Published online Jun 24, 2021. doi: 10.5306/wjco.v12.i6.404
Peer-review started: February 17, 2021
First decision: March 17, 2021
Revised: March 26, 2021
Accepted: June 2, 2021
Article in press: June 2, 2021
Published online: June 24, 2021
Processing time: 123 Days and 11 Hours
The development of breast cancer is a complex process that involves the participation of different factors. Several authors have demonstrated the overexpression of muscarinic acetylcholine receptors (mAChRs) in different tumor tissues and their role in the modulation of tumor biology, positioning them as therapeutic targets in cancer. The conventional treatment for breast cancer involves surgery, radiotherapy, and/or chemotherapy. The latter presents disadvantages such as limited specificity, the appearance of resistance to treatment and other side effects. To prevent these side effects, several schedules of drug administration, like metronomic therapy, have been developed. Metronomic therapy is a type of chemotherapy in which one or more drugs are administered at low concentrations repetitively. Recently, two chemotherapeutic agents usually used to treat breast cancer have been considered able to activate mAChRs. The combination of low concentrations of these chemotherapeutic agents with muscarinic agonists could be a useful option to be applied in breast cancer treatment, since this combination not only reduces tumor cell survival without affecting normal cells, but also decreases pathological neo-angiogenesis, the expression of drug extrusion proteins and the cancer stem cell fraction. In this review, we focus on the previous evidences that have positioned mAChRs as relevant therapeutic targets in breast cancer and analyze the effects of administering muscarinic agonists in combination with conventional chemotherapeutic agents in a metronomic schedule.
Core Tip: Muscarinic acetylcholine receptors should be considered as new targets in breast cancer therapy since they are expressed in breast tumor tissue but not in normal tissue. The addition of muscarinic agonists at low concentrations combined with traditional chemotherapeutic drugs presents promising results. The administration of these combinations in a metronomic schedule is effective to kill tumor cells, reduce tumor invasion and neoangiogenesis. Promising results have also been reported regarding the expression of drug extrusion pumps and the decrease of the cancer stem cell fraction, which would be useful to reduce resistance to traditional chemotherapy and the relapse of breast cancer.
- Citation: Español A, Salem A, Sanchez Y, Sales ME. Breast cancer: Muscarinic receptors as new targets for tumor therapy. World J Clin Oncol 2021; 12(6): 404-428
- URL: https://www.wjgnet.com/2218-4333/full/v12/i6/404.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i6.404
Cancer is a heterogeneous disease characterized by the loss of normal behavior of cells and the acquisition of new characteristics that lead to malignant transformation. These characteristics include high growth and division rate, and the ability to invade neighboring tissues and to disseminate to distant organs to generate metastases[1].
For many years, researchers thought that tumors had a clonal origin[2,3], but taking into account the high intra-tumor heterogeneity observed, they later considered the co-existence of different cell subpopulations within a tumor. One of these subpopulations, known as cancer stem cells, was identified in 1994 in an acute myoleid lymphoma[4] and later described in several solid tumors such as lung, breast, colon, prostate and brain tumors[5-8]. These cells have self-renewal and differentiation capacity, and also exhibit high expression of drug extrusion pumps, the latter of which confers them resistance to chemotherapy[9]. In addition, several authors have described that this cell population is responsible for the failure of response to cancer treatment and for cancer recurrence[10-12].
Also, it has been demonstrated that primary and metastatic tumor cells present phenotypic, genotypic and epi-genotypic differences[13]. These differences result in several changes in the expression and function of membrane protein receptors as well as in their signaling pathways. Thus, this intra-tumor heterogeneity creates a great challenge in the selection of specific biomarkers and treatments in oncology[14].
The transformation of a normal cell into a tumor one is a complex and progressive process in which the cell acquires genetic modifications like deletions or other mutations in tumor suppressor genes and/or oncogenic genes. Since these genes control cell proliferation either directly or indirectly, deletions or mutations in these genes may allow tumor cells to grow without control, disseminate and invade other tissues[15].
Tumor suppressor genes can either inhibit the cell cycle or promote apoptosis. The loss of their functionality can cause the development of cancer, as demonstrated in ovarian[16], lung[17], colorectal[18], head and neck[19], pancreatic[16], uterine[20], osteosarcoma[21], gastrointestinal[22], bladder[23] and breast[24] tumors.
The presence of several of these genes is associated with a high incidence of cancer. These genes can be divided into highly and moderately penetrant in relation to cancer. Highly penetrant genes include BRCA1, BRCA2, TP53, PTEN, CDH1, STK11 and PALB2, whereas moderately penetrant ones include CHEK2, ATM, BARD1, BRIP1, NBN, NF1, RAD51D and MSH6[25-27].
Oncogenes, on the other hand, are abnormal or mutant genes related to normal genes called proto-oncogenes, which support tumor development. From the beginning of the 1980’s, the participation of these genes has been studied and associated with tumor progression[28]. Many studies have demonstrated that proto-oncogenes affect the activity of telomerase enzyme in different tumor cell types. Due to the latter, these cells exhibit short telomeres and chromosomal instability[29]. Somatic mutations in the telomerase gene promoter have been described in gliomas[30], head and neck cancer[31], thyroid carcinoma[32], hepatocellular carcinoma[33], squamous cell carcinoma[34], bladder cancer[35], breast carcinoma[36] and melanomas[37].
In an incipient stage of tumor growth, when malignant cells exert a rapid rate of proliferation, the nutritional requirements of cells increase, leading to an angiogenic switch. The latter implies the development of new blood vessels from the ones preexisting in the tumor environment, which allows the emerging tumor to duplicate its diameter from 1 mm to 2 mm[38]. Concomitantly, changes in the cellular biological properties lead tumor cells to invade the surrounding extracellular matrix, to intravasate into the nearby blood and lymphatic vessels, and then to disseminate through the circulation to distant organs and to metastasize[39].
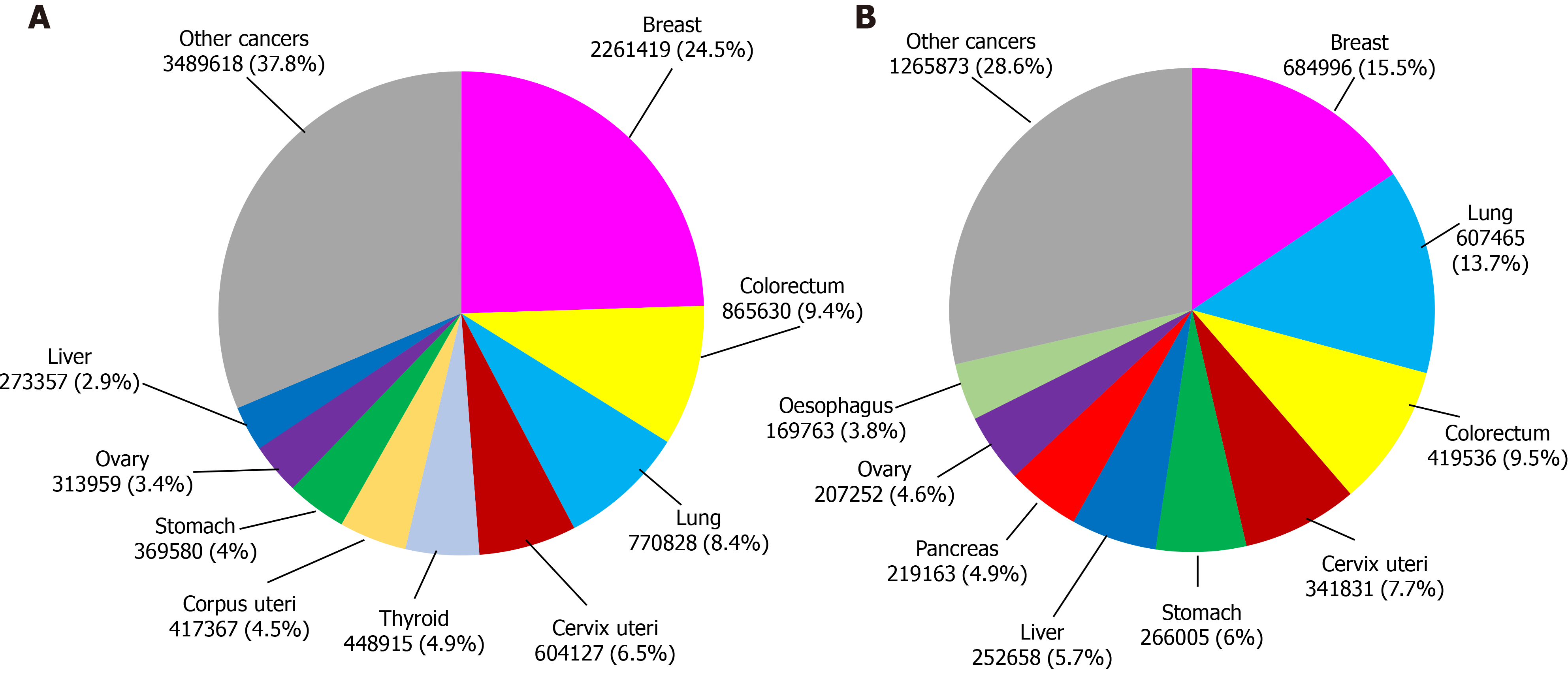
According to data from the International Agency for Research on Cancer, in 2020 this illness produced more than 19 million new cases and caused almost 10 million deaths worldwide. In particular, breast cancer has the highest incidence among all female cancer types. It represents 24.5% of the total new recorded female cancer cases and is one of the main causes of death in women, corresponding to 15.5% of deaths from this disease (Figure 1)[40].
Since life expectancy in the world population has become higher, the risk of developing tumors has also increased, considering that this parameter increases with age[41]. It is expected that the number of new cases of breast cancer in 2040 will rise to 3.2 million people and there will be more than 1 million deaths unless treatments present higher effectiveness[40].
The breast is composed of lobules (which are milk-producing glands), the ducts that connect the lobules to the nipple, and connective, fatty and lymphatic tissues. Breast cancer occurs when there is an uncontrolled growth of cells within any of these components. Although tumors can appear in any tissue of the breast, it occurs most frequently in the lobules[42].
Breast tumors exhibit particular histopathological and biological characteristics that require specific and different antitumor strategies. For these reasons, the adequate classification of tumors has a main therapeutic importance[43-45].
The different types of therapies against breast cancer include surgery, radiation and the administration of immunobiological or chemotherapeutic agents[46,47].
Surgery (tumorectomy or mastectomy) is one of the main options of treatment for patients at different stages of this disease, whereas radiotherapy is used as a complement to surgery and/or chemotherapy to reduce the probability of tumor relapse. The combination of tumorectomy and radiotherapy is used as a replacement for mastectomy in patients at the first stages of the disease.
Chemotherapy consists of the use of drugs to kill tumor cells. It can be administered as adjuvant therapy, when drugs are applied after another treatment like surgery in order to eradicate tumor cells that might have survived. Oncologists also administer neoadjuvant chemotherapy before surgery to reduce the tumor size and to be more likely to completely eliminate the tumor after the procedure[40].
As described below, according to their mechanism of action, chemotherapeutic agents can be classified into: endocrine drugs, immunological agents, DNA alkylating agents, antimetabolites and antimitotic drugs[48].
Endocrine drugs are used to treat estrogen receptor (ER) or progesterone receptor (PR) positive breast tumors. These drugs are synthetic analogs of the anti-gonadotropin-releasing hormone, anti-progestins or anti-estrogens. The latter can be divided into aromatase inhibitors and ER antagonists. The most common side effects of these drugs are: flushing sensation, nightly sweats, vaginal dryness, high blood clot risk, apoplexy, cataracts, endometrial cancer, uterus cancer, bone-mass decrease and gastrointestinal symptoms[40,49].
Immunological agents are drugs that can stimulate the patient´s immune system to detect and eliminate breast cancer cells. Immunological agents also include monoclonal antibodies against specific tumor cell proteins, and cancer treatment vaccines. Different types of immunological agents can be administered to inhibit check-points, block the suppression of the immune response and/or over-activate T-cells from patients. The most common side effects of these drugs are: fatigue, fever, shivers, weakness, nausea, vomits, dizziness, body aches and high or low blood pressure[40,50-52].
Regarding DNA alkylating agents, the ones used to treat breast cancer include cyclophosphamide and doxorubicin. Cyclophosphamide, which is an oxazaphosphorine, interferes with the duplication of DNA and RNA transcription, and its most common undesired effects are: myelosuppression, hepatotoxicity, pulmonary fibrosis, nephrotoxicity, mucosis, megaloblastic anemia, birth defects and neurotoxicity[40,53].
Doxorubicin, which is an anthracycline, is a topoisomerase II inhibitor that increases DNA degradation, preventing cell replication. Additionally, it can intercalate into DNA and promote the formation of free radicals, which in turn causes the fragmen
The antimetabolites used in breast cancer can be divided into antifolates (such as methotrexate) and pyrimidine antagonists (such as 5-FU, capecitabine or gemcitabine). Methotrexate is a competitive inhibitor of the dihydrofolate reductase which causes a decrease in pyrimidine and DNA synthesis. On the other hand, 5-FU or capecitabine can form a complex with the thymidylate synthase-folic acid, inhibiting its activity and reducing thymidine and DNA synthesis, whereas gemcitabine can incorporate a pyrimidine analog into DNA, decreasing its synthesis. The most common collateral effects of these drugs are: myelosuppression, cardiotoxicity, hepatotoxicity, pulmonary toxicity, hemolytic uremic syndrome and hyperammonemic encephalopathy[40,53].
Finally, the antimitotic drugs used in breast cancer treatment can be divided into three types: vinca alkaloids (such as vinorelbine), taxanes (such as paclitaxel and docetaxel) and non-taxane microtubule inhibitors (such as eribulin, ixabepilone and epothilone). Vinorelbine binds to B tubulin, inhibiting its polymerization into microtubules; it also prevents the formation of the mitotic spindle and arrests cells in the M phase. Taxanes produce hyper-stabilization in polymerized microtubules, thus inhibiting the degradation of the mitotic spindle, and also arrest cells in the M phase. Non-taxane microtubule inhibitors can either prevent the formation of the mitotic spindle (eribulin) or bind to B tubulin, hyper-stabilizing microtubules and producing cell arrest. Undesirable actions of these drugs are: myelosuppression, peripheral neuropathy, QT prolongation and hypersensitivity[40,54-56].
Selecting the most effective chemotherapeutic agent to treat breast tumors requires the determination of their genetic profile. Tumors are classified according to the immunohistochemical analysis of the protein expression levels of ER, PR, human epidermal growth factor receptor type 2 (HER2) and Ki-67. This analysis allows defining four breast cancer subtypes: (1) Luminal A; (2) Luminal B; (3) HER2 positive; and (4) Basal-like or triple negative[57,58].
Luminal A tumors express ER and/or PR, are HER2 negative and present low levels of Ki-67 protein, which modulates tumor cell growth[59]. This tumor subtype is the most frequently diagnosed annually and has a 5-year relative survival rate of 94%[60]. The conventional treatment for these tumors is endocrine therapy[61].
Luminal B tumors express ER and/or PR and can be positive or negative for HER2 expression. This tumor subtype has a higher cell proliferation rate than the luminal A subtype because it expresses a higher concentration of Ki-67 protein[61]. Luminal B tumors represent 10% of the total of annually diagnosed breast tumors and have a 5-year relative survival rate of 90%[60]. This tumor subtype is usually treated with endocrine therapy and in some cases with HER2 targeting drugs or other chemotherapeutic agents[61,62].
HER2 positive tumors have a high expression of this molecule and do not express ER or PR. These tumors also have a high concentration of the Ki-67 protein[59]. This tumor subtype represents 5% of all annually diagnosed breast tumors and has a 5-year relative survival rate of approximately 80%[60]. HER2 positive tumors are usually treated with HER2 targeting antibodies like trastuzumab[61] or margetuximab[63,64] alone or combined with other chemotherapeutic agents[65,66].
Triple negative tumors do not express ER, PR or HER2, but express a high concentration of Ki-67 protein and are usually very invasive and agressive[59]. This tumor subtype represents 10% of all annually diagnosed breast tumors and has a 5-year relative survival of approximately 80%[60]. These tumors can be subdivided into basal, claudin-low, normal-like and other less frequent subtypes[43,67], and, since they do not have a specific therapeutic target, they do not have any specific treatment. Although some authors indicate the use of a platinum-based agent such as cisplatin, the results are not encouraging due to the low life expectancy of the patients[68,69].
The 5-year relative survival rate varies significantly from patients with luminal A tumors, who have the best prognosis, to patients with triple negative tumors, who have the worst prognosis. The decrease in the survival rate observed in triple negative tumor patients can be explained by different factors, including higher resistance to chemotherapy and/or radiotherapy, higher relapse probability, and higher ability to metastasize in comparison with other tumor subtypes[70].
Although in recent years the diagnosis, classification and treatment of breast cancer have improved, treatment failure, recurrence and mortality are still reported world
It must be taken into account that, one of five women worldwide will develop cancer during her lifetime and one of eleven will die from this illness[40].
Muscarinic acetylcholine receptors (mAChRs) are G protein-coupled receptors (GPCRs), which belong to the superfamily of seven-transmembrane domain receptors and can modulate many functions in normal and tumor cell biology[71,72]. Regarding the latter, several authors have described an increase in the expression of different GPCRs in tumor tissues. GPCRs include thrombin receptor[73], protease-activated receptor-1[74], angiotensin II receptor type I[75], GPR161[76], GPR81[77] and leucine-rich repeat-containing G-protein-coupled receptor 5[78].
mAChRs were firstly described in the central nervous system[79] and then in the parasympathetic nervous system[80]. More recently, mAChRs, together with nicotinic receptors, have also been localized in non-neuronal cells. Also, their ligand, acetylcholine (ACh) and the enzymes that synthesize and degrade it have also been detected out of the nervous system, defining a new organization known as the non-neuronal cholinergic system[81]. In our laboratory, we described for the first time the over-expression of mAChRs of the GPCR family in breast tumor tissues and in cancer cell lines from murine and human origin[82,83].
mAChRs are metabotropic receptors and five subtypes (M1 to M5) have been identified. When activated, they can trigger different signaling pathways known as canonical or non-canonical in distinct tissues[84].
These receptors are glycoproteins with seven hydrophobic transmembrane domains connected by three extracellular and three intracellular hydrophilic loops. The domains assemble forming a structure with a pocket where the agonist binds. Also, the cytoplasmic region of the receptor couples to G protein, which is composed of three subunits: α, β and γ[85]. When a ligand binds to the receptor, guanosine diphosphate is released and replaced by guanosine triphosphate, while the subunits are dissociated into a βγ dimer and the guanosine triphosphate-bound α monomer. Depending on the α subunit type (Gαs, Gαi, Gαq, and Gα11) different downstream effectors are stimulated[86].
Agonists like ACh or the synthetic non-hydrolyzable analog carbachol can activate M1, M3 and M5 receptors, which couple to a Gαq protein, which in turn up-regulates phospholipase C activity. This enzyme cleaves phosphatidylinositol 4,5-bisphosphate into 1,2-diacylglycerol and inositol 1,4,5-triphosphate. 1,2-diacylglycerol activates protein kinase C, which stimulates downstream proteins, causing calcium influx. Inositol 1,4,5-triphosphate leads the sarcoplasmic reticulum to release stored calcium, which modulates the activation of many calcium-dependent enzymes like nitric oxide synthase[87]. Additionally, the M3 receptor can activate Ras-Raf-1-Erk-Akt through a non-canonical pathway[88].
In turn, the activation of M2 and M4 receptors, which are coupled to a Gαi/o protein, inhibits adenylyl cyclase, decreasing cyclic adenosine 3’,5’-monophosphate (cAMP) production from ATP. The decrease in cAMP concentration subsequently blocks the activation of protein kinase A[87]. M2 and M4 receptors can also regulate the activity of potassium and calcium channels[89].
The expression of the different mAChR subtypes changes throughout different body organs and tissues. This differential expression leads to diverse responses to the same stimuli. Therefore, the identification of the mAChR subtypes in different cell types is important, especially because they could have therapeutic potential. The nervous system, for example, expresses all subtypes[90-92], whereas the M1 receptor is mainly expressed in salivary glands[93], pancreas[94], bladder[95] and respiratory pathways[96]. The M2 receptor subtype is predominantly expressed in cardiac[97], digestive[98] and respiratory[99] tissues, whereas the presence of M3 receptor protein has been documented in salivary glands[93], pancreas[100], bladder[101], lung[102], colon[103] and gastric smooth muscle[104]. Finally, the M4 and M5 receptors are also expressed in the lung[105,106], while the M4 subtype is also detected in gastric tissue[107].
The expression of mAChRs in different tissues can be regulated by various stimuli. Grodzki et al[108] demonstrated that the expression of M2 receptor can be increased by the treatment of sympathetic neurons with gamma interferon. In line with these results, at our lab, we demonstrated that the de novo expression of M3 and M5 receptors can be induced by the treatment of NIH3T3 fibroblasts with interferon gamma plus lipopolysaccharide from Escherichia coli, and that this leads to an increase in the sensitivity of these cells to carbachol[109].
In several diseases, including cancer, mAChRs or their signaling pathways are differentially expressed in comparison to healthy tissues. This could be useful at the moment of considering these receptors as therapeutic targets, as described in many respiratory diseases[110]. It has been demonstrated that in pulmonary arterial hypertension, for example, the administration of M3 receptor agonists induces an anti-hypertensive therapeutic response[111]. Additionally, in chronic obstructive pulmonary disease, it has been described that the usage of muscarinic antagonists combined with beta2 adrenergic receptors reduces hyperinflation, improves dyspnea, and reduces exacerbations while improving cardiac functions[112]. This combination of muscarinic antagonists and beta2 adrenergic receptors has also been studied as a therapeutic treatment for asthma, showing promising results[113].
Alterations in the expression of mAChRs or their signaling pathways have also been detected in several diseases of the central nervous system[114-117]. However, researchers could not determine whether these alterations are either the causes or the consequences of these pathologies[118]. In spite of this, muscarinic therapy has shown promising results for these diseases. It has been documented that the treatment of patients with Alzheimer or Huntington diseases with M1 receptor agonists causes beneficial effects, reducing the symptoms of these diseases[119,120]. Furthermore, the usage of M1 antagonists in Parkinson's disease and multiple sclerosis leads to positive results, also reducing the disease symptoms[121,122].
Different authors have determined that the expression of mAChRs in malignant tissues is different from that in normal tissues. These differences comprise an increase in the expression of receptors and/or a modulation in the subtype expression pattern. In human colon cancer, for example, the expression of the M3 receptor subtype is increased more than 100-fold respect to normal tissue and its activation modulates cell proliferation, progression and invasion of this neoplasia[123]. Also, in small cell lung cancer, the M3 receptor subtype is up-regulated, promoting cell migration and invasion[124]. In human bladder, while normal tissue expresses M1, M2 and M3 receptors, tumor tissue expresses only the M2 receptor and its activation induces a decrease in cell proliferation and migration[125]. Regarding breast cancer, several studies from our laboratory have demonstrated the presence of different mAChR subtypes in breast cancer cells of murine and human origin that promote tumor growth and angiogenesis, and the absence of these receptors in normal mammary cells[126-129].
The predominant expression of different mAChR subtypes in human tumors is summarized in Table 1.
| Receptor subtypes | Malignant tissue | |
| M1 | Skin[142] | Breast[128] |
| Brain[131] | Liver[140] | |
| Prostate[136] | Esophagus[135] | |
| Cervix uteri[139] | Pancreas[147] | |
| M2 | Lung[134] | Esophagus[135] |
| Brain[143] | Bladder[125] | |
| Breast [128] | Leukemia[145] | |
| M3 | Lung [124] | Breast[148] |
| Colon[133] | Esophagus[135] | |
| Skin[142] | Liver[139] | |
| Prostate[138] | Stomach[132] | |
| Cervix uteri[139] | Leukemia[145] | |
| Head and neck[146] | ||
| M4 | Brain[131] | Breast[128] |
| Prostate[137] | Esophagus[135] | |
| Cervix uteri[139] | Oral cavity[141] | |
| M5 | Skin[130] | Breast[128] |
| Cervix uteri[139] | Esophagus[135] | |
It has been reported that the activation of mAChRs leads to the stimulation of several steps of tumor progression, involving different receptor subtypes in each tumor. Cell proliferation has been found to be induced by the M5 receptor subtype in melanoma cells[130], by the M1 and M4 receptor subtypes in glioblastoma cells[131], by the M3 receptor subtype in stomach and colon cancer[132,133], by the M2 receptor subtype in non-small cell lung cancer[134] and by all receptor subtypes in the esophagus[135].
In other types of tumor, researchers have observed not only an increase in cell proliferation, but also an increase in cell invasiveness and migration ability. This increase is induced by the M1, M3, M4 receptor subtypes in prostate tumors, by the M1, M3, M4 and M5 receptor subtypes in cervical tumors[136-139] and by the M1 and M3 receptor subtypes in hepatocellular carcinoma[124,140]. Finally, in oral cavity tumors, cell motility and dissemination have been found to be increased by the activation of the M4 receptor subtype[141].
Another important aspect to analyze is the expression and function of mAChRs in primary tumors and their metastases. Regarding the latter, two melanoma cell lines and a third one derived from a metastasis have been found to express M1 and M3 receptors. Although all of them responded to carbamylcholine, increasing cytosolic calcium levels, the metastatic cell line responded with a higher peak in calcium concentration. The authors speculated that this difference could be responsible for a higher malignance and migratory potential in metastasis[142]. In accordance with the latter observations, we have reported that the expression of mAChRs is significantly higher in the metastatic murine mammary cell line LMM3 than in the non-metastatic LM3 tumor cells that originated them[82]. However, when analyzing the function of mAChRs in tumor tissues, other authors confirmed opposite results, particularly considering the expression and function of the M2 receptor subtype. Lucianò et al[143], for example, established that two cell lines derived from a neuroblastoma with bad prognosis, SK-N-BE and SK-N-BE(2C), express the M2 receptor and that its activation inhibits the cell cycle. In human bladder tumors, Pacini et al[125] reported the expression of M2 receptor protein and found that its stimulation reduced cell proliferation and migration. These results are in line with the findings of Alessandrini et al[144], who demonstrated that the activation of the M2 receptor increases apoptosis in glioblastoma, a brain tumor with bad prognosis. Regarding leukemia, Cabadak et al[145] identified the presence of the M2 and M3 receptors in K562 cells, and linked their activation to an inhibition in cell proliferation. In head and neck cancer, Sun et al[146] reported a similar effect by M3 activation. Finally, in human pancreas tumor, Renz et al[147] demonstrated that M1 receptor activation decreases tumorigenesis.
Regarding breast cancer, we have reported the expression of all mAChR subtypes in the breast adenocarcinoma cell lines LM2 and LM3 derived from spontaneously aroused tumors in female BALB/c mice and the lack of expression of these receptors in the murine mammary non-tumorigenic cell line NMuMG[82]. Using tritiated quinuclidinyl benzilate ([3H]-QNB) in binding assays, we determined the expression mainly of the M2 and M3 receptor subtypes[126]. We also found that the addition of carbachol to tumor cells induces two opposite actions depending on the concentration of the agonist and the time of treatment. At low concentrations added for 1 h or less, we reported a stimulation in cell proliferation, migration and also in tumor angiogenesis, whereas at higher concentrations or longer periods of treatment, we observed cell death[126]. These differences due to distinct experimental conditions besides the expression of different mAChRs could explain the opposite results documented in the literature previously mentioned.
Luminal breast cancer tumors are characterized by the expression of their endocrine receptor. In MCF-7 cells, the human luminal breast cancer cell line most studied in oncology research, we identified the expression of the M3 and M4 receptors by Western blot. We also found that the short-time treatment of MCF-7 cells with carbachol activates, through the M3 receptor, a phospholipase C/protein kinase C/calcium-dependent nitric oxide synthase signaling pathway, increasing cell proliferation. Carbachol also stimulates the formation of tumor blood vessels and the expression of vascular endothelial growth factor A, concomitantly with tumor cell migration and matrix metalloproteinase-9 expression and activity[148,149]. Similarly to that reported in murine normal mammary cells, the non-tumorigenic human breast cell line MCF-10A does not express these receptors[148].
Triple negative breast tumors are characterized by the absence of a specific therapeutic target and bad prognosis. By studying two different human cell lines derived from this type of tumor, at our lab, we documented that both express mAChRs, but while MDA-MB231 cells express the M1, M2, M4 and M5 receptor subtypes, MDA-MB468 express all receptor subtypes including M3 protein[128]. Similarly to that documentated in our previous reports, short-time activation of these cell lines with carbachol stimulated proliferation in a dose-dependent manner. This effect was prevented by the presence of the muscarinic antagonist atropine, confirming the involvement of mAChRs in this effect[128]. In MDA-MB231 cells, we were able to identify the receptor involved in the mentioned effects by using small interfering RNA silencing assays, which revealed that the one responsible for the proliferative actions in this cell line is the M2 subtype[128].
Based on previous reports by other authors and our results proving that long-term and low-concentration treatment with muscarinic agonists induces a decrease in cell viability, we next decided to focus on mAChRs as therapeutic targets for breast cancer treatment in triple negative tumors[128,129].
To assign more specificity to mAChRs as blanks of action in anti-tumor therapy, it must be taken in mind that non-tumorigenic human mammary cells, like MCF-10A, or breast samples from patients with benign pathology (fibroadenoma) lack expression of mAChRs and, as a consequence, they are not sensitive to the treatment with muscarinic agonists. However, when transfected with mAChRs, normal cells acquire the ability to respond to muscarinic treatment[129]. All these results highlight the relationship between mAChRs and breast cancer development and treatment.
Many chemotherapeutic drugs are used in breast cancer treatment, but only two of them are able to bind with the active site of mAChRs: paclitaxel[82] and doxorubicin[150]. Both induce an inhibitory effect on cell proliferation in vitro similar to that observed with the cholinergic agonist carbachol[128].
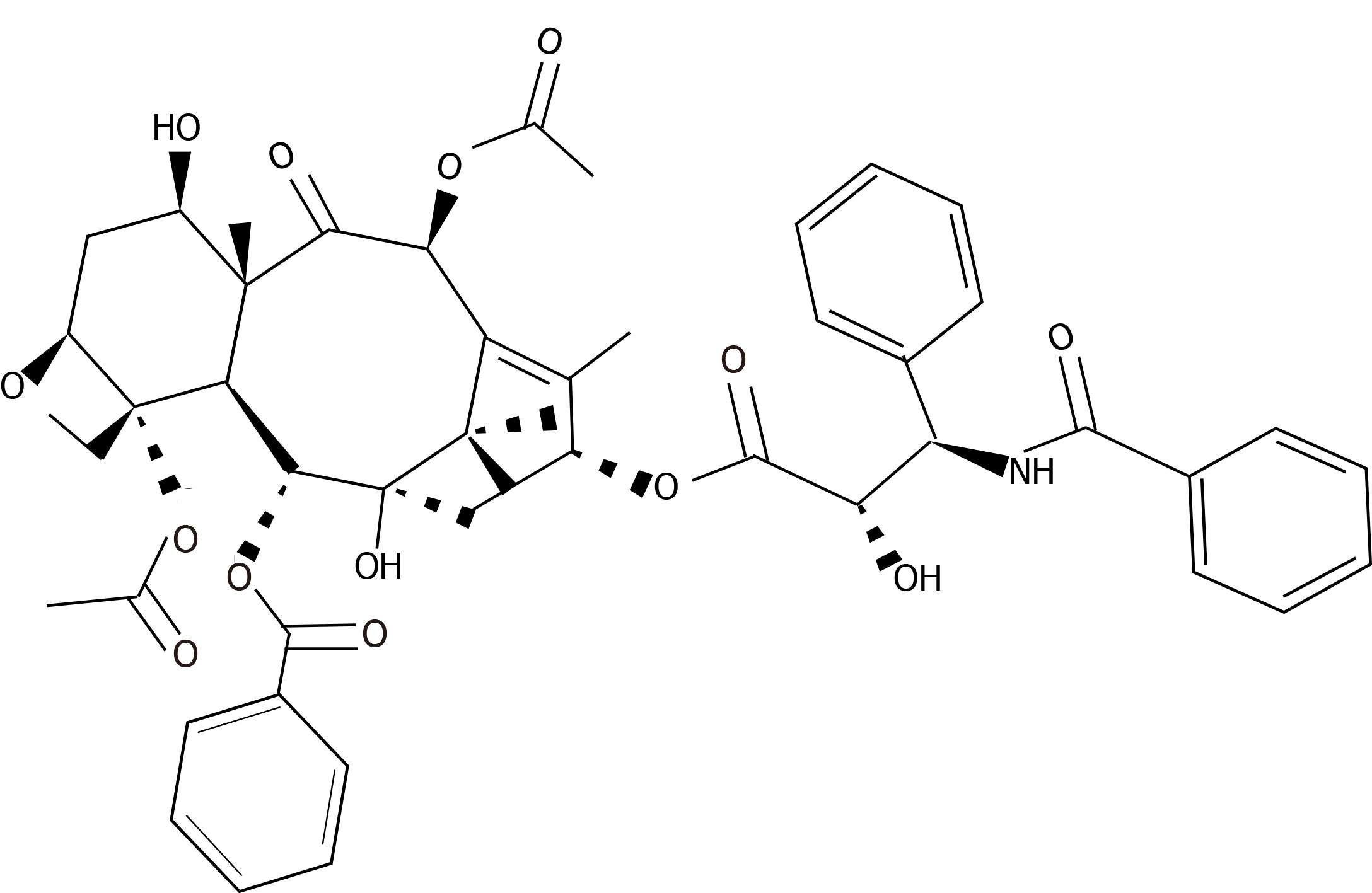
Paclitaxel is a drug of first choice in the treatment of breast cancer. It is a taxane derived from the tree Taxus brevifolia[151]. This drug was approved by the Food and Drugs Administration (FDA) for the treatment of ovarian cancer in 1992, for advanced stages of breast cancer in 1995[152], and for the early stages of breast cancer in 2001[153]. Paclitaxel is a diterpenoid pseudoalkaloid made up of an N-benzoyl phenylisoserine group and a taxane ring. In contrast with other taxanes, paclitaxel has a lateral complex chain connected to its taxane ring in C-13, which gives this drug its antitumor activity[154]. Its molecular formula is C47H5NO14 and its chemical structure is described in Figure 2.
Despite the extended use of paclitaxel in anti-tumor treatment, it has been reported that its administration at therapeutic concentrations (10-6 mol/L approximately) causes adverse effects like hepatotoxicity, leukopenia, neutropenia, anemia, thrombocytopenia, neurotoxicity, vomits, alopecia, fatigue, mucositis and diarrhea[155-158]. In addition to the low-efficiency synthesis of paclitaxel and its side effects, another problem is its insolubility in water, which affects its bioavailability. Thus, to improve its availability, it is dissolved in polyoxyethylated castor oil as a vehicle (Cremophor EL); however, this oil has been associated with several anaphylactic reactions like dyspnea with bronchospasm, hypotension and urticaria since it activates the complement system[159]. Because of this, new technologies have been developed to improve the biodistribution of this taxane, and alternative ways of administration like paclitaxel binded to albumin[160,161], paclitaxel-loaded PEG-vitamin E nanoparticles[162,163] and micellar paclitaxel[164,165] have been proposed.
The conventional dose of paclitaxel administered to treat breast cancer is 175 mg/m2 skin by intravenous inoculation for 3 h every 21 d in 6 cycles[166] and its halflife time after inoculation is 4.9 ± 3.6 h[167]. After one administration, most of the paclitaxel in the circulation binds to proteins and its systemic clearance is 700 mL/min on average[168]. Approximately 90% of the drug is degraded by hepatic metabolism by P-450 isoenzymes (CYP3A and CYP2C) and is excreted by feces, while the other 10% is excreted by urine without alterations[168-170].
Paclitaxel exerts its anti-tumor effects through different mechanisms. One of these is by acting as a cytostatic drug that binds to the β subunit of tubulin and stabilizes the polymerized microtubules, inhibiting their depolymerization[171,172]. Consequently, cells lose their ability to divide due to insufficient requirements in the G2/M mitotic control checkpoint[173]. The prolonged arrest of mitosis eventually leads to cell death[174].
Besides its antimitotic effect, paclitaxel also acts in the immune system since its administration is associated with the change in the macrophage phenotype from an M2 to an M1 profile through TLR4 (toll-like receptor 4) activation[175]. Millrud et al[176] reported that, in the presence of paclitaxel, primary human monocytes turn into proinflammatory M1 macrophages, contributing to tumor eradication.
Also, at low doses, paclitaxel can induce apoptosis in tumor cells by inactivating the B-cell lymphoma 2 protein (Bcl-2)[177-179]. Additionally, it has been described that this cytostatic drug modulates several non-coding RNAs that have a regulatory function over genes related to tumor progression[180]. Moreover, paclitaxel can modulate the release of the apoptogenic factor cytochrome that can trigger programmed cell death[181,182]. The dysregulation of these and other parameters can lead to resistance to paclitaxel treatment.
One interesting point that we have analyzed in our laboratory is the ability of paclitaxel to interact with mAChRs in a specific manner[126]. We proved that paclitaxel displaces the binding of [3H]-QNB to mAChRs expressed in murine mammary adenocarcinoma cell lines in a manner similar to that of atropine. Moreover, when we treated these cells with paclitaxel at very low concentrations (10-11 mol/L), we reported an inhibitory effect on tumor cell proliferation. This effect was prevented by the previous treatment of cells with atropine, confirming the participation of mAChRs in this action[126].
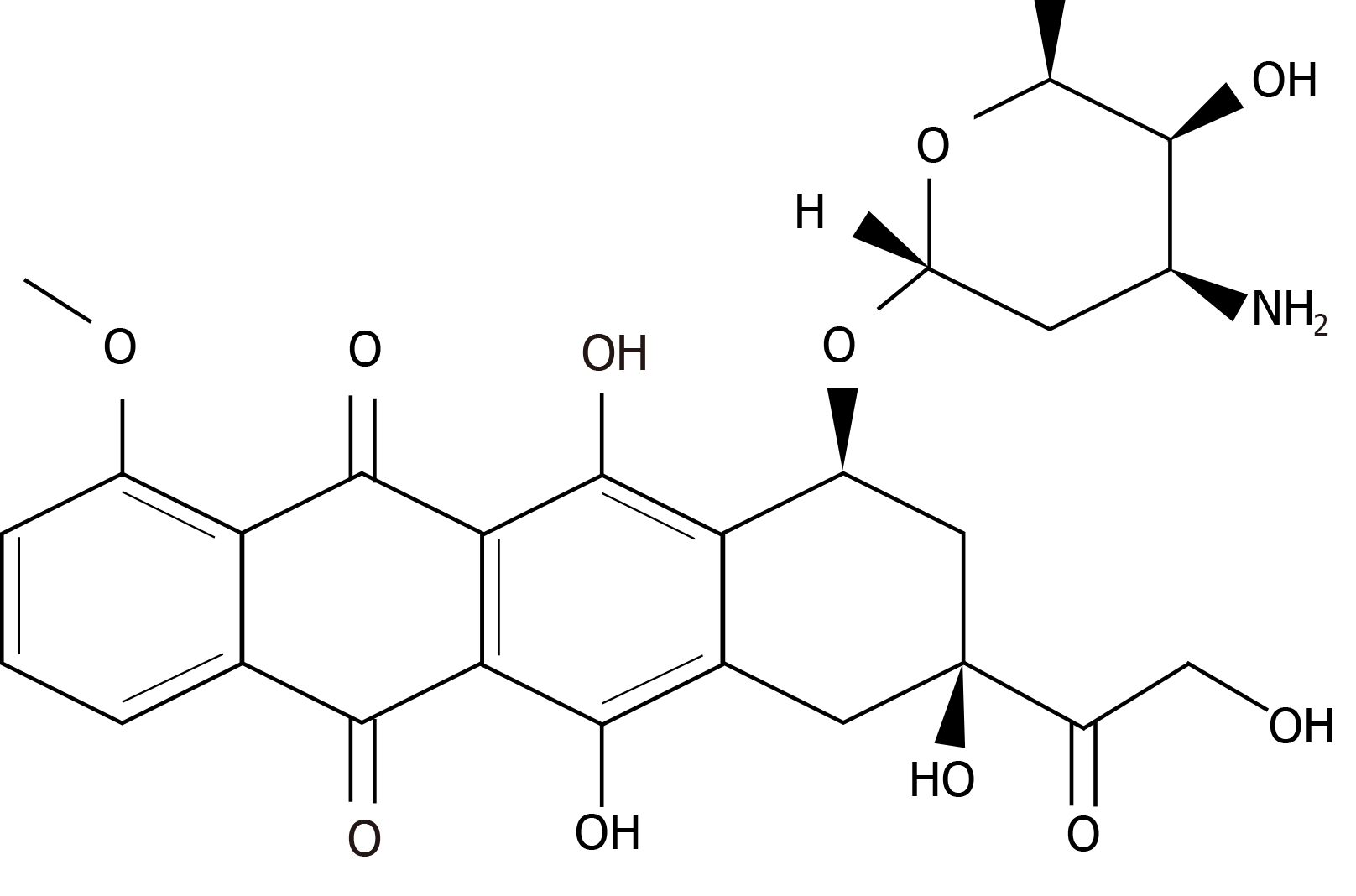
The other chemotherapeutic drug frequently used in breast cancer treatment is doxorubicin[183,184], which is an antibiotic that belongs to the family of anthracyclines and is produced by the bacterium Streptomyces peucetius. It was approved by the FDA in 1974 for metastatic breast cancer treatment[185]. Its molecular formula is C27H29NO11 and its chemical structure is shown in Figure 3.
The treatment with doxorubicin causes many side effects, including cardiotoxicity[186], infertility[187], genotoxicity[188], amenorrhea[189], thrombophlebitis[190] and lung embolism[191]. Regarding cardiotoxicity, the additional challenge is that it can occur 10 years after chemotherapy treatment, appearing as a progressive congestive cardiac failure secondary to a non-ischemic dilated cardiomyopathy, and is irrever
In breast cancer, doxorubicin is administered in 60/75 mg/m2 per dose intrave
Doxorubicin exerts its chemotherapeutic effect by three mechanisms. The first one is the inhibition of the enzyme topoisomerase II-α, which regulates the superhelical state of DNA and has a structural function regulating the tension of the DNA strands[202]. Doxorubicin stabilizes the binding of topoisomerase II-α to the cleaved DNA, preventing the replication process and leading the cell to apoptosis[203]. Given that tumor cells present a higher proliferative rate, the expression of topoisomerase II-α is high, producing a greater selectivity of doxorubicin for these cells[204].
The second mechanism of action also affects directly the DNA strands. Doxorubicin intercalates into DNA, inhibiting the activity of topoisomerase II-α, which in turn causes the inhibition of DNA synthesis[205]. Moreover, doxorubicin intercalation causes the break of the DNA in double-strand fragments and chromatin condensation, which leads to an increase in apoptosis[206]. This antibiotic can also intercalate in RNA, inhibiting the activity of RNA polymerase, although it has more affinity for DNA[207].
Finally, the third mechanism of action of doxorubicin is the production of free radicals. Doxorubicin can act as an electron acceptor, transforming its quinone to a semiquinone-free radical, which causes oxidative damage and induces the cleavage and degradation of DNA. This is a mitochondrial reaction catalyzed by the enzyme cytochrome P450 reductase in the presence of NADH dehydrogenase[208]. It has also been described that the free molecular iron can interact with doxorubicin, forming toxic free radicals and reactive nitrogen species that increase nitrosative stress and mitochondrial dysfunction, promoting apoptosis[209].
Interestingly, doxorubicin can also interact with mAChRs. It has been demonstrated that doxorubicin displaces the [3H]-QNB binding in the left atrial muscle of guinea pig hearts in a concentration-dependent manner, similarly to atropine, indicating that doxorubicin can also bind to mAChRs[150,197]. Additionally, in triple negative human tumor cells, we determined that doxorubicin exerts an inhibitory effect on proliferation, comparable to that induced by carbachol[128].
One of the most important causes in the failure of chemotherapy not only in breast cancer treatment but also in other cancers is the appearance of resistance. It can appear as a primary form, when it is present before the treatment, or acquired, when it develops after the exposure to a drug[210,211].
To improve the efficacy of chemotherapeutic agents in the treatment of breast cancer, it is important to define the mechanisms underlying resistance in this type of tumor. In breast cancer, resistance is multifactorial and implies different mechanisms and genes that exert their effects either together or separately, leading to a reduction or an inhibition of the effect of different drugs.
Regarding the factors that modulate the resistance to paclitaxel or doxorubicin, some of them are common to both drugs and others are specific to each of them. The main factors in common are the expression of drug extrusion pumps, noncoding RNAs, Bcl-2 and p53. Drug extrusion pumps are also known as ATP binding cassette (ABC) transporters. They are located in the cytoplasmic membrane and modulate the time that the drug is present inside the cell by controlling its active transport. The expression and activity of these pumps can be modified by different factors, including the chemotherapy treatment. Amawi et al[212] reported that paclitaxel treatment exerts an increase in pump activity and expression in different breast cancer cell lines, whereas Cox and Weinman[213] reported similar results in hepatocellular carcinomas treated with doxorubicin. In both cases, the increase in the expression and activity of these pumps reduced the effectiveness of the treatment. Regarding the other factors that modulate the action of paclitaxel or doxorubicin, noncoding RNAs, also called miRNA, can modulate the expression of genes that regulate apoptosis and cell survival, increasing the sensitivity or the resistance to the chemotherapeutic treatment[214-216], whereas Bcl-2 and p53 expression proteins regulate tumor growth and are important tumor markers that can be modulated by chemotherapeutic drugs, increasing the resistance to treatment[217,218].
Among paclitaxel resistance factors, the most relevant are changes in the cytoskeletal dynamics and kinetic degradation of the taxane structure. Several tumor cells express high levels of βIII-tubulin that are related to resistance to paclitaxel treatment[219,220]. When this expression is down-regulated, cells recover sensitivity to paclitaxel[221]. In this regard, Wang et al[222] described an increase in the expression of βIII-tubulin, which induces resistance because higher concentrations of the drug are needed to stabilize microtubules and block cell division.
Regarding the proteins that modulate cytoskeletal dynamics, many authors have established that an increase in the resistance to paclitaxel treatment is related to an over-expression of stathmin[223], septin[224], tubulin binding cofactor C[225] and BRCA1[226] proteins.
It is also known that enzymes of the cytochrome P450 subfamily 3A and 2C play a major role in the metabolism of taxane anticancer agents. The expression of these enzymes in solid tumors may thus play a role in the in situ metabolism of drugs as well, potentially affecting the intrinsic susceptibility of these tumors to taxane. An abnormal up-regulation of enzyme activity or expression reduces the halflife time of paclitaxel and as a consequence its efficacy in cancer patients[227].
Regarding doxorubicin, the expression of topoisomerase II-α and FOXO3 is mentioned as a main aspect in the mechanism of resistance to this drug. Doxorubicin exerts part of its chemotherapeutic effect by the binding to topoisomerase II-α, which produces DNA structure stabilization and induces apoptosis. Wang et al[222] reported that a down-regulation in topoisomerase II-α expression induces resistance to doxorubicin treatment in human breast tumor cells MCF-7. In human malignant breast samples, O'Malley et al[228] demonstrated that topoisomerase II-α expression is a good marker to determine the resistance to doxorubicin since cells with higher levels of this enzyme are more sensitive to this anthracycline.
FOXO3 is a factor associated with longevity due to its antioxidant effect[229]. In this regard, Gomes et al[230] reported that the administration of doxorubicin in breast cancer patients can increase FOXO3 expression, inducing resistance to treatment and making it a marker of bad prognosis.
Besides the previous points, it must be taken into account that the treatment with these drugs can stimulate the development of metastases. Regarding this matter, Karagiannis et al[231] demonstrated that the in vivo administration of doxorubicin or paclitaxel to murine breast tumor bearers promotes the formation of micrometastases in the lung, which is the first step necessary to produce tumor cell intravasation and the subsequent formation of metastases. Moreover, Daenen et al[232] established that paclitaxel treatment also increases vascular endothelial growth factor receptor-1 expression in lung endothelial cells, which stimulates the adhesion of circulating tumor cells and the subsequent formation of metastases.
Additionally, conventional therapeutic treatment with doxorubicin or paclitaxel can affect normal cells, inducing cell death. We and other authors have demonstrated that, at therapeutic concentrations, paclitaxel besides reducing cell viability in human breast tumor cells MCF-7 and MDA-MB231 also causes cell death in normal mammary cells MCF-10A[128,233-235].
The conventional treatment usually applied to cancer patients considers the administration of chemotherapeutic drugs at the maximum tolerated dose. Because of all the undesirable effects caused, patients need a long interval of time between cycles of treatment so that normal tissues can recover. The usage of the maximum tolerated dose is useful in tumors that rarely have a complex network of activating mutations, like acute lymphoblastic leukemia or testicular cancer. However, in other tumors like breast tumors, this conventional treatment is less effective mainly because these tumors permanently modulate the tumor microenvironment. In addition, as mentioned above, besides the toxicity induced by the administration of the maximum tolerated dose in breast cancer, development of resistance to treatment is frequently described[236,237].
One of the strategies to reduce side effects and the resistance to anti-tumor drugs is the administration of minimal doses of one or more chemotherapeutic agents, which, administered in a continuous regime or with short time intervals, improves the results of treatment. This strategy is known as metronomic therapy and its main aim is to reduce the toxicity and to increase the quality of the patients’ lives.
Metronomic therapy may be effective to inhibit tumor progression through different mechanisms that modulate not only tumor cell death but also the cancer stem cell population involved in tumor generation and metastasis[129,236]. Folkins et al[238] demonstrated that metronomic therapy with cyclophosphamide could reduce the growth of human glioma spheroids, a cancer stem cell marker. Vives et al[239] reported that the same treatment reduces the number of cancer stem precursor cells in human pancreatic tumor. Similarly, in our laboratory, we demonstrated that the treatment of human breast cancer cells MCF-7 with paclitaxel plus carbachol in a metronomic schedule causes a decrease in the cancer stem cell population[129].
The target of this kind of therapy is not only tumor cells but other cells of the tumor microenvironment[236,240-242]. Several authors have demonstrated that some chemotherapeutic drugs administered at low doses can inhibit the synthesis of pro-angiogenic factors produced by endothelial cells, which are necessary for tumor growth[236].
In addition, metronomic therapy is effective to modulate the activity of the immune system, by decreasing the number of regulatory T cells and increasing the population of cytotoxic T lymphocytes and natural killer cells[243,244].
Metronomic therapy is usually linked to repurposing drugs. The latter refers to the assignation of new uses for drugs usually administered to treat diseases other than cancer[245]. In oncology, there is an increasing interest in the prescription of non-cancer drugs for cancer treatments due to the knowledge of their pharmacokinetics/ dynamics and side effects, and because most of them are available at low cost[246].
Considering previous results obtained in our laboratory, we have recently proposed the administration of low doses of paclitaxel or doxorubicin combined with low doses of carbachol, a non-selective muscarinic agonist, or arecaidine, an M2 selective agonist, in a metronomic schedule to effectively reduce breast tumor cell viability[128]. As shown in Table 2, the effect of metronomic combinations is similar to that obtained with paclitaxel or doxorubicin administered at therapeutic concentrations (10-6 mol/L). These results position muscarinic agonists in the spectrum of repurposing drugs.
| Murine breast malignant cells | Murine breast not malignant cells |
| Paclitaxel (10-11 mol/L) + Carbachol (10-9 mol/L)[126] | |
| LM2: 68.2 ± 5.1b | NMuMG: 6.8 ± 6.6 |
| LM3: 64.6 ± 6.5b | |
| Human breast malignant cells | Human breast not malignant cells |
| Paclitaxel (10-9 mol/L) + Carbachol (10-11 mol/L)[127] | |
| MCF-7: 45.6 ± 5.8a | MCF-10A: 1.9 ± 0.3 |
| Paclitaxel (10-9 mol/L) + Carbachol (10-10 mol/L)[128] | |
| MDA-MB468: 33.4 ± 2.5c | MCF-10A: 2.9 ± 3.1 |
| Paclitaxel (10-9 mol/L) + Arecaidine propargyl ester (10-7 mol/L)[128] | |
| MDA-MB468: 26.9 ± 3.6c | MCF-10A: 3.3 ± 6.2 |
| Paclitaxel (10-8 mol/L) + Carbachol (10-11 mol/L)[128] | |
| MDA-MB231: 36.8 ± 6.2c | MCF-10A: 3.1 ± 6.2 |
| Doxorubicin (10-8 mol/L) + Carbachol (10-11 mol/L)[128] | |
| MDA-MB231: 35.3 ± 0.8c | MCF-10A: 6.1 ± 3.1 |
| Paclitaxel (10-8 mol/L) + Arecaidine propargyl ester (10-5 mol/L)[128] | |
| MDA-MB231: 35.8 ± 3.1c | MCF-10A: 8.2 ± 6.5 |
| Doxorubicin (10-8 mol/L) + Arecaidine propargyl ester (10-5 mol/L)[128] | |
| MDA-MB231: 33.3 ± 2.1c | MCF-10A: 7.8 ± 6.8 |
| Paclitaxel (10-6 mol/L)[128] | |
| MDA-MB231: 38.0 ± 6.1c | MCF-10A: 33.3 ± 1.5c |
| Doxorubicin (10-6 mol/L)[128] | |
| MDA-MB231: 41.1 ± 2.3c | MCF-10A: 45.7 ± 1.8c |
Our results focused on the presence of mAChRs in tumor cells and their absence in normal cells, giving specificity to this type of anti-tumor therapy, and indicating that it also prevents cytotoxic actions in normal cells, which could be an indicator of reduced adverse effects (Table 2).
It is important to mention that the treatment with paclitaxel plus carbachol not only reduces tumor cell viability but also prevents other important steps of tumor progression. This therapy diminishes tumor cell migration, cancer stem cell percentage, neoangiogenesis and the expression of the drug extrusion pump ABCG2[128].
The research about new antitumor therapies with drugs that increase beneficial actions and reduce adverse effects is a challenge to improve breast cancer patients’ lives. The usage of repurposing drugs, like the muscarinic agonist carbachol, which synergizes the action of traditional anti-tumor drugs might be an alternative schedule focused on mAChRs as new therapeutic targets. The presence of these receptors at high concentrations not only in breast tumors but also in other types of tumor could help to find a more specific and less aggressive manner to treat cancer patients. On the other hand, metronomic therapy is effective to kill tumor cells without affecting normal cells and also decreases pathological neo-angiogenesis and the expression of drug extrusion proteins. The latter could prevent the appearance of resistance reported in conventional chemotherapy. More in vivo experiments are needed to confirm the effectiveness of this treatment in breast cancer models and to gain information to discard systemic adverse reactions.
| 1. | Ruddon R. Cancer biology. 4th ed. University of Michigan Medical School. Ann Arbor, Michigan. Oxford University Press, 2007: 4-5. |
| 2. | Sidransky D, Frost P, Von Eschenbach A, Oyasu R, Preisinger AC, Vogelstein B. Clonal origin of bladder cancer. N Engl J Med. 1992;326:737-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 367] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 3. | Esumi M, Aritaka T, Arii M, Suzuki K, Tanikawa K, Mizuo H, Mima T, Shikata T. Clonal origin of human hepatoma determined by integration of hepatitis B virus DNA. Cancer Res. 1986;46:5767-5771. [PubMed] |
| 4. | Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3316] [Cited by in RCA: 3437] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 5. | Prewitt TW, Matthews W, Chaudhri G, Pogrebniak HW, Pass HI. Tumor necrosis factor induces doxorubicin resistance to lung cancer cells in vitro. J Thorac Cardiovasc Surg. 1994;107:43-49. [PubMed] |
| 6. | Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983-3988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7830] [Cited by in RCA: 7800] [Article Influence: 339.1] [Reference Citation Analysis (0)] |
| 7. | Vermeulen L, Todaro M, de Sousa Mello F, Sprick MR, Kemper K, Perez Alea M, Richel DJ, Stassi G, Medema JP. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc Natl Acad Sci USA. 2008;105:13427-13432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 594] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 8. | Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2- cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207-6219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 673] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 9. | Pan Y, Ma S, Cao K, Zhou S, Zhao A, Li M, Qian F, Zhu C. Therapeutic approaches targeting cancer stem cells. J Cancer Res Ther. 2018;14:1469-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 10. | Abdullah LN, Chow EK. Mechanisms of chemoresistance in cancer stem cells. Clin Transl Med. 2013;2:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 477] [Cited by in RCA: 570] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 11. | Wang W, Zhang H, Wang X, Patterson J, Winter P, Graham K, Ghosh S, Lee JC, Katsetos CD, Mackey JR, Tuszynski JA, Wong GK, Ludueña RF. Novel mutations involving βI-, βIIA-, or βIVB-tubulin isotypes with functional resemblance to βIII-tubulin in breast cancer. Protoplasma. 2017;254:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Drewa T, Styczynski J, Szczepanek J. Is the cancer stem cell population "a player" in multi-drug resistance? Acta Pol Pharm. 2008;65:493-500. [PubMed] |
| 13. | Chen C, Wang J. A physical mechanism of cancer heterogeneity. Sci Rep. 2016;6:20679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Martelotto LG, Ng CK, Piscuoglio S, Weigelt B, Reis-Filho JS. Breast cancer intra-tumor heterogeneity. Breast Cancer Res. 2014;16:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 255] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 15. | Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19834] [Cited by in RCA: 19782] [Article Influence: 760.8] [Reference Citation Analysis (0)] |
| 16. | Kang R, Xie Y, Zhang Q, Hou W, Jiang Q, Zhu S, Liu J, Zeng D, Wang H, Bartlett DL, Billiar TR, Zeh HJ 3rd, Lotze MT, Tang D. Intracellular HMGB1 as a novel tumor suppressor of pancreatic cancer. Cell Res. 2017;27:916-932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 17. | Zhang ZL, Wang NN, Ma QL, Chen Y, Yao L, Zhang L, Li QS, Shi MH, Wang HF, Ying Z. Somatic and germline mutations in the tumor suppressor gene PARK2 impair PINK1/Parkin-mediated mitophagy in lung cancer cells. Acta Pharmacol Sin. 2020;41:93-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Chen MS, Lo YH, Chen X, Williams CS, Donnelly JM, Criss ZK 2nd, Patel S, Butkus JM, Dubrulle J, Finegold MJ, Shroyer NF. Growth Factor-Independent 1 Is a Tumor Suppressor Gene in Colorectal Cancer. Mol Cancer Res. 2019;17:697-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Lin LH, Chang KW, Cheng HW, Liu CJ. SMAD4 Somatic Mutations in Head and Neck Carcinoma Are Associated With Tumor Progression. Front Oncol. 2019;9:1379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Yuan M, Yao L, Abulizi G. Tumor-suppressor gene SOX1 is a methylation-specific expression gene in cervical adenocarcinoma. Medicine (Baltimore). 2019;98:e17225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Li M, Jin X, Li H, Wu G, Wang S, Yang C, Deng S. Key genes with prognostic values in suppression of osteosarcoma metastasis using comprehensive analysis. BMC Cancer. 2020;20:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Sun Q, Uddin MN, Li M, Wang X, Lai M. Computational Identification of Tumor Suppressor Genes Based on Gene Expression Profiles in Normal and Cancerous Gastrointestinal Tissues. J Oncol. 2020;2020:2503790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Chang S, Yim S, Park H. The cancer driver genes IDH1/2, JARID1C/ KDM5C, and UTX/ KDM6A: crosstalk between histone demethylation and hypoxic reprogramming in cancer metabolism. Exp Mol Med. 2019;51:1-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 24. | Zhang Y, Stovall DB, Wan M, Zhang Q, Chou JW, Li D, Sui G. SOX7 Target Genes and Their Contribution to Its Tumor Suppressive Function. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Han MR, Zheng W, Cai Q, Gao YT, Zheng Y, Bolla MK, Michailidou K, Dennis J, Wang Q, Dunning AM, Brennan P, Chen ST, Choi JY, Hartman M, Ito H, Lophatananon A, Matsuo K, Miao H, Muir K, Sangrajrang S, Shen CY, Teo SH, Tseng CC, Wu AH, Yip CH, Kang D, Xiang YB, Easton DF, Shu XO, Long J. Evaluating genetic variants associated with breast cancer risk in high and moderate-penetrance genes in Asians. Carcinogenesis. 2017;38:511-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Li J, Li H, Makunin I; kConFab Investigators; Thompson BA, Tao K, Young EL, Lopez J, Camp NJ, Tavtigian SV, John EM, Andrulis IL, Khanna KK, Goldgar D, Chenevix-Trench G. Panel sequencing of 264 candidate susceptibility genes and segregation analysis in a cohort of non-BRCA1, non-BRCA2 breast cancer families. Breast Cancer Res Treat. 2017;166:937-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Wood ME, McKinnon W, Garber J. Risk for breast cancer and management of unaffected individuals with non-BRCA hereditary breast cancer. Breast J. 2020;26:1528-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Tabin CJ, Bradley SM, Bargmann CI, Weinberg RA, Papageorge AG, Scolnick EM, Dhar R, Lowy DR, Chang EH. Mechanism of activation of a human oncogene. Nature. 1982;300:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 971] [Cited by in RCA: 993] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 29. | Srinivas N, Rachakonda S, Kumar R. Telomeres and Telomere Length: A General Overview. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 203] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 30. | Shaughnessy M, Njauw CN, Artomov M, Tsao H. Classifying Melanoma by TERT Promoter Mutational Status. J Invest Dermatol. 2020;140:390-394.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Yilmaz I, Erkul BE, Ozturk Sari S, Issin G, Tural E, Terzi Kaya Terzi N, Karatay H, Celik M, Ulusan M, Bilgic B. Promoter region mutations of the telomerase reverse transcriptase (TERT) gene in head and neck squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;130:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Liu R, Li Y, Chen W, Cong J, Zhang Z, Ma L, Chu L, Xiao H, Zhang Y, Liu Y, Xu Y, Yu Q, Yang X, Sun C. Mutations of the TERT promoter are associated with aggressiveness and recurrence/distant metastasis of papillary thyroid carcinoma. Oncol Lett. 2020;20:50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Kwa WT, Effendi K, Yamazaki K, Kubota N, Hatano M, Ueno A, Masugi Y, Sakamoto M. Telomerase reverse transcriptase (TERT) promoter mutation correlated with intratumoral heterogeneity in hepatocellular carcinoma. Pathol Int. 2020;70:624-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Erkul E, Yilmaz I. TERT promoter mutation in patients with second primary of tongue squamous cell carcinoma. Oral Oncol. 2021;114:105089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Batista R, Lima L, Vinagre J, Pinto V, Lyra J, Máximo V, Santos L, Soares P. TERT Promoter Mutation as a Potential Predictive Biomarker in BCG-Treated Bladder Cancer Patients. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Shimoi T, Yoshida M, Kitamura Y, Yoshino T, Kawachi A, Shimomura A, Noguchi E, Yunokawa M, Yonemori K, Shimizu C, Kinoshita T, Ichimura K, Fukuda T, Fujiwara Y, Tamura K. TERT promoter hotspot mutations in breast cancer. Breast Cancer. 2018;25:292-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Yuan G, Song J, Li N, Song Q, Li Y, Du Y, Wang X, Jiao Y, Wu L. Telomere Maintenance Associated Mutations in the Genetic Landscape of Gynecological Mucosal Melanoma. Front Oncol. 2020;10:1707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Folkman J, Merler E, Abernathy C, Williams G. Isolation of a tumor factor responsible for angiogenesis. J Exp Med. 1971;133:275-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1165] [Cited by in RCA: 1098] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 39. | Saxena M, Christofori G. Rebuilding cancer metastasis in the mouse. Mol Oncol. 2013;7:283-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 40. | International Agency for Research on Cancer. Data visualization tools for exploring the global cancer burden in 2020. [cited 30 January 2021]. In: International Agency for Research on Cancer [Internet]. Available from: https://gco.iarc.fr/today/home. |
| 41. | Diaby V, Tawk R, Sanogo V, Xiao H, Montero AJ. A review of systematic reviews of the cost-effectiveness of hormone therapy, chemotherapy, and targeted therapy for breast cancer. Breast Cancer Res Treat. 2015;151:27-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 42. | Winters S, Martin C, Murphy D, Shokar NK. Breast Cancer Epidemiology, Prevention, and Screening. Prog Mol Biol Transl Sci. 2017;151:1-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 347] [Article Influence: 38.6] [Reference Citation Analysis (1)] |
| 43. | Dai X, Li T, Bai Z, Yang Y, Liu X, Zhan J, Shi B. Breast cancer intrinsic subtype classification, clinical use and future trends. Am J Cancer Res. 2015;5:2929-2943. [PubMed] |
| 44. | Tang Y, Wang Y, Kiani MF, Wang B. Classification, Treatment Strategy, and Associated Drug Resistance in Breast Cancer. Clin Breast Cancer. 2016;16:335-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 191] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 45. | O'Brien KM, Cole SR, Engel LS, Bensen JT, Poole C, Herring AH, Millikan RC. Breast cancer subtypes and previously established genetic risk factors: a bayesian approach. Cancer Epidemiol Biomarkers Prev. 2014;23:84-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Nakajima N, Oguchi M, Kumai Y, Yoshida M, Inoda H, Yoshioka Y, Iwase T, Ito Y, Akiyama F, Ohno S. Clinical outcomes and prognostic factors in patients with stage II-III breast cancer treated with neoadjuvant chemotherapy followed by surgery and postmastectomy radiation therapy in the modern treatment era. Adv Radiat Oncol. 2018;3:271-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Akram M, Siddiqui SA. Breast cancer management: past, present and evolving. Indian J Cancer. 2012;49:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 48. | Peart O. Breast intervention and breast cancer treatment options. Radiol Technol. 2015;86:535M-558M; quiz 559-62. [PubMed] |
| 49. | Abotaleb M, Kubatka P, Caprnda M, Varghese E, Zolakova B, Zubor P, Opatrilova R, Kruzliak P, Stefanicka P, Büsselberg D. Chemotherapeutic agents for the treatment of metastatic breast cancer: An update. Biomed Pharmacother. 2018;101:458-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 50. | Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, Ruddy K, Tsang J, Cardoso F. Breast cancer. Nat Rev Dis Primers. 2019;5:66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 999] [Cited by in RCA: 1957] [Article Influence: 279.6] [Reference Citation Analysis (0)] |
| 51. | Esteva FJ, Hubbard-Lucey VM, Tang J, Pusztai L. Immunotherapy and targeted therapy combinations in metastatic breast cancer. Lancet Oncol. 2019;20:e175-e186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 323] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 52. | Guo ZS, Lu B, Guo Z, Giehl E, Feist M, Dai E, Liu W, Storkus WJ, He Y, Liu Z, Bartlett DL. Vaccinia virus-mediated cancer immunotherapy: cancer vaccines and oncolytics. J Immunother Cancer. 2019;7:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 229] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 53. | Falzone L, Salomone S, Libra M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front Pharmacol. 2018;9:1300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 578] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 54. | Shetty N, Gupta S. Eribulin drug review. South Asian J Cancer. 2014;3:57-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Shen F, Long D, Yu T, Chen X, Liao Y, Wu Y, Lin X. Vinblastine differs from Taxol as it inhibits the malignant phenotypes of NSCLC cells by increasing the phosphorylation of Op18/stathmin. Oncol Rep. 2017;37:2481-2489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 56. | Weaver BA. How Taxol/paclitaxel kills cancer cells. Mol Biol Cell. 2014;25:2677-2681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 707] [Cited by in RCA: 1047] [Article Influence: 95.2] [Reference Citation Analysis (0)] |
| 57. | Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, Perou CM, Ellis MJ, Nielsen TO. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736-750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1415] [Cited by in RCA: 1596] [Article Influence: 93.9] [Reference Citation Analysis (0)] |
| 58. | Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, Perou CM, Nielsen TO. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14:1368-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 883] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 59. | He L, Lv Y, Song Y, Zhang B. The prognosis comparison of different molecular subtypes of breast tumors after radiotherapy and the intrinsic reasons for their distinct radiosensitivity. Cancer Manag Res. 2019;11:5765-5775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 60. | Howlader N, Noone A, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis D, Chen H, Feuer E, Cronin K. SEER cancer statistics review, 1975-2017. [cited 15 April 2020]. In: National Cancer Institute [Internet]. Available from: https://seer.cancer.gov/csr/1975_2017/. |
| 61. | Curigliano G, Burstein HJ, Winer EP, Gnant M, Dubsky P, Loibl S, Colleoni M, Regan MM, Piccart-Gebhart M, Senn HJ, Thürlimann B; St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2017, André F, Baselga J, Bergh J, Bonnefoi H, Brucker SY, Cardoso F, Carey L, Ciruelos E, Cuzick J, Denkert C, Di Leo A, Ejlertsen B, Francis P, Galimberti V, Garber J, Gulluoglu B, Goodwin P, Harbeck N, Hayes DF, Huang CS, Huober J, Hussein K, Jassem J, Jiang Z, Karlsson P, Morrow M, Orecchia R, Osborne KC, Pagani O, Partridge AH, Pritchard K, Ro J, Rutgers EJT, Sedlmayer F, Semiglazov V, Shao Z, Smith I, Toi M, Tutt A, Viale G, Watanabe T, Whelan TJ, Xu B. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017;28:1700-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 816] [Article Influence: 90.7] [Reference Citation Analysis (0)] |
| 62. | Kamigaki S, Arai T, Miwa H, Fukunaga M, Ohsato H, Imamura H, Sohta Y, Kaze C, Furukawa H. [A case of luminal B recurrent breast cancer with liver and lymph node metastases successfully treated with combination therapy of S-1 plus trastuzumab]. Gan To Kagaku Ryoho. 2010;37:1321-1323. [PubMed] |
| 63. | Tarantino P, Morganti S, Uliano J, Giugliano F, Crimini E, Curigliano G. Margetuximab for the treatment of HER2-positive metastatic breast cancer. Expert Opin Biol Ther. 2021;21:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 64. | Catenacci DVT, Kang YK, Park H, Uronis HE, Lee KW, Ng MCH, Enzinger PC, Park SH, Gold PJ, Lacy J, Hochster HS, Oh SC, Kim YH, Marrone KA, Kelly RJ, Juergens RA, Kim JG, Bendell JC, Alcindor T, Sym SJ, Song EK, Chee CE, Chao Y, Kim S, Lockhart AC, Knutson KL, Yen J, Franovic A, Nordstrom JL, Li D, Wigginton J, Davidson-Moncada JK, Rosales MK, Bang YJ; CP-MGAH22-5 Study Group. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): a single-arm, phase 1b-2 trial. Lancet Oncol. 2020;21:1066-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 65. | Catenacci DV, Rosales M, Chung HC, H Yoon H, Shen L, Moehler M, Kang YK. MAHOGANY: margetuximab combination in HER2+ unresectable/metastatic gastric/gastroesophageal junction adenocarcinoma. Future Oncol. 2021;17:1155-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 66. | Tolaney SM, Wardley AM, Zambelli S, Hilton JF, Troso-Sandoval TA, Ricci F, Im SA, Kim SB, Johnston SR, Chan A, Goel S, Catron K, Chapman SC, Price GL, Yang Z, Gainford MC, André F. Abemaciclib plus trastuzumab with or without fulvestrant vs trastuzumab plus standard-of-care chemotherapy in women with hormone receptor-positive, HER2-positive advanced breast cancer (monarcHER): a randomised, open-label, phase 2 trial. Lancet Oncol. 2020;21:763-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 186] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 67. | Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI, He X, Perou CM. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12:R68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1584] [Cited by in RCA: 1609] [Article Influence: 100.6] [Reference Citation Analysis (0)] |
| 68. | Hill DP, Harper A, Malcolm J, McAndrews MS, Mockus SM, Patterson SE, Reynolds T, Baker EJ, Bult CJ, Chesler EJ, Blake JA. Cisplatin-resistant triple-negative breast cancer subtypes: multiple mechanisms of resistance. BMC Cancer. 2019;19:1039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 69. | Silver DP, Richardson AL, Eklund AC, Wang ZC, Szallasi Z, Li Q, Juul N, Leong CO, Calogrias D, Buraimoh A, Fatima A, Gelman RS, Ryan PD, Tung NM, De Nicolo A, Ganesan S, Miron A, Colin C, Sgroi DC, Ellisen LW, Winer EP, Garber JE. Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J Clin Oncol. 2010;28:1145-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 760] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 70. | Nakshatri H, Qi G, You J, Kerry B, Schneider B, Zon R, Buck C, Regnier F, Wang M. Intrinsic subtype-associated changes in the plasma proteome in breast cancer. Proteomics Clin Appl. 2009;3:1305-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 71. | Zhang N, Sun P, Xu Y, Li H, Liu H, Wang L, Cao Y, Zhou K, TinghuaiWang. The GPER1/SPOP axis mediates ubiquitination-dependent degradation of ERα to inhibit the growth of breast cancer induced by oestrogen. Cancer Lett. 2021;498:54-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 72. | Hsu LH, Chu NM, Lin YF, Kao SH. G-Protein Coupled Estrogen Receptor in Breast Cancer. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 73. | Even-Ram S, Uziely B, Cohen P, Grisaru-Granovsky S, Maoz M, Ginzburg Y, Reich R, Vlodavsky I, Bar-Shavit R. Thrombin receptor overexpression in malignant and physiological invasion processes. Nat Med. 1998;4:909-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 338] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 74. | McEachron TA, Church FC, Mackman N. Regulation of thrombin-induced plasminogen activator inhibitor-1 in 4T1 murine breast cancer cells. Blood Coagul Fibrinolysis. 2011;22:576-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 75. | Rhodes DR, Ateeq B, Cao Q, Tomlins SA, Mehra R, Laxman B, Kalyana-Sundaram S, Lonigro RJ, Helgeson BE, Bhojani MS, Rehemtulla A, Kleer CG, Hayes DF, Lucas PC, Varambally S, Chinnaiyan AM. AGTR1 overexpression defines a subset of breast cancer and confers sensitivity to losartan, an AGTR1 antagonist. Proc Natl Acad Sci USA. 2009;106:10284-10289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 76. | Feigin ME, Xue B, Hammell MC, Muthuswamy SK. G-protein-coupled receptor GPR161 is overexpressed in breast cancer and is a promoter of cell proliferation and invasion. Proc Natl Acad Sci USA. 2014;111:4191-4196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 77. | Lee YJ, Shin KJ, Park SA, Park KS, Park S, Heo K, Seo YK, Noh DY, Ryu SH, Suh PG. G-protein-coupled receptor 81 promotes a malignant phenotype in breast cancer through angiogenic factor secretion. Oncotarget. 2016;7:70898-70911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 78. | Chen Z, Xue C. G-Protein-Coupled Receptor 5 (LGR5) Overexpression Activates β-Catenin Signaling in Breast Cancer Cells via Protein Kinase A. Med Sci Monit Basic Res. 2019;25:15-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 79. | CURTIS DR, RYALL RW. NICOTINIC AND MUSCARINIC RECEPTORS OF RENSHAW CELLS. Nature. 1964;203:652-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Bebbington A, Brimblecombe RW. Muscarinic receptors in the peripheral and central nervous systems. Adv Drug Res. 1965;2:143-172. [PubMed] |
| 81. | Grando SA, Kist DA, Qi M, Dahl MV. Human keratinocytes synthesize, secrete, and degrade acetylcholine. J Invest Dermatol. 1993;101:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 201] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 82. | Español A, Eiján AM, Mazzoni E, Davel L, Jasnis MA, Sacerdote De Lustig E, Sales ME. Nitric oxide synthase, arginase and cyclooxygenase are involved in muscarinic receptor activation in different murine mammary adenocarcinoma cell lines. Int J Mol Med. 2002;9:651-657. [PubMed] |
| 83. | Rimmaudo LE, de la Torre E, Sacerdote de Lustig E, Sales ME. Muscarinic receptors are involved in LMM3 tumor cells proliferation and angiogenesis. Biochem Biophys Res Commun. 2005;334:1359-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 84. | Chen J, Cheuk IWY, Shin VY, Kwong A. Acetylcholine receptors: Key players in cancer development. Surg Oncol. 2019;31:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 85. | Wess J. Molecular biology of muscarinic acetylcholine receptors. Crit Rev Neurobiol. 1996;10:69-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 369] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 86. | Predescu DV, Crețoiu SM, Crețoiu D, Pavelescu LA, Suciu N, Radu BM, Voinea SC. G Protein-Coupled Receptors (GPCRs)-Mediated Calcium Signaling in Ovarian Cancer: Focus on GPCRs activated by Neurotransmitters and Inflammation-Associated Molecules. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 87. | Bui T, Duong H. Muscarinic Agonists. 2020 Dec 4. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. [PubMed] |
| 88. | Köse M. GPCRs and EGFR - Cross-talk of membrane receptors in cancer. Bioorg Med Chem Lett. 2017;27:3611-3620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 89. | Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279-290. [PubMed] |
| 90. | Gurwitz D, Razon N, Sokolovsky M, Soreq H. Expression of muscarinic binding sites in primary human brain tumors. Brain Res. 1984;316:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 91. | Braun T, Schofield PR, Shivers BD, Pritchett DB, Seeburg PH. A novel subtype of muscarinic receptor identified by homology screening. Biochem Biophys Res Commun. 1987;149:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 92. | Bonner TI, Young AC, Brann MR, Buckley NJ. Cloning and expression of the human and rat m5 muscarinic acetylcholine receptor genes. Neuron. 1988;1:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 607] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 93. | Shida T, Tokunaga A, Kondo E, Ueda Y, Ohno K, Saika T, Kiyama H, Tohyama M. Expression of muscarinic and nicotinic receptor mRNA in the salivary gland of rats: a study by in situ hybridization histochemistry. Brain Res Mol Brain Res. 1993;17:335-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 94. | Peralta EG, Ashkenazi A, Winslow JW, Smith DH, Ramachandran J, Capon DJ. Distinct primary structures, ligand-binding properties and tissue-specific expression of four human muscarinic acetylcholine receptors. EMBO J. 1987;6:3923-3929. [PubMed] |
| 95. | Mimata H, Nomura Y, Emoto A, Latifpour J, Wheeler M, Weiss RM. Muscarinic receptor subtypes and receptor-coupled phosphatidylinositol hydrolysis in rat bladder smooth muscle. Int J Urol. 1997;4:591-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 96. | Russell M, Winitz S, Johnson GL. Acetylcholine muscarinic m1 receptor regulation of cyclic AMP synthesis controls growth factor stimulation of Raf activity. Mol Cell Biol. 1994;14:2343-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 97. | Fukuda K, Kubo T, Akiba I, Maeda A, Mishina M, Numa S. Molecular distinction between muscarinic acetylcholine receptor subtypes. Nature. 1987;327:623-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 124] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 98. | Doods HN, Entzeroth M, Ziegler H, Mayer N, Holzer P. Pharmacological profile of selective muscarinic receptor antagonists on guinea-pig ileal smooth muscle. Eur J Pharmacol. 1994;253:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 99. | Emala CW, Aryana A, Levine MA, Yasuda RP, Satkus SA, Wolfe BB, Hirshman CA. Expression of muscarinic receptor subtypes and M2-muscarinic inhibition of adenylyl cyclase in lung. Am J Physiol. 1995;268:L101-L107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 100. | Hootman SR, Zukerman J, Kovalcik SA. Muscarinic receptors in isolated guinea pig pancreatic ducts. Biochem Pharmacol. 1993;46:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 101. | Harriss DR, Marsh KA, Birmingham AT, Hill SJ. Expression of muscarinic M3-receptors coupled to inositol phospholipid hydrolysis in human detrusor cultured smooth muscle cells. J Urol. 1995;154:1241-1245. [PubMed] |
| 102. | Williams CL, Lennon VA. Activation of M3 muscarinic acetylcholine receptors inhibits voltage-dependent calcium influx in small cell lung carcinoma. J Biol Chem. 1990;265:1443-1447. [PubMed] |
| 103. | Frucht H, Jensen RT, Dexter D, Yang WL, Xiao Y. Human colon cancer cell proliferation mediated by the M3 muscarinic cholinergic receptor. Clin Cancer Res. 1999;5:2532-2539. [PubMed] |
| 104. | Hanack C, Pfeiffer A. Upper gastrointestinal porcine smooth muscle expresses M2- and M3-receptors. Digestion. 1990;45:196-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 105. | Lazareno S, Buckley NJ, Roberts FF. Characterization of muscarinic M4 binding sites in rabbit lung, chicken heart, and NG108-15 cells. Mol Pharmacol. 1990;38:805-815. [PubMed] |
| 106. | Matthiesen S, Bahulayan A, Kempkens S, Haag S, Fuhrmann M, Stichnote C, Juergens UR, Racké K. Muscarinic receptors mediate stimulation of human lung fibroblast proliferation. Am J Respir Cell Mol Biol. 2006;35:621-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 107. | So I, Yang DK, Kim HJ, Min KW, Kang TM, Kim SJ, Kim KW, Park KH, Jeon JH, Choi KH, Kim IG. Five subtypes of muscarinic receptors are expressed in gastric smooth muscles of guinea pig. Exp Mol Med. 2003;35:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 108. | Grodzki AC, Ghogha A, Mangini L, Fryer AD, Lein PJ. IFNγ Increases M2 Muscarinic Receptor Expression in Cultured Sympathetic Neurons. Curr Neurobiol. 2011;2:23-29. [PubMed] |
| 109. | Español AJ, Maddaleno MO, Lombardi MG, Cella M, Martínez Pulido P, Sales ME. Treatment with LPS plus INF-γ induces the expression and function of muscarinic acetylcholine receptors, modulating NIH3T3 cell proliferation: participation of NOS and COX. Br J Pharmacol. 2014;171:5154-5167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 110. | Alves-Lopes R, Neves KB, Touyz RM. Muscarinic Receptor Type-3 in Hypertension and Cholinergic-Adrenergic Crosstalk: Genetic Insights and Potential for New Antihypertensive Targets. Can J Cardiol. 2019;35:555-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 111. | Menegatti R, Carvalho FS, Lião LM, Villavicencio B, Verli H, Mourão AA, Xavier CH, Castro CH, Pedrino GR, Franco OL, Oliveira-Silva I, Ashpole NM, Silva ON, Costa EA, Fajemiroye JO. Novel choline analog 2-(4-((1-phenyl-1H-pyrazol-4-yl)methyl)piperazin-1-yl)ethan-1-ol produces sympathoinhibition, hypotension, and antihypertensive effects. Naunyn Schmiedebergs Arch Pharmacol. 2019;392:1071-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 112. | Hohlfeld JM, Vogel-Claussen J, Biller H, Berliner D, Berschneider K, Tillmann HC, Hiltl S, Bauersachs J, Welte T. Effect of lung deflation with indacaterol plus glycopyrronium on ventricular filling in patients with hyperinflation and COPD (CLAIM): a double-blind, randomised, crossover, placebo-controlled, single-centre trial. Lancet Respir Med. 2018;6:368-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 113. | Page C, Cazzola M. Bifunctional drugs for the treatment of asthma and chronic obstructive pulmonary disease. Eur Respir J. 2014;44:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 114. | Vogt BA. Cingulate cortex in Parkinson's disease. Handb Clin Neurol. 2019;166:253-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 115. | Lebois EP, Thorn C, Edgerton JR, Popiolek M, Xi S. Muscarinic receptor subtype distribution in the central nervous system and relevance to aging and Alzheimer's disease. Neuropharmacology. 2018;136:362-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (8)] |
| 116. | Gatta V, Mengod G, Reale M, Tata AM. Possible Correlation between Cholinergic System Alterations and Neuro/Inflammation in Multiple Sclerosis. Biomedicines. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 117. | Lee J, Hwang YJ, Shin JY, Lee WC, Wie J, Kim KY, Lee MY, Hwang D, Ratan RR, Pae AN, Kowall NW, So I, Kim JI, Ryu H. Epigenetic regulation of cholinergic receptor M1 (CHRM1) by histone H3K9me3 impairs Ca(2+) signaling in Huntington's disease. Acta Neuropathol. 2013;125:727-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 118. | Tata AM, Velluto L, D'Angelo C, Reale M. Cholinergic system dysfunction and neurodegenerative diseases: cause or effect? CNS Neurol Disord Drug Targets. 2014;13:1294-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 119. | Scarpa M, Hesse S, Bradley SJ. M1 muscarinic acetylcholine receptors: A therapeutic strategy for symptomatic and disease-modifying effects in Alzheimer's disease? Adv Pharmacol. 2020;88:277-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 120. | Nickols HH, Conn PJ. Development of allosteric modulators of GPCRs for treatment of CNS disorders. Neurobiol Dis. 2014;61:55-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (17)] |
| 121. | Erosa-Rivero HB, Bata-García JL, Alvarez-Cervera FJ, Heredia-López FJ, Góngora-Alfaro JL. The potency and efficacy of anticholinergics to inhibit haloperidol-induced catalepsy in rats correlates with their rank order of affinities for the muscarinic receptor subtypes. Neuropharmacology. 2014;81:176-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 122. | Deshmukh VA, Tardif V, Lyssiotis CA, Green CC, Kerman B, Kim HJ, Padmanabhan K, Swoboda JG, Ahmad I, Kondo T, Gage FH, Theofilopoulos AN, Lawson BR, Schultz PG, Lairson LL. A regenerative approach to the treatment of multiple sclerosis. Nature. 2013;502:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 434] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 123. | Cheng K, Shang AC, Drachenberg CB, Zhan M, Raufman JP. Differential expression of M3 muscarinic receptors in progressive colon neoplasia and metastasis. Oncotarget. 2017;8:21106-21114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 124. | Lin G, Sun L, Wang R, Guo Y, Xie C. Overexpression of muscarinic receptor 3 promotes metastasis and predicts poor prognosis in non-small-cell lung cancer. J Thorac Oncol. 2014;9:170-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 125. | Pacini L, De Falco E, Di Bari M, Coccia A, Siciliano C, Ponti D, Pastore AL, Petrozza V, Carbone A, Tata AM, Calogero A. M2muscarinic receptors inhibit cell proliferation and migration in urothelial bladder cancer cells. Cancer Biol Ther. 2014;15:1489-1498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 126. | Español AJ, Jacob G, Dmytrenko G, Sales ME. Muscarinic activation enhances the anti-proliferative effect of paclitaxel in murine breast tumor cells. Anticancer Agents Med Chem. 2013;13:1273-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 127. | Español AJ, Salem A, Rojo D, Sales ME. Participation of non-neuronal muscarinic receptors in the effect of carbachol with paclitaxel on human breast adenocarcinoma cells. Roles of nitric oxide synthase and arginase. Int Immunopharmacol. 2015;29:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 128. | Español AJ, Salem A, Di Bari M, Cristofaro I, Sanchez Y, Tata AM, Sales ME. The metronomic combination of paclitaxel with cholinergic agonists inhibits triple negative breast tumor progression. Participation of M2 receptor subtype. PLoS One. 2020;15:e0226450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 129. | Salem AR, Martínez Pulido P, Sanchez F, Sanchez Y, Español AJ, Sales ME. Effect of low dose metronomic therapy on MCF-7 tumor cells growth and angiogenesis. Role of muscarinic acetylcholine receptors. Int Immunopharmacol. 2020;84:106514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 130. | Kohn EC, Alessandro R, Probst J, Jacobs W, Brilley E, Felder CC. Identification and molecular characterization of a m5 muscarinic receptor in A2058 human melanoma cells. Coupling to inhibition of adenylyl cyclase and stimulation of phospholipase A2. J Biol Chem. 1996;271:17476-17484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 131. | Liu H, Xia J, Wang T, Li W, Song Y, Tan G. Differentiation of human glioblastoma U87 cells into cholinergic neuron. Neurosci Lett. 2019;704:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 132. | Yu H, Xia H, Tang Q, Xu H, Wei G, Chen Y, Dai X, Gong Q, Bi F. Acetylcholine acts through M3 muscarinic receptor to activate the EGFR signaling and promotes gastric cancer cell proliferation. Sci Rep. 2017;7:40802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 133. | Tolaymat M, Larabee SM, Hu S, Xie G, Raufman JP. The Role of M3 Muscarinic Receptor Ligand-Induced Kinase Signaling in Colon Cancer Progression. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 134. | Zhao Q, Yue J, Zhang C, Gu X, Chen H, Xu L. Inactivation of M2 AChR/NF-κB signaling axis reverses epithelial-mesenchymal transition (EMT) and suppresses migration and invasion in non-small cell lung cancer (NSCLC). Oncotarget. 2015;6:29335-29346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 135. | Wang J, Krysiak PS, Laurier LG, Sims SM, Preiksaitis HG. Human esophageal smooth muscle cells express muscarinic receptor subtypes M(1) through M(5). Am J Physiol Gastrointest Liver Physiol. 2000;279:G1059-G1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 136. | Yin QQ, Xu LH, Zhang M, Xu C. Muscarinic acetylcholine receptor M1 mediates prostate cancer cell migration and invasion through hedgehog signaling. Asian J Androl. 2018;20:608-614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 137. | Witte LP, Teitsma CA, de la Rosette JJ, Michel MC. Muscarinic receptor subtype mRNA expression in the human prostate: association with age, pathological diagnosis, prostate size, or potentially interfering medications? Naunyn Schmiedebergs Arch Pharmacol. 2014;387:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 138. | Song W, Yuan M, Zhao S. Variation of M3 muscarinic receptor expression in different prostate tissues and its significance. Saudi Med J. 2009;30:1010-1016. [PubMed] |
| 139. | Parnell EA, Calleja-Macias IE, Kalantari M, Grando SA, Bernard HU. Muscarinic cholinergic signaling in cervical cancer cells affects cell motility via ERK1/2 signaling. Life Sci. 2012;91:1093-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 140. | Zhang L, Wu LL, Huan HB, Chen XJ, Wen XD, Yang DP, Xia F. Sympathetic and parasympathetic innervation in hepatocellular carcinoma. Neoplasma. 2017;64:840-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 141. | Chiu CC, Chen BH, Hour TC, Chiang WF, Wu YJ, Chen CY, Chen HR, Chan PT, Liu SY, Chen JY. Betel quid extract promotes oral cancer cell migration by activating a muscarinic M4 receptor-mediated signaling cascade involving SFKs and ERK1/2. Biochem Biophys Res Commun. 2010;399:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 142. | Nagy D, Kosztka L, Pap P, Nagy Z, Rusznák Z, Csernoch L, Szücs G. Cytoplasmic Ca2+ concentration changes evoked by muscarinic cholinergic stimulation in primary and metastatic melanoma cell lines. Melanoma Res. 2011;21:12-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 143. | Lucianò AM, Perciballi E, Fiore M, Del Bufalo D, Tata AM. The Combination of the M2 Muscarinic Receptor Agonist and Chemotherapy Affects Drug Resistance in Neuroblastoma Cells. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 144. | Alessandrini F, Cristofaro I, Di Bari M, Zasso J, Conti L, Tata AM. The activation of M2 muscarinic receptor inhibits cell growth and survival in human glioblastoma cancer stem cells. Int Immunopharmacol. 2015;29:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 145. | Cabadak H, Aydin B, Kan B. Regulation of M2, M3, and M4 muscarinic receptor expression in K562 chronic myelogenous leukemic cells by carbachol. J Recept Signal Transduct Res. 2011;31:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 146. | Sun F, Li D, Wang C, Peng C, Zheng H, Wang X. Acacetin-induced cell apoptosis in head and neck squamous cell carcinoma cells: Evidence for the role of muscarinic M3 receptor. Phytother Res. 2019;33:1551-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 147. | Renz BW, Tanaka T, Sunagawa M, Takahashi R, Jiang Z, Macchini M, Dantes Z, Valenti G, White RA, Middelhoff MA, Ilmer M, Oberstein PE, Angele MK, Deng H, Hayakawa Y, Westphalen CB, Werner J, Remotti H, Reichert M, Tailor YH, Nagar K, Friedman RA, Iuga AC, Olive KP, Wang TC. Cholinergic Signaling via Muscarinic Receptors Directly and Indirectly Suppresses Pancreatic Tumorigenesis and Cancer Stemness. Cancer Discov. 2018;8:1458-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 233] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 148. | Fiszman GL, Middonno MC, de la Torre E, Farina M, Español AJ, Sales ME. Activation of muscarinic cholinergic receptors induces MCF-7 cells proliferation and angiogenesis by stimulating nitric oxide synthase activity. Cancer Biol Ther. 2007;6:1106-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 149. | Pelegrina LT, Lombardi MG, Fiszman GL, Azar ME, Morgado CC, Sales ME. Immunoglobulin g from breast cancer patients regulates MCF-7 cells migration and MMP-9 activity by stimulating muscarinic acetylcholine receptors. J Clin Immunol. 2013;33:427-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 150. | Temma K, Akera T, Chugun A, Ohashi M, Yabuki M, Kondo H. Doxorubicin: an antagonist of muscarinic receptors in guinea pig heart. Eur J Pharmacol. 1992;220:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 151. | Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93:2325-2327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2767] [Cited by in RCA: 2559] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 152. | O'Shaughnessy JA, Cowan KH. Current status of paclitaxel in the treatment of breast cancer. Breast Cancer Res Treat. 1995;33:27-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 153. | Goodman J, Walsh V. The story of Taxol: nature and politics in the pursuit of an anti-cancer drug. Cambridge University Press, 2001: 120-121. |
| 154. | Alves RC, Fernandes RP, Eloy JO, Salgado HRN, Chorilli M. Characteristics, Properties and Analytical Methods of Paclitaxel: A Review. Crit Rev Anal Chem. 2018;48:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 155. | Marupudi NI, Han JE, Li KW, Renard VM, Tyler BM, Brem H. Paclitaxel: a review of adverse toxicities and novel delivery strategies. Expert Opin Drug Saf. 2007;6:609-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 373] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 156. | Banerji A, Lax T, Guyer A, Hurwitz S, Camargo CA Jr, Long AA. Management of hypersensitivity reactions to Carboplatin and Paclitaxel in an outpatient oncology infusion center: a 5-year review. J Allergy Clin Immunol Pract. 2014;2:428-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 157. | Brewer JR, Morrison G, Dolan ME, Fleming GF. Chemotherapy-induced peripheral neuropathy: Current status and progress. Gynecol Oncol. 2016;140:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 196] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 158. | Chou PL, Huang YP, Cheng MH, Rau KM, Fang YP. Improvement of Paclitaxel-Associated Adverse Reactions (ADRs) via the Use of Nano-Based Drug Delivery Systems: A Systematic Review and Network Meta-Analysis. Int J Nanomedicine. 2020;15:1731-1743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 159. | Weiss RB, Donehower RC, Wiernik PH, Ohnuma T, Gralla RJ, Trump DL, Baker JR Jr, Van Echo DA, Von Hoff DD, Leyland-Jones B. Hypersensitivity reactions from taxol. J Clin Oncol. 1990;8:1263-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 889] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 160. | Pippen J, Paul D, Vukelja S, Clawson A, Iglesias J. Dose-dense doxorubicin and cyclophosphamide followed by dose-dense albumin-bound paclitaxel plus bevacizumab is safe as adjuvant therapy in patients with early stage breast cancer. Breast Cancer Res Treat. 2011;130:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 161. | Viúdez A, Ramírez N, Hernández-García I, Carvalho FL, Vera R, Hidalgo M. Nab-paclitaxel: a flattering facelift. Crit Rev Oncol Hematol. 2014;92:166-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 162. | Danhier F, Lecouturier N, Vroman B, Jérôme C, Marchand-Brynaert J, Feron O, Préat V. Paclitaxel-loaded PEGylated PLGA-based nanoparticles: in vitro and in vivo evaluation. J Control Release. 2009;133:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 425] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 163. | Lu J, Huang Y, Zhao W, Chen Y, Li J, Gao X, Venkataramanan R, Li S. Design and characterization of PEG-derivatized vitamin E as a nanomicellar formulation for delivery of paclitaxel. Mol Pharm. 2013;10:2880-2890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 164. | Vergote I, Bergfeldt K, Franquet A, Lisyanskaya AS, Bjermo H, Heldring N, Buyse M, Brize A. A randomized phase III trial in patients with recurrent platinum sensitive ovarian cancer comparing efficacy and safety of paclitaxel micellar and Cremophor EL-paclitaxel. Gynecol Oncol. 2020;156:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 165. | Park IH, Sohn JH, Kim SB, Lee KS, Chung JS, Lee SH, Kim TY, Jung KH, Cho EK, Kim YS, Song HS, Seo JH, Ryoo HM, Lee SA, Yoon SY, Kim CS, Kim YT, Kim SY, Jin MR, Ro J. An Open-Label, Randomized, Parallel, Phase III Trial Evaluating the Efficacy and Safety of Polymeric Micelle-Formulated Paclitaxel Compared to Conventional Cremophor EL-Based Paclitaxel for Recurrent or Metastatic HER2-Negative Breast Cancer. Cancer Res Treat. 2017;49:569-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 166. | Nabholtz JM, Gelmon K, Bontenbal M, Spielmann M, Catimel G, Conte P, Klaassen U, Namer M, Bonneterre J, Fumoleau P, Winograd B. Multicenter, randomized comparative study of two doses of paclitaxel in patients with metastatic breast cancer. J Clin Oncol. 1996;14:1858-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 278] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 167. | Rowinsky EK, Donehower RC. The clinical pharmacology of paclitaxel (Taxol). Semin Oncol. 1993;20:16-25. [PubMed] |
| 168. | de Weger VA, Beijnen JH, Schellens JH. Cellular and clinical pharmacology of the taxanes docetaxel and paclitaxel--a review. Anticancer Drugs. 2014;25:488-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 169. | Backman JT, Filppula AM, Niemi M, Neuvonen PJ. Role of Cytochrome P450 2C8 in Drug Metabolism and Interactions. Pharmacol Rev. 2016;68:168-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 192] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 170. | Lee MY, Apellániz-Ruiz M, Johansson I, Vikingsson S, Bergmann TK, Brøsen K, Green H, Rodríguez-Antona C, Ingelman-Sundberg M. Role of cytochrome P450 2C8*3 (CYP2C8*3) in paclitaxel metabolism and paclitaxel-induced neurotoxicity. Pharmacogenomics. 2015;16:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 171. | Slichenmyer WJ, Von Hoff DD. Taxol: a new and effective anti-cancer drug. Anticancer Drugs. 1991;2:519-530. [PubMed] |
| 172. | Luo Y, Li D, Ran J, Yan B, Chen J, Dong X, Liu Z, Liu R, Zhou J, Liu M. End-binding protein 1 stimulates paclitaxel sensitivity in breast cancer by promoting its actions toward microtubule assembly and stability. Protein Cell. 2014;5:469-479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 173. | Lobert S, Jefferson B, Morris K. Regulation of β-tubulin isotypes by micro-RNA 100 in MCF7 breast cancer cells. Cytoskeleton (Hoboken). 2011;68:355-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 174. | Alqahtani FY, Aleanizy FS, El Tahir E, Alkahtani HM, AlQuadeib BT. Paclitaxel. Profiles Drug Subst Excip Relat Methodol. 2019;44:205-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 175. | Wanderley CW, Colón DF, Luiz JPM, Oliveira FF, Viacava PR, Leite CA, Pereira JA, Silva CM, Silva CR, Silva RL, Speck-Hernandez CA, Mota JM, Alves-Filho JC, Lima-Junior RC, Cunha TM, Cunha FQ. Paclitaxel Reduces Tumor Growth by Reprogramming Tumor-Associated Macrophages to an M1 Profile in a TLR4-Dependent Manner. Cancer Res. 2018;78:5891-5900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 280] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 176. | Millrud CR, Mehmeti M, Leandersson K. Docetaxel promotes the generation of anti-tumorigenic human macrophages. Exp Cell Res. 2018;362:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 177. | Mellor HR, Rouschop KM, Wigfield SM, Wouters BG, Harris AL. Synchronised phosphorylation of BNIP3, Bcl-2 and Bcl-xL in response to microtubule-active drugs is JNK-independent and requires a mitotic kinase. Biochem Pharmacol. 2010;79:1562-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 178. | Chae S, Kim YB, Lee JS, Cho H. Resistance to paclitaxel in hepatoma cells is related to static JNK activation and prohibition into entry of mitosis. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1016-G1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 179. | Hossain M, Banik NL, Ray SK. Synergistic anti-cancer mechanisms of curcumin and paclitaxel for growth inhibition of human brain tumor stem cells and LN18 and U138MG cells. Neurochem Int. 2012;61:1102-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 180. | Asghari F, Haghnavaz N, Shanehbandi D, Khaze V, Baradaran B, Kazemi T. Differential altered expression of let-7a and miR-205 tumor-suppressor miRNAs in different subtypes of breast cancer under treatment with Taxol. Adv Clin Exp Med. 2018;27:941-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 181. | Ren X, Zhao B, Chang H, Xiao M, Wu Y, Liu Y. Paclitaxel suppresses proliferation and induces apoptosis through regulation of ROS and the AKT/MAPK signaling pathway in canine mammary gland tumor cells. Mol Med Rep. 2018;17:8289-8299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 182. | Zhang X, Wu X, Zhang F, Mo S, Lu Y, Wei W, Chen X, Lan L, Lu B, Liu Y. Paclitaxel induces apoptosis of esophageal squamous cell carcinoma cells by downregulating STAT3 phosphorylation at Ser727. Oncol Rep. 2017;37:2237-2244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 183. | Batist G, Barton J, Chaikin P, Swenson C, Welles L. Myocet (liposome-encapsulated doxorubicin citrate): a new approach in breast cancer therapy. Expert Opin Pharmacother. 2002;3:1739-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 115] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 184. | Giordano SH, Lin YL, Kuo YF, Hortobagyi GN, Goodwin JS. Decline in the use of anthracyclines for breast cancer. J Clin Oncol. 2012;30:2232-2239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 185. | Cortazar P, Justice R, Johnson J, Sridhara R, Keegan P, Pazdur R. US Food and Drug Administration approval overview in metastatic breast cancer. J Clin Oncol. 2012;30:1705-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 186. | Zeiss CJ, Gatti DM, Toro-Salazar O, Davis C, Lutz CM, Spinale F, Stearns T, Furtado MB, Churchill GA. Doxorubicin-Induced Cardiotoxicity in Collaborative Cross (CC) Mice Recapitulates Individual Cardiotoxicity in Humans. G3 (Bethesda). 2019;9:2637-2646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 187. | Samare-Najaf M, Zal F, Safari S. Primary and Secondary Markers of Doxorubicin-Induced Female Infertility and the Alleviative Properties of Quercetin and Vitamin E in a Rat Model. Reprod Toxicol. 2020;96:316-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 188. | Roychoudhury S, Kumar A, Bhatkar D, Sharma NK. Molecular avenues in targeted doxorubicin cancer therapy. Future Oncol. 2020;16:687-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 189. | Shin JJ, Choi YM, Jun JK, Lee KH, Kim TY, Han W, Im SA. Amenorrhea and Menopause in Patients with Breast Cancer after Chemotherapy. J Breast Cancer. 2019;22:624-634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 190. | Patel SR, Burgess MA, Papadopolous NE, Sidhu G, Gray R, Plager C, Jenkins J, Benjamin RS. Phase II study of CI-980 (NSC 635370) in patients with previously treated advanced soft-tissue sarcomas. Invest New Drugs. 1998;16:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 191. | Rivankar S. An overview of doxorubicin formulations in cancer therapy. J Cancer Res Ther. 2014;10:853-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 525] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 192. | Lipshultz SE, Lipsitz SR, Sallan SE, Dalton VM, Mone SM, Gelber RD, Colan SD. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629-2636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 524] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 193. | Feijen EAM, Leisenring WM, Stratton KL, Ness KK, van der Pal HJH, van Dalen EC, Armstrong GT, Aune GJ, Green DM, Hudson MM, Loonen J, Oeffinger KC, Robison LL, Yasui Y, Kremer LCM, Chow EJ. Derivation of Anthracycline and Anthraquinone Equivalence Ratios to Doxorubicin for Late-Onset Cardiotoxicity. JAMA Oncol. 2019;5:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 191] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 194. | Bernstein D, Fajardo G, Zhao M, Urashima T, Powers J, Berry G, Kobilka BK. Differential cardioprotective/cardiotoxic effects mediated by beta-adrenergic receptor subtypes. Am J Physiol Heart Circ Physiol. 2005;289:H2441-H2449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 195. | Figueredo VM. Chemical cardiomyopathies: the negative effects of medications and nonprescribed drugs on the heart. Am J Med. 2011;124:480-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 196. | Vasić M, Lončar-Turukalo T, Tasić T, Matić M, Glumac S, Bajić D, Popović B, Japundžić-Žigon N. Cardiovascular variability and β-ARs gene expression at two stages of doxorubicin - Induced cardiomyopathy. Toxicol Appl Pharmacol. 2019;362:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 197. | Chugun A, Uchide T, Temma K, Kennedy RH, Klimberg SV, Hara Y, Sasaki T, Akera T. Doxorubicin affects the cardiac muscarinic system in the rat. J Vet Med Sci. 2001;63:1315-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 198. | Johnson-Arbor K, Dubey R. Doxorubicin. Treasure Island (FL): StatPearls Publishing, 2020. |
| 199. | Gil P, Favre R, Durand A, Iliadis A, Cano JP, Carcassonne Y. Time dependency of adriamycin and adriamycinol kinetics. Cancer Chemother Pharmacol. 1983;10:120-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 200. | Bugat R, Robert J, Herrera A, Pinel MC, Huet S, Chevreau C, Boussin G, Roquain J, Carton M. Clinical and pharmacokinetic study of 96-h infusions of doxorubicin in advanced cancer patients. Eur J Cancer Clin Oncol. 1989;25:505-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 201. | Sallustio BC, Boddy AV. Is there scope for better individualisation of anthracycline cancer chemotherapy? Br J Clin Pharmacol. 2021;87:295-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 202. | Bjornsti MA, Kaufmann SH. Topoisomerases and cancer chemotherapy: recent advances and unanswered questions. F1000Res. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 203. | Mizutani H, Tada-Oikawa S, Hiraku Y, Kojima M, Kawanishi S. Mechanism of apoptosis induced by doxorubicin through the generation of hydrogen peroxide. Life Sci. 2005;76:1439-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 364] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 204. | Delgado JL, Hsieh CM, Chan NL, Hiasa H. Topoisomerases as anticancer targets. Biochem J. 2018;475:373-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 330] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 205. | Aubel-Sadron G, Londos-Gagliardi D. Daunorubicin and doxorubicin, anthracycline antibiotics, a physicochemical and biological review. Biochimie. 1984;66:333-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 170] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 206. | Chen NT, Wu CY, Chung CY, Hwu Y, Cheng SH, Mou CY, Lo LW. Probing the dynamics of doxorubicin-DNA intercalation during the initial activation of apoptosis by fluorescence lifetime imaging microscopy (FLIM). PLoS One. 2012;7:e44947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 207. | Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1572] [Cited by in RCA: 1658] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 208. | Meredith AM, Dass CR. Increasing role of the cancer chemotherapeutic doxorubicin in cellular metabolism. J Pharm Pharmacol. 2016;68:729-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 222] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 209. | Yalcintepe L, Halis E. Modulation of iron metabolism by iron chelation regulates intracellular calcium and increases sensitivity to doxorubicin. Bosn J Basic Med Sci. 2016;16:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 210. | Maloney SM, Hoover CA, Morejon-Lasso LV, Prosperi JR. Mechanisms of Taxane Resistance. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 211. | Nishio K, Nakamura T, Koh Y, Suzuki T, Fukumoto H, Saijo N. Drug resistance in lung cancer. Curr Opin Oncol. 1999;11:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 212. | Amawi H, Sim HM, Tiwari AK, Ambudkar SV, Shukla S. ABC Transporter-Mediated Multidrug-Resistant Cancer. Adv Exp Med Biol. 2019;1141:549-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 179] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 213. | Cox J, Weinman S. Mechanisms of doxorubicin resistance in hepatocellular carcinoma. Hepat Oncol. 2016;3:57-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 214. | Hu Y, Xu K, Yagüe E. miR-218 targets survivin and regulates resistance to chemotherapeutics in breast cancer. Breast Cancer Res Treat. 2015;151:269-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 215. | Tormo E, Pineda B, Serna E, Guijarro A, Ribas G, Fores J, Chirivella E, Climent J, Lluch A, Eroles P. MicroRNA Profile in Response to Doxorubicin Treatment in Breast Cancer. J Cell Biochem. 2015;116:2061-2073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 216. | García-Vazquez R, Ruiz-García E, Meneses García A, Astudillo-de la Vega H, Lara-Medina F, Alvarado-Miranda A, Maldonado-Martínez H, González-Barrios JA, Campos-Parra AD, Rodríguez Cuevas S, Marchat LA, López-Camarillo C. A microRNA signature associated with pathological complete response to novel neoadjuvant therapy regimen in triple-negative breast cancer. Tumour Biol. 2017;39:1010428317702899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 217. | Hamilton A, Larsimont D, Paridaens R, Drijkoningen M, van de Vijver M, Bruning P, Hanby A, Houston S, Treilleux I, Guastalla JP, Van Vreckem A, Sylvester R, Piccart M. A study of the value of p53, HER2, and Bcl-2 in the prediction of response to doxorubicin and paclitaxel as single agents in metastatic breast cancer: a companion study to EORTC 10923. Clin Breast Cancer. 2000;1:233-40; discussion 241-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 218. | Pilco-Ferreto N, Calaf GM. Influence of doxorubicin on apoptosis and oxidative stress in breast cancer cell lines. Int J Oncol. 2016;49:753-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 229] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 219. | Li W, Zhai B, Zhi H, Li Y, Jia L, Ding C, Zhang B, You W. Association of ABCB1, β tubulin I, and III with multidrug resistance of MCF7/DOC subline from breast cancer cell line MCF7. Tumour Biol. 2014;35:8883-8891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 220. | Pusztai L. Markers predicting clinical benefit in breast cancer from microtubule-targeting agents. Ann Oncol. 2007;18 Suppl 12:xii15-xii20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 221. | Zhuo Y, Guo Q. [Down-regulated βIII-tubulin expression can reverse paclitaxel resistance in A549/taxol cells lines]. Zhongguo Fei Ai Za Zhi. 2014;17:581-587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 222. | Wang J, Song Y, Xu S, Zhang Q, Li Y, Tang D, Jin S. Down-regulation of ICBP90 contributes to doxorubicin resistance. Eur J Pharmacol. 2011;656:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 223. | Alli E, Bash-Babula J, Yang JM, Hait WN. Effect of stathmin on the sensitivity to antimicrotubule drugs in human breast cancer. Cancer Res. 2002;62:6864-6869. [PubMed] |
| 224. | Targa B, Klipfel L, Cantaloube I, Salameh J, Benoit B, Poüs C, Baillet A. Septin filament coalignment with microtubules depends on SEPT9_i1 and tubulin polyglutamylation, and is an early feature of acquired cell resistance to paclitaxel. Cell Death Dis. 2019;10:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 225. | Hage-Sleiman R, Herveau S, Matera EL, Laurier JF, Dumontet C. Tubulin binding cofactor C (TBCC) suppresses tumor growth and enhances chemosensitivity in human breast cancer cells. BMC Cancer. 2010;10:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 226. | Chabalier C, Lamare C, Racca C, Privat M, Valette A, Larminat F. BRCA1 downregulation leads to premature inactivation of spindle checkpoint and confers paclitaxel resistance. Cell Cycle. 2006;5:1001-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 116] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 227. | van Eijk M, Boosman RJ, Schinkel AH, Huitema ADR, Beijnen JH. Cytochrome P450 3A4, 3A5, and 2C8 expression in breast, prostate, lung, endometrial, and ovarian tumors: relevance for resistance to taxanes. Cancer Chemother Pharmacol. 2019;84:487-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 228. | O'Malley FP, Chia S, Tu D, Shepherd LE, Levine MN, Bramwell VH, Andrulis IL, Pritchard KI. Topoisomerase II alpha and responsiveness of breast cancer to adjuvant chemotherapy. J Natl Cancer Inst. 2009;101:644-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 229. | Morris BJ, Willcox DC, Donlon TA, Willcox BJ. FOXO3: A Major Gene for Human Longevity--A Mini-Review. Gerontology. 2015;61:515-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 266] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 230. | Gomes AR, Zhao F, Lam EW. Role and regulation of the forkhead transcription factors FOXO3a and FOXM1 in carcinogenesis and drug resistance. Chin J Cancer. 2013;32:365-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 231. | Karagiannis GS, Pastoriza JM, Wang Y, Harney AS, Entenberg D, Pignatelli J, Sharma VP, Xue EA, Cheng E, D'Alfonso TM, Jones JG, Anampa J, Rohan TE, Sparano JA, Condeelis JS, Oktay MH. Neoadjuvant chemotherapy induces breast cancer metastasis through a TMEM-mediated mechanism. Sci Transl Med. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 387] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 232. | Daenen LG, Roodhart JM, van Amersfoort M, Dehnad M, Roessingh W, Ulfman LH, Derksen PW, Voest EE. Chemotherapy enhances metastasis formation via VEGFR-1-expressing endothelial cells. Cancer Res. 2011;71:6976-6985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 233. | Robles SJ, Buehler PW, Negrusz A, Adami GR. Permanent cell cycle arrest in asynchronously proliferating normal human fibroblasts treated with doxorubicin or etoposide but not camptothecin. Biochem Pharmacol. 1999;58:675-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 234. | Nestal de Moraes G, Vasconcelos FC, Delbue D, Mognol GP, Sternberg C, Viola JP, Maia RC. Doxorubicin induces cell death in breast cancer cells regardless of Survivin and XIAP expression levels. Eur J Cell Biol. 2013;92:247-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 235. | Calaf GM, Ponce-Cusi R, Carrión F. Curcumin and paclitaxel induce cell death in breast cancer cell lines. Oncol Rep. 2018;40:2381-2388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 236. | Kareva I, Waxman DJ, Lakka Klement G. Metronomic chemotherapy: an attractive alternative to maximum tolerated dose therapy that can activate anti-tumor immunity and minimize therapeutic resistance. Cancer Lett. 2015;358:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 180] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 237. | Bracha S, Walshaw R, Danton T, Holland S, Ruaux C, Obradovich J. Evaluation of toxicities from combined metronomic and maximal-tolerated dose chemotherapy in dogs with osteosarcoma. J Small Anim Pract. 2014;55:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 238. | Folkins C, Man S, Xu P, Shaked Y, Hicklin DJ, Kerbel RS. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 2007;67:3560-3564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 280] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 239. | Vives M, Ginestà MM, Gracova K, Graupera M, Casanovas O, Capellà G, Serrano T, Laquente B, Viñals F. Metronomic chemotherapy following the maximum tolerated dose is an effective anti-tumour therapy affecting angiogenesis, tumour dissemination and cancer stem cells. Int J Cancer. 2013;133:2464-2472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 240. | Hanahan D, Bergers G, Bergsland E. Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest. 2000;105:1045-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 597] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 241. | Kerbel RS, Klement G, Pritchard KI, Kamen B. Continuous low-dose anti-angiogenic/ metronomic chemotherapy: from the research laboratory into the oncology clinic. Ann Oncol. 2002;13:12-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 135] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 242. | Cazzaniga ME, Dionisio MR, Riva F. Metronomic chemotherapy for advanced breast cancer patients. Cancer Lett. 2017;400:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 243. | Ge Y, Domschke C, Stoiber N, Schott S, Heil J, Rom J, Blumenstein M, Thum J, Sohn C, Schneeweiss A, Beckhove P, Schuetz F. Metronomic cyclophosphamide treatment in metastasized breast cancer patients: immunological effects and clinical outcome. Cancer Immunol Immunother. 2012;61:353-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 184] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 244. | Kareva I. A Combination of Immune Checkpoint Inhibition with Metronomic Chemotherapy as a Way of Targeting Therapy-Resistant Cancer Cells. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 245. | Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP. Repurposing drugs in your medicine cabinet: untapped opportunities for cancer therapy? Future Oncol. 2015;11:181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 246. | Sales ME, Español AJ, Salem AR, Pulido PM, Sanchez Y, Sanchez F. Role of Muscarinic Acetylcholine Receptors in Breast Cancer: Design of Metronomic Chemotherapy. Curr Clin Pharmacol. 2019;14:91-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Argentina
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lamballe F S-Editor: Gao CC L-Editor: A P-Editor: Xing YX