Published online Nov 24, 2020. doi: 10.5306/wjco.v11.i11.959
Peer-review started: August 5, 2020
First decision: September 17, 2020
Revised: October 1, 2020
Accepted: October 19, 2020
Article in press: October 19, 2020
Published online: November 24, 2020
Processing time: 104 Days and 20 Hours
Colorectal cancer (CRC) is the third leading cause of cancer-related death in males and females in the United States. Approximately, 20%-22% of patients have metastatic disease at the time of presentation, and 50%-60% will develop metastasis over the course of their disease. Despite advances in systemic therapies, there remains a paucity of effective third- and later-line therapies for patients with ongoing disease progression. However, rechallenging chemo-resistant CRC tumors with previously administered therapies is an emerging concept that may be a life-prolonging option for heavily treated metastatic colorectal cancer (mCRC).
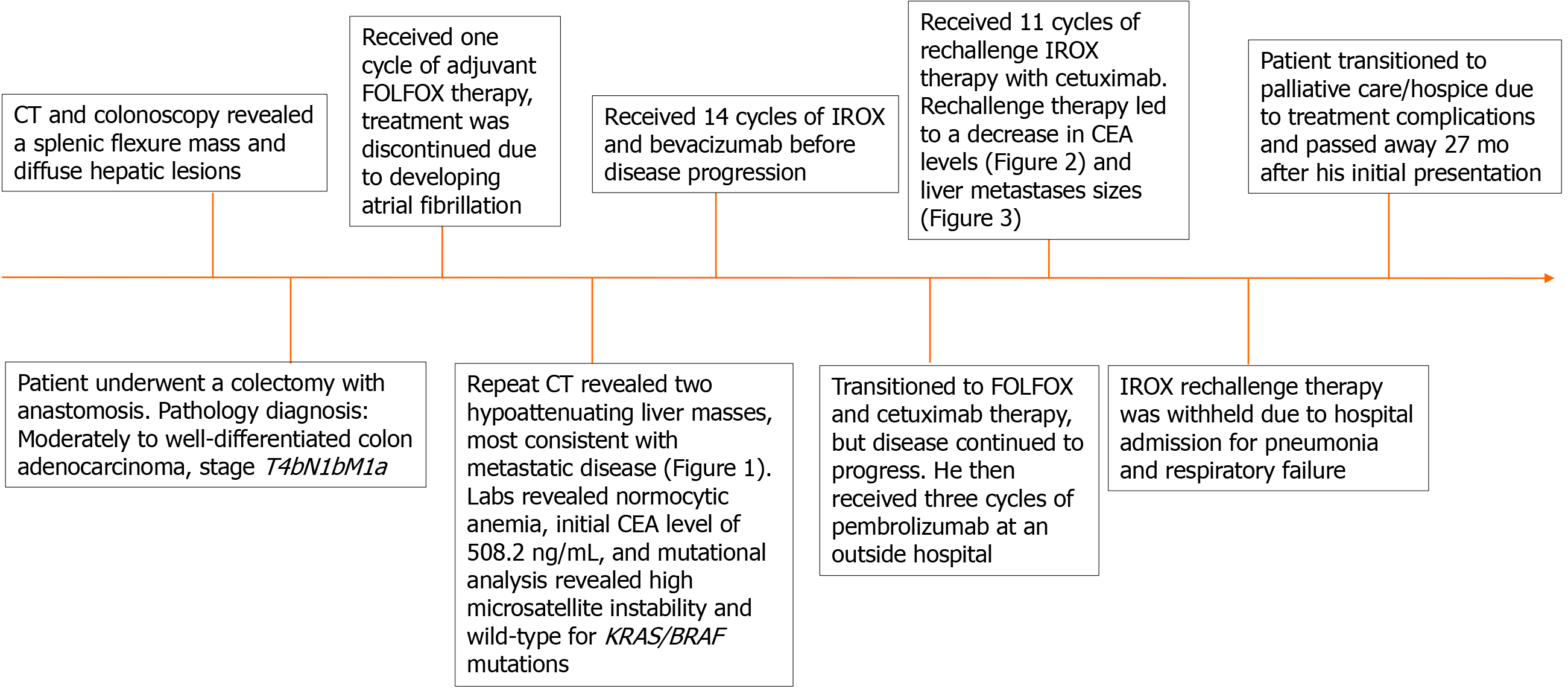
A 41-year-old man with no previous medical history initially presented with worsening diffuse abdominal tenderness. Computed tomography was significant for a splenic flexure mass and hepatic lesions concerning for metastatic disease. He underwent a colectomy with anastomosis. Postoperative pathology was diagnostic for moderately to well-differentiated adenocarcinoma (T4bN1bM1a). He received adjuvant 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX), but therapy was discontinued due to the development of atrial fibrillation. Additional workup indicated a carcinoembryonic antigen level of 508.2 ng/mL, and mutational analysis found that the tumor was microsatellite instability-high and KRAS/BRAF wild-type. He was started on irinotecan with oxaliplatin (IROX), and bevacizumab (14 cycles), developed disease progression, was transitioned to FOLFOX and cetuximab, and then eventually three cycles of pembrolizumab. Following disease progression, he was rechallenged with IROX therapy, as he previously responded well to oxaliplatin-based therapy. The IROX rechallenge provided this patient with a ten-month survival benefit, decreased metastatic burden, and marked improvement in his clinical condition.
Rechallenge of previous lines of well-tolerated systemic chemotherapy regimens may be a valuable therapeutic strategy in patients with heavily-treated mCRC.
Core Tip: Despite advances in therapeutic strategies, colorectal cancer (CRC) remains a deadly disease. There are limited options for patients with chemo-refractory mCRC. We present a case of a patient with heavily treated metastatic colorectal cancer (mCRC) that was responsive to oxaliplatin-based rechallenge therapy. Oxaliplatin rechallenge therapy provided this patient with a ten-month survival benefit, marked improvement in his clinical condition, performance status, and quality of life. This case highlights the importance of considering rechallenge therapy in patients with chemo-refractory mCRC. Monitoring for oxaliplatin-associated peripheral sensory neuropathy should be considered for patients who are candidates for oxaliplatin rechallenge therapy.
- Citation: Reddy TP, Khan U, Burns EA, Abdelrahim M. Chemotherapy rechallenge in metastatic colon cancer: A case report and literature review . World J Clin Oncol 2020; 11(11): 959-967
- URL: https://www.wjgnet.com/2218-4333/full/v11/i11/959.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i11.959
Colorectal cancer (CRC) is the third most common cancer and the third leading cause of cancer death in the United States[1]. An estimated 147950 new CRC cases and 53200 deaths are expected to occur in 2020[1]. Approximately 20%-22% of CRC patients present with metastatic disease at initial diagnosis and 50%-60% will develop metastasis throughout their disease[1]. Metastatic colorectal cancer (mCRC) carries a poor prognosis, with a 5-year overall survival (OS) rate of 14%[1]. The current recommended first-line systemic chemotherapy regimens include 5-fluorouracil, leucovorin, irinotecan (FOLFIRI), 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX), capecitabine plus oxaliplatin (XELOX), and 5-fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI)[2-5]. Biologic agents such as the vascular endothelial growth factor inhibitor bevacizumab and epidermal growth factor receptor (EGFR) inhibitor cetuximab may be used as adjuncts to systemic chemotherapy in mCRC[6,7].
Despite advances in cytotoxic and targeted therapy, treatment resistance remains a significant barrier to the management of mCRC. Resistance can be primary (poor initial response) or secondary (loss of initial response). Many patients exhibit rapid disease progression with third- and fourth-lines therapies. The therapeutic options for these patients remain limited and typically consist of regorafenib or trifluridine-tipiracil (FTD/TPI)[8,9]. The role of rechallenge therapy with chemotherapy, biologic agents, or combination therapy in patients who have developed secondary resistance, particularly previously responding patients, is not clear. We present a case that challenges the dogma of irreversible secondary resistance and supports the potential of rechallenge chemotherapy as a life-prolonging treatment option in heavily-treated mCRC.
A 41-year-old man presented for a second opinion regarding his worsening abdominal pain.
The patient initially presented to a hospital in Qatar with an 8-mo history of diffuse abdominal pain. A computed tomography (CT) scan and colonoscopy revealed a splenic flexure mass and diffuse hepatic lesions concerning for metastatic disease. The patient then received another opinion at a hospital in Bangkok, Thailand, where a repeat CT scan and colonoscopy confirmed the initial diagnosis. The patient had a 17 pack-year cigarette smoking history. He denies alcohol consumption or a family history of cancer. During his clinical visits in Qatar and Thailand, his physical exam was unremarkable, and he denied weight loss, constipation, diarrhea, hematochezia, or melena. In Qatar, he underwent a colectomy with anastomosis. The pathologic diagnosis was moderate to well-differentiated colonic adenocarcinoma, stage T4bN1bM1a. He then received adjuvant treatment with FOLFOX chemotherapy, however after the first cycle, he developed atrial fibrillation, and treatment was discontinued. Afterward, he presented to our hospital for further workup and recommendations.
The patient has no past medical history.
The patient has no personal or family history.
The vitals on admission was within normal reference range and his physical examination was unremarkable.
Labs indicated a normocytic anemia, and an elevated carcinoembryonic antigen (CEA) level (508.2 ng/mL). Blood chemistries, urinalysis, urine cultures, coagulation times including international normalized ratio, prothrombin, and partial thromboplastin times were within normal limits. Electrocardiogram was within normal limits.
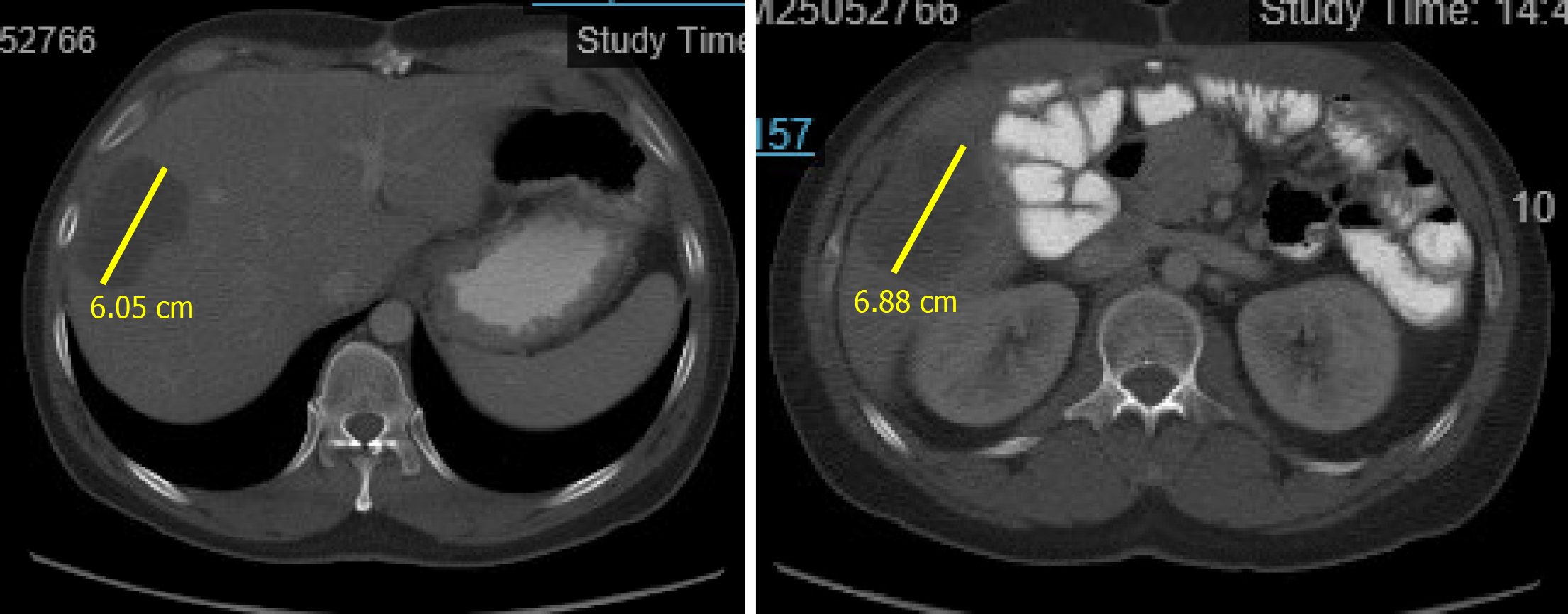
An initial imaging evaluation with CT of the abdomen and pelvis revealed two hypoattenuating masses in the liver. One 6.1 cm superior mass was in the right lateral lobe of the liver and a 6.9 cm inferior mass was in the lower tip of the right lobe (Figure 1). These masses were most compatible with liver metastasis from invasive CRC. The portal vein was patient, bile ducts were not dilated, and the gallbladder was normal.
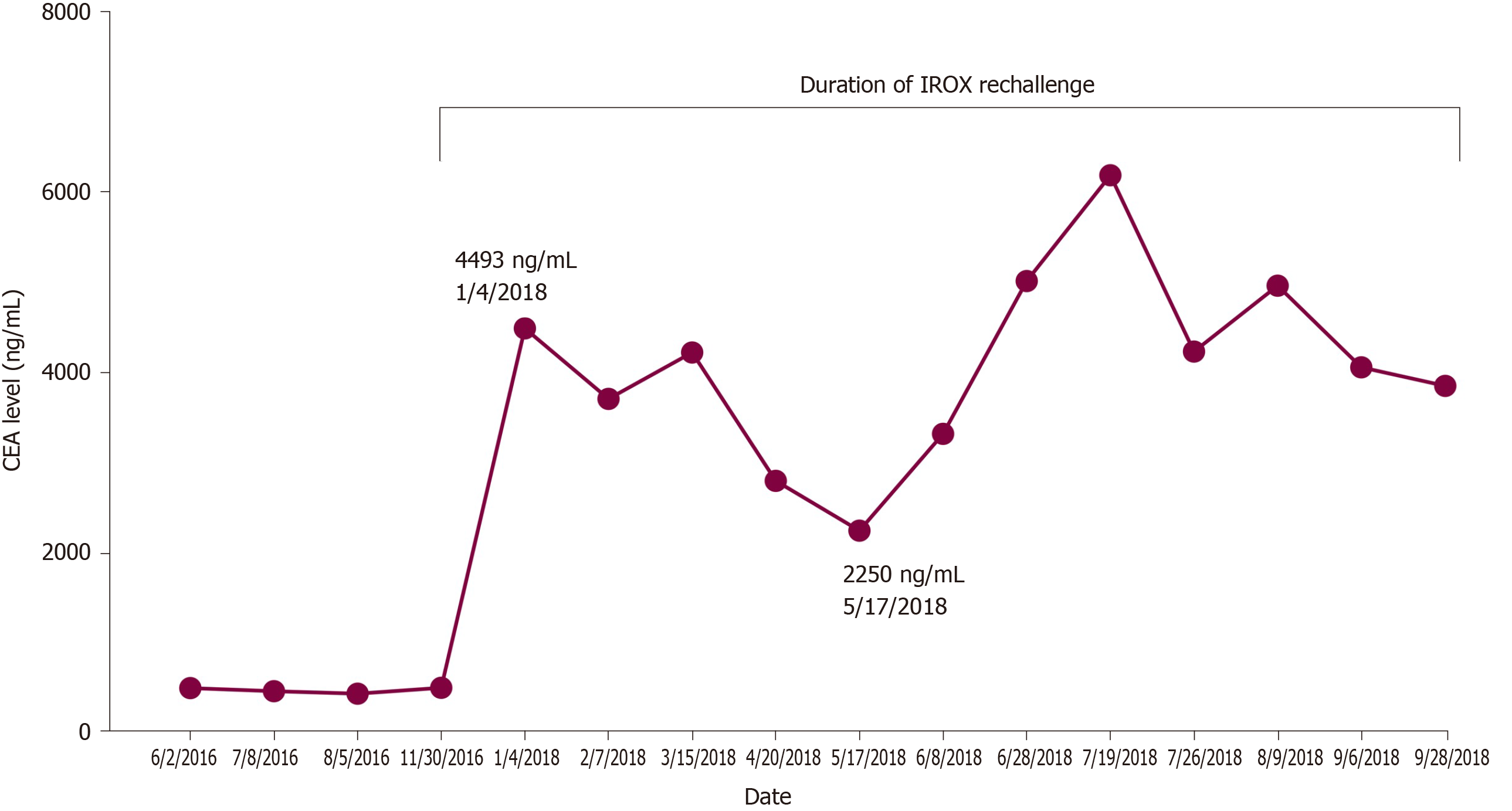
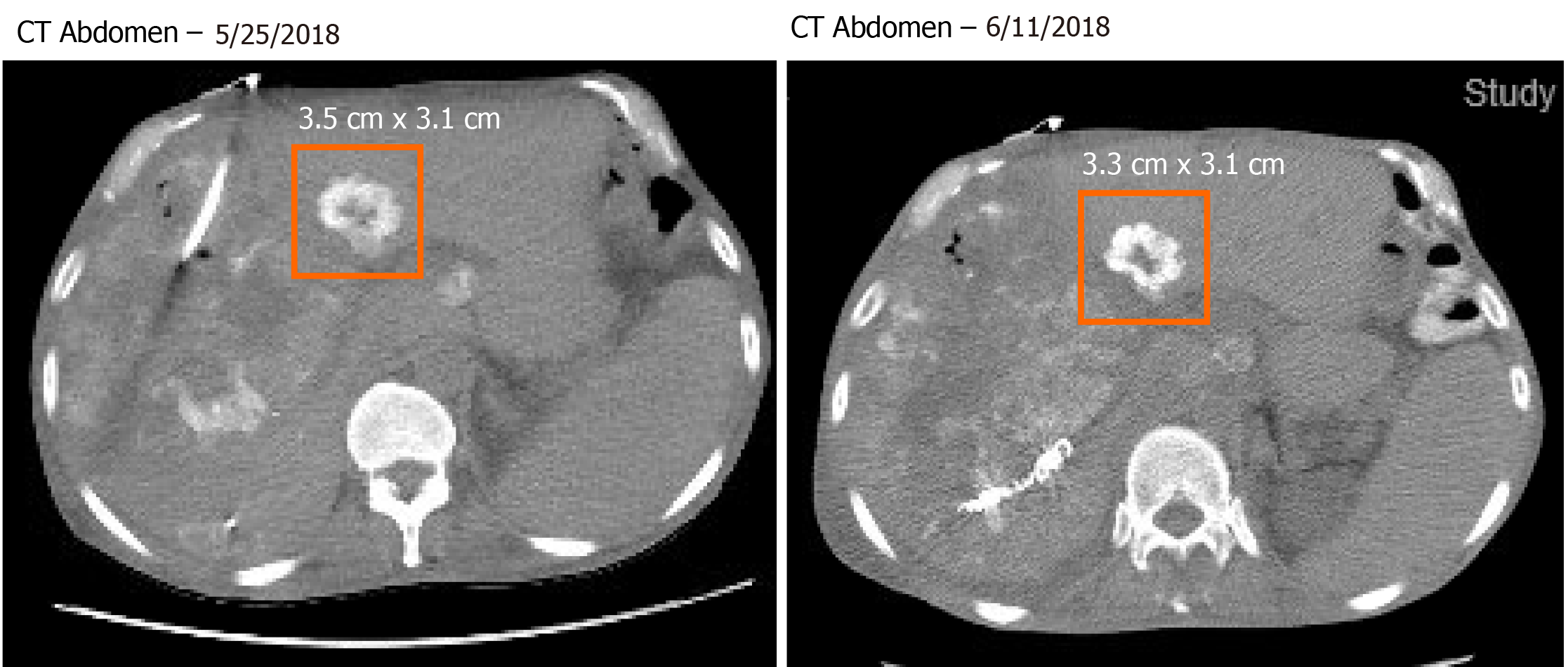
Pathology slides of tumor tissue samples were obtained from the patient’s hospitalization in Bangkok and reviewed. The pathology report indicated invasive colonic adenocarcinoma, moderately differentiated (low-grade). The mutational analysis found that the patient had high microsatellite instability and wildtype (WT) KRAS and BRAF mutations. Given his history of atrial fibrillation on FOLFOX, he was started on irinotecan and oxaliplatin (IROX) plus bevacizumab. The patient had a partial response to IROX plus bevacizumab therapy but after 14 cycles of treatment, he developed disease progression and was transitioned to FOLFOX plus cetuximab. His disease progressed on FOLFOX and cetuximab, and thereafter the patient was lost to follow up for one year. During that time, he received three cycles of pembrolizumab from an outside hospital. Unfortunately, staging workup after 3 cycles of pembrolizumab indicated enhanced disease progression, particularly increased enlargement of metastatic liver lesions. In early 2018, we discussed with the patient about three potential chemotherapy options: (1) FTD/TPI; (2) Regorafenib; and (3) Rechallenge with IROX. The decision was made to rechallenge with IROX therapy based on the patient’s previous response to this regimen. Therefore, the patient received rechallenge therapy with IROX plus cetuximab every two weeks (11 cycles total). He tolerated the treatment well and showed a marked improvement in his clinical condition, performance status, and quality of life. Furthermore, a substantial reduction in the CEA level from 4493 ng/mL to 2250 ng/mL was observed after four months of treatment (Figure 2). Follow-up CT scans showed a decrease in liver metastasis size shown in the scans obtained at five and six months after rechallenge therapy (Figure 3). The diagnostic workup, disease management, and treatment are also summarized in Figure 4.
The final diagnosis was chemo-resistant metastatic KRAS/BRAF WT, microsatellite instability-high colorectal carcinoma (T4bN1bM1a).
The patient continued to receive IROX plus cetuximab rechallenge chemotherapy for a total of nine months. The treatment was eventually withheld due to hospital admission for pneumonia and respiratory failure.
After noting treatment complications, the patient and his family chose to transition to palliative care and hospice. He passed away 27 mo after his initial presentation to our hospital. The oxaliplatin rechallenge therapy provided this patient with a ten-month survival benefit.
Limited cytotoxic agents are available for the treatment of mCRC. Unfortunately, there are a significant number of patients that still progress past the third and fourth line of therapy, who may respond to other therapeutic options. Rechallenge therapy with either chemotherapy/biologic therapy alone or combination therapy has not been fully investigated as a viable option for patients with disease progression. In this case, oxaliplatin-based chemotherapy rechallenge demonstrated a viable alternative for this patient with heavily-pretreated mCRC. Despite progression on multiple lines of chemotherapy, immunotherapy, and investigational therapy, IROX rechallenge provided this patient with an additional ten-month survival benefit. The rationale of reattempting a previous line of systemic chemotherapy stems from the possibility that subsequent therapies following the development of chemoresistance may sensitize patients to the primary therapy by promoting the growth of sensitive clones[10]. Another possibility is epigenetic alterations may result in tumor resistance, which may reverse following a “drug holiday”[10]. While mechanisms that result in chemoresistance are well known, the cellular mechanisms and predictive factors associated with response to rechallenge therapy need to further elucidated.
Findings from this case are consistent with previous studies demonstrating the promise of oxaliplatin-based chemotherapy rechallenge in the third- or fourth-line setting for mCRC. These previous studies are summarized in Table 1. Suenaga et al[11] performed a single-arm, open-label, phase II clinical trial (RE-OPEN) to examine the safety and efficacy of reintroducing oxaliplatin in patients with mCRC refractory to standard chemotherapy. The eligible patients in this study had previously received oxaliplatin and irinotecan and achieved stable disease or response, followed by disease progression ≥ 6 mo during the first oxaliplatin-based therapy. The primary endpoint was disease control rate (DCR) after 12 wk of re-challenge therapy and the secondary endpoints were safety, overall response rate, and progression-free survival (PFS). Oxaliplatin was reintroduced by treating patients with the FOLFOX6 regimen. The study found that the DCR after 12 wk of rechallenge therapy was 39.4% (95%CI 21.8-57.0) and the response rate (complete and partial response) was 6.1%. The median PFS and OS were 3.2 and 9.8 mo, respectively. A concern with rechallenging patients with oxaliplatin is the risk of developing peripheral sensory neuropathy (PSN) during the oxaliplatin rechallenge. However, in this study, the incidence of grade 1 and 3 PSN events was 53.1% and 0%, respectively[11].
| Ref. | Prior lines of chemotherapy | Patient cohort | Treatment regimen | PFS (months) | OS (months) |
| Suenaga et al[11] | Oxaliplatin-based therapy: First-line: 26 (78.8%); Second-line 7 (21.2%). Molecular-targeted therapy: None: 1 (3.0%); Bevacizumab: 27 (81.8%); Cetuximab or Panitumumab: 19 (57.6%) | 33 patients, previously received oxaliplatin and irinotecan, had stable disease, and disease progression ≥ 6 mo | FOLFOX6: 33 (100%) | 3.2 | 9.8 |
| Yang et al[13] | Oxaliplatin-based therapy: First-line: 76 (80.0%); Second-line 19 (20.0%). Oxaliplatin rechallenge therapy: Third-line: 78 (82.1%); Fourth-line: 13 (13.7%); Fifth or more: 4 (4.2%). Control arm: Anti-EGFR + irinotecan: 29 (100%) | Rechallenge arm: 95 patients received who received oxaliplatin in the first/second-line and were rechallenged as a third or later line of therapy | mFOLFOX6: 70 (73.7%); XELOX: 19 (20%); Other: 6 (6.3) | 1.7a | 12.2 |
| Control arm: 29 patients received anti-EGFR and irinotecan therapy | Anti-EGFR + irinotecan: 29 (100%) | 11.4 | |||
| Köstek et al[14] | All patients received two lines of chemotherapy of any of the following combinations: (FOLFIRI, FOLRFIRI/XELIRI, FOLFOX/XELOX, capecitabine, FUFA/capecitabine) monotherapy or combined with biologic agents bevacizumab/cetuximab/panitumab | Regorafenib arm: 73 | Regorafenib | 3.4 | 6.6 |
| Rechallenge arm: 31; rechallenge therapy was identified as re-using a regimen that was previously administered to patients and had obtained disease control | FOLFOX + cetuximab: 8, FOLFOX + bevacizumab: 6, FOLFOX: 4, FOLFIRI: 2, FOLFIRI + cetuximab: 3, capecitabine: 2, FOLFOX + panitumab: 1, FOLFIRI + bevacizumab: 1, FUFA + bevacizumab: 1, capecitabine + bevacizumab: 1, XELOX + bevacizumab: 1, FOLFIRINOX: 1 | 9.2 | 12.0 | ||
| Fernandes et al[16] | Patients with documented progression to regimens containing oxaliplatin, irinotecan, and 5-FU (most patients received at least three regimens) | Rechallenge arm: 21 patients who were rechallenged with either FOLFIRINOX or FOLFOXIRI | FOLFIRINOX: 13 (61.9%); FOLFOXIRI: 8 (38.1%) | 4.0 | 8.6 |
| Townsend et al[17] | Patients identified to received oxaliplatin previously. Prior lines of chemotherapy before oxaliplatin rechallenge (information on prior regimen was not provided): 4 lines for 2 patients, 3 lines for 6 patients, 2 lines for 8 patients, 1 line for 4 patients | Rechallenge arm: 20 patients who were rechallenged with FOX therapy | FOX: 20 | 3.7 | 7.8 |
| Sgouros et al[18] | Patients previously treated with IROX with refractory disease (median prior lines of chemotherapy: 3) | Rechallenge arm: 25 patients who were rechallenged with IROX therapy | Irinotecan (180 mg/m2/135 mg/m2) and oxaliplatin (85 mg/m2/65 mg/m2) every two weeks | 3.0 | 7.0 |
A follow-up to the RE-OPEN trial was a phase I clinical trial (LUPIN study), in which the study examined the safety of rechallenging oxaliplatin with FTD/TPI[12]. The patient cohort of interest in this study were patients with mCRC, whose tumors acquired resistance to prior chemotherapy with oxaliplatin and irinotecan[12]. The study concluded that a safe rechallenge regimen would be oxaliplatin 85 mg/m2 on days 1 and 15 every four weeks and FTD/TPI 35 mg/m2 bid on days 1-5 and 15-19. The LUPIN study concluded that FTD/TPI could potentially replace 5-fluorouracil in combination with oxaliplatin. The efficacy of this novel combinatorial approach of FTD/TPI with oxaliplatin for mCRC needs to be further evaluated.
Comparable results were found in a retrospective study performed by Yang et al[13] in 2018. In this study, patients with mCRC, who progressed from oxaliplatin, fluoropyrimidine, and irinotecan as first and second-line chemotherapy, were re-challenged with an oxaliplatin-based therapy. The control arm in this study was mCRC patients treated with anti-EGFR biologic therapy and irinotecan. The OS for oxaliplatin rechallenge arm and control arm was 12.2 and 11.4 mo, respectively (no significant difference between both treatment arms, P > 0.05). Multivariate analysis found that patients who obtained disease control with oxaliplatin rechallenge had a better time to treatment failure (6.1 vs 1.7 mo, P < 0.001) and OS (15.7 vs 6.3 mo, P < 0.001) compared to patients with progressive disease. This study showed that rechallenge with oxaliplatin-containing chemotherapy yielded equivalent tumor control and survival benefit to that of anti-EGFR antibodies with irinotecan in the third- or later-line setting in mCRC[13].
Köstek et al[14] found that chemotherapy rechallenge (FOLFOX alone, FOLFOX with either cetuximab or bevacizumab, FOLFIRI alone or with cetuximab or bevacizumab, capecitabine plus bevacizumab, FOLFIRINOX, XELOX plus bevacizumab, or folinic acid and 5-fluorouracil plus bevacizumab) was more effective than regorafenib in the third-line treatment of mCRC patients. The PFS and OS with rechallenge therapy were 9.2 mo and 12 mo vs 3.4 mo and 6.6 mo with regorafenib, respectively. Another supporting study was a retrospective analysis that investigated the feasibility and efficacy of oxaliplatin rechallenge in mCRC patients previously treated with adjuvant or palliative oxaliplatin-based chemotherapy, who had remained disease-free or progression-free for at least 6 mo after the last dose of oxaliplatin-based therapy[15]. Sixty-five patients were rechallenged with FOLFOX and 45 patients were rechallenged with XELOX. The median PFS and OS in this study with oxaliplatin rechallenge were 5.9 mo and 18.5 mo, respectively[15].
Fernandes et al[16] conducted a retrospective study to evaluate the benefit of rechallenging patients with mCRC to 5-fluorouracil, irinotecan, and oxaliplatin therapy (FOLFIRINOX or FOLFOXIRI). Twenty-one patients were retrospectively analyzed, with a response rate was 38% and 24% of patients had stable disease after rechallenge therapy. The median OS was 8.6 mo and only one patient had experienced grade 5 neutropenic sepsis. Another retrospective study had examined a South Australian mCRC database for patients who were rechallenged with FOX therapy (oxaliplatin and fluoropyrimidine)[17]. The study included 20 patients and discovered that for this patient cohort, the response rate was 18% and 48% of patients had stable disease after oxaliplatin rechallenge. The median PFS and OS were 3.7 and 7.8 mo, respectively[17]. Another study reported a comparable OS to rechallenge therapy, a 7-mo median OS, and 32% DCR[18]. This particular study investigated oxaliplatin-based chemotherapy rechallenge in mCRC patients previously treated with oxaliplatin, irinotecan, bevacizumab, cetuximab, or panitumumab therapies (if wild-type KRAS)[18]. Though these retrospective studies support the rationale of oxaliplatin rechallenge as another third/fourth-line option for mCRC, the concern with such studies is that there is no formal assessment as to whether oxaliplatin rechallenge leads to worsening PSN in this patient cohort.
If considering chemotherapy rechallenge with an oxaliplatin-based regimen, clinicians should be wary of the development of PSN, which may lead to dose reduction of therapy, premature cessation of treatment, and significantly impair quality of life. To investigate whether oxaliplatin rechallenge results in new or worsening PSN, Besora et al[19] conducted a retrospective clinical study of 106 patients who were rechallenged with FOLFOX4/6, XELOX, or TOMOX. PSN was graded according to the National Cancer Institute-Common Toxicity Criteria for Adverse Events[20]. The study found that before oxaliplatin rechallenge, the frequencies of oxaliplatin-associated grade 1 and 2 PSN were 23.8% and 8.5%, respectively. After oxaliplatin rechallenge, 39.6% and 22.6% of patients developed grade 1 and 2 PSN, respectively; No patients developed grade 3 PSN. About 31% of all patients in this study experienced worsening PSN symptoms, whereas 68.9% of patients had the same PSN grade as before rechallenge therapy. This study sheds light on how oxaliplatin rechallenge may be a safe option, albeit neurological monitoring using scales such as the total neuropathy score, should be considered for mCRC patients who may undergo rechallenge therapy. Furthermore, a balance between rechallenge therapy to improve survival vs its impact on worsening PSN on quality of life for patients is crucial.
There are limited therapeutic options for mCRC that has progressed past the third and fourth lines of therapy. Rechallenging a chemo-resistant tumor with a previous well-tolerated and responsive line of chemotherapy may be a life-prolonging therapeutic approach for mCRC. This case demonstrates that rechallenge with IROX may offer a valid treatment option for mCRC patients with chemo-resistant disease, particularly in select patients with previous favorable response to oxaliplatin-based chemotherapy. IROX may serve as a viable option for rechallenge therapy, as seen in this case. However, neurological monitoring should be considered for mCRC patients who may undergo oxaliplatin-based rechallenge therapy. Further studies to elucidate the cellular mechanisms and predictive factors associated with enhanced response to rechallenge therapy in mCRC are warranted.
| 1. | Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2015, National Cancer Institute. Bethesda, MD. 2018; Available from: https://seer.cancer.gov/archive/csr/1975_2015/. |
| 2. | de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938-2947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3005] [Cited by in RCA: 2838] [Article Influence: 109.2] [Reference Citation Analysis (1)] |
| 3. | Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1779] [Cited by in RCA: 1729] [Article Influence: 78.6] [Reference Citation Analysis (2)] |
| 4. | Cassidy J, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzén F, Saltz L. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26:2006-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 644] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 5. | Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W, Fanchini L, Cortesi E, Picone V, Vitello S, Chiara S, Granetto C, Porcile G, Fioretto L, Orlandini C, Andreuccetti M, Masi G; Gruppo Oncologico Nord Ovest. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 902] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 6. | Fakih MG. Metastatic colorectal cancer: current state and future directions. J Clin Oncol. 2015;33:1809-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 385] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 7. | Edwards MS, Chadda SD, Zhao Z, Barber BL, Sykes DP. A systematic review of treatment guidelines for metastatic colorectal cancer. Colorectal Dis. 2012;14:e31-e47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D; CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2194] [Cited by in RCA: 2213] [Article Influence: 170.2] [Reference Citation Analysis (0)] |
| 9. | Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero J, Komatsu Y, Sobrero A, Boucher E, Peeters M, Tran B, Lenz HJ, Zaniboni A, Hochster H, Cleary JM, Prenen H, Benedetti F, Mizuguchi H, Makris L, Ito M, Ohtsu A; RECOURSE Study Group. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 1064] [Article Influence: 96.7] [Reference Citation Analysis (0)] |
| 10. | Ioannou P, Talesnik J. Platelet antiaggregatory substances inhibit arachidonic acid induced coronary constriction. Can J Physiol Pharmacol. 1986;64:398-405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Suenaga M, Mizunuma N, Matsusaka S, Shinozaki E, Ozaka M, Ogura M, Yamaguchi T. Phase II study of reintroduction of oxaliplatin for advanced colorectal cancer in patients previously treated with oxaliplatin and irinotecan: RE-OPEN study. Drug Des Devel Ther. 2015;9:3099-3108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Suenaga M, Wakatsuki T, Mashima T, Ogura M, Ichimura T, Shinozaki E, Nakayama I, Osumi H, Ota Y, Takahari D, Chin K, Seimiya H, Yamaguchi K. A phase I study to determine the maximum tolerated dose of trifluridine/tipiracil and oxaliplatin in patients with refractory metastatic colorectal cancer: LUPIN study. Invest New Drugs. 2020;38:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Yang Q, Huang Y, Jiang Z, Wang H, Li W, Zhang B, Xie D. Rechallenge of oxaliplatin-containing regimens in the third- or later-line therapy for patients with heavily treated metastatic colorectal cancer. Onco Targets Ther. 2018;11:2467-2473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Köstek O, Hacıoğlu MB, Sakin A, Demir T, Sarı M, Ozkul O, Araz M, Doğan AF, Demircan NC, Uzunoğlu S, Çiçin İ, Erdoğan B. Regorafenib or rechallenge chemotherapy: which is more effective in the third-line treatment of metastatic colorectal cancer? Cancer Chemother Pharmacol. 2019;83:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Kim JJ, Kang J, Hong YS, Kim KP, Kim SY, Kim TW, Kim JE. Oxaliplatin rechallenge in metastatic colorectal cancer patients after prior oxaliplatin treatment. Med Oncol. 2018;35:65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Fernandes GDS, Braghiroli MI, Artioli M, Paterlini ACCR, Teixeira MC, Gumz BP, Girardi DDM, Braghiroli OFM, Costa FP, Hoff PM. Combination of Irinotecan, Oxaliplatin and 5-Fluorouracil as a Rechallenge Regimen for Heavily Pretreated Metastatic Colorectal Cancer Patients. J Gastrointest Cancer. 2018;49:470-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Townsend AR, Bishnoi S, Broadbridge V, Beeke C, Karapetis CS, Jain K, Luke C, Padbury R, Price TJ. Rechallenge with oxaliplatin and fluoropyrimidine for metastatic colorectal carcinoma after prior therapy. Am J Clin Oncol. 2013;36:49-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Sgouros J, Aravantinos G, Dragasis S, Koutsounas K, Antoniou G, Belechri M, Antonopoulos M, Visvikis A, Res H, Samantas E. Reintroduction of irinotecan and oxaliplatin as a combination (IROX regimen) in heavily pretreated colorectal cancer patients - A single-center experience. FCO. 2013;4:13-18. |
| 19. | Besora S, Santos C, Izquierdo C, Martinez-Villacampa MM, Bruna J, Velasco R. Rechallenge with oxaliplatin and peripheral neuropathy in colorectal cancer patients. J Cancer Res Clin Oncol. 2018;144:1793-1801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Velasco R, Bruna J, Briani C, Argyriou AA, Cavaletti G, Alberti P, Frigeni B, Cacciavillani M, Lonardi S, Cortinovis D, Cazzaniga M, Santos C, Kalofonos HP. Early predictors of oxaliplatin-induced cumulative neuropathy in colorectal cancer patients. J Neurol Neurosurg Psychiatry. 2014;85:392-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Guo XZ S-Editor: Huang P L-Editor: A P-Editor: Wang LL