomotic leaks, abscesses, and wound’s problems, surgery should be postponed for at least 2 wk after SEMS placement. However, long delays in surgery could increase the complications rate related to SEMS. Therefore, surgery is suggested approximately 14 d after SEMS insertion[7,17].
Three randomized controlled trials compared SEMS and decompressive stoma as palliative treatments for malignant bowel obstructions[36-38]. Palliative situations included patients unfit for surgery, as well as patients with inoperable primary lesions or metastatic disease. Given its effectiveness and the enhanced quality of life (QoL) that comes from avoiding a stoma, colonic stenting has been judged to be superior in both investigations. In a randomized prospective trial, Fiori et al[37,38] found that the mortality and morbidity rates following palliative stenting and colostomies were comparable. However, in the stenting group a shorter hospital stay, a faster return to oral intake, and a shorter operating time were recorded. On the other hand, a Dutch trial with a similar study design was prematurely stopped because of the unacceptable high mortality rate due to perforations in the stenting group. The authors hypothesized that the unpredictable high frequency of perforation in the nonsurgical arm could be associated with the type of stent used at that time[39].
Data about the effects and safety of systemic chemotherapy alone or in association with biological agents (anti-VEGF or anti-EGFR) combined with palliative stenting in metastatic colorectal cancer (mCRC) patients are lacking.
In a metanalysis including 837 mCRC patients, patients treated with SEMS had similar overall survival compared to surgery-treated patients (7.64 mo vs 7.88 mo respectively), shorter time before starting chemotherapy (33.36 d vs 15.53 d, P < 0.00001) and lower 30-d mortality (4.2% vs 10.5%, P = 0.01)[40]. Tumor response to chemotherapy could increase the rate of complications related to stent placement, such as stent migration or late perforation, but, on the other hand, could reduce the risk of obstruction by maintaining its luminal patency, especially in a palliative setting. A multicenter retrospective study included 38 mCRC patients treated with only chemotherapy; major complications related to stenting were: Perforation (8%), stent migration (5%), and re-obstruction secondary to tumor ingrowth (13%)[41]. A retrospective trial including 72 mCRC patients compared long-term outcomes of palliative SEMS in patients treated with chemotherapy or with best supportive care. In the chemotherapy group, there was a higher rate of late migration (20% vs 2.4%, P = 0.018, for chemo
The introduction of bevacizumab improved outcome of mCRC patients[44], although data about its effect on stent placement are still controversial. Moreover, some authors raised the hypothesis of an increased risk to develop SEMS-related complications (such as perforation) in patients on bevacizumab[45,46]. Conversely, other authors demonstrated that the addition of bevacizumab to chemotherapy was not related to a higher perforation rate in comparison to chemotherapy alone[47,48]. In an Italian retrospective, multicenter study including 91 mCRC patients treated with chemotherapy plus anti-VEGF or anti-EGFR agents, no correlation between chemotherapy with or without biological therapy, K-RAS status or risk of SEMS-related complications was shown[46].
These studies had several limitations: Retrospective nature, different outcomes and small sample size, patients with heterogeneous characteristics and different settings. At the state of the art more prospective and randomized trials to define the outcome and safety of the association of SEMS placement and systemic treatment are needed.
Colonic stenting is a well-recognized palliative approach for treating malignant left-sided colonic obstruction, with high rates of technical and clinical success. Especially in patients with poor general condition and limited life expectancy, it may allow for an early hospital discharge, an improved QoL and prolonged survival in comparison to surgery.
SEMS placement as a BTS has the advantage to convert an ES into an elective one, reducing preoperative morbidity, allowing for adequate oncological staging, good colonic preparation and faster initiation of chemotherapy. Although numerous prospective and retrospective investigations have highlighted serious concerns about tumor seeding after endoscopic colorectal stent placement, particularly in cases of perforation, recent high quality studies displayed encouraging results. Operator expertise remains a key element to ensure accurate stent placement and restoration of bowel function with a low rate of complications. For this reason, this approach should be considered a standard practice only in experienced high-volume referral centers and clinicians should carefully select the patients fit for an endoscopic decompressing approach before starting the procedure.
In conclusion, further evidence from prospective, ideally randomized trials on the probability of tumor recurrence following stenting is necessary to show the long-term safety of stenting as a BTS. Until then, the evident short-term advantages, combined with the high mortality rate in frail and elderly patients, should be weighed against the potential long-term threats of tumor recurrence.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68596] [Article Influence: 13719.2] [Reference Citation Analysis (201)] |
| 2. | Sagar J. Colorectal stents for the management of malignant colonic obstructions. Cochrane Database Syst Rev. 2011;2011:CD007378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Baer C, Menon R, Bastawrous S, Bastawrous A. Emergency Presentations of Colorectal Cancer. Surg Clin North Am. 2017;97:529-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Shimura T, Joh T. Evidence-based Clinical Management of Acute Malignant Colorectal Obstruction. J Clin Gastroenterol. 2016;50:273-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Frago R, Ramirez E, Millan M, Kreisler E, del Valle E, Biondo S. Current management of acute malignant large bowel obstruction: a systematic review. Am J Surg. 2014;207:127-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 6. | Pisano M, Zorcolo L, Merli C, Cimbanassi S, Poiasina E, Ceresoli M, Agresta F, Allievi N, Bellanova G, Coccolini F, Coy C, Fugazzola P, Martinez CA, Montori G, Paolillo C, Penachim TJ, Pereira B, Reis T, Restivo A, Rezende-Neto J, Sartelli M, Valentino M, Abu-Zidan FM, Ashkenazi I, Bala M, Chiara O, De' Angelis N, Deidda S, De Simone B, Di Saverio S, Finotti E, Kenji I, Moore E, Wexner S, Biffl W, Coimbra R, Guttadauro A, Leppäniemi A, Maier R, Magnone S, Mefire AC, Peitzmann A, Sakakushev B, Sugrue M, Viale P, Weber D, Kashuk J, Fraga GP, Kluger I, Catena F, Ansaloni L. 2017 WSES guidelines on colon and rectal cancer emergencies: obstruction and perforation. World J Emerg Surg. 2018;13:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 208] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 7. | van Hooft JE, Veld JV, Arnold D, Beets-Tan RGH, Everett S, Götz M, van Halsema EE, Hill J, Manes G, Meisner S, Rodrigues-Pinto E, Sabbagh C, Vandervoort J, Tanis PJ, Vanbiervliet G, Arezzo A. Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2020. Endoscopy. 2020;52:389-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 222] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 8. | Kim HJ, Choi GS, Park JS, Park SY, Jun SH. Higher rate of perineural invasion in stent-laparoscopic approach in comparison to emergent open resection for obstructing left-sided colon cancer. Int J Colorectal Dis. 2013;28:407-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Kim SJ, Kim HW, Park SB, Kang DH, Choi CW, Song BJ, Hong JB, Kim DJ, Park BS, Son GM. Colonic perforation either during or after stent insertion as a bridge to surgery for malignant colorectal obstruction increases the risk of peritoneal seeding. Surg Endosc. 2015;29:3499-3506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Sabbagh C, Browet F, Diouf M, Cosse C, Brehant O, Bartoli E, Mauvais F, Chauffert B, Dupas JL, Nguyen-Khac E, Regimbeau JM. Is stenting as "a bridge to surgery" an oncologically safe strategy for the management of acute, left-sided, malignant, colonic obstruction? Ann Surg. 2013;258:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 206] [Article Influence: 15.8] [Reference Citation Analysis (2)] |
| 11. | Maruthachalam K, Lash GE, Shenton BK, Horgan AF. Tumour cell dissemination following endoscopic stent insertion. Br J Surg. 2007;94:1151-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 194] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 12. | Arezzo A, Passera R, Lo Secco G, Verra M, Bonino MA, Targarona E, Morino M. Stent as bridge to surgery for left-sided malignant colonic obstruction reduces adverse events and stoma rate compared with emergency surgery: results of a systematic review and meta-analysis of randomized controlled trials. Gastrointest Endosc. 2017;86:416-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 151] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 13. | Golder AM, McMillan DC, Horgan PG, Roxburgh CSD. Determinants of emergency presentation in patients with colorectal cancer: a systematic review and meta-analysis. Sci Rep. 2022;12:4366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 14. | Bakker IS, Snijders HS, Grossmann I, Karsten TM, Havenga K, Wiggers T. High mortality rates after nonelective colon cancer resection: results of a national audit. Colorectal Dis. 2016;18:612-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Bonin EA, Baron TH. Update on the indications and use of colonic stents. Curr Gastroenterol Rep. 2010;12:374-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | van Halsema EE, van Hooft JE. Does short-term morbidity and stoma reduction outweigh a potential long-term risk of colonic stent placement? Gastrointest Endosc. 2017;86:427-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Lee JM, Byeon JS. Colorectal Stents: Current Status. Clin Endosc. 2015;48:194-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Ohki T, Yoshida S, Yamamoto M, Isayama H, Yamada T, Matsuzawa T, Saito S, Kuwai T, Tomita M, Shiratori T, Shimada M, Hirakawa T, Koizumi K, Saida Y. Determining the difference in the efficacy and safety of self-expandable metallic stents as a bridge to surgery for obstructive colon cancer among patients in the CROSS 0 group and those in the CROSS 1 or 2 group: a pooled analysis of data from two Japanese prospective multicenter trials. Surg Today. 2020;50:984-994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Matsuzawa T, Ishida H, Yoshida S, Isayama H, Kuwai T, Maetani I, Shimada M, Yamada T, Saito S, Tomita M, Koizumi K, Hirata N, Sasaki T, Enomoto T, Saida Y. A Japanese prospective multicenter study of self-expandable metal stent placement for malignant colorectal obstruction: short-term safety and efficacy within 7 days of stent procedure in 513 cases. Gastrointest Endosc. 2015;82:697-707.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Lee HJ, Hong SP, Cheon JH, Kim TI, Kim WH, Park SJ. Clinical Outcomes of Self-Expandable Metal Stents for Malignant Rectal Obstruction. Dis Colon Rectum. 2018;61:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Mashar M, Mashar R, Hajibandeh S. Uncovered versus covered stent in management of large bowel obstruction due to colorectal malignancy: a systematic review and meta-analysis. Int J Colorectal Dis. 2019;34:773-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Kuwai T, Yamaguchi T, Imagawa H, Yoshida S, Isayama H, Matsuzawa T, Yamada T, Saito S, Shimada M, Hirata N, Sasaki T, Koizumi K, Maetani I, Saida Y. Factors related to difficult self-expandable metallic stent placement for malignant colonic obstruction: A post-hoc analysis of a multicenter study across Japan. Dig Endosc. 2019;31:51-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Park JK, Lee MS, Ko BM, Kim HK, Kim YJ, Choi HJ, Hong SJ, Ryu CB, Moon JH, Kim JO, Cho JY, Lee JS. Outcome of palliative self-expanding metal stent placement in malignant colorectal obstruction according to stent type and manufacturer. Surg Endosc. 2011;25:1293-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Dahdaleh FS, Sherman SK, Poli EC, Vigneswaran J, Polite BN, Sharma MR, Catenacci DV, Maron SB, Turaga KK. Obstruction predicts worse long-term outcomes in stage III colon cancer: A secondary analysis of the N0147 trial. Surgery. 2018;164:1223-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Webster PJ, Aldoori J, Burke DA. Optimal management of malignant left-sided large bowel obstruction: do international guidelines agree? World J Emerg Surg. 2019;14:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Tejero E, Mainar A, Fernández L, Tobío R, De Gregorio MA. New procedure for the treatment of colorectal neoplastic obstructions. Dis Colon Rectum. 1994;37:1158-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 177] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | van Hooft JE, Bemelman WA, Oldenburg B, Marinelli AW, Lutke Holzik MF, Grubben MJ, Sprangers MA, Dijkgraaf MG, Fockens P; collaborative Dutch Stent-In study group. Colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction: a multicentre randomised trial. Lancet Oncol. 2011;12:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 318] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 28. | Arezzo A, Balague C, Targarona E, Borghi F, Giraudo G, Ghezzo L, Arroyo A, Sola-Vera J, De Paolis P, Bossotti M, Bannone E, Forcignanò E, Bonino MA, Passera R, Morino M. Colonic stenting as a bridge to surgery versus emergency surgery for malignant colonic obstruction: results of a multicentre randomised controlled trial (ESCO trial). Surg Endosc. 2017;31:3297-3305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 128] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 29. | Arezzo A, Forcignanò E, Bonino MA, Balagué C, Targarona E, Borghi F, Giraudo G, Ghezzo L, Passera R, Morino M; collaborative ESCO study group. Long-term Oncologic Results After Stenting as a Bridge to Surgery Versus Emergency Surgery for Malignant Left-sided Colonic Obstruction: A Multicenter Randomized Controlled Trial (ESCO Trial). Ann Surg. 2020;272:703-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 30. | Cirocchi R, Arezzo A, Sapienza P, Crocetti D, Cavaliere D, Solaini L, Ercolani G, Sterpetti AV, Mingoli A, Fiori E. Current Status of the Self-Expandable Metal Stent as a Bridge to Surgery Versus Emergency Surgery in Colorectal Cancer: Results from an Updated Systematic Review and Meta-Analysis of the Literature. Medicina (Kaunas). 2021;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Wang X, He J, Chen X, Yang Q. Stenting as a bridge to resection versus emergency surgery for left-sided colorectal cancer with malignant obstruction: A systematic review and meta-analysis. Int J Surg. 2017;48:64-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Foo CC, Poon SHT, Chiu RHY, Lam WY, Cheung LC, Law WL. Is bridge to surgery stenting a safe alternative to emergency surgery in malignant colonic obstruction: a meta-analysis of randomized control trials. Surg Endosc. 2019;33:293-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 33. | Veld JV, Amelung FJ, Borstlap WAA, van Halsema EE, Consten ECJ, Siersema PD, Ter Borg F, van der Zaag ES, de Wilt JHW, Fockens P, Bemelman WA, van Hooft JE, Tanis PJ; Dutch Snapshot Research Group. Comparison of Decompressing Stoma vs Stent as a Bridge to Surgery for Left-Sided Obstructive Colon Cancer. JAMA Surg. 2020;155:206-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 34. | Zhang J, Zhu H, Yang W, Liu X, Zhang D, Jiang X, Yang L, Zhou Z. Endoscopic stent versus diverting stoma as a bridge to surgery for obstructive colorectal cancer: a systematic review and meta-analysis. Langenbecks Arch Surg. 2022;407:3275-3285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | CReST Collaborative Group. Colorectal Endoscopic Stenting Trial (CReST) for obstructing left-sided colorectal cancer: randomized clinical trial. Br J Surg. 2022;109:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 36. | Xinopoulos D, Dimitroulopoulos D, Theodosopoulos T, Tsamakidis K, Bitsakou G, Plataniotis G, Gontikakis M, Kontis M, Paraskevas I, Vassilobpoulos P, Paraskevas E. Stenting or stoma creation for patients with inoperable malignant colonic obstructions? Surg Endosc. 2004;18:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 142] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 37. | Fiori E, Lamazza A, De Cesare A, Bononi M, Volpino P, Schillaci A, Cavallaro A, Cangemi V. Palliative management of malignant rectosigmoidal obstruction. Colostomy vs. endoscopic stenting. A randomized prospective trial. Anticancer Res. 2004;24:265-268. [PubMed] |
| 38. | Fiori E, Lamazza A, Schillaci A, Femia S, Demasi E, Decesare A, Sterpetti AV. Palliative management for patients with subacute obstruction and stage IV unresectable rectosigmoid cancer: colostomy versus endoscopic stenting: final results of a prospective randomized trial. Am J Surg. 2012;204:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 39. | van Hooft JE, Fockens P, Marinelli AW, Timmer R, van Berkel AM, Bossuyt PM, Bemelman WA; Dutch Colorectal Stent Group. Early closure of a multicenter randomized clinical trial of endoscopic stenting versus surgery for stage IV left-sided colorectal cancer. Endoscopy. 2008;40:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 213] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 40. | Zhao XD, Cai BB, Cao RS, Shi RH. Palliative treatment for incurable malignant colorectal obstructions: a meta-analysis. World J Gastroenterol. 2013;19:5565-5574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 41. | Cézé N, Charachon A, Locher C, Aparicio T, Mitry E, Barbieux JP, Landi B, Dorval E, Moussata D, Lecomte T. Safety and efficacy of palliative systemic chemotherapy combined with colorectal self-expandable metallic stents in advanced colorectal cancer: A multicenter study. Clin Res Hepatol Gastroenterol. 2016;40:230-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | Han JP, Hong SJ, Kim SH, Choi JH, Jung HJ, Cho YH, Ko BM, Lee MS. Palliative self-expandable metal stents for acute malignant colorectal obstruction: clinical outcomes and risk factors for complications. Scand J Gastroenterol. 2014;49:967-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Scotti GB, Sapienza P, Lapolla P, Crocetti D, Tarallo M, Brachini G, Mingoli A, Fiori E. Endoscopic Stenting and Palliative Chemotherapy in Advanced Colorectal Cancer: Friends or Foes? In Vivo. 2022;36:1053-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7832] [Cited by in RCA: 7792] [Article Influence: 354.2] [Reference Citation Analysis (8)] |
| 45. | Bong JW, Lee JL, Kim CW, Yoon YS, Park IJ, Lim SB, Yu CS, Kim TW, Kim JC. Risk Factors and Adequate Management for Complications of Bevacizumab Treatment Requiring Surgical Intervention in Patients With Metastatic Colorectal Cancer. Clin Colorectal Cancer. 2018;17:e639-e645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Fuccio L, Correale L, Arezzo A, Repici A, Manes G, Trovato C, Mangiavillano B, Manno M, Cortelezzi CC, Dinelli M, Cennamo V, de Bellis M; KRASTENT Study Group. Influence of K-ras status and anti-tumour treatments on complications due to colorectal self-expandable metallic stents: a retrospective multicentre study. Dig Liver Dis. 2014;46:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 47. | Park YE, Park Y, Park SJ, Cheon JH, Kim WH, Kim TI. Outcomes of stent insertion and mortality in obstructive stage IV colorectal cancer patients through 10 year duration. Surg Endosc. 2019;33:1225-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Imbulgoda A, MacLean A, Heine J, Drolet S, Vickers MM. Colonic perforation with intraluminal stents and bevacizumab in advanced colorectal cancer: retrospective case series and literature review. Can J Surg. 2015;58:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: European Society of Gastrointestinal Endoscopy (ESGE), 61320133; Italian Society of Digestive Endoscopy (SIED); Italian Society of Neurogastroenterology and Motility (SINGEM).
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chow WK, Taiwan; Gu GL, China S-Editor: Gong ZM L-Editor: A P-Editor: Zhang XD