Published online Mar 5, 2026. doi: 10.4292/wjgpt.v17.i1.113444
Revised: September 15, 2025
Accepted: December 19, 2025
Published online: March 5, 2026
Processing time: 169 Days and 17 Hours
Based on the traditional Chinese medicine theory that "acid can astringe and consolidate", Xiaozhiling Injection is a multi-award-winning traditional Chinese medicine preparation. It is developed from gallnut and alum, with tannic acid and potassium aluminum sulfate as its main components. Clinically, it demonstrates excellent efficacy and high safety in treating mid-grade hemorrhoids. Characterized by minimal invasiveness, few dietary restrictions, wide age adaptability, and mild postoperative reactions, this injection has shown distinct therapeutic advantages. Since its introduction decades ago, its sclerotherapy technique has been widely adopted both in China and internationally. Clinical follow-up studies have indicated relatively low recurrence rates over the long term, confirming its stable efficacy and low complication rate. Additionally, research using animal models has further verified the effectiveness of Xiaozhiling Injection and explored its mechanisms through pathological and immunohistochemical analyses.
To investigate the mechanism underlying the therapeutic effect of Xiaozhiling injection by observing the presence of internal hemorrhoids during different periods.
An internal hemorrhoid model was successfully established, and intervention with Xiaozhiling injection was investigated 8 hours, 3 days, 1 week, 2 weeks, 1 month, and 4 months after injection. By observing the appearance of the anus, performing laser speckle blood flow imaging, determining the rectal submucosa thickness, observing pathological morphology, and conducting immunohistochemical staining of anorectal tissues from rats, the changes in various indices were evaluated at different time points after Xiaozhiling injection.
The degree of internal hemorrhoidal mucosal eminence in the anuses of the rats gradually decreased. Laser speckle blood flow imaging revealed that the anorectal hemoperfusion flow first increased but then decreased, and microvascular dilation first increased but then decreased. Microscopy revealed vascular dilation of the submucosal interstitium, and the thickness of the submucosa first increased but then decreased. Immunohistochemical staining for myeloperoxidase indicated that the chronic inflammatory reaction first increased but then stabilized, CD61 expression indicated platelet aggregation, martius-scarlet-blue expression indicated red blood cell mud-like stasis, and CD42b expression indicated that platelet activation first increased but then decreased.
Xiaozhiling injection can stimulate the proliferation of collagen fibres in the submucosa and make loose connective tissue compact and neat, thus exerting therapeutic effects. This study is expected to provide a new direction for the minimally invasive treatment of internal haemorrhoids.
Core Tip: Xiaozhiling injection exerts its prominent therapeutic efficacy by potently stimulating the proliferation of collagen fibres within the submucosal layer of the hemorrhoidal tissue, which in turn remodels the originally loose and disorganized connective tissue into a compact, well-aligned structure. This targeted tissue-modulating mechanism not only alleviates the key symptoms of internal haemorrhoids but also holds great promise for paving a novel, minimally invasive therapeutic avenue for this prevalent anorectal disorder.
- Citation: Huang SY, Lin WG, Lan H, Xu ZG, Zheng XX, Liu XB, Cheng YM, Li ZF, Ke MH. Mechanism of Xiaozhiling injection in the treatment of internal hemorrhoids based on changes in perianal blood flow and rectal submucosal fibers. World J Gastrointest Pharmacol Ther 2026; 17(1): 113444
- URL: https://www.wjgnet.com/2150-5349/full/v17/i1/113444.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v17.i1.113444
On the basis of the traditional Chinese medicine theory that “acid can be astringent and astringent can be fixed and removed”, Xiaozhiling, a famous remedy for anorectal diseases in integrated Chinese and Western medicine, is com
Croton oil (6%; Beijing Huawei Ruike Chemical Co., Ltd., Beijing, China), pyridine (Beijing Lambolide Trading Co., Ltd., Beijing, China), ether (Guangzhou Xilong Science Co., Ltd., Guangzhou, China), isoflurane for inhalation (Shanghai Baxter Healthcare Corporation, Shanghai, China), 4% neutral formaldehyde fixing solution, immunohistochemical reagents [myeloperoxidase (MPO), CD61, martius-scarlet-blue (MSB), and CD42b], hematoxylin-eosin (HE) staining reagents, Masson staining reagent, and Xiaozhiling injection (Beijing China Resources High-tech Natural Medicine Co., Ltd., Beijing, China) were used.
BIOHIT pipettes (20-200 μL), a microtome (micromMH325), push machine (Leica, Germany), embedding machine (LeicaEG1150H, Germany), Olympus BX45 optical microscope, microscope acquisition head (LG500, Korea), BS-420 Mindray automatic biochemical instrument, Laser Speckle Flow Imaging System (PericamPSiSystem2.0, Sweden), single-arm brain stereotaxator (Shenzhen Rayward Life Technology Co., Ltd., Shenzhen, China), and small animal air anesthesia machine (Shenzhen Reward Life Technology Co., Ltd., Shenzhen, China) were used.
Experimental animals: A total of 42 healthy male specific pathogen-free 8-week-old SD rats weighing 180-220 g were purchased from SCXK Laboratory Animal Co., Ltd. Shanghai Jieshijie Laboratory Animal Co., Ltd (2018-0004; Shanghai, China). The rats were raised in the Laboratory Animal Center of Fujian University of Chinese Medicine (SYXK; 2019-0007; Fujian Province, China). Normal maintenance feed was provided by the Laboratory Animal Center of Fujian University of Chinese Medicine (SYXK; 2019-0007; Fujian Province, China). The environment had a 12-hour/12-hour light/dark cycle and constant humidity, and the temperature was controlled at 22-25 °C. The experiments were carried out after the rats were allowed to adapt to the environment for 1 week. All the procedures were performed following the experimental ethics requirements of the Fujian Academy of Traditional Chinese Medicine (approval number: No. FJATCM-IAEC2018015). The treatment of the rats followed the Guiding Opinions on the Treatment of Laboratory Animals[9]. An internal hemorrhoid model was successfully established in rats according to a previously published method[8].
Sample size determination: Forty-two rats were randomly divided into 7 groups: Group A (group A), 8 hours after drug injection (group B), 3 days after drug injection (group C), 1 week after drug injection (group D), 2 weeks after drug injection (group E), 1 month after drug injection (group F), and 4 months after drug injection (group G); each group included 6 animals. Random numbers were generated using the standard = RAND function in Microsoft Excel. The sample size for each group (n = 6) was determined on the basis of a combination of established standards in the field of experimental rodent morphology and a preliminary power analysis. A sample size of 5-8 animals per group is widely adopted and considered sufficient for detecting significant morphological and immunohistochemical changes in similar rat model studies.
Anesthesia was performed by inhalation of isoflurane (induced anesthesia concentration of 3%-3.5%, continuous anesthesia of 1.5%-2.5%). The limbs of the rats were fixed in the supine position, the perianal and rectal mucosa were disinfected by a routine method involving iodophor, and the rectal mucosa of the rats was fully exposed. A 1-mL syringe was used to remove the hemorrhoidal solution at different points of the mucosa. Afterward, a 5-length needle was injected into the hemorrhoidal bulge (submucosa) parallel to the anal canal and rectum. After no blood could be drawn into the needle, Xiaozhiling was injected until the mucosa became pale and full. A total of 0.1-0.2 mL of Xiaozhiling was injected at each point, and the total amount that was injected ranged from 0.3-0.5 mL. After injection, a cotton swab was inserted into the anal passage to ensure that the liquid was evenly and fully absorbed. After the operation, the perianal and rectal mucosa were disinfected, and the rats were returned to their cages. Laser speckle flow imaging and pa
After the rats received an overdose of isoflurane anesthesia, abdominal aorta blood samples were collected, 3 mL of serum was prepared, and the levels of liver and kidney function indices, such as alanine aminotransferase, glutamic oxalate amino acid transferase, alkaline phosphatase, urea, and creatinine, in the serum were measured. These indices were detected via a BS-420 Mindrays (Shenzhen Mindray Biomedical Electronics Co., LTD, Shenzhen, China) automatic biochemical analyzer.
Statistical analyses were performed using SPSS (version 26.0; IBM Corp., Armonk, NY, United States). Continuous data are presented as the means ± SD. Prior to comparative analysis, the normality of all the datasets was assessed using the Shapiro–Wilk test, and the homogeneity of variance was confirmed with Levene’s test. For data satisfying both normality and homogeneity of variance, one-way analysis of variance was employed to compare differences among multiple groups. The least significant difference post hoc test was subsequently used for pairwise comparisons between groups. A P-value of less than 0.05 was considered to indicate statistical significance, and a P-value of less than 0.01 was considered to indicate statistical significance.
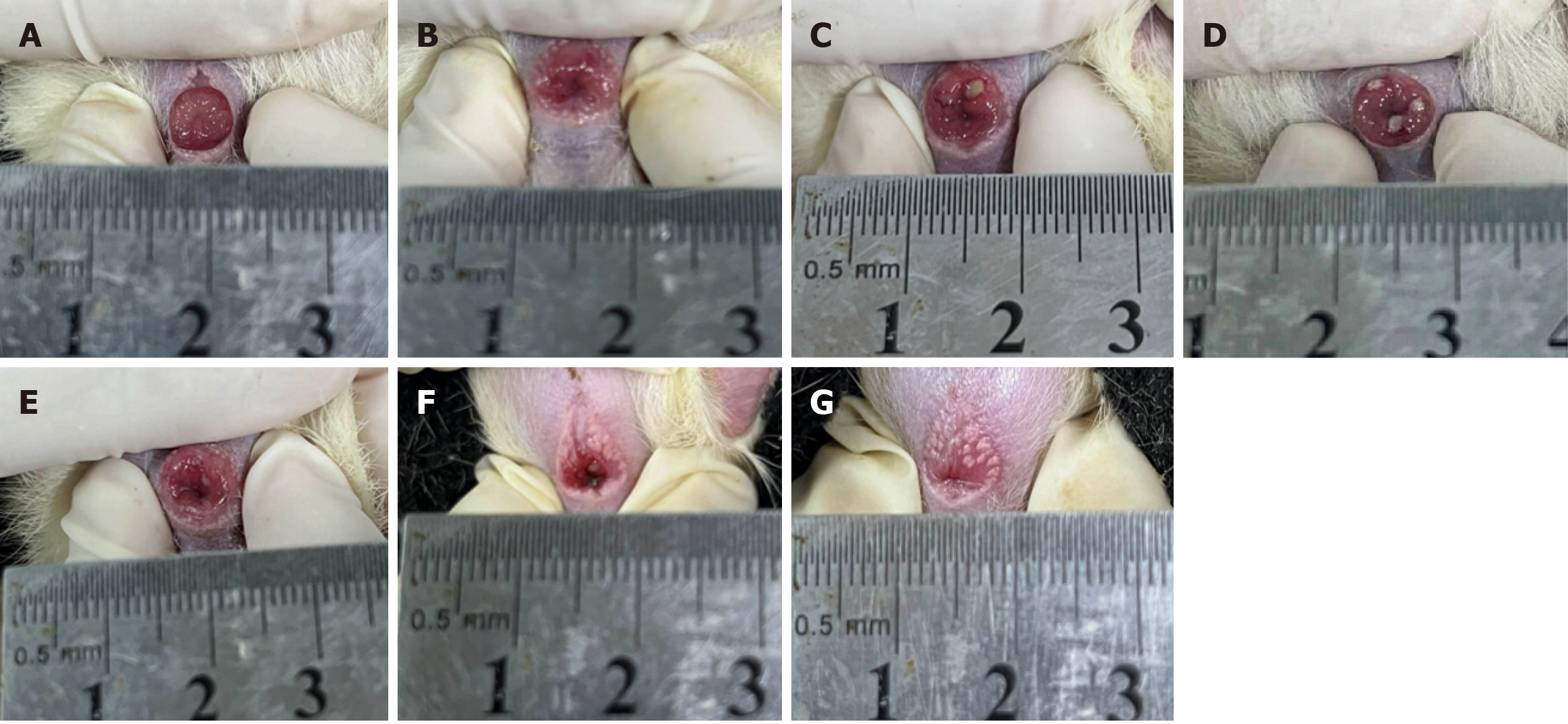
Figure 1 and Table 1 shows the symptoms and signs of rats treated with Xiaozhiling for different periods after internal hemorrhoid establishment.
| Group | Injection site mucosa | Haematochezia | Perianal oedema | Perianal ulcer |
| (A) | Hyperaemia | Yes | Yes | No |
| (B) | Bleached bulge | Little | Obvious | No |
| (C) | Some necrotic tissue | Obvious | Obvious | Some ulcer spots |
| (D) | Obvious necrotic tissue | Obvious | Reduced | Partially ulcerated surface |
| (E) | Reduced necrotic tissue | Little | No | Reduced surface ulceration |
| (F) | Normal | No | No | No |
| (G) | Normal | No | No | No |
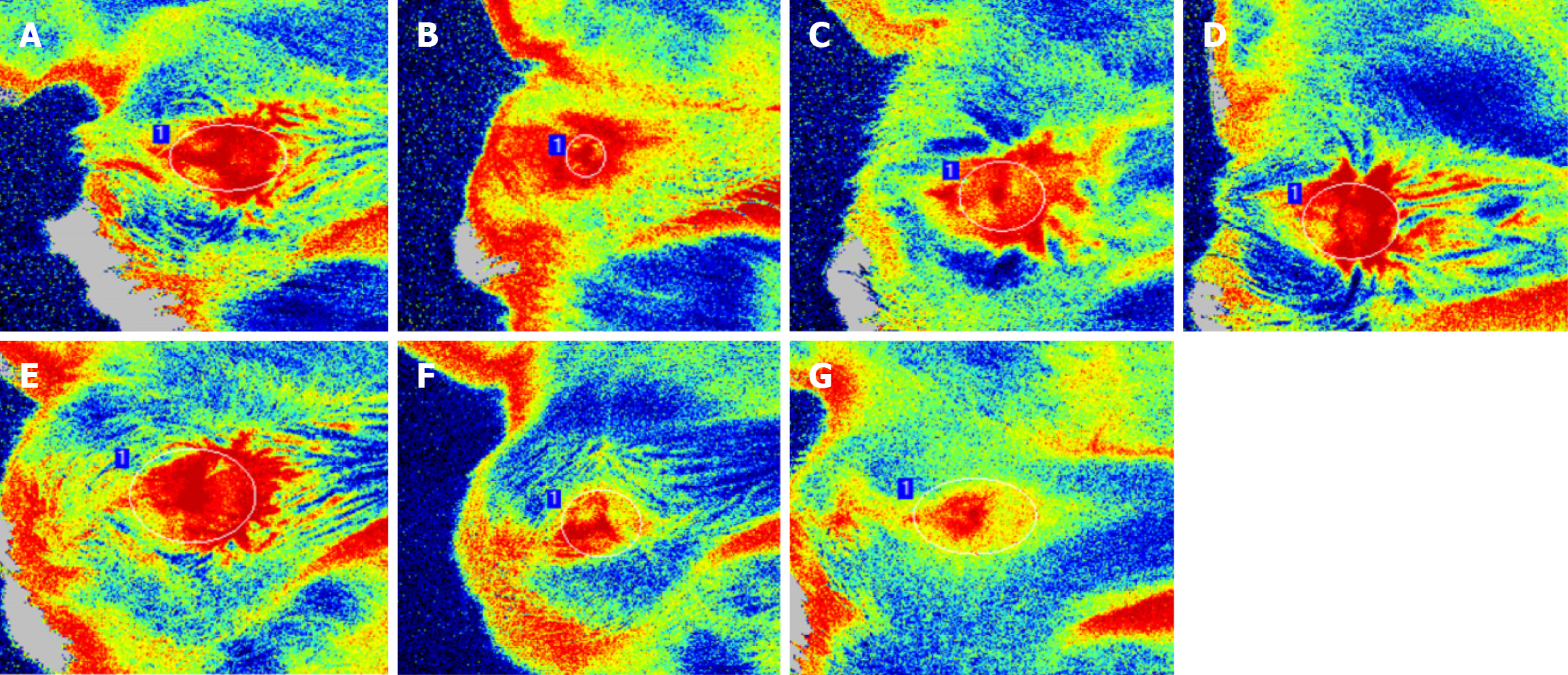
According to the blood flow images (Figure 2) obtained by laser speckle flow imaging, the perianal microvessels were dilated after the internal hemorrhoid model was successfully established. The dilatation was reduced at 8 hours after injection, after which the perianal microvascular dilatation gradually increased over time. Dendritic bifurcation neovascularization was observed at 1-2 weeks after injection, but was ameliorated at 1 month after injection, and the degree of vascular dilatation at 4 months after injection was lower than that in the control group. Xiaozhiling first increased perianal microcirculation and then ameliorated perianal microvascular dilation.
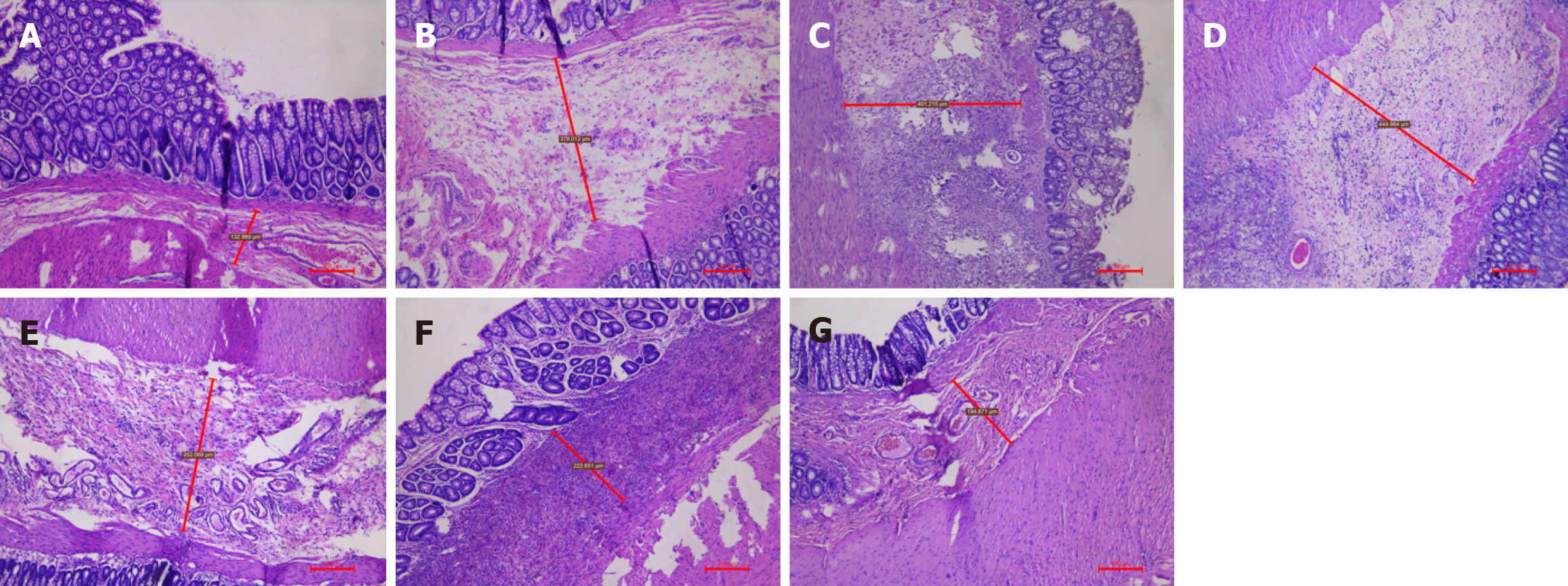
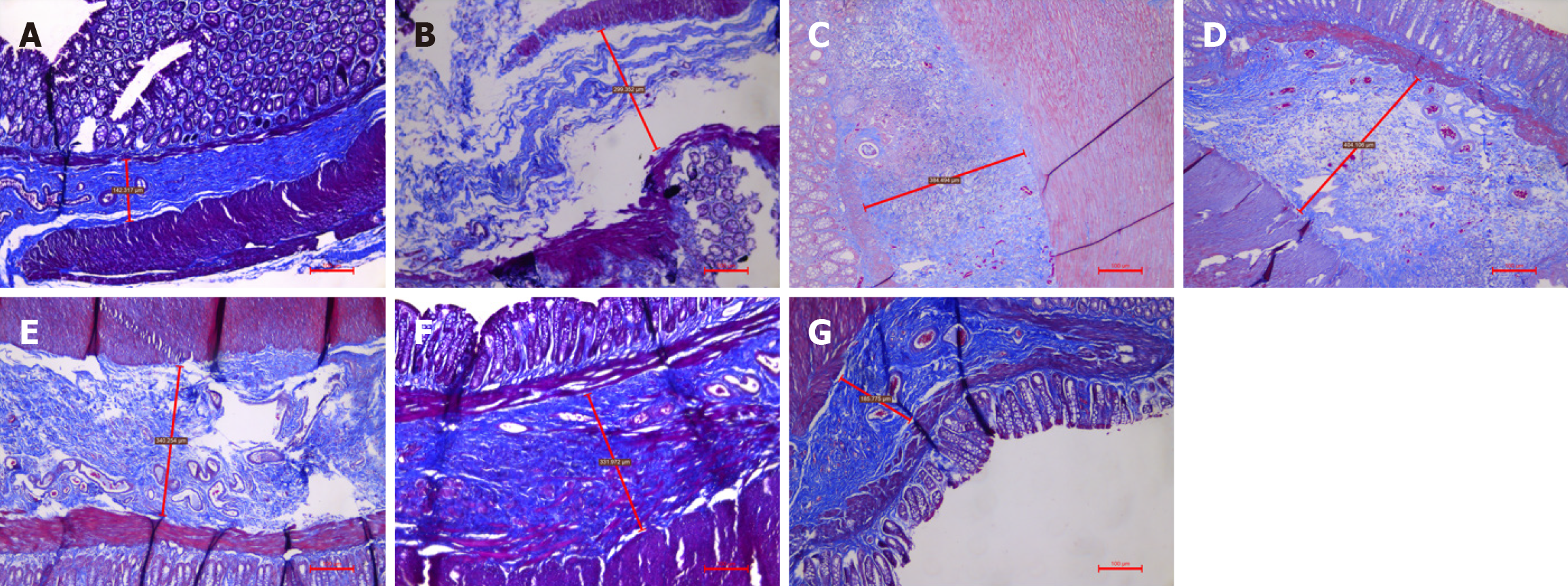
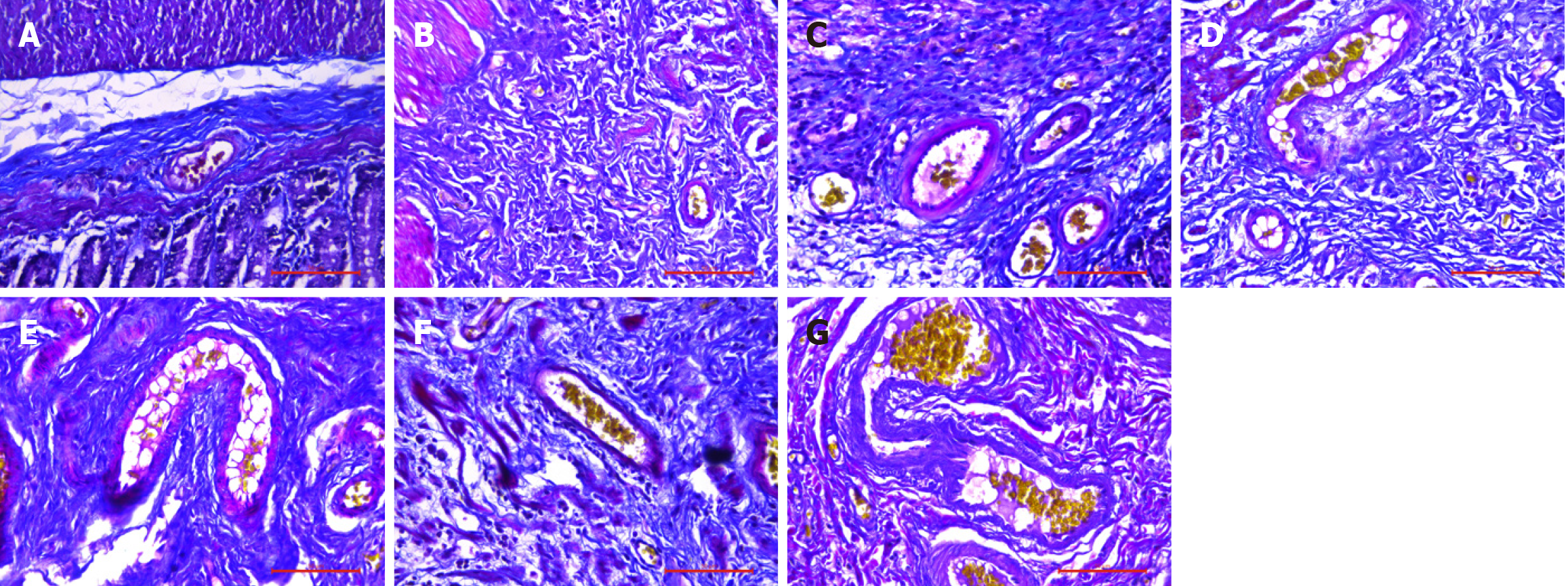
HE staining and Masson staining of samples from the A-G groups revealed that the submucosal thickness exhibited a unimodal increase over time. HE staining (Figure 3) and Masson staining (Figures 4, 5, 6, 7 and 8) revealed that the submucosal collagen fibers were densely arranged and that the blood vessels were dilated after treatment. Rapid thickening of the submucosa, loose tissue, enlarged space, loose arrangement of collagen fibers, increased numbers of inflammatory cells, fibromuscular hyperplasia of interstitial tissue, and structural relaxation were observed at 8 hours after Xiaozhiling injection. The thickness of the submucosa peaked at 1 week but then gradually decreased. The blood vessels were still in a dilated state, and the collagen fibers were rearranged and gradually tightened. After treatment, the submucosal thickness continued to decrease between 1 month and 4 months, the vascular dilation gradually improved, the submucosal arrangement became dense again, and its characteristics gradually became similar to those of the blank group.
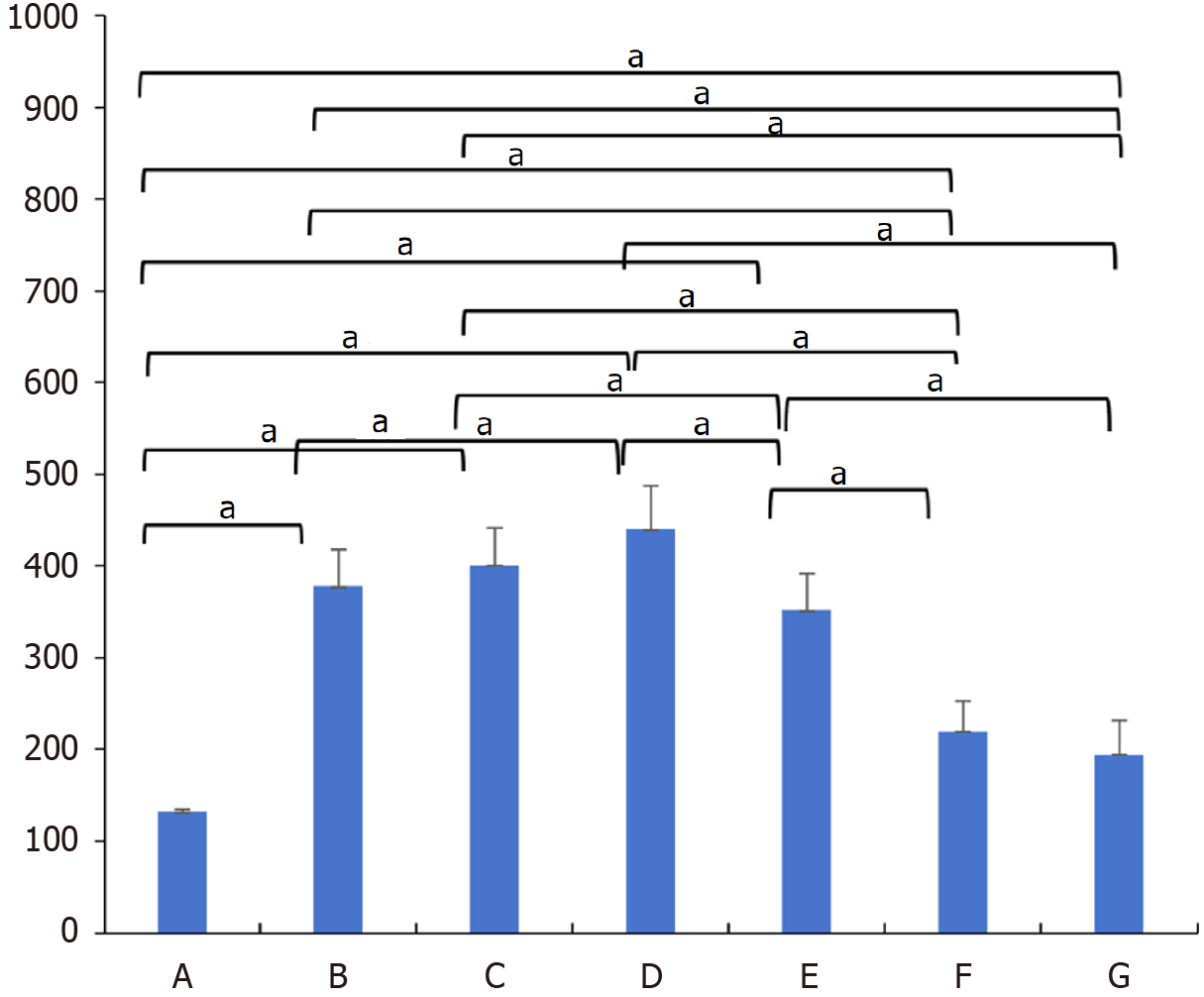
According to the results in Table 2 and Figure 9, there were significant differences in the submucosal thickness between the different groups (F = 62.381; P < 0.001). Postinjection comparisons revealed that the submucosal thickness of the model group was significantly lower than that at 8 hours, 3 days, 1 week, 2 weeks, 1 month, and 4 months after injection. The submucosal thickness at 8 hours after injection was significantly lower than that at 1 week after injection, and that at 8 hours after injection was significantly greater than that at 1 month and 4 months after injection. The submucosal thickness at 3 days after injection was significantly greater than that at 2 weeks, 1 month, and 4 months after injection. The submucosal thickness at 1 week after injection was significantly greater than that at 2 weeks, 1 month, and 4 months after injection. The submucosal thickness at 2 weeks after injection was significantly greater than that at 1 month and 4 months after injection.
| Group | A (1) | B (2) | C (3) | D (4) | E (5) | F (6) | G (7) |
| Submucosal thickness | 132.02 ± 3.2211 | 378.111 ± 39.9672 | 401.056 ± 40.0113 | 440.631 ± 47.4143 | 352.146 ± 39.9873 | 219.707 ± 32.484 | 194.825 ± 37.0134 |
| F | 62.381 | ||||||
| P value | 0.001 | ||||||
| LSD | (1) < (2), (1) < (3), (1) < (4), (1) < (5), (1) < (6), (1) < (7), (2) < (4), (2) > (6), (2) > (7), (3) > (5), (3) > (6), (3) > (7), (4) > (5), (4) > (6), (4) > (7), (5) > (6), (5) > (7) | ||||||
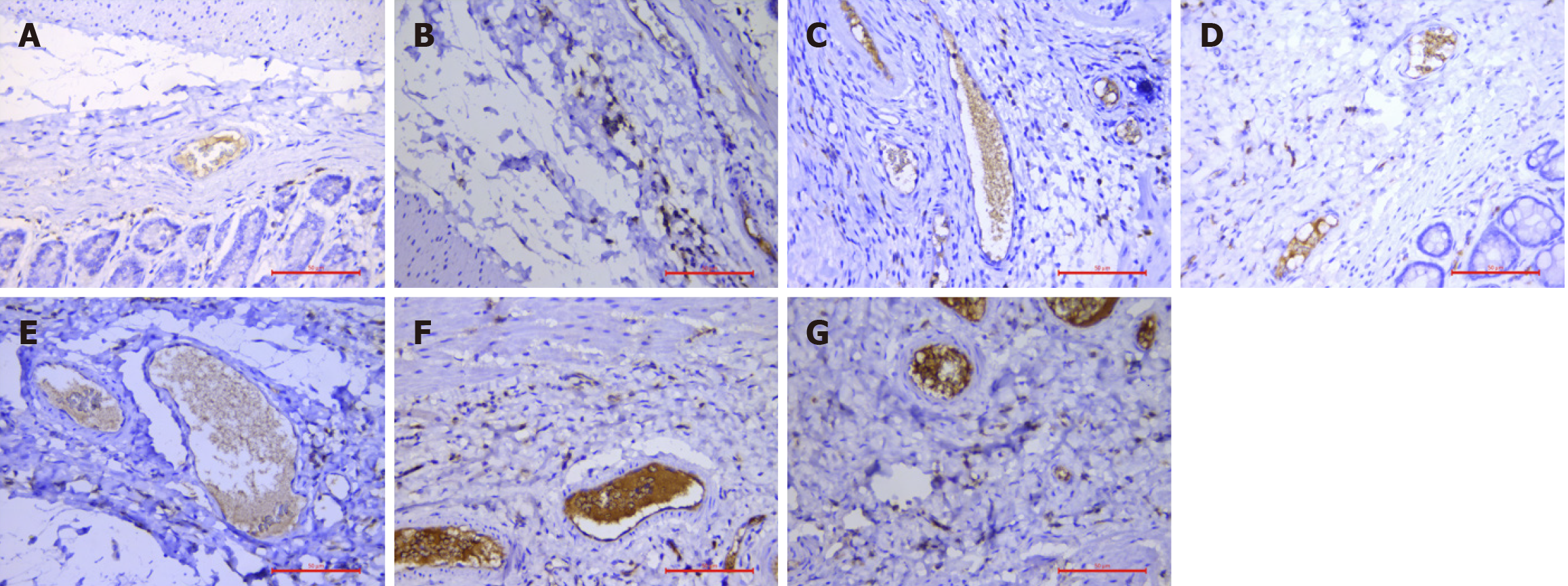
MPO is a peroxidase that is produced by the oxidation reaction of azocyanine granules in neutrophils and monocytes[10]. Positive reactions manifest as dark brown or yellow-brown particles in the cytoplasm of cells, and the overexpression of MPO is associated with inflammation and oxidative stress, which are believed to be closely related to the occurrence and development of chronic tissue injury and disease[11,12]. When the local anti-inflammatory system of rats was activated after drug treatment, the expression of MPO significantly increased and was a good indicator of the inflammatory response at different time points after Xiaozhiling injection. Pathology revealed that in the submucosa and vascular lumen of the model group, staining for neutrophils and monocytes was scattered and weak. However, with increasing time after drug intervention, the numbers of neutrophils and monocytes in the submucosa and vascular lumen increased continuously from 3 days to 4 months, indicating that in the anti-inflammatory stage, neutrophils and monocytes secrete anti-inflammatory factors such as MPO to trigger the body’s repair response.
The thrombus tissue at the site of positive CD61 expression was brown and yellow in color. Yang et al[13] observed thrombosis formation, mechanization, and recalculation in a rat model of deep vein thrombosis that was established with the “stenosis method” and reported that CD61 expression was scattered throughout the thrombus as early as 3 hours after surgery. Peak CD61 expression was observed 1 day after the operation. The expression of CD61 began to decrease and reached its lowest level at 2 weeks post-operation. CD61 expression subsequently returned to the newly formed platelets at the site of the institutionalized thrombus and secondary stenosis of lumen formation, but this expression level was lower than the peak that was observed on the first postoperative day. Pathology revealed that positive CD61 expression in the submucosa and its vascular lumen was extremely weak in the model group, whereas positive CD61 expression in the submucosa and its vascular lumen increased with time after drug intervention, and positive CD61 expression increased significantly at 4 months after injection, indicating that platelet aggregation was gradually induced by the chronic inflammatory response.
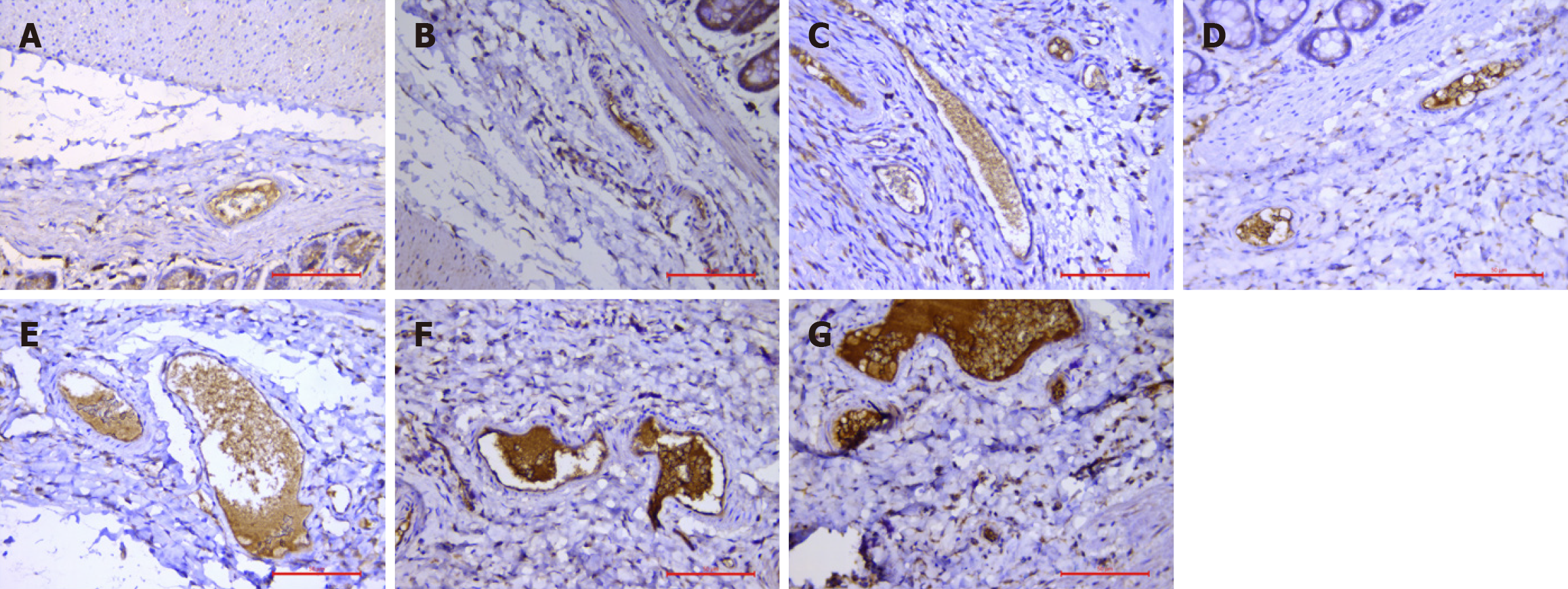
MSB staining revealed collagen fibers (blue staining), red blood cells (yellow staining), platelets (blue-gray staining), and fresh fibrin (red staining)[14]. According to red blood cell flow characteristics, such as deformability, aggregation, and adhesion, are important features are necessary for the survival of red blood cells[15]. Aggregation means that red blood cells accumulate to form roll-like aggregates when blood flow is slow; under these conditions, red blood cell aggregation increases, and the blood is in a highly viscous state, so roll-like aggregates easily form. Red blood cell aggregation is characterized as follows: (1) Mild red blood cell aggregation: Red blood cells are adherent, and abnormal proteins lead to an imbalance in the surface charge of red blood cells, which generally causes 3-5 red blood cells to be connected in series; (2) Moderate red blood cell aggregation: Red blood cells form rouleaux that resemble coins in a string; and (3) Severe red blood cell aggregation: Red blood cells clump, resulting in the formation of large blood stasis clots. MSB staining of samples after drug intervention revealed mild red blood cell aggregation in the lumen at 8 hours to 3 days after injection, moderate red blood cell aggregation at 1-2 weeks, and clumpy moderate and severe red blood cell aggregation at 1-4 months, indicating that the internal hemorrhoid model of rats exhibited slow hemorheology and “mud flow”-like stasis of red blood cells after Xiaozhiling injection.
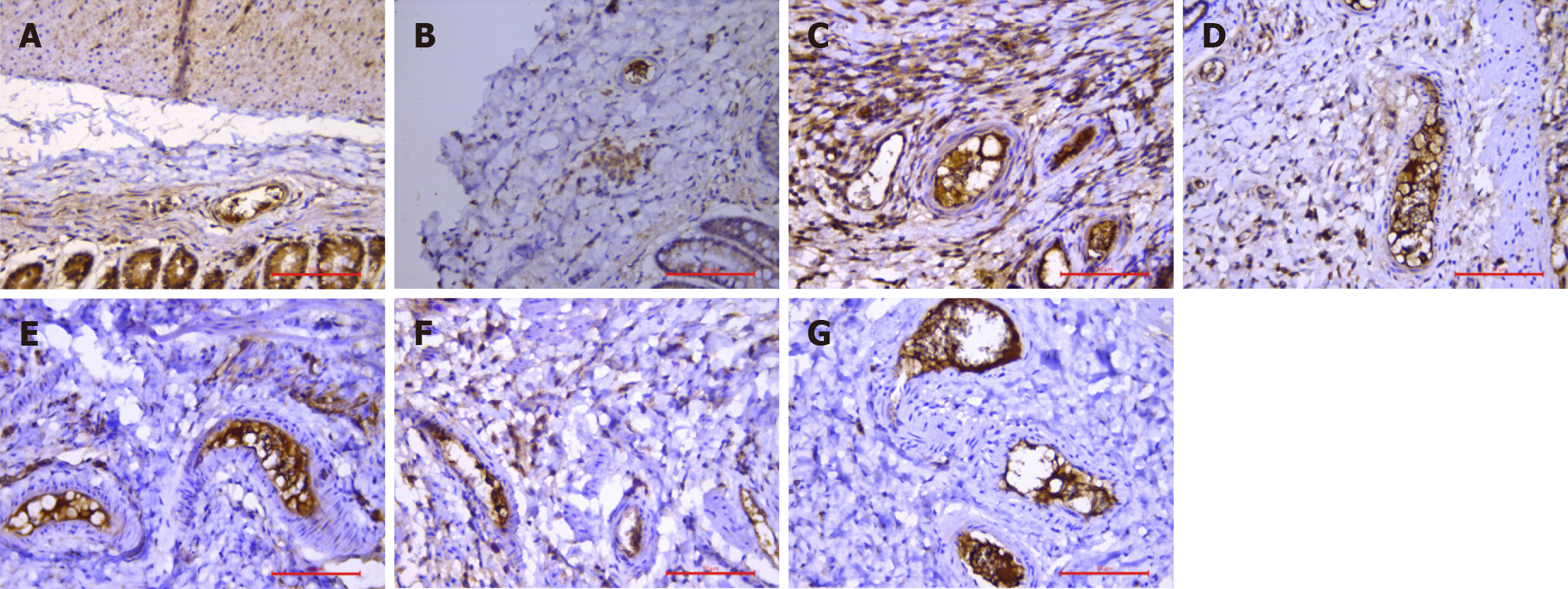
CD42b is a transmembrane glycoprotein that is present on the surface of endothelial cells and platelets. When the body is subjected to stimuli, such as thromboembolism and endothelial injury, cells reduce their CD42b secretion, affecting the function of coagulation and fibrinolytic systems, promoting platelet activation and aggregation, and accelerating thrombosis[16]. Studies[17] have shown that CD42b is connected to the membrane skeleton system and that platelet activation causes changes in the membrane distribution of CD42b, resulting in a decrease in its levels. Therefore, we believe that platelet activation is positively correlated with a decrease in CD42b levels. Pathology revealed weak CD42b expression in the submucosa and its vascular lumen in the model group. In the internal hemorrhoid model, the positive expression of CD42b in the submucosa and its vascular lumen first decreased but then increased with time, indicating that the platelet activity in the rats first increased but then decreased after drug treatment, which was closely related to the development of blood stasis after the injection of Xiaozhiling.
Liver and kidney function were assessed after the study, and no abnormalities were found. There were no deaths due to drug treatment in any of the groups.
Xiaozhiling is a sclerosing agent that has astringent and solidifying functions, and it can play a role in constricting blood vessels in the hemorrhoidal area, promoting aseptic inflammation of the inner wall of blood vessels[18] proliferation of fiber tissues in the hemorrhoidal nucleus, and causing local intravascular thrombosis, venous thrombosis and proliferative endarteritis; thus, this treatment reduces the hemorrhoidal nucleus[19]. Studied the effects of Xiaozhiling on blood vessels and blood and microcirculatory blood flow in animal experiments with rabbits, and their results revealed that Xiaozhiling can reduce hemoperfusion flow, thrombosis, and anticoagulation. In a previous study[20], the authors injected different doses of Xiaozhiling into muscle tissue and reported that the injection of large doses of Xiaozhiling into muscle tissue caused hyperplasia of the tissue. When tissue hyperplasia reaches a certain level, the blood vessels are compressed, and the tissue to which these vessels supply blood may become necrotic. Cai[21] injected 0.5 mL of Xiaozhiling liquid under the rectal mucosa of rabbits, and reported that many muscle fibers formed at the injection site according to three-dimensional postlight microscopy. However, 14 days after our team injected Xiaozhiling into rabbits with rectal intramucosal prolapse[22], chronic inflammatory cell infiltration and fibrous tissue proliferation were observed at the injection site.
In this study, laser speckle blood flow imaging combined with immunohistochemical staining revealed that after Xiaozhiling injection, blood flow was significantly reduced because of local blood vessel ischemia, hypoxia, and occlusion. Less blood vessel dilation was observed 8 hours after injection than before. In addition, vascular compensatory hyperplasia, branching-like vascular bundles, and compensatory blood flow increased; 3 days to 2 weeks after injection, the blood flow gradually increased. Most thrombi consisted of aggregated platelets, fibrin, and bound red blood cells. Platelets are predominant in arterial thrombosis, whereas fibrin is the main component of venous thrombosis. Excessive activation and aggregation of platelets are the main causes of arterial thrombosis[23]. When blood vessels are damaged, activated platelets in the blood vessels migrate to the damaged part of the blood vessels to adhere and aggregate, forming thrombi to repair the damaged blood vessels. The aggregation and adhesion of red blood cells and endothelial cells are important factors in microvascular obstruction and microthrombus formation[24]. The positive expression of MSB and CD42b indicated that platelet activation and fibrin expression increased, which further indicated that Xiaozhiling injection accelerated blood stasis. After drug treatment, the microcirculation dysfunction of the perianal tissue in the rats resulted in slow blood flow, tissue hypoxia, damage to the microvascular intima, widening of the endothelial space, and infiltration of plasma water into the tube wall, resulting in increased hematocrit, increased erythrocyte aggregation, decreased erythrocyte deformability, and increased blood viscosity, eventually leading to the formation of small blood stasis clots in the perianal area. After Xiaozhiling injection, the area of blood stasis in the lumen increased, and the degree of coloration increased. The exacerbation of blood stasis further proved that Xiaozhiling injection accelerated submucous blood stasis and that the silted blood flow accelerated the establishment of collateral circulation in the hemorrhoidal area, which is consistent with the findings of the author’s team.
In terms of changes in the thickness of the submucosa, this study revealed that the submucosa rapidly thickened at 8 hours after Xiaozhiling injection, the tissue exhibited a loose shape according to Masson staining, and the thickness peaked one week after injection. The aseptic inflammatory response that occurred after Xiaozhiling injection was presumed to be a period of inflammatory activity. During the period from 1 week to 4 months after injection, the thickness of the submucosa continued to decrease, and this time was thought to be a period of remission of inflammation; additionally, the layer boundary of the affected intestinal wall returned to normal, Masson staining continued to increase, and the tissue was dense. Aluminum ions in Xiaozhiling can strongly affect inflammation, increase the permeability of blood vessels, cause the accumulation and infiltration of white blood cells, and eventually cause tissue fibrosis through aseptic inflammation, resulting in tissue adhesion[25]. Tannic acid in the gallnut coagulates histamine and constricts blood vessels through strong acid retraction.
In this study, we evaluated a current approach to treating hemorrhoids. Simple ligation of blood vessels in the hemorrhoidal area or injection of vascular sclerosing agents into the blood vessels in the hemorrhoidal area are used to treat hemorrhoids[26]. Since collateral circulation is still observed around the hemorrhoidal area, whether the current treatment approach is consistent with the nature of hemorrhoids warrants further discussion. During circumferential resection of the superior hemorrhoidal mucosa, although the lateral branch circulation area is effectively removed, adverse postoperative reactions, such as anal stenosis, are likely to occur because of the greater tissue damage associated with this approach[27]. On the basis of selective hemorrhoidal superior mucosal resection, this improvement can reduce rectovaginal fistula and rectal stenosis[28], but according to the findings of this study, protrusion can easily occur in the mucosal bridge area after surgery, which is considered due to “compensatory hemorrhoids” that arise after the establishment of collateral circulation. The Xiaozhiling injection method involves massaging the injection site after injection into obviously raised hemorrhoids to promote diffusion of the liquid into the submucosa. By “treating hemorrhoids with inflammation”, submucosal collagen fiber hyperplasia, loose and dense connective tissue, and perianal blood supply were reduced, thus reducing hemorrhoids, and no deaths occurred during this treatment. Multisite injection not only effectively affects the collateral circulation but also does not destroy the structure surrounding the hemorrhoidal area; thus, this approach is not only a safe and minimally invasive treatment method but also achieves the intended therapeutic effects. The evaluation of any sclerosing agent fundamentally relies on a comparative analysis of its efficacy and safety profile against established alternatives. While the present study demonstrated the efficacy of Xiaozhiling in inducing localized fibrosis and vascular occlusion in a rat model, interpreting these findings within the broader context of widely used sclerosing agents provides deeper insight.
Internationally common agents include polidocanol, sodium tetradecyl sulfate, and phenol-based formulations. In comparison to these agents, Xiaozhiling offers a distinct mechanistic advantage. Unlike single-component sclerosants such as polidocanol or sodium tetradecyl sulfate, Xiaozhiling is a compound formulation whose therapeutic effects arise from the synergistic interaction of its constituents. Song et al[19] showed that aluminum potassium sulfate facilitates rapid protein coagulation and endothelial damage, whereas tannic acid has both astringent and anti-inflammatory effects. Compared with the intense inflammatory reactions that are sometimes associated with detergent-type sclerosants such as polidocanol, this multitarget action may promote more controlled and organized fibrosis. It is plausible that this modulated tissue response could correlate with a reduced long-term recurrence rate - a significant clinical advantage. Furthermore, the localized tissue effects observed in our study - characterized by well-demarcated fibrotic areas with minimal surrounding inflammation - suggest a favorable tolerability profile. Therefore, beyond its efficacy, the clinical value of Xiaozhiling may lie in its ability to achieve potent sclerosing effects while potentially offering a wider therapeutic window, thereby mitigating the common trade-off between efficacy and safety often encountered with other injectable treatments.
Xiaozhiling injection can stimulate the proliferation of collagen fibres in the submucosa and make loose connective tissue compact and neat, thus exerting therapeutic effects. This study is expected to provide a new direction for the minimally invasive treatment of internal haemorrhoids.
| 1. | Zhang TT, Shi XW, Zhuang HY. [Meta-analysis of Xiaozhilin injection in the treatment of internal hemorrhoids]. Zhongguo Minjian Liaofa. 2024;32:79-83. [DOI] [Full Text] |
| 2. | Zhang X, Bai JS. [Brief discussion on the pathogenesis, diagnosis and treatment of hemorrhoids]. Zhongguo Gangchangbing Zazhi. 2019;9:72-74. |
| 3. | Chinese Medical Doctor Association Anorectal Physicians Branch, Chinese Medical Association Surgery Branch Colorectal Surgery Group. Chinese expert consensus on hemorrhoidal injection therapy (2023). Zhonghua Weichang Waike Zazhi. 2023;26:1103-1111. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Shi ZQ. [Xiaozhiling four-step injection for treatment of Ⅲ and Ⅳ stage hemorrhoids -- occlusion of upper rectal artery branch and disappearance of hemorrhoid sclerosis]. Zhongguo Zhongxiyi Jiehe Zazhi. 1998;201. [DOI] [Full Text] |
| 5. | Chen ZQ. [Curative effect of Xiaozhiling injection on rectal mucosal prolapse in children: a report of 42 cases: Effect observation]. Zhongguo Gangchangbing Zazhi. 2019;39:37-38. |
| 6. | Chen Q, Li HS. [A review of the domestic and international development trend of Xiaozhiling over the 40 years since its inception]. Jiangsu Zhongyiyao. 2020;52:76-80. [DOI] [Full Text] |
| 7. | Abe T, Kunimoto M, Hachiro Y, Ohara K, Inagaki M. Long-term Outcomes of Aluminum Potassium Sulfate and Tannic Acid Sclerotherapy for Prolapsed Hemorrhoids: A Single-Center, Observational Study. Dis Colon Rectum. 2022;65:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Ke MH, Huang S, Lin H, Xu Z, Li X, Li Z, Chen F, Wu H. Establishment and study of a rat internal haemorrhoid model. Sci Rep. 2023;13:21385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 9. | Shi XP, Zong AN, Tao J, Wang LZ. [Research on guiding opinions on treating experimental animals well]. Zhongguo Yike Daxue Xuebao. 2007;4:493. |
| 10. | Guilpain P, Servettaz A, Batteux F, Guillevin L, Mouthon L. Natural and disease associated anti-myeloperoxidase (MPO) autoantibodies. Autoimmun Rev. 2008;7:421-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Feng Y, Zhu J, Pan B, Yang Y. [Relationship between dysregulation of intestinal bifidobacteria, enterococci and eubacteria and serum TLR4, MPO expression and disease activity in ulcerative colitis patients]. Linchuang Yixue. 2023;11:9-12. [DOI] [Full Text] |
| 12. | Wang YT, Qin YJ, Li TY. [Research progress on the development of myeloperoxidase inhibitors]. Nanjing Yike Daxue Xuebao (Ziran Kexue Ban). 2023;43:1756-1763. [DOI] [Full Text] |
| 13. | Yang CT, Zuo M, Wang SJ, Liu X, Ma RF, Qi Q, Bi HT, Li YM, Zhang GZ. Estimation on Formation Time of Thrombus. Fa Yi Xue Za Zhi. 2018;34:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Chen LQ, Liu J, Liu LB, Liu CM. [Study on the protective effect of nicodil on coronary microcirculation disturbance in rats based on Nrf2/ARE pathway]. Zhongguo Laonianxue Zazhi. 2023;43:4492-4496. [DOI] [Full Text] |
| 15. | Zhu NN, Jin XJ, Guo JR. [The research progress of erythrocyte morphology, function and physicochemical properties in diabetes mellitus]. Zhongguo Tangniaobing Zazhi. 2019;27:863-865. [DOI] [Full Text] |
| 16. | Feng Y, Li YM, Cai LT, Li J, Zhang Y. [Correlation between cd42b, cd62p, Hcy, hs-CRP and the severity of coronary artery disease in patients with acute myocardial infarction]. Wenzhou Yike Daxue Xuebao. 2019;12:930-933. [DOI] [Full Text] |
| 17. | Li X, Guo DX, Li X. [Expression of CD42b, hs-CRP and CD61 in elderly patients with chronic left heart failure]. Xiandai Zhongxiyi Jiehe Zazhi. 2013;22:2737-2738. [DOI] [Full Text] |
| 18. | The Coloproctology Society of Chinese Association of Integrative Medicine. [Chinese guidelines for the diagnosis and treatment of hemorrhoids]. Jiezhichang Gangmen Waike. 2020;26:519-533. [DOI] [Full Text] |
| 19. | Zhang Y, Liu JX, Ma SS. [Effect of Xiaozhiling injection on vascular system]. Shengli Kexue. 1982;2:32. [DOI] [Full Text] |
| 20. | Ke MH, Ye L, Chen LW, Wei L, Zheng MX, Wan D. [Experimental study on Xiaozhiling injection for the treatment of rectal intramucous prolapse in rabbits]. Yatai Chuantong Yiyao. 2015;11:7-9. |
| 21. | Cai YH. [Xiaozhiling anal pad suspension curing effect of animal experimental study]. Xin Zhongyi. 2015;47:233-234. [DOI] [Full Text] |
| 22. | Ke MH, Wu HS, Cheng YM, Xu ZG. [Experimental study on different doses of Xiaozhiling injected into muscle tissue of rats]. Zhongguo Yixue Gongcheng. 2021;7:1-5. [DOI] [Full Text] |
| 23. | He H, Zhang YJ, Wu DD, Xie XL, Xin JY, Guligena S, An DQ. [Research on effect of Tianxiangdan for inhibiting platelet aggregation and improving carotid thrombosis]. Xinjiang Yike Daxue Xuebao. 2022;3:318-322. |
| 24. | Song Y, Huang Z, Xu J, Ren D, Wang Y, Zheng X, Shen Y, Wang L, Gao H, Hou J, Pang Z, Qian J, Ge J. Multimodal SPION-CREKA peptide based agents for molecular imaging of microthrombus in a rat myocardial ischemia-reperfusion model. Biomaterials. 2014;35:2961-2970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Chen Q. [Construction of a predictive model for recurrence of rectal prolapse treated with Xiaozhiling injection, evaluation of its efficacy, and integrated pharmacology study on its mechanism of prolapse fixation]. Doctoral Thesis, China Academy of Chinese Medical Sciences. 2021. Available from: https://d.wanfangdata.com.cn/thesis/CiBUaGVzaXNOZXdTMjAyNTA2MTMyMDI1MDYxMzE2MTkxNhIIWTM4NzU0MDcaCGk4ZjM0ajgx. |
| 26. | Sun HB, Wang DW, Tang XL, Pan HY, Li LQ, Meng QH. [Clinical progress of hemorrhoid treatment]. Xiandai Shengwu Yixue Jinzhan. 2016;16:3597-3600. [DOI] [Full Text] |
| 27. | Iida Y, Saito H, Takashima Y, Saitou K, Munemoto Y. Procedure for prolapse and hemorrhoids (PPH) with low rectal anastomosis using a PPH 03 stapler: low rate of recurrence and postoperative complications. Int J Colorectal Dis. 2017;32:1687-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Zhang G, Liang R, Wang J, Ke M, Chen Z, Huang J, Shi R. Network meta-analysis of randomized controlled trials comparing the procedure for prolapse and hemorrhoids, Milligan-Morgan hemorrhoidectomy and tissue-selecting therapy stapler in the treatment of grade III and IV internal hemorrhoids(Meta-analysis). Int J Surg. 2020;74:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/