©The Author(s) 2025.
World J Clin Oncol. Dec 24, 2025; 16(12): 111086
Published online Dec 24, 2025. doi: 10.5306/wjco.v16.i12.111086
Published online Dec 24, 2025. doi: 10.5306/wjco.v16.i12.111086
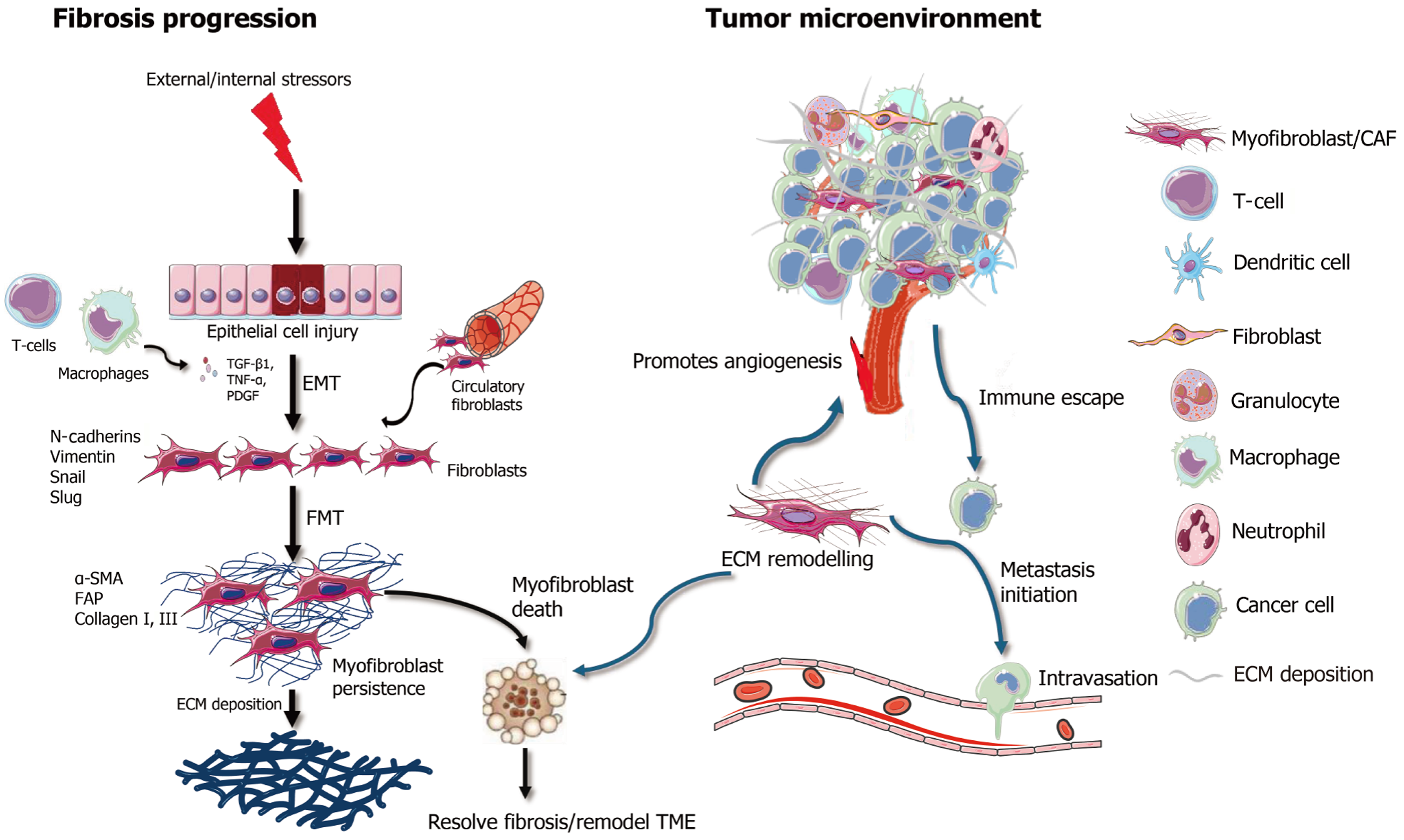
Figure 1 Myofibroblasts/cancer associated fibroblasts in fibrosis and tumor microenvironment.
Left panel: Upon external or internal stressors, epithelial cell injury triggers epithelial-to-mesenchymal transition, mediated by inflammatory cytokines (e.g., transforming growth factor, tumor necrosis factor, platelet derived growth factor) released by immune cells such as macrophages. This leads to fibroblast activation through fibroblast-to-myofibroblast transition, marked by the expression of α-smooth muscle actin, fibroblast activator protein, and collagen I/III, which contribute to excessive extracellular matrix deposition. Persistent myofibroblast activation leads to fibrosis, in contrast inducing myofibroblast death could resolve fibrosis. Right panel: Within the tumor microenvironment, cancer-associated myofibroblasts promote tumor progression by enhancing angiogenesis, supporting immune evasion, initiating metastasis, and remodeling the excessive extracellular matrix. The cellular interactions involve multiple components, including T cells, macrophages, dendritic cells, and cancer cells. Myofibroblast depletion or reprogramming may thus serve as a therapeutic strategy to mitigate fibrosis and recondition the tumor microenvironment. TGF-β1: Transforming growth factor; TNF-α: Tumor necrosis factor α; PDGF: Platelet derived growth factor; EMT: Epithelial-to-mesenchymal transition; FMT: Fibroblast-to-myofibroblast transition; α-SMA: α-smooth muscle actin; FAP: Fibroblast activator protein; TME: Tumor microenvironment; ECM: Excessive extracellular matrix; CAF: Cancer-associated myofibroblast.
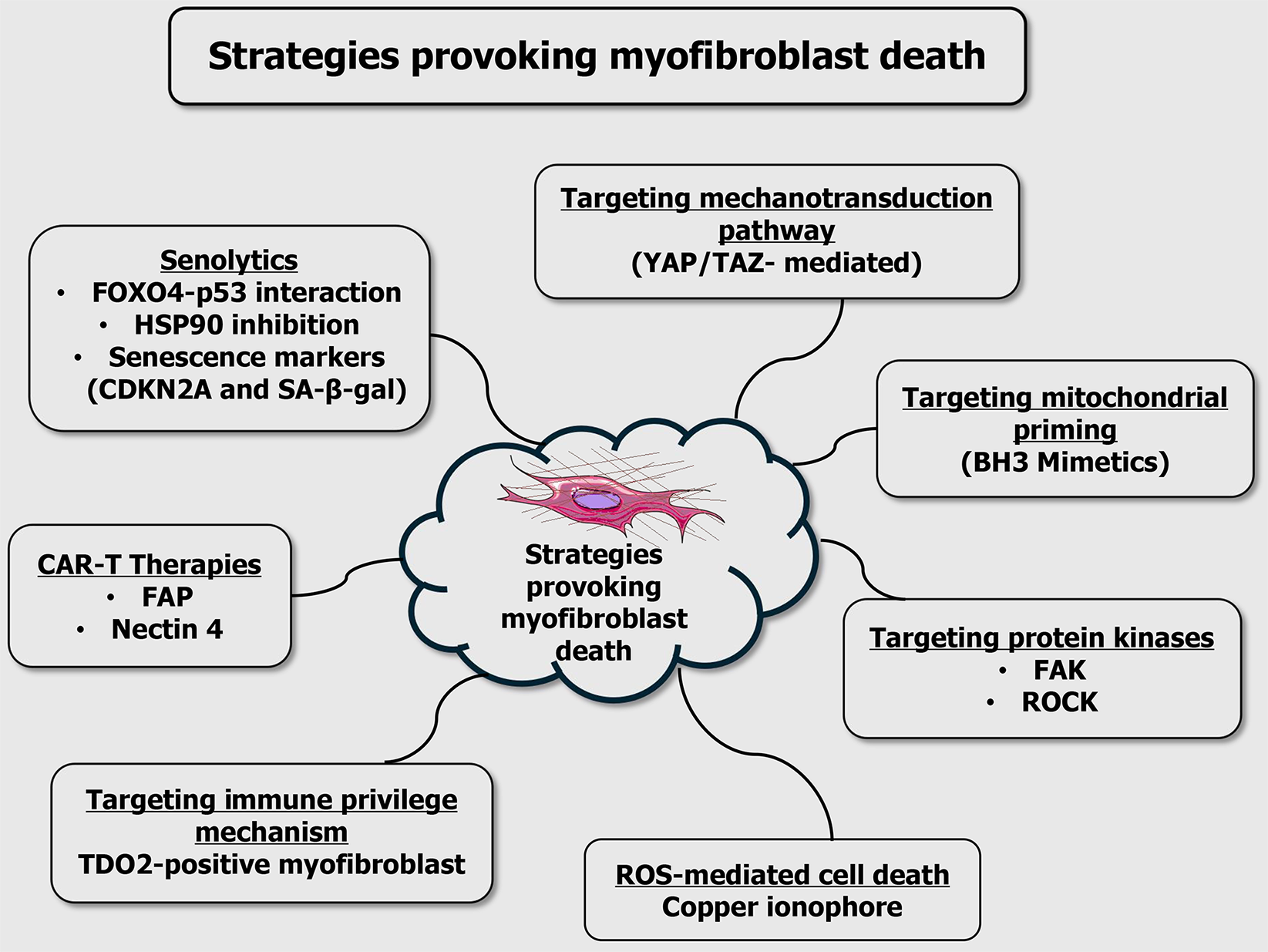
Figure 2 Strategies provoking myofibroblast death.
Key approaches include: (1) Senolytics: Elimination of senescent myofibroblasts by disrupting the forkhead box O4-p53 interaction, inhibiting heat shock protein 90, or targeting senescence markers like cyclin dependent kinase inhibitor 2A and senescence-associated β-galactosidase; (2) Chimeric antigen receptor T cell therapies: Engineered T cells targeting fibroblast-specific antigens such as fibroblast activation protein and nectin-4; (3) Immune privilege targeting: Disruption of immunosuppressive mechanisms, including tryptophan 2,3-dioxygenase-expressing myofibroblasts; (4) Mechanotransduction inhibition: Blocking Yes-associated protein and transcriptional coactivator with PDZ-binding motif signaling pathways that respond to extracellular matrix stiffness; (5) Mitochondrial priming: Sensitizing cells to apoptosis using BCL-2 homology 3 mimetics; (6) Protein kinase inhibition: Targeting focal adhesion kinase and Rho-associated coiled-coil containing protein kinase, which regulate cytoskeletal remodeling and survival signalling; and (7) Reactive oxygen species: Mediated cell death - induction of oxidative stress via agents such as copper ionophores. Collectively, these strategies offer promising avenues for the selective elimination of pathogenic myofibroblasts in fibrotic diseases and cancer. FOXO4: Forkhead box O4; HSP90: Heat shock protein 90; CDKN2A: Cyclin dependent kinase inhibitor 2A; SA-β-gal: Senescence-associated β-galactosidase; CAR-T: Chimeric antigen receptor T; FAP: Fibroblast activation protein; TDO2: Tryptophan 2,3-dioxygenase; YAP/TAZ: Yes-associated protein and transcriptional coactivator with PDZ-binding motif; BH3: B-cell lymphoma 2 homology 3; FAK: Focal adhesion kinase; ROCK: Rho-associated coiled-coil containing protein kinase; ROS: Reactive oxygen species.
- Citation: Shalini T, Sudhandiran G. Provoking myofibroblast death: Strategies to resolve fibrosis and remodel tumor microenvironment. World J Clin Oncol 2025; 16(12): 111086
- URL: https://www.wjgnet.com/2218-4333/full/v16/i12/111086.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i12.111086