Published online Nov 24, 2020. doi: 10.5306/wjco.v11.i11.945
Peer-review started: May 8, 2020
First decision: July 25, 2020
Revised: August 30, 2020
Accepted: September 22, 2020
Article in press: September 22, 2020
Published online: November 24, 2020
Processing time: 194 Days and 5.4 Hours
Patients in the acute phase of rehabilitation after vestibular tumor surgery are dysfunctional in basic daily activities. Balance, gait impairments, and falls are prevalent with vestibular loss.
To determine the degree of balance disorders after vestibular tumor surgery, the susceptibility to falls and to assess motor tasks using the Functional Gait Assessment (FGA) scale for functional gait, as part of the vestibular rehabilitation program during hospital stay.
Patients who achieved a score higher than 25 points on the Mini-Mental State Examination and higher than 8 points on the Barthel index were included in the study. They were evaluated with the Berg Balance Scale the second day after surgery, during their hospital stay, at discharge, and three months after surgery. Throughout their hospitalization, patients took part in the vestibular rehabilitation program, focusing on multiple motor tasks included in the FGA.
All patients progressed clinically and statistically significant differences in functional activities of daily living were observed during hospitalization, before discharge to the home environment (median = 11; P = 0.0059) and three months after vestibular tumor surgery (median = 8; P = 0.0058). After discharge from hospital, four patients were at risk of falls, and two patients were at risk at three months.
Our study showed a positive effect of the use of FGA tasks as part of a rehabilitation program on functional activities of daily living in patients after vestibular tumor surgery. Nevertheless, we suggest further research to include a larger sample and a control group to overcome the deficiencies of our study.
Core Tip: This was a prospective pilot study of 10 patients who had problems with balance after surgical removal of a tumor from the cerebellopontine angle of the brain with the aim of using multitasking Functional Gait Assessment exercises as part of vestibular rehabilitation strategies for targeting better recovery and improvement of balance.
- Citation: Kos N, Brcar M, Velnar T. Functional Gait Assessment scale in the rehabilitation of patients after vestibular tumor surgery in an acute hospital. World J Clin Oncol 2020; 11(11): 945-958
- URL: https://www.wjgnet.com/2218-4333/full/v11/i11/945.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i11.945
Vestibular schwannomas or acoustic neuromas are benign intracranial tumors of the vestibular nerve, which are usually treated by surgical resection[1,2]. The most commonly used surgical procedure in our clinical department is the retrosigmoid suboccipital approach[3]. It offers direct access to the tumor between the cerebellar hemisphere and the petrous bone with good visualization of the lower nerves and the inner auditory meatus, which is normally obscured by the tumor. The danger, however, is damage to the 7th and 8th nerve complex, which can be avoided by a meticulous operative technique and intraoperative monitoring[4,5].
In all cases, it is not possible to preserve the acoustic nerve, which is embedded in the tumor and is therefore destroyed during surgery. The facial nerve may be morphologically preserved[6,7]. Following resection, patients may experience vertigo, nausea, and a range of symptoms that include deficits in gaze stability, mobility, and balance[8,9]. Vestibular rehabilitation may be useful in reducing the severity and minimizing the impact of these symptoms[10,11]. Other problems, secondary to vestibular disorders, can also arise, such as nausea and vomiting, fatigue, and reduced ability to focus or concentrate[12]. Symptoms due to vestibular disorders can diminish the quality of life and impact all aspects of daily living. They also contribute to emotional problems such as anxiety and depression. Additionally, one of the consequences of having a vestibular disorder is that symptoms frequently cause people to adopt a sedentary lifestyle to avoid bringing on, or worsening, dizziness, and imbalance. As a result, decreased muscle strength and flexibility, increased joint stiffness and reduced stamina can occur[13,14].
Vestibular rehabilitation therapy (VRT) is a specialized exercise-based program primarily designed to reduce vertigo and dizziness, gaze instability, imbalance, and falls. For most patients with vestibular loss, the deficit is permanent as the amount of restoration of vestibular function is very small. However, after vestibular system damage, patients can feel better and function can return through compensation. This occurs because the brain learns to use other senses (vision and somatosensory; body sense) to substitute for the deficient vestibular system. The health of particular parts of the nervous system (brainstem and cerebellum, visual and somatosensory sensations) is important in determining the extent of recovery that can be gained through compensation. For many, compensation occurs naturally over time, but for patients in the acute stage of recovery, whose symptoms do not reduce and who continue to have difficulty returning to daily activities, VRT can help with recovery by promoting compensation[15].
The VRT goal is to use a problem-oriented approach to promote compensation. This is achieved by customizing exercises to address each patient’s specific problems. Therefore, before an exercise program can be designed, a comprehensive clinical examination is needed to identify problems related to the vestibular disorder[16,17].
Depending on the vestibular-related problems identified, three principal methods of exercise can be implemented: (1) Habituation; (2) Gaze stabilization; and (3) Balance training[18].
Habituation exercises are used to treat symptoms of dizziness that are produced by self-motion or produced due to visual stimuli[19,20]. Habituation exercise is indicated for patients, who report increased dizziness when they move, especially when they make quick head movements, or when they change positions such as when they bend over or look up to reach above their heads. The goal of habituation exercises is to reduce the dizziness through repeated exposure to specific movements or visual stimuli that provoke patients’ dizziness. These exercises are designed to mildly or at the most moderately, provoke the patients’ symptoms of dizziness. The increase in symptoms should only be temporary, and before continuing onto other exercises or tasks the symptoms should return completely to the baseline level. Over time and with good compliance and perseverance, the intensity of the patient’s dizziness will decrease as the brain learns to ignore the abnormal signals it is receiving from the inner ear[21].
Gaze stabilization exercises are used to improve control of eye movements so that vision is clear during head movement. These exercises are appropriate for patients who report problems seeing clearly because their visual world appears to bounce or jump around, such as when reading or when trying to identify objects in the environment, especially when moving around. There are two types of eye and head exercises used to promote gaze stability. The choice of which exercises to use depends on the type of vestibular disorder and extent of the disorder. One type of gaze stability exercise incorporates fixating on an object while patients repeatedly move their heads back and forth or up and down for a couple of minutes. The other type of gaze stability exercise is designed to use vision and somatosensation (body sense) as substitutes for the damaged vestibular system. Gaze shifting and remembered target exercises use sensory substitution to promote gaze stability. These exercises are particularly helpful for a patient with poor to no vestibular function[22].
Balance training exercises are used to improve steadiness so that daily activities for self-care, work, and leisure can be performed successfully. Exercises used to improve balance should be designed to address each patient’s specific underlying balance problems[23]. Also, the exercises need to be moderately challenging but safe enough, to ensure that patients do not fall while doing them. Features of the balance exercises that are manipulated to make them challenging are also included in the Functional Gait Assessment (FGA) scale, which is focused on the movement of the head and trunk with vertical and horizontal turns during walking with specific activities requiring high-level function, such as gait on a level surface, change in gait speed, backward gait, step up and downstairs, stepping over obstacles, turning around, gait with eyes closed and gait with a narrow base of support. Additionally, the importance of FGA is increasingly emphasized during the patient’s hospital stay and especially when they are released to the home environment[24,25]. Balance exercises should be designed to reduce environmental barriers and fall risk. For example, the exercises should help improve patients’ ability to walk outside on uneven ground or walk in the dark. Ultimately, balance training exercises are designed to help improve standing, bending, reaching, turning, walking, and if required, other more demanding activities like running, so that patients can safely and confidently return to their daily activities[26,27]. Our main goal in this study was to use the FGA scale as part of vestibular therapy to improve the dynamic performances by new, learned strategies, that lead to the best optimal functional recovery. Due to the patient’s balance impairment, relatively short hospitalization, and the fact that they are left on their own, leave without the possibility of rehabilitation treatment, the need for such a vestibular rehabilitation treatment, with included specific FGA activities during walking, should be initiated in the hospital.
Patients are seen by a specialist physiatrist and by a licensed physical or occupational therapist with advanced post-graduate training. Vestibular rehabilitation training begins the day after the patient’s vestibular surgery with a comprehensive clinical assessment that includes collecting and documenting the type and intensity of postoperative symptoms and the precipitating circumstances. Acute stage rehabilitation aims to evaluate cognitive function[28], sensory and somatosensory abilities[29,30] and to prevent secondary complications[31], recover sensorimotor function, achieve interaction between postural control and selective movement to establish coordinated movement patterns and finally to achieve independence in the activities of daily living and independence in performing functional gait[32,33]. In the acute post-surgery phase, gait function assessment is meant for clinical use only and is a part of functional assessment. For this purpose, the Barthel index is often used. It does not, however, provide adequate information/data for planning the rehabilitation program and measuring the functional outcome of the program[34]. Balance assessment is a part of the rehabilitation program in the early phase of rehabilitation and is implemented in different positions and activities, such as sitting, sitting to standing, and walking. The assessment of balance during gait is important for the identification of gait patterns and the introduction of suitable walking aids[35]. One of the most commonly used balance assessment tests in the hospital is a 14-item Berg Balance Scale (BBS), taken from the activities of daily living and represents the general mobility of the patients. The scale is organized in such a way that tasks move from less demanding to increasingly demanding functional abilities. The rating scale is for each of the five-level assignments (grades from 0 to 4). The total number of points is 56. The predictive value of the BBS for falls and the estimation of equilibrium indicate that scores below 45 points suggest an imbalance and thus an increased risk for falls. A clinically significant change between two BBS measurements indicating an improvement in equilibrium is 8 points[36,37].
As vestibular rehabilitation should focus on adaptation, particularly for the restoration of the dynamic functions, our focus was to improve patients’ gaze of stability, balance control, and gait with challenging FGA tasks that require constant adjustment of postural stability. To confirm that statement, a more detailed analysis of the randomized-controlled trials investigating vestibular rehabilitation following the resection of an acoustic neuroma has provided greater insight into the efficacy of various components of vestibular rehabilitation interventions[38,39]. So far, we have not implemented such a demanding vestibular rehabilitation program in the hospital, and we would like to believe that the application of this type of high-level balance intervention during gait in patients could be useful in reducing the severity and minimizing the impact of their balance symptoms. Usually, physiotherapists treat patient’s balance disorders in the acute hospital stage according to their methods and concepts, without implemented complex functional tasks, during gait. In patient’s implementation of functional training in the early postoperative period, we try to eliminate, reduce or prevent defects of systems, that are important to the balance; develop an active balance-specific movement, sensory and cognitive strategy; and practice functional tasks by changing the complexity of posture tasks during standing and walking[40]. The regular hospital rehabilitation program does not implement various postural tasks included in the FGA, such as head-eye movements with various body postures and activities, and maintaining balance with a support base reduced with various orientations of the head and trunk, while performing gait and exposing patients gradually to various sensory and motor environments[41,42].
The purpose of this pilot study was to determine patients’ balance disorders, their susceptibility to falls, and to investigate the acceptability of the BBS by reporting its score distribution (mean/mode score, minimal and maximal score). We also want to determine the adequacy of the FGA scale and its challenging tasks in patients after vestibular surgery during hospitalization.
A prospective nonrandomized study involving 10 patients admitted to the Department of Neurosurgery in Ljubljana from January 2016 to June 2017 for planned vestibular schwannoma surgery was conducted.
All patients underwent surgery using the retrosigmoid suboccipital approach, which is used in our clinical department. Before the operation, the patients were placed in the contralateral park-bench position and draped and prepped. The monitoring electrodes for all three facial branches were placed. The surgical approach then included osteoplastic or osteoclastic trepanation with exposure of the dura and transverse and sigmoid sinuses, and the bone was drilled over the sigmoid sinus to expose the mastoid air cells, which were sealed with bone wax. After relieving the cerebrospinal fluid (CSF) pressure through the opening of the cisterna magna, the tumor was visualized and devascularisation and detachment followed. In addition to the classical operative technique, an ultrasonic aspirator was also used. Monitoring was continuously carried out during the operation, to identify the facial nerve. In all cases, it was not possible to preserve the acoustic nerve, which was embedded in the tumor and destroyed. The facial nerve was morphologically preserved. No postoperative complications were observed such as CSF leak, wound healing complications, or bone infection[6,7]. In all patients, tumor removal was complete.
The following inclusion criteria were taken into account: (1) Patient’s first surgery of the cerebellopontine angle; (2) Ability to follow instructions; (3) Ability to participate (achieving at least 25 out of 30 possible in the Mini-Mental State Examination [MMSE]); and (4) Ability to walk the distance of 6 m without excessive fatigue with the help of a physiotherapist or a suitable walking aid (achieving at least 8 out of 20 possible in Barthel Index [BI])[28,34]. The MMSE and BI were carried out on the second day after surgery. Patients with pre-existing hemiplegia, paraplegia or paraparesis; previous head injury or lower limb damage; those with cardiorespiratory or febrile conditions and blind patients were excluded from the study.
In the first postoperative days, patients experienced dizziness, headache, and nausea. Before vestibular therapy, nausea, vomiting, and vertigo were prevented or reduced by medication, such as antiemetics or anti-vertigo drugs. According to a previous report[43], vestibular therapy must proceed from the head to locomotion in a top-down strategy; thus, the patients first performed Cawthorne-Cooksey (CC) exercises, which involved eye and head movements in the lying, sitting and standing position like as shown in (Table 1). The CC exercises are a graduated set of simple exercises to reduce dizziness and restore the patient’ balance. They can also help to reduce sensitivity to motion, relax the neck and shoulder muscles, train the eyes to move independently of the head, practice the head movements that cause dizziness, help in the development of vestibular compensation, improve general coordination and encourage natural unprompted movement. The exercises were originally developed to help compensate for stable vestibular loss in one ear, such as following acoustic neuroma surgery. The purpose of these exercises was to build up a tolerance mechanism and the more diligently and regularly they were carried out by the patients, the sooner their symptoms would disappear[44]. In the beginning, all ten patients performed CC exercises three times a day for 10 min, with their eyes open and slow head movements in a supine position in bed, and only when they felt safe in a sitting and standing position, they moved to the next level of performing more challenging tasks involving dynamic stability of the body and limb movement. They then progressed to the next level with quick eye and head movements with their eyes closed. Patients reported mild to moderate discomfort while performing the exercises, also because of their awareness to endure dizziness. On the first day after surgery, we assisted patients in the exercises, and in the following days, they performed the exercises in the form of written instructions independently.
| In bed–supine position | Sitting position | Standing position |
| Eye movements, head immobile, at first slow, then quick | ||
| Up and down (1) | Repeat as the previous section | Repeat as in the previous section |
| Side to side (2) | Repeat as the previous section | Repeat as in the previous section |
| Repeat (1) and (2), focusing on finger | Shrug shoulders and rotate | Repeat as in the previous section |
| Focusing on the finger, moving about 3 feet to 2 inches away and back | Bend forward and pick up objects from the ground | Change from a sit to stand position with eyes open, then shut |
| Head movements, at first slow then quick, later with eyes closed | ||
| Bending forward and backward | Rotate head and shoulders slowly, then fast; first with eyes open, then closed | Throw the ball from hand to hand (above eye level) |
| Turn side to side | Rotate head, shoulders, and trunk with eyes open, then closed | Throw the ball from hand to hand under knees |
| Change from sitting to standing and turn around in between |
On the second day after surgery, when patients were able to walk the distance of 6 m (with the assistance of a physiotherapist or a suitable walking aid), the initial assessment of the patients’ static and dynamic balance in the sitting and standing position was carried out using the BBS. On the day of discharge from hospital and three months after surgery, patients were re-tested using the BBS.
Based on the initial assessments of balance in the sitting and standing positions, the level of patients’ balance impairments and the risk of falls were determined. Patients were divided into two groups according to the initial BBS results and their level of equilibrium impairment. Patients in the first group were those who reached a BBS value ranging from 21 to 40 points and had a truncated but acceptable balance; thus, they needed help during basic daily activities. These patients were able to walk only with a walking aid and the help of the physiotherapist. Patients in the second group included those with 41 or more points on the BBS and were independent during a short gait distance. However, postural instability and dynamic balance disorders were detected in patients performing functional gait. As none of the patients had an initial BBS score of more than 45 points, all patients were at risk for falls. They also completed the BBS assessment in full, with all the required tasks. Based on the BBS initial scoring, patients were included in a specially designed exercise protocol, with an emphasized requirement for postural adjustments and dynamic balance, while performing functional gait. The exercise protocol included 10 gait-related FGA activities and was organized as a 50 m marked polygon in the hallway section of the neurosurgery department, twice a day for 20 min with 5 repetitions of each functional item during a two-week hospital recovery period. The first group of exercises emphasized rotary head movement and body axis rotation; the second group emphasized a resizing of the support surface and stabilization of the body; the third group of exercises required a reduced proprioceptive inflow. All patients were motivated and focused on performing more demanding postural activities during exercise. No one suffered a fall, although the group with the poorer balance used a walking aid and physiotherapist assistance during functional gait. Patients in the second group were able to perform functional gait tasks independently under the therapist’s supervision. Table 2 shows the functional FGA activities, performed by the patients in the first and second groups. The order of function tasks shown is as it was during exercise[41,42].
| Type of exercises | Purpose of the exercises |
| Walking with a glass full of water | Double attention |
| Walking with a change of speed | Stabilization of the body with a reduced support surface |
| Turning around its axis | Vestibular-ocular stabilization |
| Walking by turning the head in a horizontal plane (left/right) | Vestibular-ocular stabilization during walking |
| Walking by tilting the head in a vertical plane (up/down) | Vestibular-ocular stabilization during walking |
| Walking over obstacles | Thesis transfer and depth assessment |
| Walking backward | Decreased proprioceptive inflow |
| Walking with eyes closed | Decreased proprioceptive inflow |
| Tandem walking or walking heel toes forward | Reduced support surface |
| Walking up and downstairs | Thesis transfer and depth assessment |
On patients’ discharge from hospital to the home environment, they received relevant and thorough instructions on how to perform balance exercises at home in both printed and multimedia DVD format. The video clip was recorded outdoors-by the sea to bring the daily implementation of balance activities closer to the patient. Balance exercises for the home environment were composed of three sets; in a sitting and standing position and during gait. Each balance set consisted of 10 progressively challenging postural activities with five repetitions and progressed from stable positions to position changes and finally to upright positions, with various maneuvers to challenge balance, with eyes closed, feet together, picking up an object, turning, alternate stepping, narrowed base of support and functional gait. In addition, they were asked to fill in a specially designed logbook of balance exercise and a logbook of falls to provide feedback on the execution of therapeutic exercises and the potential occurrence of falls. Three months after surgery, all participating patients were invited for re-testing using the BBS.
The research was designed as a pilot study and will serve as a base for further research with a larger number of patients with the same diagnosis and a group of control patients with balance disorders. The patients will be treated by physiotherapists according to their concepts and methods, without challenging tasks during functional gait and their BBS results recorded. The BBS score the day before the operation will also be added as a control for all patients in the acute stage. The Medical Ethics Committee of the Republic of Slovenia approved the research design.
Descriptive statistics were calculated for the variables considered. The non-parametric Wilcoxon test for paired samples was used to compare the mean value of the BBS score at admission and discharge and three months thereafter. The statistical significance was set at P = 0.05. We used the R studio package (R version 3.6.1.).
Ten patients who underwent schwannoma surgery were included in the study: Six females and four males aged between 18 and 57 years (average 39.5 years). The average hospital stay was 10.5 d (minimum of 7 d, maximum 14 d).
Five patients had left-sided impairment, four had right-sided impairment and one patient did not exhibit clear lateralization of symptoms. The visual and auditory systems were affected in all participants: Five of them had suffered left-sided facial paralysis and deafness in the left ear, four had suffered right-sided facial paralysis, and deafness in the right ear, whereas one patient had symptoms of double vision and partial deafness in the left ear. The gustatory, olfactory, tactile, and vestibular systems were partially affected in all patients. Vestibular impairment in these patients consisted of disturbances in detecting changes in direction and speed of the head movement as well as changes in body position; acceleration, inhibition. Tactile impairment was shown to be a changed sense of touch, pain, pressure, and temperature. All patients had difficulties with swallowing, asymmetry in facial mimic, and speech problems as speech was not fluent and incomprehensible. Two patients experienced impairment of the proprioceptive system, and had trouble identifying the position and movement of the body or its parts. Despite all recorded sensory deficits, none of the patients reported any problems during testing and all of them completed the tests. The only symptom reported was early fatigue.
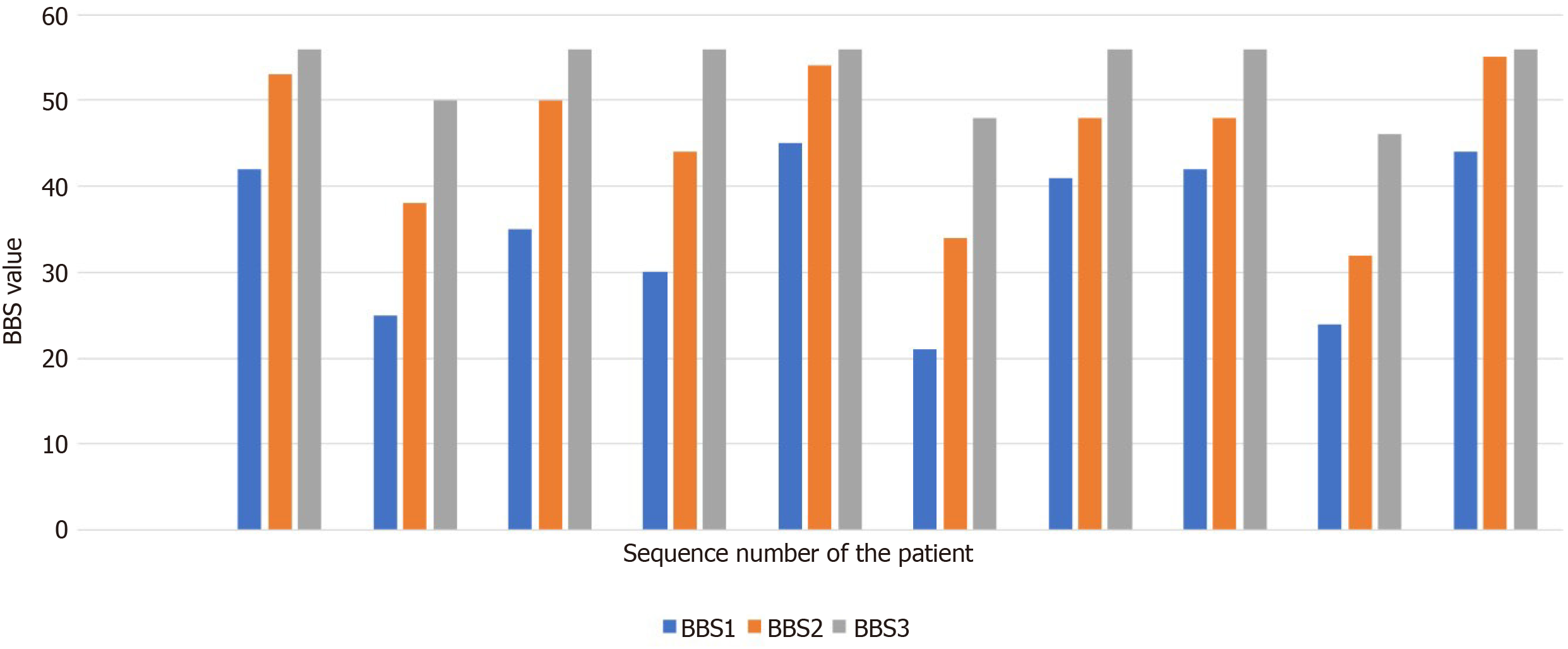
During the first BBS assessment, on the second day after surgery, none of the ten patients were able to perform gait without an adequate walking aid and they all needed help. Five patients who scored an initial BBS assessment ranging from 41 to 44 points required therapeutic readiness or supervision during gait; two patients needed an adequate walking aid with therapeutic supervision and scored 31 to 40 points on the BBS and; three patients scored 21 to 30 points on the BBS and needed a walking aid and help from the therapist during gait.
None of the patients exceeded the 46-point fall limit, although everyone was at risk for falls. After patient discharge from hospital to the home environment, six patients were able to perform gait without a walking aid; two needed supervision by the therapist and a suitable walking aid and two required an adequate walking aid and assistance from the therapist. Four patients were at risk for falls, and six exceeded the fall limit. Three months after surgery, functional gait in eight patients was independent and they were not at risk for falls, two patients still needed supervision during gait and were at risk for falls. Table 3 shows the use of walking aids, assistance by the therapist, and supervision during patients’ gait on the first BBS evaluation (the fourth day after surgery), the second BBS assessment (at discharge to the home environment), and the third BBS assessment (three months after surgery).
| Walking aid | First assessment (on the second day of surgery) | Second assessment (at discharge from hospital) | Third assessment (three months after surgery) |
| Without walking aid | 0 | 6 | 8 |
| Supervision | 5 | 0 | 2 |
| Walking aid and supervision | 2 | 2 | 0 |
| Walking aid and therapist assistance | 3 | 2 | 0 |
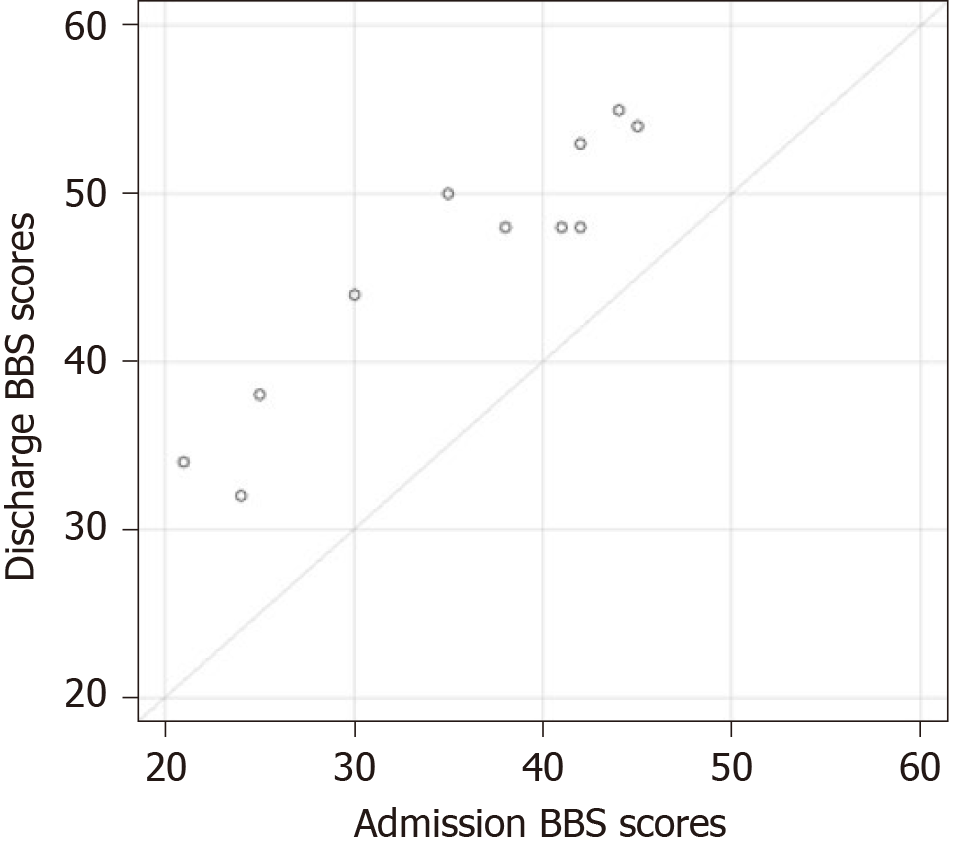
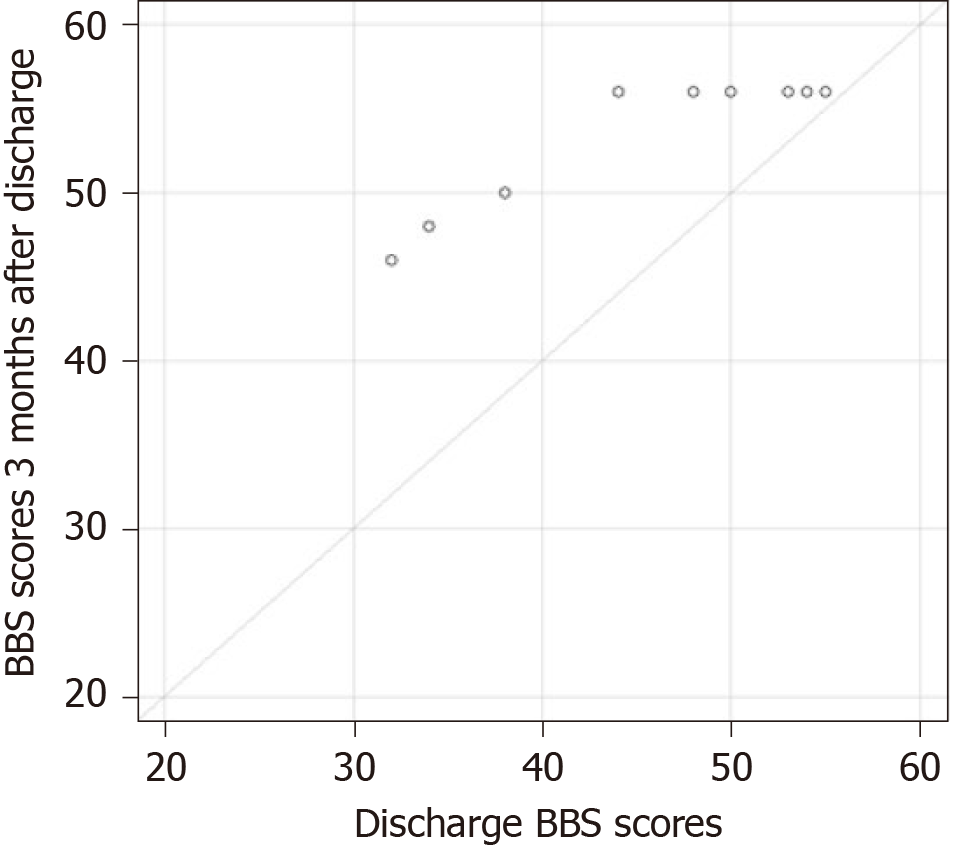
Figure 1 shows the balance assessment using the BBS in each of the ten patients, during the first assessment (on the fourth day after surgery), second assessment (at discharge from the hospital), and third assessment (three months after surgery). In all patients, the progression assessment was statistically significant, both at the time of rehabilitation (median = 11; P = 0.0059) and three months after discharge (median = 8; P = 0.0058). The median progression was not statistically different (P = 0.2012). Figure 2 shows a flow chart of the progress of BBS assessment during rehabilitation treatment. Figure 3 is a diagram of the progress of BBS assessment three months after discharge from the hospital.
In the early postoperative period, patients who have undergone vestibular tumor surgery experience balance impairments and postural instability, which are the main predictors of quality of life and have been shown to increase fall risk[45]. The most commonly used functional clinical assessment in patients with vestibular loss is the BBS. If the balance deficits in patients are identified early, the determination of appropriate vestibular treatment and effective strategies to prevent or decrease the negative effects of postural instability can be provided[46,47].
The purpose of our pilot study was to evaluate the improvement of functional balance in each patient and thus reduce the risk of falls in the hospital through the application of functional FGA exercises[48], which were implemented as part of the vestibular rehabilitation program to regain patients’ ability to walk safely and to mimic their daily activities. Each movement challenging task emphasizes while performing functional gait, different aspects of the balance characteristics of patients with vestibular loss[49,50]. The vestibular rehabilitation interventions should be initiated in the early postoperative period of patients’ recovery, as this critical plastic time window is characterized by major structural and functional changes. The efficacy of VRT might benefit from coinciding with the early stage of plastic event expression and may contribute to stabilizing, guiding, and shaping the newly formed functional connections in the deafferented vestibular nuclei and associated neuronal networks. This is in agreement with the top-down approach to vestibular compensation proposed by Balaban et al[51]. Adaptation, as a recovery mechanism of VRT, was used in our study and requires a dynamic interaction between the patient and the environment. This means that adaptation is a qualitative variation of the response, resulting from positive learning[52]. We can select a new reference frame for posture control and orientation perception and this can be done by re-weighting of the remaining sensory cues[53]. The lost dynamic vestibular function in patients can be replaced by new behavioral strategies involving several neuronal networks distributed in the brain, which reorganize to mimic the lost function[54]. Transferring newly learned skills with FGA functional gait training from the hospital into daily life is easier and faster if exercises are as close as possible to daily activities[55]. All patients in the study were motivated, fully focused, and were able to complete the FGA items. Ensuring patient safety during functional FGA activities was our major concern during treatment assessment[56,57].
The statistical analysis in our study showed a statistically significant functional balance improvement on the BBS scale at discharge from hospital (median = 11; P = 0.0059) and three months after discharge from hospital to the home environment (median = 8; P = 0.0058) in this sample of patients. This statistical analysis confirmed our hypothesis of the BBS scale being an appropriate functional balance assessment test in the early rehabilitation treatment, as it is also highly sensitive to changes that occur during rehabilitation programs and provides an adequate assessment of patients’ postural instability.
In the initial BBS assessment, 30% of patients had very poor balance (BBS value from 21 to 30) and needed the help of a therapist and a walking aid during gait; 20% of patients had a truncated, but acceptable balance (BBS value from 31 to 40) and needed therapist supervision and an appropriate walking aid, and 50% of patients had good balance (BBS value from 41 to 45) and were independent during gait under the supervision of a physical therapist. All were at risk for falls. According to the initial BBS assessment, patients had difficulties in changing positions (such as positions with a smaller base of support or higher center of gravity) or in more challenging situations (such as increased range of motion or speed of movements). Patients with balance disorders require comprehensive assistance or more time to complete daily activities, thus they reduce the speed of performing daily tasks[45]. None of the patients were able to perform the most challenging items in the BBS, such as the "tandem stance", "one-legged stance", "alternating foot" and "look behind" items. The challenging items identified will help us better understand patients’ balance decline sequence and guide screening and intervention programs[47].
The specificity of the chosen exercises was based on the principle of the autonomous phase of motor learning, the ability to focus on the influx from the proprioceptive system and the ability to implement motion strategies that, after a period of longer repetition, would almost completely become automatic, with a minimum degree of cognitive control. We predicted that patients would be able to transfer these newly learned movement strategies from exercise to daily life[55-57]. To achieve physical well-being we also advised them to take on some recreational activities such as dancing, yoga, or walking. Before discharge, the patients’ family was acquainted with information on internal and environmental factors important for fall prevention. They were given instructions, both in written and multimedia format (DVD), regarding balance-improving exercises in the sitting and standing position and during gait. Additionally, they were asked to keep a record of home performing FGA exercises and all the falls in specially designed logs in their home environment. Before patients were discharged to the home environment, we detected a clinically significant change in improving balance in all 10 patients. According to the second BBS evaluation, 60% of patients scored 48 to 55 points and were independent during gait and not at risk for falls; 40% of patients scored 32 to 44 points on the BBS; they were at risk for falls and during gait needed a suitable walking aid and the therapist’s assistance or supervision. Thus, 60% of patients evaluated using the BBS had high scores, which indicated the effect of the ceiling of the BBS scale. A ceiling effect occurs when the highest score on the scale does not capture or discriminate between differences in the upper and the attribute being measured[46,47]. Patients with the highest BBS scores were able to perform the most challenging BBS items such as tandem stance and one-legged stance.
Three months after discharge, according to the BBS estimate, 80% of patients had the highest score and a ceiling effect, which shows that the BBS functional test was not challenging enough to assess balance sufficiently and detect balance impairments across the full spectrum of patients with vestibular loss[57,58]. According to Steffen and Seney[59,60], the BBS score must change by at least 5 points to show a true change in balance. Therefore, 30% of patients after discharge and 70% of patients three months after discharge in our study were evaluated to have more than 52 points and were unable to show any progress in balance using the BBS. This lack of ability to identify balance impairments in patients with vestibular disorders three months after discharge may prevent important further intervention measures.
The BBS includes standing up, turning around and bending over, tandem stance, and one-legged stance, which are the most common ways falls occur in people after vestibular loss[46,47]. However, unlike the situations where falls occur, the BBS items allow full attention to be allocated to these tasks, possibly missing those who would lose their balance under nontested circumstances. It has been shown that attention allocation can drastically change balance deficits in patients after vestibular loss, whether it is a dual-task situation or the individual perceives that he or she is being observed[47]. The patients were fully focused and many were able to complete the BBS items, although these same tasks are problematic under normal circumstances.
This encouraged us to develop a special hospital rehabilitation program with FGA postural adjustments included while performing functional gait and can also have an improved impact on the more static items in the BBS.
Statistical analysis also showed that the median progression in balance after BBS did not differ significantly at the time of hospitalization and after discharge (P = 0.2012). This is the subject of further research with a control group of patients and, above all, a larger group of patients with vestibular disorders. We are not convinced of the significant impact on the progression of improvement of functional balance by the specifically designed FGA postural tasks in the home environment and the impact of normal recovery in patients after vestibular surgery.
Implemented multitasking FGA exercises, as a part of the vestibular rehabilitation program for functional gait, proved helpful in the acute period of hospital stay as the functional independence of patients while engaging in basic daily activities improved at the time of discharge and three months after discharge. The BBS is considered the reference standard for the determination of fall risk and evaluation of balance impairments in patients after vestibular tumor surgery. The FGA dynamic gait items were used as a part of the vestibular rehabilitation program to improve the patients’ static BBS items and to determine their internal and critical external risk factors for falls. However, given the small number of cases and lack of a control group, we cannot with certainty conclude that the FGA Scale tasks provide sufficient positive effects on early-stage rehabilitation in patients after vestibular tumor surgery. We believe future research with a larger sample size and the inclusion of a control group would provide an insight into the effects of the implementation of the FGA scale tasks on early-stage rehabilitation programs and might also provide useful information on the guidelines for early stage rehabilitation in patients after vestibular tumor surgery.
Patients in the acute phase of rehabilitation after vestibular tumor surgery usually experience vertigo, nausea, and a range of symptoms that include deficits in gaze stability, mobility, and balance. In addition to these problems, secondary problems such as vomiting, fatigue and reduced ability to focus or concentrate can occur. These symptoms can diminish the quality of life and impact all aspects of daily living and contribute to emotional problems sometimes causing anxiety or depression. Due to these symptoms, relatively short hospitalization, and the fact that they are left on their own, without the possibility of rehabilitation treatment, there is a need for such a vestibular rehabilitation treatment, including specific Functional Gait Assessment (FGA) activities during walking, and should be initiated in the hospital.
The purpose of our pilot study was to evaluate the improvement in functional balance in each patient and thus reduce the risk of falls. Experimental functional FGA exercises were chosen as a part of the vestibular rehabilitation program to regain patients’ ability to walk safely and to mimic their daily activities. Furthermore, this topic was chosen as the regular hospital rehabilitation program does not implement various postural tasks included in the FGA. We believe future research should focus on attempting to develop the most effective vestibular rehabilitation program for patients with vestibular loss in the early postoperative period.
The main goals of this study were to improve dynamic performance, to determine patients’ balance disorders; their susceptibility to falls, and to investigate the acceptability of the Berg Balance Scale (BBS) by reporting its score distribution. Most of the patients in the study, evaluated with the BBS showed statistically significant clinical progress in functional activities of daily living. The routine collection and reporting of BBS balance outcomes before and after surgery should be considered for all patients with vestibular loss, given the profound impact on the overall quality of life and for further quality studies in this area.
Our study was conducted at the Department of Neurosurgery in Ljubljana between January 2016 and June 2017. We included patients who underwent vestibular schwannoma surgery and scored higher than 25 points on the Mini-Mental State Examination and higher than 8 points on the Barthel index during the initial examination. We used the BBS for evaluation on the second day after surgery, during their hospital stay, at discharge, and three months after surgery. A vestibular rehabilitation program, focusing on multiple motor tasks included in the FGA scale was formed and implemented in the acute stage of rehabilitation. Before discharge, the patients were provided with instructions regarding balance-improving exercises in sitting and standing positions and during gait. Additionally, they were asked to keep a record of FGA-based exercise performance as well as falls in their home environment. Descriptive statistics were used for the variables considered. The non-parametric Wilcoxon test for paired samples was used to compare the mean value of the BBS scale at admission and discharge and three months after discharge. The statistical significance was set at P = 0.05. We used the R studio package (R version 3.6.1.).
Ten patients were included in the research: Six females and four males aged between 18 and 57 years (average 39.5 years). The average hospital stay was 10.5 d (minimum of 7 d, maximum 14 d). Analysis showed a statistically significant improvement in functional balance on the BBS scale at discharge from hospital (median = 11; P = 0.0059) and three months after discharge from hospital (median = 8; P = 0.0058) in these patients. The statistical analysis confirmed our hypothesis that the BBS scale is an appropriate functional balance assessment test in early rehabilitation, as it is also highly sensitive to changes that occur during rehabilitation programs and provides an adequate assessment of patients' postural instability. Statistical analysis also showed that the median progression in balance after BBS did not significantly differ at the time of hospitalization and after discharge (P = 0.2012). Larger numbers and more homogeneous cases in potential future studies would confirm or refute the results of our study.
Multitasking FGA exercises, as a part of the vestibular rehabilitation program for functional gait, proved helpful in the acute postoperative period as the functional independence of patients improved at the time of discharge and three months after discharge. However, given the small number of cases and lack of a control group, we cannot with certainty conclude that FGA scale tasks provide sufficient positive effects on early-stage rehabilitation in patients after vestibular tumor surgery. There are several key priorities for future research. Randomized controlled trials with larger sample sizes and more rigorous methodologies are needed to investigate the effects of each motor element of the FGA scale. Investigation of FGA intervention parameters, based on routine collection of balance outcomes, the timing and duration of FGA interventions, and the minimum or optimal "dosage" requirement to achieve effectiveness should be considered. The effects of education and social reinforcement also require further investigation.
Our study may be a good baseline for further research and to establish the Slovenian rehabilitation register for patients after surgical removal of vestibular tumors. The register should include a valid rehabilitation protocol for all Slovenian hospitals in primary care.
| 1. | Peng KA, Chen BS, Lorenz MB, Lekovic GP, Schwartz MS, Slattery WH, Wilkinson EP. Revision Surgery for Vestibular Schwannomas. J Neurol Surg B Skull Base. 2018;79:528-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Sheth SA, Tirino JL, Martuza RL. Vestibular schwannoma: suboccipital approach. Neurosurg Focus. 2014;36:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Charabi S, Tos M, Thomsen J, Charabi B, Mantoni M. Vestibular schwannoma growth: the continuing controversy. Laryngoscope. 2000;110:1720-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Hillman TA, Chen DA, Fuhrer R. An alternative treatment for facial nerve tumors: short-term results of radiotherapy. Ear Nose Throat J. 2008;87:574-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Rahman MA, Hafiz A, Islam KT, Mitra PK, Mahmud E, Barua KK. Surgical outcomes of cerebellopontine angle tumors in 48 Cases. Bangladesh J Neurosurg. 2020;9:117-220. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Perneczky A, Reisch R. Retrosigmoidal approach. In: Tschabischer M. Keyhole approaches in neurosurgery: concept and surgical technique. Volume 1. New York: Springer Wien, 2008: 159-178. |
| 7. | Cohen HS. A review on screening tests for vestibular disorders. J Neurophysiol. 2019;122:81-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Curthoys IA, Halmagy GM. Vestibular compensation-recovery after unilateral vestibular loss. In: Herdman SJ, Clendaniel RA. Vestibular Rehabilitation: contemporary perspectives in rehabilitation. 4th ed Pennsylvania: F.A. Davis Company, 2014: 50-58. |
| 9. | Renga V. Clinical Evaluation of Patients with Vestibular Dysfunction. Neurol Res Int. 2019;2019:3931548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Smith PF, Zheng Y. From ear to uncertainty: vestibular contributions to cognitive function. Front Integr Neurosci. 2013;7:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Furman JM, Whitney SL. Central causes of dizziness. Phys Ther. 2000;80:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Brainard A, Gresham C. Prevention and treatment of motion sickness. Am Fam Physician. 2014;90:41-46. [PubMed] |
| 13. | Swartz R, Longwell P. Treatment of vertigo. Am Fam Physician. 2005;71:1115-1122. [PubMed] |
| 14. | Dieterich M, Brandt T. Vestibular lesions of central vestibular pathways. In: Herdman SJ, Clendaniel RA. Vestibular Rehabilitation: contemporary perspectives in rehabilitation. 4th ed. Pennsylvania: F.A. Davis Company, 2014: 59-84. |
| 15. | Keshner EA, Galgon AK. Postural abnormalities in vestibular disorders. In: Herdman SJ, Clendaniel RA. Vestibular Rehabilitation: contemporary perspectives in rehabilitation. 4th ed. Pennsylvania: F.A. Davis Company, 2014: 85-109. |
| 16. | Herdman SJ. Vestibular rehabilitation. Curr Opin Neurol. 2013;26:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Ojha S, Clamp PJ. A Systematic Review of Interventions for Balance Dysfunction in Patients With Vestibular Schwannoma. Otol Neurotol. 2020;41:e295-e303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Stineman MG, Kwong PL, Kurichi JE, Prvu-Bettger JA, Vogel WB, Maislin G, Bates BE, Reker DM. The effectiveness of inpatient rehabilitation in the acute postoperative phase of care after transtibial or transfemoral amputation: study of an integrated health care delivery system. Arch Phys Med Rehabil. 2008;89:1863-1872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Horak FB. Postural compensation for vestibular loss and implications for rehabilitation. Restor Neurol Neurosci. 2010;28:57-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Pavlou M, Lingeswaran A, Davies RA, Gresty MA, Bronstein AM. Simulator based rehabilitation in refractory dizziness. J Neurol. 2004;251:983-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Pavlou M, Quinn C, Murray K, Spyridakou C, Faldon M, Bronstein AM. The effect of repeated visual motion stimuli on visual dependence and postural control in normal subjects. Gait Posture. 2011;33:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Yelnik A, Bonan I. Clinical tools for assessing balance disorders. Neurophysiol Clin. 2008;38:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm (Vienna). 2007;114:1339-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 504] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 24. | Wrisley DM, Marchetti GF, Kuharsky DK, Whitney SL. Reliability, internal consistency, and validity of data obtained with the functional gait assessment. Phys Ther. 2004;84:906-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 405] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 25. | Leddy AL, Crowner BE, Earhart GM. Functional gait assessment and balance evaluation system test: reliability, validity, sensitivity, and specificity for identifying individuals with Parkinson disease who fall. Phys Ther. 2011;91:102-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 219] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 26. | Lord SR, Menz HB, Sherrington C. Home environment risk factors for falls in older people and the efficacy of home modifications. Age Ageing. 2006;35 Suppl 2:ii55-ii59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 159] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Norris D, Clark MS, Shipley S. The Mental Status Examination. Am Fam Physician. 2016;94:635-641. [PubMed] |
| 28. | Maravita A, Spence C, Driver J. Multisensory integration and the body schema: close to hand and within reach. Curr Biol. 2003;13:R531-R539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 351] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 29. | Levin MF, Panturin E. Sensorimotor integration for functional recovery and the Bobath approach. Motor Control. 2011;15:285-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Katz S, Arish N, Rokach A, Zaltzman Y, Marcus EL. The effect of body position on pulmonary function: a systematic review. BMC Pulm Med. 2018;18:159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 31. | Rothwell JC, Rosenkranz K. Role of afferent input in motor organization in health and disease. IEEE Eng Med Biol Mag. 2005;24:40-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Parker AM, Lord RK, Needham DM. Increasing the dose of acute rehabilitation: is there a benefit? BMC Med. 2013;11:199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | MAHONEY FI, BARTHEL DW. FUNCTIONAL EVALUATION: THE BARTHEL INDEX. Md State Med J. 1965;14:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 438] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 34. | Tyson SF, Connell LA. How to measure balance in clinical practice. A systematic review of the psychometrics and clinical utility of measures of balance activity for neurological conditions. Clin Rehabil. 2009;23:824-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 35. | Mancini M, Horak FB. The relevance of clinical balance assessment tools to differentiate balance deficits. Eur J Phys Rehabil Med. 2010;46:239-248. [PubMed] |
| 36. | Lima CA, Ricci NA, Nogueira EC, Perracini MR. The Berg Balance Scale as a clinical screening tool to predict fall risk in older adults: a systematic review. Physiotherapy. 2018;104:383-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 37. | Chae R, Mcdermott M, Muacevic A, Adler JR, Sharon JD. Vestibular migraine following radiosurgery for vestibular schwannoma. Cureus. 2020;12:e8569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Vereeck L, Wuyts FL, Truijen S, De Valck C, Van de Heyning PH. The effect of early customized vestibular rehabilitation on balance after acoustic neuroma resection. Clin Rehabil. 2008;22:698-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 39. | Tsuchiya A, Ora H, Hao Q, Ono Y, Sato H, Kameda K, Miyake Y. Body Movement Synchrony Predicts Degrees of Information Exchange in a Natural Conversation. Front Psychol. 2020;11:817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Zijlstra A, Mancini M, Chiari L, Zijlstra W. Biofeedback for training balance and mobility tasks in older populations: a systematic review. J Neuroeng Rehabil. 2010;7:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 41. | Han BI, Song HS, Kim JS. Vestibular rehabilitation therapy: review of indications, mechanisms, and key exercises. J Clin Neurol. 2011;7:184-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 42. | Whitney SL, Sparto PJ. Principles of vestibular physical therapy rehabilitation. NeuroRehabilitation. 2011;29:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 43. | Hain TC. Neurophysiology of vestibular rehabilitation. NeuroRehabilitation. 2011;29:127-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Muir SW, Berg K, Chesworth B, Speechley M. Use of the Berg Balance Scale for predicting multiple falls in community-dwelling elderly people: a prospective study. Phys Ther. 2008;88:449-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 242] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 45. | Blum L, Korner-Bitensky N. Usefulness of the Berg Balance Scale in stroke rehabilitation: a systematic review. Phys Ther. 2008;88:559-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 774] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 46. | Alqahtani BA, Sparto PJ, Whitney SL, Greenspan SL, Perera S, Brach JS. Psychometric properties of instrumented postural sway measures recorded in community settings in independent living older adults. BMC Geriatr. 2020;20:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Beninato M, Fernandes A, Plummer LS. Minimal clinically important difference of the functional gait assessment in older adults. Phys Ther. 2014;94:1594-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 48. | Lin JH, Hsu MJ, Hsu HW, Wu HC, Hsieh CL. Psychometric comparisons of 3 functional ambulation measures for patients with stroke. Stroke. 2010;41:2021-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 49. | Pollock C, Eng J, Garland S. Clinical measurement of walking balance in people post stroke: a systematic review. Clin Rehabil. 2011;25:693-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 50. | Balaban CD, Hoffer ME, Gottshall KR. Top-down approach to vestibular compensation: translational lessons from vestibular rehabilitation. Brain Res. 2012;1482:101-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 51. | Lacour M, Helmchen C, Vidal PP. Vestibular compensation: the neuro-otologist's best friend. J Neurol. 2016;263 Suppl 1:S54-S64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 198] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 52. | Borel L, Lopez C, Péruch P, Lacour M. Vestibular syndrome: a change in internal spatial representation. Neurophysiol Clin. 2008;38:375-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 53. | Macdougall HG, Curthoys IS. Plasticity during Vestibular Compensation: The Role of Saccades. Front Neurol. 2012;3:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 54. | Thieme H, Ritschel C, Zange C. Reliability and validity of the functional gait assessment (German version) in subacute stroke patients. Arch Phys Med Rehabil. 2009;90:1565-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Walker ML, Austin AG, Banke GM, Foxx SR, Gaetano L, Gardner LA, McElhiney J, Morris K, Penn L. Reference group data for the functional gait assessment. Phys Ther. 2007;87:1468-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 56. | Kornetti DL, Fritz SL, Chiu YP, Light KE, Velozo CA. Rating scale analysis of the Berg Balance Scale. Arch Phys Med Rehabil. 2004;85:1128-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 57. | Haley SM, Fragala-Pinkham MA. Interpreting change scores of tests and measures used in physical therapy. Phys Ther. 2006;86:735-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 699] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 58. | Steffen T, Seney M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item short-form health survey, and the unified Parkinson disease rating scale in people with parkinsonism. Phys Ther. 2008;88:733-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 606] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 59. | Boulgarides LK, McGinty SM, Willett JA, Barnes CW. Use of clinical and impairment-based tests to predict falls by community-dwelling older adults. Phys Ther. 2003;83:328-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 221] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 60. | Van Holle V, Deforche B, Van Cauwenberg J, Goubert L, Maes L, Van de Weghe N, De Bourdeaudhuij I. Relationship between the physical environment and different domains of physical activity in European adults: a systematic review. BMC Public Health. 2012;12:807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 202] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Slovenia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lin JM S-Editor: Gao CC L-Editor: Webster JR P-Editor: Wang LL