Published online Mar 5, 2026. doi: 10.4292/wjgpt.v17.i1.113728
Revised: October 7, 2025
Accepted: December 3, 2025
Published online: March 5, 2026
Processing time: 162 Days and 20.3 Hours
Primary gastric lymphoma is relatively rare, accounting for 5% of primary gastric neoplasms, with the two most common lymphoma subtypes being diffuse large B-cell lymphoma and mucosa-associated lymphoid tissue lymphoma. Many patients with gastrointestinal lymphoma have a delayed presentation involving vague sy
We present a case of a 71-year-old male with disseminated primary gastric high-grade B-cell lymphoma with bony metastases. This was identified after the patient presented to the emergency department on three separate occasions with vague symptomatology, including chest, abdominal and back pain, and loose stools. Chest X-ray identified an atraumatic left rib fracture, and this was further explored with an outpatient computed tomography chest, which showed left fifth, seventh and eighth rib fractures, with ill-defined lucencies suspicious for underlying lesions. Computed tomography lumbar spine showed possible metastatic disease in the lumbar vertebrae. Positron emission tomography scan revealed non-specific mild to moderate heterogeneous uptake in the stomach, with disseminated, mixed, predominantly lytic bone metastases throughout the axial and appendicular skeletons. Gastroscopy subsequently identified multiple 10 mm to 20 mm semi-sessile polyps in the stomach, and biopsy confirmed high-grade “double-hit” B-cell lymphoma with melocytomatosis oncogene and BCL6 rearrangements. The patient was treated with rituximab, etoposide, prednisolone, vincristine, cyclophosphamide, doxorubicin and intrathecal methotrexate.
This case report highlights an uncommon presentation of high-grade primary gastric lymphoma and re-emphasises the aggressive nature of the disease.
Core Tip: “Double-hit” primary gastric high-grade B-cell lymphoma is a rare variant of B-cell lymphoma that can present quite aggressively and is associated with significant morbidity and potential mortality. Endoscopically, these lesions can present as a polypoid mass, ulceration or prominent mucosal folds, and, in the absence of clinical suspicion, can be easily missed. Timely diagnosis via endoscopy and treatment is important to ensure positive outcomes; however, the literature is limited with regard to the prognosis of “double-hit” primary gastric high-grade B-cell lymphoma compared to its nodal and extra-nodal counterparts.
- Citation: Fu MY, Jia K, Lee C, O'Neill RS, Thilakanathan C, Turner I. Double-hit primary high-grade gastric B-cell lymphoma presenting with pancytopaenia and atraumatic back pain: A case report. World J Gastrointest Pharmacol Ther 2026; 17(1): 113728
- URL: https://www.wjgnet.com/2150-5349/full/v17/i1/113728.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v17.i1.113728
Gastrointestinal lymphoma is a primary lymphoma of the gastrointestinal tract, which accounts for 1%-4% of all gastrointestinal malignancies[1]. Approximately 40% of extranodal lymphomas involve the gastrointestinal system[2]. The most common sites affected are the stomach, accounting for 65% of gastrointestinal lymphoma cases, and the duodenum, accounting for 20%[3]. Primary gastric lymphoma specifically accounts for 5% of primary gastric neoplasms, with the two most common subtypes of gastrointestinal lymphoma being diffuse large B-cell lymphoma (DLBCL) and mucosa-associated lymphoid tissue lymphoma[4]. Burkitt’s lymphoma, follicular lymphoma and mantle cell lymphoma are rarely seen in the gastrointestinal tract. T-cell/natural killer cell lymphomas are also less common, although an association between enteropathy-associated T-cell lymphoma and coeliac disease has been identified[1].
Primary gastric lymphoma has been noted to occur more commonly in males over the age of 50. Risk factors include infection with Helicobacter pylori[5], Epstein-Barr virus, hepatitis B virus, human T-lymphotropic virus 1 and/or Campylobacter jejuni, as well as inflammatory bowel disease and immune suppression, for instance in transplant recipients[6]. For gastric DLBCL, atrophic gastritis has also been identified as a risk factor[7]. Many patients with gastrointestinal lymphoma have a delayed presentation involving vague symptomatology, which may include dyspepsia, abdominal pain, nausea, anorexia, weight loss, anaemia, obstruction and malabsorption[3,8]. Up to 30% of patients with gastric DLBCL experience gastrointestinal bleeding[9]. With regards to gastric lymphoma, there are three main endoscopic injury patterns these being ulceration, diffuse infiltration with enlargement of the mucosal folds, and a polypoid mass, none of which are specific for the diagnosis of gastric lymphoma[10].
Diagnosis of gastric lymphoma is primarily achieved through histopathological, immunohistochemical and fluorescence in situ hybridisation (FISH) analysis of biopsied gastric mucosa. Typically, there is diffuse proliferation of large atypical lymphoid cells, with cells expressing CD19, CD20, CD22 and paired box protein 5; and Ki67 is usually high. 30%-40% of gastric DLBCL cases harbour B-cell lymphoma-6 (BCL6) gene translocation at chromosome 3q27, and the translocation partner is most commonly the immunoglobulin heavy chain (IGH) gene. In addition, 30% of gastric DLBCL patients harbour BCL2 translocation, while 15%-25% have melocytomatosis oncogene (MYC) translocation. In contrast, all variants of Burkitt’s lymphoma harbour MYC translocation at 8q24, frequently to the IgH chain gene at 14q32.
Treatment of gastric lymphoma previously centred around surgery for localised disease, but in recent years, there has been a paradigm shift towards treatment focusing on chemotherapy and radiotherapy[11]. The chemotherapy regimen for DLBCL is usually R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), although vincristine is occasionally omitted due to the perceived risk of gastric perforation. Central nervous system prophylaxis, for example, with intrathecal methotrexate, is usually reserved for high-grade lymphoma (Burkitt’s lymphoma or “double-hit” high-grade B-cell lymphoma, where cellular MYC (c-MYC) and BCL2 and/or BCL6 rearrangement is identified by cytogenetic testing or FISH)[12].
The prognosis of patients with gastric DLBCL is usually determined by disease staging using the Ann Arbour system and the patient’s age, although other factors affecting prognosis include: Tumour bulk, elevated lactate dehydrogenase, male sex, low body mass index, elevated serum free light chains, and concordant bone marrow involvement[13,14]. Reports of primary gastric high-grade “double-hit” B-cell lymphoma are scarce, and its clinical characteristics, diagnostic challenges, and prognosis remain poorly defined. Here, we present a case of a patient with primary gastric high-grade “double-hit” B-cell lymphoma initially deemed to be histopathologically suggestive of primary gastric Burkitt’s lymphoma, which was identified in a patient who presented to our institution with atraumatic back pain and pancytopaenia.
A 71-year-old male presented to our institution with a three-week history of atraumatic chest and lower back pain, on the background of a recent diagnosis of pathological rib fractures.
The patient originally presented to the emergency department (ED) three weeks prior to the current presentation, with a three-day history of chest pain, left lower quadrant abdominal pain and loose stools. He was treated with a course of oral ciprofloxacin for possible colitis. Chest X-ray at this time incidentally identified an atraumatic left rib fracture, and the patient was discharged from the ED with a referral to obtain a computed tomography (CT) chest to further investigate this. Ten days later, the patient re-presented to the ED with persistent chest and now lower back pain, which was also atraumatic. There was no bladder or bowel dysfunction, or saddle anaesthesia. Lumbar spine X-ray showed lumbosacral osteophytes but was otherwise normal. The patient had not yet completed the CT chest, and hence, he was discharged with analgesia and a plan to complete the CT chest in the outpatient setting.
The patient completed the CT chest two days following discharge from the ED. This scan identified left fifth, seventh and eighth rib fractures with pleural and extrapleural thickening, and ill-defined lucencies suspicious for underlying lesions. There was also a suspicious right lower lobe posterior basal spiculated lung nodule. The patient was sub
The patient had a history of ischaemic heart disease, with previous acute myocardial infarction and cardiac arrest four years prior, resulting in coronary artery bypass surgery, tissue aortic valve and mitral valve annuloplasty and dual-chamber pacemaker insertion. He also had a history of hypertension, hyperlipidaemia, type 2 diabetes mellitus with chronic kidney disease, gout and a previous prostatectomy for prostate cancer. In terms of his regular medications on admission, the patient was taking dapagliflozin 10 mg mane, metformin-XR 1000 mg twice daily, pantoprazole 40 mg mane, metoprolol succinate 47.5 mg nocte, rosuvastatin 10 mg nocte, sacubitril/valsartan 24.3 mg/25.7 mg nocte, allopurinol 100 mg mane, and spironolactone 12.5 mg nocte. He had no allergies. He was a previous social smoker but had ceased 42 years prior to presentation. He reported occasional alcohol consumption. He lived in a retirement village and was independent with his activities of daily living, mobilising with a walking stick.
There was no significant family history.
Initial examination was significant for L3-5 spinal tenderness on palpation. The patient was haemodynamically stable. There was no significant lower limb neurological deficit identified. The abdomen was soft and non-tender. His chest was clear with equal air entry. Heart sounds were dual with no murmur.
Initial biochemical assessment revealed bicytopaenia with a haemoglobin level of 101 g/L, white cell count of 3.9 × 109/L with predominant lymphopaenia at 0.8 × 109/L, and thrombocytopaenia at 44 × 109/L. The patient was also hypercalcaemic with a corrected calcium level of 3.10 mmol/L. On later testing, Epstein-Barr virus immunoglobulin G was positive, cytomegalovirus immunoglobulin M and immunoglobulin G were negative, and human immunodeficiency virus was negative.
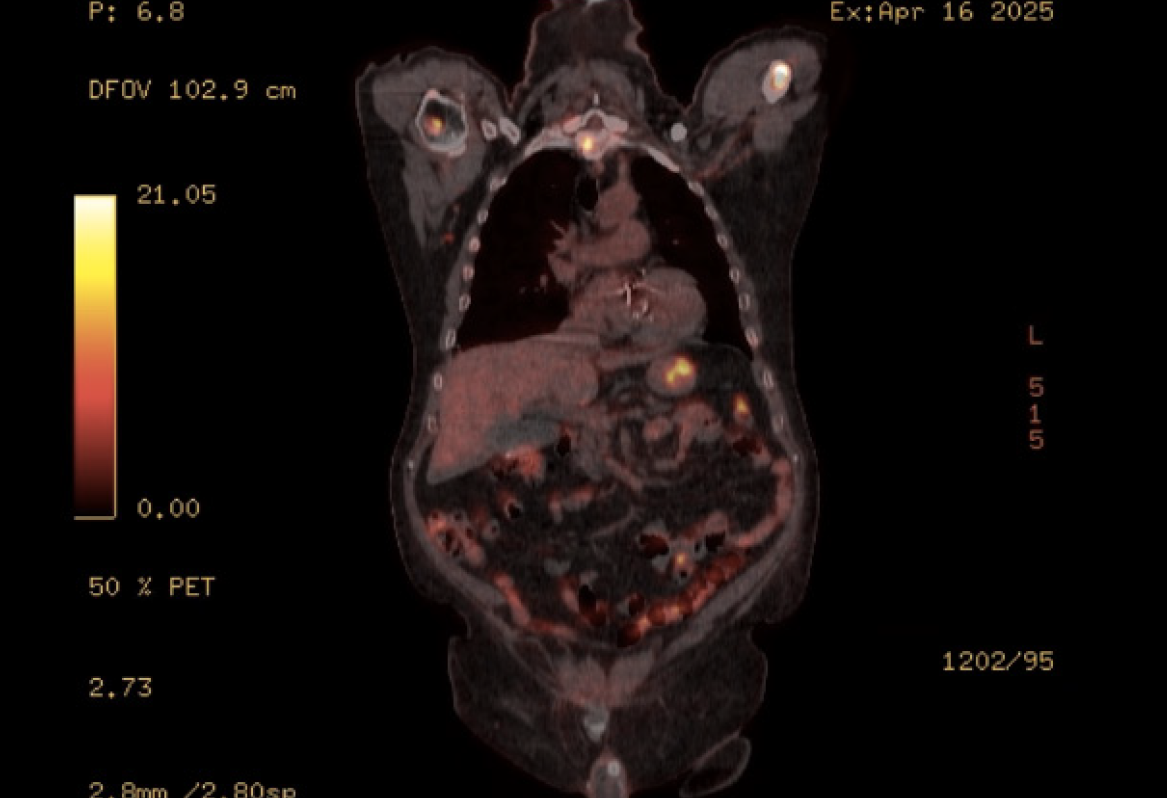
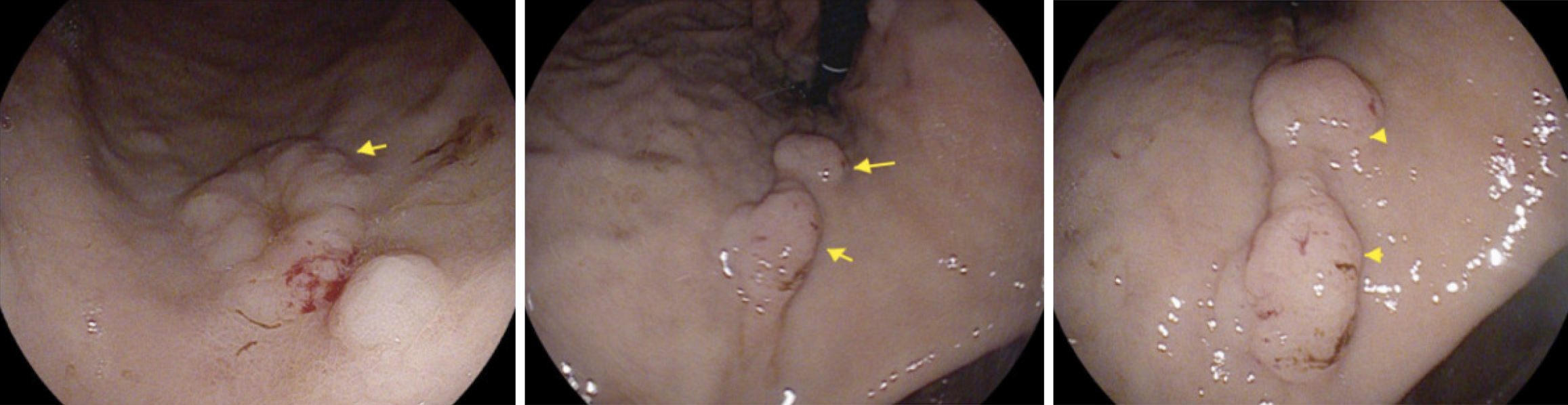
PET scan revealed disseminated, mixed, predominantly lytic bone metastases throughout the axial and appendicular skeletons, including throughout the spine, pelvis, bilateral mid to proximal humeri and femora, bilateral ribs, sternum and bilateral scapulae. There was no definite fluorodeoxyglucose-avid primary neoplasm, particularly in the lung. There was non-specific mild to moderate heterogeneous uptake in the stomach, most intensely in the proximal stomach along the greater curvature, and distal stomach along the lesser curvature and pylorus (Figure 1). There was no significant focal fluorodeoxyglucose uptake seen elsewhere to suggest other metastatic disease. Following this PET result, the patient underwent a gastroscopy with gastric nodule biopsies to further investigate the malignant process. Multiple 10-20 mm semi-sessile polyps were noted in the gastric fundus, antrum, greater and lesser curvature and the incisura (Figure 2). There were no other overt lesions identified.
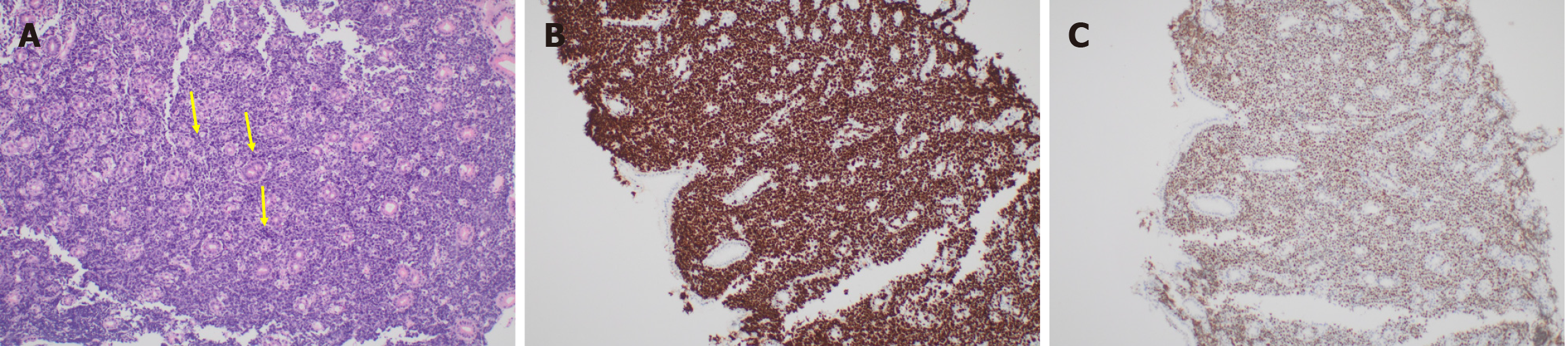
The histopathology from the gastric mucosal biopsies showed features that were initially suggestive of Burkitt’s lymphoma. There was diffuse infiltration by discohesive malignant cells that expanded the lamina propria and infiltrated between the glands. The neoplastic cells had a high nuclear/cytoplasmic ratio with hyperchromatic atypical nuclei, occasional prominent nucleoli and a limited amount of cytoplasm (Figure 3A). There was brisk mitotic activity with many abnormal forms. Ki67 was reported as 100% (Figure 3B). Immunohistochemical stains revealed that the neoplastic cells were CD20, CD19, CD10, BCL6 and c-MYC positive (Figure 3C). Negative stains included BCL2, CD3, CD5, CD21, Mum-1, CD30 and cyclin D1. No Helicobacter pylori microorganisms were identified on immunohistochemical stain. Further FISH analysis showed MYC and BCL6 rearrangement with a lack of IgH/MYC rearrangement, which is specific for Burkitt’s lymphoma. Hence, the favoured diagnosis was high-grade “double-hit” B-cell lymphoma with MYC and BCL6 rearrangements.
Bone marrow biopsy showed a mildly hypercellular aspirate with mildly increased trilineage haematopoiesis. There was no evidence of marrow involvement. There were reduced iron stores. Trephine was hypercellular with increased trilineage haematopoiesis and increased fibrosis. There was no evidence of involvement by lymphoproliferative disorder or non-haematopoietic malignancy.
Lumbar puncture showed no evidence of cerebral involvement.
The clinical and radiological findings were consistent with primary gastric high-grade “double-hit” B-cell lymphoma with bony metastases.
The patient was reviewed by the Haematology team and discussed at the lymphoma multidisciplinary team meeting. He was commenced on dose-adjusted rituximab, etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin chemotherapy. He also subsequently had a lumbar puncture with intrathecal methotrexate.
Chemotherapy was complicated by thrombocytopenia, possibly reflecting idiopathic thrombocytopaenic purpura. Human leukocyte antigen (HLA) testing showed HLA class I antibodies, not requiring HLA-matched platelets. He was treated with intravenous immunoglobulins and avatrombopag. Unfortunately, the patient also experienced rectal bleeding in the setting of haemorrhoids and profound thrombocytopenia, which resolved with tranexamic acid administration. He was also diureticized for fluid overload in the setting of multiple blood product transfusions on the background of heart failure with preserved ejection fraction. With this, the patient made a significant recovery and was discharged from the hospital with a view to ongoing outpatient treatment. On discharge, he was reviewed monthly in the outpatient haematology clinic with regular blood tests. His post-discharge course was complicated by anaemia necessitating transfusions, which subsequently resolved. Four months post-discharge, he had an outpatient PET scan, which was consistent with radiological remission with improvement in his cell counts with a haemoglobin of 85 g/L, white cell count of 1.6 × 109/L, and improvement in his thrombocytopenia with a platelet count of 92 × 109/L.
Primary gastric lymphoma represents 40% of extranodal lymphomas and is the second most common malignancy of the stomach. Although primary gastric lymphomas possess the ability to spread or transform, the disease itself is relatively indolent, with most cases remaining confined to the stomach for years after diagnosis. With an increasing incidence of the disease, a better understanding of diagnosis and treatment has developed, translating to a change in treatment paradigm from surgery to systemic therapy, along with an improved prognosis and quality of life. Although gastric DLBCL is a well-known entity, with advances in molecular profiling, the concept of “double-hit” lymphomas (DHL) and “triple-hit” lymphomas (THL) is a relatively new phenomenon[12]. These specific subtypes of high-grade B-cell lymphoma are morphologically and biologically distinct from other B-cell lymphoma subtypes, in that they harbour c-MYC and BCL2 and/or BCL6 rearrangements, and have a high proliferation rate, with large cells (that may be over twice the size of a lymphocyte). There is a spectrum of morphology associated with DHL/THL, with some similar to DLBCL or Burkitt’s lymphoma, as demonstrated in the presented case, while others have blastoid, lymphoblastic morphology or grey zone features[15].
DHL/THL are a relatively rare subtype of primary gastric high-grade lymphoma[16]. In the case of nodal lymphoma, double rearrangement, or “double-hit”, of the MYC and BCL2 genes and the double expression of the MYC and BCL2 proteins have been previously associated with poor prognosis[17]. Despite it being well documented in the case of localised DLBCL and nodal DLBCL, making up 6%-14% of DLBCL cases, its clinical significance in the context of primary gastric lymphoma is poorly understood[18-20]. In a study by Choi et al[21], there were only 2 cases with MYC and BCL6 rearrangement in the stomach among 101 cases of B-cell lymphoma in the gastrointestinal tract. He et al[22] evaluated 188 cases of primary gastric B-cell lymphoma, which showed no cases involving DHL. In contrast to this, “double-hit” primary gastric lymphoma has been reported to make up to 15%-25% of DHL/THL cases[23], with Guo et al[15] performing a small retrospective study where they identified 15 cases of primary DHL/THL in a cohort of 57 patients, of which 8 were primary gastric in origin. With regards to their presentation, the main manifestations at disease onset were abdominal pain, bloating, vomiting, melaena or a change in stool frequency and consistency. Despite its documented poor prognosis in the context of nodal DLBCL, there is conflicting evidence with reference to prognosis in primary gastric high-grade B-cell lymphoma, with Kawajiri et al[24] reporting no difference in patient outcomes, namely overall and progression free survival, in those with “double-hit” primary gastric lymphoma compared with the other cases of primary gastric DLBCL included in their retrospective study. Rosenthal and Younes[25] challenged this with patients diagnosed with gastrointestinal DHL/THL having a worse prognosis compared to those with gastrointestinal DLBCL. Further large prospective studies should aim to determine what patient and tumor factors influence patient outcomes in those diagnosed with “double-hit” primary high-grade gastric B-cell lymphoma.
Alongside its rarity, the morphologically and biologically distinct nature of DHL/THL has implications for their investigation and treatment. Patients with high-grade B-cell lymphoma more often present with high-risk clinical features and advanced-stage disease, which is rapidly progressive, has high rates of central nervous system involvement, is usually refractory to conventional therapy and as such conveys a poorer prognosis. Furthermore, DHL/THL must be treated with more intensive chemotherapy due to their perceived poor prognosis. The mainstay of therapy for DLBCL, R-CHOP, does not typically yield a good response. Instead, treatment with rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin has been shown to lead to better event-free survival compared to R-CHOP.
This case highlights an uncommon presentation of a “double-hit” primary gastric B-cell lymphoma. The patient was initially diagnosed with Burkitt’s lymphoma based on the histopathological analysis of the gastric specimen; however, following FISH testing, was subsequently diagnosed as a high-grade “double-hit” primary gastric B-cell lymphoma. This demonstrates the critical importance of FISH testing in these patients, as it can dictate the treatment and ultimately the prognosis. It is vital that ongoing research should aim to determine what patient factors predispose to the development of “double-hit” primary gastric B-cell lymphoma, as well as whether it is indeed associated with a poorer prognosis compared to those who lack the “double-hit” or “triple-hit” molecular phenotype. The fact that the presented patient had a normal gastroscopy less than one year prior to his diagnosis highlights the aggressive nature of the disease compared to its more indolent counterparts.
Primary gastric lymphoma is a relatively common site for extranodal lymphoma, with DLBCL being commonly implicated. High-grade “double-hit” and “triple-hit” B-cell lymphoma has emerged as a relatively new entity over the past decade, likely in the context of advanced molecular analysis of tumour tissue. This case report highlights an uncommon presentation of a high-grade “double-hit” primary gastric B-cell lymphoma that presented with back pain secondary to metastatic disease. It highlights the aggressive behaviour of the disease and reaffirms that studies are required to further characterise this disease entity.
| 1. | Ghimire P, Wu GY, Zhu L. Primary gastrointestinal lymphoma. World J Gastroenterol. 2011;17:697-707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 245] [Cited by in RCA: 282] [Article Influence: 18.8] [Reference Citation Analysis (3)] |
| 2. | Ferreri AJ, Montalbán C. Primary diffuse large B-cell lymphoma of the stomach. Crit Rev Oncol Hematol. 2007;63:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Bautista-Quach MA, Ake CD, Chen M, Wang J. Gastrointestinal lymphomas: Morphology, immunophenotype and molecular features. J Gastrointest Oncol. 2012;3:209-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 66] [Reference Citation Analysis (0)] |
| 4. | Lewis RB, Mehrotra AK, Rodríguez P, Manning MA, Levine MS. From the radiologic pathology archives: gastrointestinal lymphoma: radiologic and pathologic findings. Radiographics. 2014;34:1934-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Kuo SH, Yeh KH, Chen LT, Lin CW, Hsu PN, Hsu C, Wu MS, Tzeng YS, Tsai HJ, Wang HP, Cheng AL. Helicobacter pylori-related diffuse large B-cell lymphoma of the stomach: a distinct entity with lower aggressiveness and higher chemosensitivity. Blood Cancer J. 2014;4:e220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Thomas JA, Allday MJ, Crawford DH. Epstein-Barr virus-associated lymphoproliferative disorders in immunocompromised individuals. Adv Cancer Res. 1991;57:329-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer. 1972;29:252-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 8. | Rackner VL, Thirlby RC, Ryan JA Jr. Role of surgery in multimodality therapy for gastrointestinal lymphoma. Am J Surg. 1991;161:570-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Medina-Franco H, Germes SS, Maldonado CL. Prognostic factors in primary gastric lymphoma. Ann Surg Oncol. 2007;14:2239-2245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Taal BG, Burgers JM. Primary non-Hodgkin's lymphoma of the stomach: endoscopic diagnosis and the role of surgery. Scand J Gastroenterol Suppl. 1991;188:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Roukos DH, Hottenrott C, Encke A, Baltogiannis G, Casioumis D. Primary gastric lymphomas: a clinicopathologic study with literature review. Surg Oncol. 1994;3:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Horn H, Ziepert M, Becher C, Barth TF, Bernd HW, Feller AC, Klapper W, Hummel M, Stein H, Hansmann ML, Schmelter C, Möller P, Cogliatti S, Pfreundschuh M, Schmitz N, Trümper L, Siebert R, Loeffler M, Rosenwald A, Ott G; German High-Grade Non-Hodgkin Lymphoma Study Group. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood. 2013;121:2253-2263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 414] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 13. | Rohatiner A, d'Amore F, Coiffier B, Crowther D, Gospodarowicz M, Isaacson P, Lister TA, Norton A, Salem P, Shipp M. Report on a workshop convened to discuss the pathological and staging classifications of gastrointestinal tract lymphoma. Ann Oncol. 1994;5:397-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 359] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | Polyatskin IL, Artemyeva AS, Krivolapov YA. [Revised WHO classification of tumors of hematopoietic and lymphoid tissues, 2017 (4th edition):lymphoid tumors]. Arkh Patol. 2019;81:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 15. | Guo J, Cai Y, Wang Z, Xu J, Chen H, Zhang J, Xu X, Rao H, Tian S. Double/triple hit lymphoma in the gastrointestinal tract: clinicopathological features, PD-L1 expression and screening strategy. Mod Pathol. 2022;35:1667-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Cox MC, Di Napoli A, Scarpino S, Salerno G, Tatarelli C, Talerico C, Lombardi M, Monarca B, Amadori S, Ruco L. Clinicopathologic characterization of diffuse-large-B-cell lymphoma with an associated serum monoclonal IgM component. PLoS One. 2014;9:e93903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Hu S, Xu-Monette ZY, Tzankov A, Green T, Wu L, Balasubramanyam A, Liu WM, Visco C, Li Y, Miranda RN, Montes-Moreno S, Dybkaer K, Chiu A, Orazi A, Zu Y, Bhagat G, Richards KL, Hsi ED, Choi WW, Zhao X, van Krieken JH, Huang Q, Huh J, Ai W, Ponzoni M, Ferreri AJ, Zhou F, Slack GW, Gascoyne RD, Tu M, Variakojis D, Chen W, Go RS, Piris MA, Møller MB, Medeiros LJ, Young KH. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood. 2013;121:4021-31; quiz 4250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 546] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 18. | Li M, Zhang QL, Zhao W, Huang X, Gong LP, Shi QF, Liu CL, Gao ZF. [The incidence of high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements in diffuse large B-cell lymphoma]. Zhonghua Xue Ye Xue Za Zhi. 2021;42:124-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 19. | Li S, Lin P, Medeiros LJ. Advances in pathological understanding of high-grade B cell lymphomas. Expert Rev Hematol. 2018;11:637-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Scott DW, King RL, Staiger AM, Ben-Neriah S, Jiang A, Horn H, Mottok A, Farinha P, Slack GW, Ennishi D, Schmitz N, Pfreundschuh M, Nowakowski GS, Kahl BS, Connors JM, Gascoyne RD, Ott G, Macon WR, Rosenwald A. High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with diffuse large B-cell lymphoma morphology. Blood. 2018;131:2060-2064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 179] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 21. | Choi SY, Kim SJ, Kim WS, Kim K, Ko YH. Aggressive B cell lymphomas of the gastrointestinal tract: clinicopathologic and genetic analysis. Virchows Arch. 2011;459:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | He M, Chen K, Li S, Zhang S, Zheng J, Hu X, Gao L, Chen J, Song X, Zhang W, Wang J, Yang J. Clinical Significance of "Double-hit" and "Double-protein" expression in Primary Gastric B-cell Lymphomas. J Cancer. 2016;7:1215-1225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Zhang J, Weng Z, Huang Y, Li M, Wang F, Wang Y, Rao H. High-grade B-Cell Lymphoma With MYC, BCL2, and/or BCL6 Translocations/Rearrangements: Clinicopathologic Features of 51 Cases in a Single Institution of South China. Am J Surg Pathol. 2020;44:1602-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Kawajiri A, Maruyama D, Maeshima AM, Nomoto J, Makita S, Kitahara H, Miyamoto KI, Fukuhara S, Suzuki T, Munakata W, Tajima K, Itami J, Taniguchi H, Kobayashi Y, Tobinai K. Impact of the double expression of MYC and BCL2 on outcomes of localized primary gastric diffuse large B-cell lymphoma patients in the rituximab era. Blood Cancer J. 2016;6:e477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Rosenthal A, Younes A. High grade B-cell lymphoma with rearrangements of MYC and BCL2 and/or BCL6: Double hit and triple hit lymphomas and double expressing lymphoma. Blood Rev. 2017;31:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/