Published online Mar 5, 2026. doi: 10.4292/wjgpt.v17.i1.112788
Revised: September 1, 2025
Accepted: November 24, 2025
Published online: March 5, 2026
Processing time: 189 Days and 1.4 Hours
Inflammatory bowel disease (IBD) management has evolved with the advent of Janus kinase inhibitors (JAKi), oral small molecules that modulate cytokine-driven inflammation via the Janus kinase-signal transducer and activator of transcription pathway. This narrative review synthesizes current evidence for JAKi in ulcera
Core Tip: Janus kinase inhibitors are reserved for moderate-to-severe inflammatory bowel disease (IBD) after biologic failure or steroid dependence. Carefully selecting patients based on their cardiovascular, thromboembolic, and infection-related risks is of paramount importance. Tofacitinib is an excellent choice for patients with moderate to severe ulcerative colitis (UC) and acute severe UC. Upadacitinib is currently approved for both refractory UC and Crohn’s disease (CD); however, emerging data suggest its beneficial role in acute severe UC. Limited evidence exists for the combination of small molecules and other biologicals in patients with difficult-to-treat IBD and peri-anal CD. Close monitoring of side effects is advocated in such patients. More data is warranted on their role in extraintestinal manifestations, pediatric and pregnant patients with IBD.
- Citation: Malakar S, Giri S, Jena A, Nath P. Updated review of Janus kinase inhibitors for the management of inflammatory bowel disease. World J Gastrointest Pharmacol Ther 2026; 17(1): 112788
- URL: https://www.wjgnet.com/2150-5349/full/v17/i1/112788.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v17.i1.112788
Ulcerative colitis (UC) and Crohn’s disease (CD) are characterized by intestinal ulceration secondary to the altered innate and adaptive immune system, and gut dysbiosis in the background of genetic susceptibility[1-3]. It often follows a chronic relapsing-remitting course[4,5]. Immunosuppression is the mainstay of treatment in patients with inflammatory bowel disease (IBD). Janus kinase inhibitors (JAKi) are novel, orally administered small molecules. By inhibiting Janus kinase (JAK), they attenuate various cytokine-dependent pathways in the inflammatory cascades[6-9]. JAKi has been widely used in connective tissue disorders and autoimmune diseases. More data pertaining to JAKi comes from rheumatological diseases[10]. Intravenous or subcutaneous biologicals like tissue necrosis factor inhibitors [anti-tumor necrosis factor α (anti-TNF-α)] require therapeutic dose monitoring[11]. In long-term use, reactivation of tuberculosis (TB) in ende
Recently, various landmark trials have been instrumental in demonstrating the excellent safety and efficacy profile of JAKi against UC and CD[17]. Tofacitinib, upadacitinib, and filgotinib have been found to be effective compared to pla
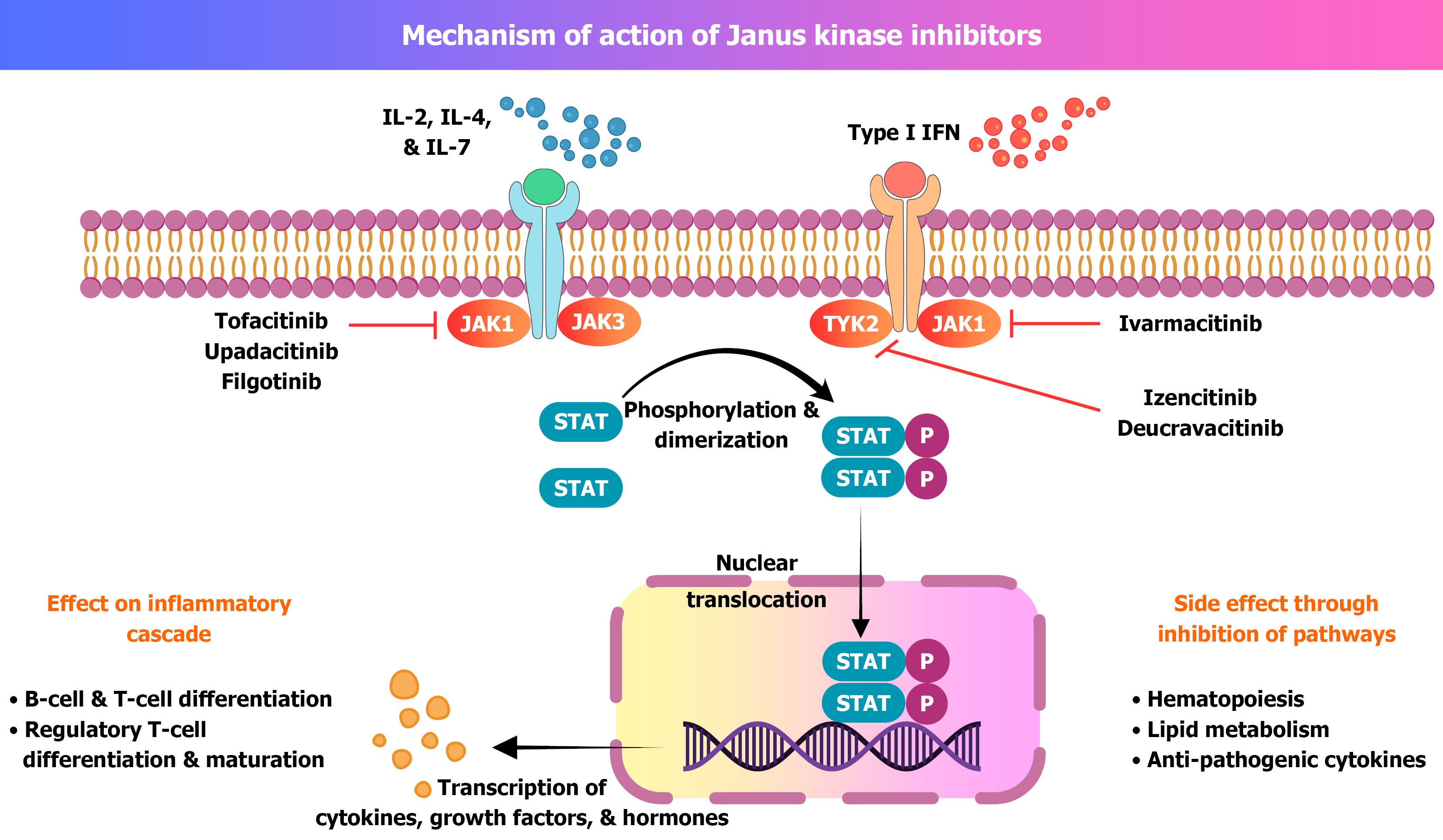
Tofacitinib was introduced in 2019. It was the 1st approved JAKi for IBD[22,23]. JAKs are the non-receptor tyrosine kinases. They are linked to the cytoplasmic part of type I and type II cytokine receptors. There are four cytokine isoforms, i.e., JAK-1, JAK-2, JAK-3, and TYK[22-25]. The isoforms are linked to the transcription of different cytokines, hormones, interleukins (ILs), and growth factors. Phosphorylation of JAK leads to activation of the signal transducer and activator of transcription (STAT) pathway[24,25] (Figure 1). In the context of IBD, the JAK-STAT pathway is important as it is responsible for cytokine and IL production, as well as the recruitment of inflammatory cells into the intestinal lumen. Recruitment of inflammatory cells leads to damage to intestinal cells and produces ulceration[25].
Tofacitinib preferentially inhibits JAK-1 and JAK-2, whereas upadacitinib and filgotinib selectively inhibit JAK-1 (Table 1). Peficitinib inhibits all four isomers of JAK. Newer JAKi TD-1473 acts on intestinally restricted JAK-1, JAK-2 and JAK-3[26,27]. JAK-STAT pathways are also involved in erythropoietin and other growth factors production. Use of JAKi is associated with the theoretical risks of developing anaemia and cytopenia[24-28].
| Parameter | Tofacitinib | Upadacitinib | Filgotinib |
| JAK selectivity | JAK1 > JAK3 > JAK2 | JAK1 > JAK2 > JAK3 > TYK2 | JAK1 > JAK2 > JAK3 |
| Approved indication | Moderate to severe UC | Moderate to severe UC and CD | Moderate to severe UC (EMA-approved only) |
| Induction dosing | 10 mg BID for 8 weeks | 45 mg OD for 8-12 weeks | 200 mg OD |
| Maintenance dosing | 5 mg BID (or 10 mg if needed short-term) | 15 mg or 30 mg OD based on response | 200 mg OD |
| Efficacy (induction) | Clinical remission: 18%-20% (OCTAVE 1 & 2) | Clinical remission: 26%-34% (UC trials), 44% (CD-CELEST) | Clinical remission: 26.1% vs 15.3% placebo (SELECTION) |
| Efficacy (maintenance) | 34%-41% remission at 52 weeks (OCTAVE sustain) | 42%-52% remission in UC; 40%-47% in CD (CELEST-OLE) | 37.2% remission at 58 weeks |
| Real-world data | Established role in UC, encouraging data in ASUC, however no benefit in CD | Effective in UC and CD, including tofacitinib-refractory patients | Emerging for UC, limited data in CD |
| Pregnancy category | Category C (not recommended) | Category D (not recommended) | Contraindicated (reproductive toxicity in animal studies) |
| Regulatory approval | FDA & EMA approved for UC | FDA & EMA approved for UC and CD | EMA approved; FDA rejected application |
The landmark studies on tofacitinib, OCTAVE and OCTAVE sustain have established its role in patients with moderate to severe UC[29-35]. In the OCTAVE Sustain trial, remission at 52 weeks was seen in 34.2% of patients receiving 5 mg tofacitinib and 41% in the 10 mg tofacitinib group. Tofacitinib was found to be more effective than placebo for managing moderate to severe UC. Around half of the patients in the OCTAVE and OCTAVE sustain group were biological-ex
| Ref. | Number of patients | Dose | Response | Adverse events |
| OCTAVE 1[34] | 476 | 10 mg BID | Remission at 8 weeks: 18.2% | AEs: 60%; SAEs: 3.4% |
| OCTAVE 2[34] | 429 | 10 mg BID | Remission at 8 weeks: 16.6% | AEs: 54.1%; SAEs: 4.2% |
| OCTAVE sustain[34,35] | 397 | 198: 5 mg BID; 197: 10 mg BID | Remission at 52 weeks: 34.3% vs 40.6% | AEs: 72%-80%; SAEs: 5.1%-5.6% |
| Ollech et al[36] | 30 | 10 mg BID | At 8 weeks clinical response and remission: 40% and 20% respectively | Not mentioned |
| Giri et al[37] | 47 | 10 mg BID | After 8 weeks: 70.2% achieved clinical remission. By 24 weeks 60% patients were at remission | AEs: 21.2% (two tuberculosis, one cytomegalovirus, and one HZV) |
| Hoffmann et al[38] | 38 | 10 mg BID | At 8 weeks: Steroid free remission was seen in 29% of patients | Three patients developed SAEs (pneumonia, colectomy, and colonic perforation) |
| Sandborn et al[23] | 194 | 10 mg BID: 33; 15 mg BID: 49 | 10 mg BID: Clinical response at 8 weeks: 42% with 10 mg BID | Nasopharyngitis (six patients), post-operative abscess, anal abscess (one patients), neutropenia (three patients) |
Contemporary real-world data have yielded similar results. A prospective study by Ollech et al[36] included 30 patients with moderate to severe UC (Table 2). Most of them (96.7%) previously failed on biological therapy and had extensive colitis (86.6%). At eight weeks, clinical, biochemical response (faecal calprotectin < 250 μg/g stool) and endo
Positioning tofacitinib in the algorithm of the treatment for moderate to severe UC has been a challenge. However, recent data did not show any statistically significant differences between tofacitinib, anti-TNF and ustekinumab for outcomes in patients with biological-naïve moderate to severe UC[38-40]. A network meta-analysis suggests that for biological-naïve patients, IFX ranked the highest for induction of clinical remission (odds ratio vs placebo, 4.07; 95% confidence interval: 2.67-6.21). Patients who received anti-TNF responded equally to tofacitinib and ustekinumab[40]. They were superior to vedolizumab and adalimumab[29,40,41].
Intravenous steroid remains the cornerstone of therapy in patients with ASUC[42]. 30%-40% of patients with ASUC may not respond to steroids and require other salvage therapies[43]. Tofacitinib has been used as a salvage therapy for patients with ASUC, not responding to steroids or even biologicals[44,45]. Overall efficacy ranges from 60%-70%[46-51]. A systematic review by Jena et al[46] included 21 patients with ASUC. Tofacitinib showed efficacy of 75% as the first-line therapy, 85.7% as the second-line therapy and 67% as the third-line therapy. A randomised controlled trial (RCT) from India has assessed the efficacy of tofacitinib combined with steroids in patients with ASUC (Table 3). While continuing steroids, at day seven, a higher response was seen in patients who received tofacitinib compared to placebo (83% vs 58%; P = 0.007). The need for rescue therapy was lower in patients receiving tofacitinib and steroids. Another series has also reported the usefulness of tofacitinib in patients with ASUC. Of eight patients with ASUC[52], seven patients responded to tofacitinib. One of the eight patients developed varicella zoster (VZ) infection, and another one required colectomy (Table 3). Other reports are listed in Table 3[53]. An open-label interventional study included 24 patients with ASUC. Among them, eight patients were anti-TNF-experienced, two received vedolizumab, and two received ustekinumab. At seven days, clinical response was seen in 58.3% of patients. The mean number of days to achieve clinical response was 2.4 days. In six months, colectomy was required in 25% patients[54].
| Ref. | Evidence | Number of patients | Anti-TNF-experienced | Response rate |
| Berinstein et al[44] | Case series | 4 | 2 patients | 75%; one patient failed to respond |
| Berinstein et al[45] | Case control | 40 | 100% | Lower rate of 90% colectomy compared to the control group |
| Uzzan et al[47] | Retrospective | 55 | 98% | Clinical response: 40% at week 6; clinical remission: 46% at week 14; colectomy free survival 74% at 6 months |
| Singh et al[48] | RCT | 53 | Not available | Day 7 response rate of tofacitinib: 83%; need of rescue therapy lower in tofacitinib group |
| Honap et al[49] | Case series | 7 (5 ASUC) | 100% | Three patients had colectomy-free survival |
| Sedano et al[50] | Case report | 1 | 100% | Complete remission |
| Kotwani et al[51] | Case series | 4 | 100% | All patients had complete remission |
| Malakar et al[52] | Case series | 8 | 12.5% | 87.5% patients responded by the fifth day of treatment |
| Bercier and Niland[53]; Narula et al[54] | Case report; open label interventional trial | 1, 24 | 100%, 33.3% | Complete remission; day-7 clinical response was seen in 58.3% with colectomy rate of 24% |
Tofacitinib was the 1st JAKi to be tried in patients with moderate to severe CD. In a large multicenter RCT in 2017, at eight weeks, the proportion of patients with clinical remission was 43.5% and 43% in the 5 mg and 10 mg twice a day groups, respectively. It was not statistically significant as compared to the placebo group (36.7%; P > 0.05)[55]. After the RCT failed to demonstrate the efficacy of tofacitinib in patients with moderate to severe CD, the major focus shifted to esta
In a phase 3 RCT, upadacitinib was found to be safe and effective in patients with moderately severe UC. 319 patients in the UC1 group and 354 patients in the UC2 group received 45 mg/day upadacitinib for 8 weeks. After 8 weeks, 26% of patients in UC1 and 34% of patients in the UC2 group achieved clinical response, which was statistically significant com
| Ref. | Number of patients | Clinical response at 8 weeks | Clinical remission on maintenance | Major adverse events |
| Danese et al[56], UC1 | 319 | 26% | UC3: 30 mg group: 52%; 15 group: 43% | Nasopharyngitis (5%) and creatine phosphokinase elevation (4%) |
| UC2 | 345 | 34% | UC3: 30 mg group: 52%; 15 group: 43% | Same as above |
| Sandborn et al[57], phase 2b | 250 | At 8 weeks clinical remission, 14.3%, 13.5% and 19.6% in 15 mg, 30 mg and 45 mg group | Endoscopic remission: 30.6%, 27% and 35.7% in 15 mg, 30 mg and 45 mg group | One patient herpes zoster, one pulmonary thromboembolism and deep venous thrombosis |
| Friedberg et al[58], Panaccione et al[59], U-ACTIVATE | 44; 369 (15 mg OD: 142 patients and 30 mg OD: 227 patients) | At 4 weeks clinical response: 60% and remission: 77%; not available | At 8 weeks clinical response: 67% and clinical remission: 83%; at 48 weeks, 81% of patients maintained clinical remission in 15 mg OD and 30 mg OD groups; at 96 weeks 47% of patients in 15 mg OD group and 45% patients in 30 mg OD group had endoscopic remission | Acne (23%); treatment emergent adverse events: 15 mg OD: 238.5 events per 100 patient years; 30 mg OD: Group: 233.4 events per 100 patient-years. Most common adverse events were hepatic dysfunction, rise in the CPK and lymphopenia |
The landmark CELEST trial, published in 2020, assessed the safety and efficacy of upadacitinib in patients with moderate to severe CD[60,61]. In this double-blind RCT, adults with non-response or intolerance to immunosuppressant or anti-TNF-α were randomised into a 1:1:1:1:1 group to receive either placebo or 3 mg, 6 mg, 12 mg or 24 mg of upadacitinib OD (Table 5). Patients completing 16 weeks of therapy were re-randomised for another 36 weeks of maintenance therapy. The upadacitinib group achieved higher clinical and endoscopic remission at 16 weeks compared to placebo (clinical response: 13% in 3 mg BD; 27% in 6 mg BD; 11% in 12 mg BD; 22% in 24 mg BD; 14% in 24 mg OD and 11% in placebo group; endoscopic remission: 10% in 3 mg BD; 8% in 6 mg BD; 8% in 12 mg BD; 22% in 24 mg BD and 14% in 24 mg OD and 0% in the placebo group; P ≤ 0.05) (Table 5). The remission rate was maintained at 52 weeks (Table 5). CELEST-OLE was the extension trial of CELEST. It recruited patients who responded to upadacitinib at 12 weeks. At 52 weeks, clinical remission was seen in 37.3% of patients receiving a 15 mg/day dose and 47.6% of patients receiving a 30 mg/kg dose. Endoscopic remission was seen in 40.1% and 28.6% of patients, respectively (Table 5). However, higher doses were associated with an increased number of HZ infections (4% in the 10 mg/day group vs 7.2% in the 15 mg/day group). In the U-EXCEL and U-EXCEED trials, patients with moderate to severe CD disease were randomly assigned to receive 45 mg OD upadacitinib or placebo (2:1). Patients who had a clinical response were included in the U-ENDURE maintenance trial (upadacitinib 15 mg vs 30 mg vs placebo). At 12 weeks, a significantly higher number of patients achieved clinical remission in the upadacitinib group as compared to the placebo (U-EXCEL: 49.5% vs 29.1%; U-EXCEED: 38.9% vs 21.1%). After 52 weeks (U-ENDURE), 37.3% and 47.5% patients had clinical remission in the 15 mg OD and 20 mg OD groups, respectively, as compared to the placebo (7.3%). HZ infection was common in the 15 mg and 30 mg groups[60].
| Drugs | Induction of remission | Induction rates | Long term outcome | Adverse events |
| Tofacitinib[55] | 180 | At 8 weeks clinical remission: 43.5% and 43% with 5 mg and 10 mg group | At 26 weeks, clinical response was maintained in 55.8% and 39.5% patients in 5 mg and 10 mg group respectively | Severe adverse events at 26 weeks 11.7% in 5 mg group and 9.8% in 10 mg group |
| Upadacitinib, Friedberg et al[58] | 40 | At 4 weeks clinical response: 60%; clinical remission: 87% | At 8 weeks clinical response 76%; clinical remission: 70.6% | Acne (23%) |
| Loftus et al[60], U-EXCEL, U-EXCEED and U-ENDURE | U-EXCEL: 526; U-EXCEED: 495; U-ENDURE: 502 | At 12 weeks clinical response was seen in U-EXCEL: 49.5% (vs 29.1% in placebo); U-EXCEED: 38.9% (vs 21.1% in placebo) | At 52 weeks higher patients on upadacitinib had clinical remission vs placebo (15 mg OD: 37.3%; 30 mg OD: 47.6%, 7.3% in placebo) | Herpes zoster; 30 mg group: 7.2 events per 100 person year; 15 mg group: 4.0 events per 100 person year; gastrointestinal perforation: 0.6-0.9 events per 100 person-year |
| Upadacitinib[61] | CELEST: 220; CELEST OLE: 107 | At 12 weeks CLE: Clinical remission: 44.4%; endoscopic response: 40.2% | CELEST OLE: At 52 weeks; clinical: 15 mg: 37.3%; 30 mg: 47.6%; endoscopic: 35.5% with 10 mg/day; 40.1%; remission: 15 mg/day: 19.1%; 30 mg/day: 28.6% | Herpes zoster: 4% in 10 mg group; and 7.2% in 15 mg group |
Previously, tofacitinib was used to treat CD. But it did not meet primary and secondary endpoints and failed to prove its clinical efficacy against CD. The earlier-mentioned study by Friedberg et al[58] has shown excellent results with up
After tofacitinib failed to show clinical efficacy in patients with moderate to severe CD, upadacitinib holds the position as a suitable JAKi in patients with CD. However, against UC, upadacitinib and tofacitinib both have excellent safety and efficacy. The role of tofacitinib or upadacitinib in patients with prior JAK2i exposure is evolving. A comparative real-world data has demonstrated the superior efficacy of upadacitinib in patients with tofacitinib-refractory UC. In that study, 81 patients received upadacitinib, and among them, 30% of patients had previously experienced tofacitinib. After inverse probability of treatment-weighted logistic regression, patients who received upadacitinib were shown to have a higher steroid-free clinical remission[62].
Indirect comparison of both drugs was done from the data of major placebo-controlled trials, which include U-ACHIEVE, U-ACCOMPLISH, GEMINI-1, UNIFI, and OCTAVE. After matching baseline characteristics, upadacitinib was found to be better than tofacitinib or vedolizumab for patients with active UC[60]. Recent meta-analysis has established the superiority of upadacitinib in achieving clinical, endoscopic and histological remission[63,64].
Filgotinib is approved by the European Medicines Agency (EMA) for adult patients with moderate to severe UC who failed to respond to biologicals[27,28,30,65,66]. It is an oral once-daily pill with a half-life of 6 hours. Filgotinib has a long duration of action as its metabolite has a half-life of 27 hours. The safety and efficacy of filgotinib were evaluated in the SELECTION trial[65]. Patients received 100 mg OD, 200 mg OD, or placebo. Patients who received a 200 mg/day dose only performed better compared to placebo (Table 6). At 58 weeks filgotinib group achieved better outcomes than the placebo with a comparable rate of AEs (Table 6). In moderate to severe CD, filgotinib has been used with acceptable safety and efficacy (Table 6). In the FITZROY trial, 47% of patients demonstrated clinical remission after 10 weeks[66].
| Ref. | Number of patients | Response at 10 weeks | Long term results | Adverse events |
| SELECTION, Feagan et al[65] | UC; 100 mg/day: 277; 200 mg/day: 245; placebo: 137 | At 10 weeks; clinical remission was seen in 26.1% of patients compared to 15.3% in placebo | At 58 weeks; 37.2% patients in 200 mg/day group had clinical remission compared to 11.2% | HZ 2 patients in 100 mg group and 4 patients in 200 mg group |
| FITZROY, Vermeire et al[66] | CD; 130 in 200 mg/day; 44 in placebo | At 10 weeks 47% in filgotinib group achieved clinical remission | At week 20, 50%-71% maintained clinical remission | Serious infection in 3% of patients in filgotinib group |
Other newer JAKi include peficitinib, izencitinib, ivarmacitinib, brepocitinib, OST-122, ritlecitinib and deucravacitinib[67-72]. Their mechanism of action and major role in IBD are described in Table 7[73,74].
Tofacitinib is currently recommended for managing patients with moderate to severe UC not responding to steroids, azathioprine, or biologicals. The American Gastroenterological Association recommends the use of upadacitinib and tofacitinib in moderate to severe UC[75]. Induction dose is 10 mg twice daily (BID) for at least 8 weeks, followed by a maintenance dose of 5 mg BID. Patients who fail to respond to the initial induction treatment, a 10 mg BID dose, can be extended for an additional eight weeks[29]. Therapeutic failure is considered if patients do not respond to a 10 mg BID dose after 16 weeks of therapy. A higher dose for the long-term period is associated with a higher incidence of AEs[29,76-82]. Upadicitinib is the only JAK-2i recommended in patients with moderate to severe CD. Based on the results of the previous RCT, 45 mg once daily for 12 weeks is used for the induction of remission. Responders received 15 mg OD or a 30 mg dose subsequently for the next 52 weeks[29,61-63]. Filgotinib is approved by the EMA for use in moderate to severe UC, but not by the Food and Drug Administration[29]. Generic JAKis are cheap and readily available in countries like India (tofacitinib)[77].
Upadacitinib has been used in moderate to severe UC that is not responding to anti-TNF therapy. Recently, upadacitinib has been tried off-label in patients with ASUC. Upadacitinib in ASUC is associated with rapid and sustained impro
Approved therapies for pediatric IBD are limited[81]. Anti-TNF and vedolizumab are reserved for refractory IBD in the pediatric population[81]. There have been a few reported cases and series documenting the safety of JAKi in pediatric patients with IBD. Miller et al[82] reported a case of refractory UC who were salvaged with upadacitinib. Based on current data, JAKi are not recommended in pediatric refractory IBD[82,83].
The reported prevalence of extraintestinal manifestations (EIM) ranges from 6%-47% in patients with IBD[84,85]. The prevalence and frequency of EIMs increase with longer disease duration, and they are more common in patients with CD[85]. Currently, anti-TNF therapy is the cornerstone in managing such patients; however, JAKi and anti-IL 12/23 are emerging as disease-modifying agents in patients with EIM[79-81]. Upregulation of JAK-STAT pathways is mostly found in various EIMs. Tofacitinib is approved for use in patients with rheumatoid arthritis and psoriatic arthritis[86-91]. Response rate ranges from 42% to 70%. A post-hoc analysis of the OCTAVE sustain study has demonstrated that tofacitinib can improve UC-related peripheral arthritis[92]. A report has demonstrated the efficacy of tofacitinib in patients with pyoderma gangrenosum associated with IBD[93]. Filgotinib has also been used in RA, PsA, and ankylosing spondylitis with a response rate of 64%-80%, 80% and 75%, respectively[94-97]. Among JAKis, only tofacitinib has shown promising results in various EIMs. After a multidisciplinary discussion (dermatologist and immunologist), they can be used in patients with IBD and EIM[98].
Combination therapies target different pathways of the inflammatory cascade in patients with IBD. Various combinations, including anti-TNF, vedolizumab, ustekinumab, and anti-IL-23, have been tried[99]. JAKi has been used in com
AEs associated with JAKi include overwhelming immunosuppression leading to infectious complications, cytopenia, elevated liver enzymes, vascular thrombosis, and dyslipidemia (Table 8)[104-106]. VZ infection is a well-known infectious complication associated with tofacitinib and upadacitinib. Headache (7.8%), nasopharyngitis (7.4%), arthritis (2.9%), and worsening colitis (2.3%) were the most reported AEs in the OCTAVE trial[34,35]. HZ virus and cytomegalovirus (CMV) infections were reported in 0.25%-0.6% of patients. In the OCTAVE sustain trial, HZ virus infection was reported in 1.5% and 5.1% of patients receiving 5 mg BD and 10 mg BD tofacitinib, respectively (Table 8)[34,35,107-109]. Other AEs include non-melanoma skin cancer, basal cell carcinoma, a rise in muscle enzymes, lymphopenia, dyslipidemia, and lymphoma[100-102]. In the OCTAVE sustain trial, after 52 weeks of treatment with tofacitinib, total cholesterol and triglycerides were elevated in 22% and 7% of patients, respectively (Table 8). A rise in CPK was seen in 27% of such patients. On long-term follow-up, around 10% of patients discontinued drugs for intolerable side effects, though serious AEs were present in only 5.1%-5.6% of patients[34-36,106]. In the TROPIC consortium, one of 76 patients developed venous thromboembolism[110].
| Adverse events | Frequency | Caution | Action |
| Bacterial infections[34,35,109] | Serious infection: 2.81 per 100 person-years; serious infection: 0.5%-1%; any infection: 36%-40% | Pneumonia, urinary tract infection and sepsis are most common | Proper screening and antibiotics |
| Varicella zoster reactivation[35,36,109] | OCTAVE sustain: 1.5%-5.1%; overall with JAKi: 2.67 per 100 person-year | More common in Asian population, old age, past IFX-failure, steroid experienced and 10 mg BID dose | Vaccination with Shingrix or GlaxoSmithKline 3-4 weeks prior to the treatment |
| Reactivation or infection with tuberculosis[37,112,113] | Tofacitinib: TB: Crude IR 0.21, 95%CI: 0.14-0.30 was the most common OI (n = 26); median time between drug start and diagnosis was 64 weeks (range 15-161 weeks); 2/47 | Test for latent tuberculosis by IGRA, Mantoux and chest imaging | Treat for LTBI infection |
| Venous thrombosis[56,114] | Tofacitinib: DVT: 0.04 events/100 PYs of exposure (95%CI: 0.00-0.23); PTE: 0.16 PY (95%CI: 0.04-0.41); one DVT and one PTE of 250 patients with UC receiving tofacitinib | History of malignancy, old age and prior history of DVT are the risk factors | Avoid or use with caution in patients with history of malignancy, history of DVT or old aged patients |
| Dyslipidemia[34,35] | Tofacitinib: 28%-30% | Not associated with major cardiovascular events | Baseline lipids then 4-8 weeks after therapy. And 6 monthly afterwards |
| Acne[57,111] | Upadacitinib: 15.9%; tofacitinib: 4.3%; filgotinib: 1.9% | History of acne vulgaris is a risk factor for JAKi-associated acne | 40% of patients with JAKi-associated acne can be managed with pharmacotherapy and 0.8% patients may require dose reduction |
| Bone marrow suppression[34,35,118] | 2/633 patients | JAK-STAT pathway involved in erythropoietin production, so JAKi may cause anemia | Blood count in baseline then 4-8 weeks after therapy. And 3 monthly afterwards; < 8 g/dL or drop > 2 g/dL: Stop until hemoglobin improves; ANC: < 500: Stop tofacitinib |
| Major adverse cardiovascular events[119] | Tofacitinib: Increased risk of MACE compared to placebo (OR = 5, 95%CI: 1.7-10) | Risk factors for MACE: Age > 49 years, with hypertension, and the total cholesterol to HDL cholesterol ratio | Use of statin in after proper cardiac risk evaluation using ASCVD risk calculator |
| Malignancy[116,118,120] | Over risk of malignancy: 4.2% most commonly: NMSC: IR of NMSC was 0.51 per 100 PYE; Others: Lung (22%) > breast (17.7%) > lymphoma (9.3%) | Past NMSC, anti-TNF failure and azathioprine use and age are the risk factors | Avoid in patients with history of NMSC |
| Hepatotoxicity[118] | 3/633 patients | May cause an asymptomatic rise in liver enzymes or worsen pre-existing liver disease | CTP A: No dose reduction; CTP B: 5 mg BID; CTP C: Contraindicated; blood count at baseline, then 4-8 weeks after therapy, and 3 monthly afterwards |
JAKi-associated acne is a well-known cosmetic side effect in patients with IBD. In a multi-centre retrospective study including 2183 JAKi-treated patients, the crude prevalence rate of acne was 15.9% in the upadacitinib group, 4.3% in the tofacitinib group and 1.9% in the filgotinib group. 40% patients with JAKi-associated acne were managed with pharmacological therapy, whereas only 18 patients required a dose reduction of JAKi[111].
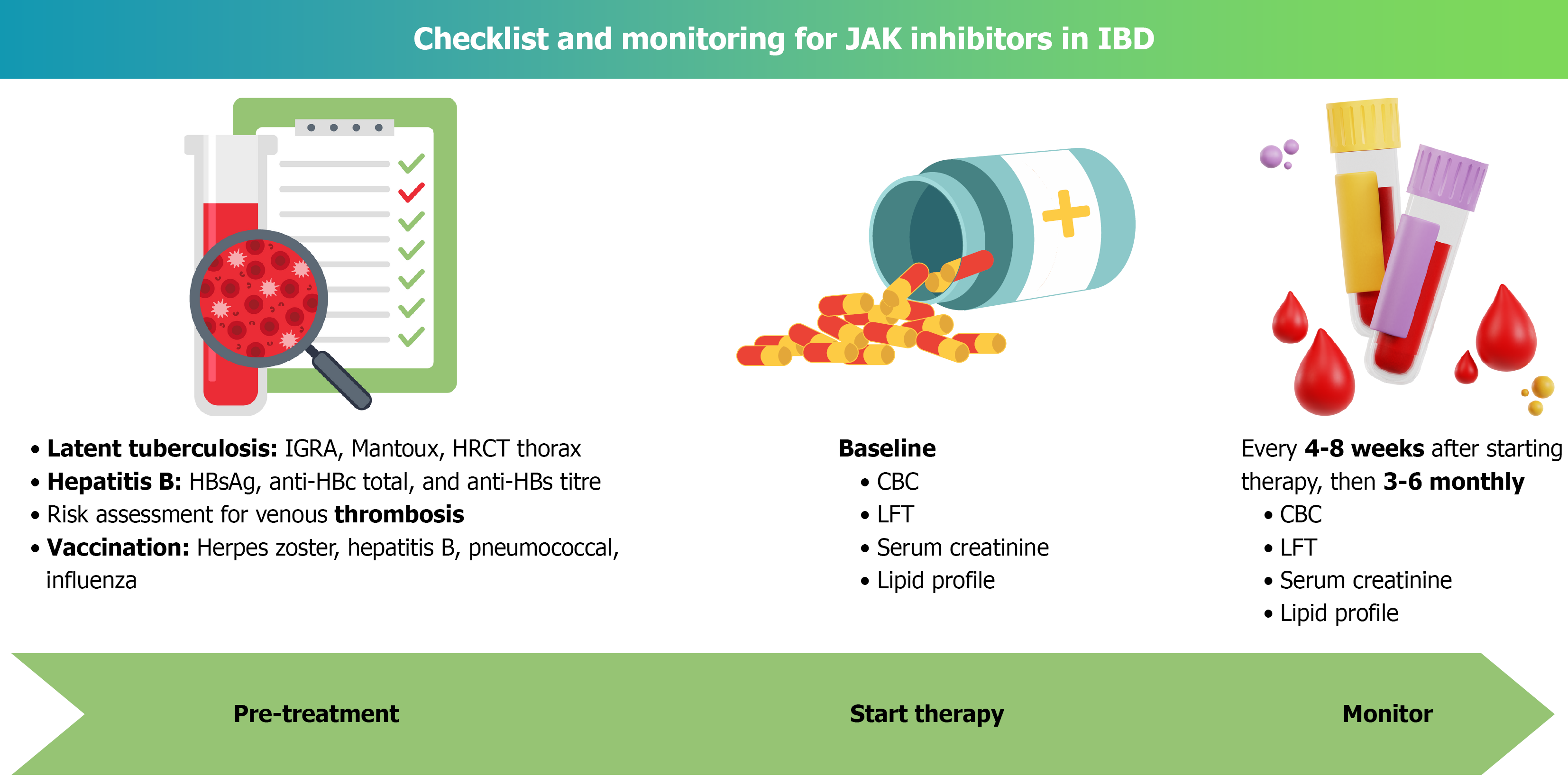
Data on the long-term complication rate with upadacitinib originated mostly from the clinical and real-world studies in rheumatological diseases (Table 8). Median duration of onset of all AEs with upadacitinib is 93 days[112-114]. Risk factors of HZ virus infection include old age (> 50 years) and prior infection. 95% of those infections are non-fatal and self-limiting after cessation of the drugs (Figure 2). A recent systematic review and meta-analysis have demonstrated the overall safety profile of upadacitinib; however, it was associated with an increased risk of hepatic disorder, neutropenia, acne, HZ infection, and increased CPK level. Upadacitinib use was not associated with renal dysfunction, non-melanoma skin cancer, cardiac events or thromboembolic complications[115-120].
Pre-clinical animal studies suggest that tofacitinib crosses the placental barrier and can be associated with fetal malformations. Most data on the feto-maternal outcome following tofacitinib exposure originates from patients with rheumatological diseases[121-123]. 47 pregnancies from a rheumatological cohort who were exposed to tofacitinib were assessed for feto-maternal complications. Seven patients had spontaneous abortions, and one case of pulmonary valve stenosis was reported. Eight of them underwent medical termination of pregnancy. Spontaneous abortion has been reported from a UC cohort who were exposed to tofacitinib[121-123]. Most data comes from retrospective studies. Currently, the use of tofacitinib is not recommended in pregnancy and breastfeeding[29]. Upadacitinib is not currently recommended in lactating mothers with IBD; however, recent data from lactating mothers receiving upadacitinib have shown that the median upadacitinib milk concentration was below the theoretical cut-off for acceptable excretion in breastmilk[124]. Large prospective data is warranted to establish its safety and efficacy during pregnancy and lactation.
JAKi are relatively newer molecules for managing IBD. The majority of data on the long-term safety and efficacy have emerged from patients with rheumatological diseases like RA and psoriatic arthritis. Unlike 5-aminosalicylic acid, their impact on colorectal carcinogenesis in patients with IBD is unknown[125,126]. Non-melanoma skin cancers have been associated with the use of JAKi; long-term data on patients with IBD would solve the concerns. Acute infectious complications are avoidable if proper screening of latent infection is carried out or risk factors are identified and managed properly before the initiation of JAKi. VZ reactivation is a common AE reported in landmark trials as well as contemporary real-world data (Table 8)[34,35]. CMV belongs to the same family (herpesvirus). So, there is a theoretical risk of reactivation of CMV in patients with UC with JAKi[127,128]. However, data is scarce regarding the issue. There have been cases of reactivation of TB with the use of tofacitinib[37]. With its widespread use in the Asian sub-continent, the risk of reactivation of latent TB will be a major concern[37]. The data on its teratogenicity and safety in breastfeeding are yet to be established[122-124]. Many phase II and clinical trials are ongoing to assess the newer JAKi, including peficitinib, izencitinib, ivarmacitinib, brepocitinib, OST-122, ritlecitinib and deucravacitinib[105]. There is a lack of large data on the safety and efficacy of JAKi in pediatric patients and patients with EIM[76,77]. JAKis are being used with other therapies like IFX, vedolizumab and ustekinumab, though comparative data are lacking[99-101]. Large multicentre prospective data is warranted to assess the safety of combination therapy in IBD.
Despite the risk of infectious and other non-infectious AEs, JAKi are an excellent choice for patients with IBD (Table 8). After properly screening patients for the risk of infections, JAKi can be started safely[129]. Patients on JAKi should be closely monitored for cytopenia, HZ rash, elevated liver enzymes and other infectious complications (Figure 2, Table 8). Well-designed RCTs are warranted to establish the role of tofacitinib in steroid-refractory ASUC. Prospective real-world data with a long follow-up on JAKi in patients with IBD can answer the concerns regarding its long-term safety, efficacy, and implications of colonic carcinogenesis.
Tofacitinib and upadacitinib are safe and effective in managing biologics-experienced patients with IBD. Emerging data suggest that tofacitinib can be used as a salvage therapy in steroid-refractory or steroid-dependent UC and ASUC. Tofacitinib is ineffective in patients with CD. Whereas, conversely, upadacitinib has been used in patients with tofacitinib-experienced UC. Combining JAKi with other immunosuppressive therapies should be avoided, as it may lead to profound immunosuppression. Bacterial, viral, and mycobacterial infections and reactivation remain the major concerns while using JAKi. Proper screening and vaccinations can prevent such complications. Patients should be monitored carefully for other side effects. With expanding data on their efficacy and safety, JAKi are poised to transform the therapeutic landscape of IBD. Future research should continue to explore gut-selective agents to maximise benefit and minimise systemic risks.
| 1. | Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20:91-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 751] [Cited by in RCA: 1132] [Article Influence: 94.3] [Reference Citation Analysis (31)] |
| 2. | Chang JT. Pathophysiology of Inflammatory Bowel Diseases. N Engl J Med. 2020;383:2652-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 892] [Article Influence: 148.7] [Reference Citation Analysis (0)] |
| 3. | Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2019] [Cited by in RCA: 1980] [Article Influence: 132.0] [Reference Citation Analysis (2)] |
| 4. | Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014;13:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 743] [Article Influence: 57.2] [Reference Citation Analysis (2)] |
| 5. | Weimers P, Munkholm P. The Natural History of IBD: Lessons Learned. Curr Treat Options Gastroenterol. 2018;16:101-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Cai Z, Wang S, Li J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front Med (Lausanne). 2021;8:765474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 368] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 7. | Luo H, Cao G, Luo C, Tan D, Vong CT, Xu Y, Wang S, Lu H, Wang Y, Jing W. Emerging pharmacotherapy for inflammatory bowel diseases. Pharmacol Res. 2022;178:106146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 8. | Ben Ghezala I, Charkaoui M, Michiels C, Bardou M, Luu M. Small Molecule Drugs in Inflammatory Bowel Diseases. Pharmaceuticals (Basel). 2021;14:637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Hazel K, O'Connor A. Emerging treatments for inflammatory bowel disease. Ther Adv Chronic Dis. 2020;11:2040622319899297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 10. | O'Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis. 2013;72 Suppl 2:ii111-ii115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 344] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 11. | Wu Y, Lin B, Thilakanathan C, Lehmann P, Xuan W, Mohsen W, Toong C, Williams AJ, Ng W, Connor S. Therapeutic drug monitoring in inflammatory bowel disease reduces unnecessary use of infliximab with substantial associated cost-savings. Intern Med J. 2021;51:739-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Giri S, Bhrugumalla S, Shukla A, Gangadhar S, Reddy S, Angadi S, Shinde L, Kale A. Risk of tuberculosis with anti-TNF therapy in Indian patients with inflammatory bowel disease despite negative screening. Arab J Gastroenterol. 2025;26:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Roda G, Jharap B, Neeraj N, Colombel JF. Loss of Response to Anti-TNFs: Definition, Epidemiology, and Management. Clin Transl Gastroenterol. 2016;7:e135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 557] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 14. | Justiniano CF, Aquina CT, Becerra AZ, Xu Z, Boodry CI, Swanger AA, Monson JRT, Fleming FJ. Postoperative Mortality After Nonelective Surgery for Inflammatory Bowel Disease Patients in the Era of Biologics. Ann Surg. 2019;269:686-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Palacio FGM, de Souza LMP, Moreira JPL, Luiz RR, de Souza HSP, Zaltman C. Hospitalization and surgery rates in patients with inflammatory bowel disease in Brazil: a time-trend analysis. BMC Gastroenterol. 2021;21:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Jefremow A, Neurath MF. Novel Small Molecules in IBD: Current State and Future Perspectives. Cells. 2023;12:1730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 17. | Weissman S, Saleem S, Aldulaimi D. Landmark studies and emerging strategies for the management of acute severe ulcerative colitis. Gastroenterol Hepatol Bed Bench. 2019;12:179-182. [PubMed] |
| 18. | Spiewak TA, Patel A. User's guide to JAK inhibitors in inflammatory bowel disease. Curr Res Pharmacol Drug Discov. 2022;3:100096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Wodeyar AM, Pansuriya N, Saeed S, Lakhani A, Sartaj S, Keerthi NSJ, Guntur Bhuvika Raji A, S B, Wahane V, Thapa Y, Abriha F. Upadacitinib in Crohn's Disease: A Comprehensive Systematic Review of Efficacy and Safety. Cureus. 2023;15:e50657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 20. | Wlassits R, Müller M, Fenzl KH, Lamprecht T, Erlacher L. JAK-Inhibitors - A Story of Success and Adverse Events. Open Access Rheumatol. 2024;16:43-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 21. | Hoisnard L, Lebrun-Vignes B, Maury S, Mahevas M, El Karoui K, Roy L, Zarour A, Michel M, Cohen JL, Amiot A, Claudepierre P, Wolkenstein P, Grimbert P, Sbidian E. Adverse events associated with JAK inhibitors in 126,815 reports from the WHO pharmacovigilance database. Sci Rep. 2022;12:7140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 113] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 22. | D'Amico F, Parigi TL, Fiorino G, Peyrin-Biroulet L, Danese S. Tofacitinib in the treatment of ulcerative colitis: efficacy and safety from clinical trials to real-world experience. Therap Adv Gastroenterol. 2019;12:1756284819848631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 23. | Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, Niezychowski W; Study A3921063 Investigators. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 644] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 24. | Wood NJ. IBD: Could oral Janus kinase inhibitor be a new drug treatment for active ulcerative colitis? Nat Rev Gastroenterol Hepatol. 2012;9:557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Feagan B. Update on Tofacitinib for Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y). 2016;12:572-574. [PubMed] |
| 26. | Shawky AM, Almalki FA, Abdalla AN, Abdelazeem AH, Gouda AM. A Comprehensive Overview of Globally Approved JAK Inhibitors. Pharmaceutics. 2022;14:1001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 228] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 27. | Herrera-deGuise C, Serra-Ruiz X, Lastiri E, Borruel N. JAK inhibitors: A new dawn for oral therapies in inflammatory bowel diseases. Front Med (Lausanne). 2023;10:1089099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 28. | Antonioli L, Armuzzi A, Fantini MC, Fornai M. JAK inhibitors: an evidence-based choice of the most appropriate molecule. Front Pharmacol. 2024;15:1494901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 29. | Gordon H, Biancone L, Fiorino G, Katsanos KH, Kopylov U, Al Sulais E, Axelrad JE, Balendran K, Burisch J, de Ridder L, Derikx L, Ellul P, Greuter T, Iacucci M, Di Jiang C, Kapizioni C, Karmiris K, Kirchgesner J, Laharie D, Lobatón T, Molnár T, Noor NM, Rao R, Saibeni S, Scharl M, Vavricka SR, Raine T. ECCO Guidelines on Inflammatory Bowel Disease and Malignancies. J Crohns Colitis. 2023;17:827-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 204] [Reference Citation Analysis (0)] |
| 30. | Lefevre PLC, Vande Casteele N. Clinical Pharmacology of Janus Kinase Inhibitors in Inflammatory Bowel Disease. J Crohns Colitis. 2020;14:S725-S736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Eichner A, Wohlrab J. Pharmacology of inhibitors of Janus kinases - Part 1: Pharmacokinetics. J Dtsch Dermatol Ges. 2022;20:1485-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 32. | Sandborn WJ, Feagan BG, Loftus EV Jr, Peyrin-Biroulet L, Van Assche G, D'Haens G, Schreiber S, Colombel JF, Lewis JD, Ghosh S, Armuzzi A, Scherl E, Herfarth H, Vitale L, Mohamed MF, Othman AA, Zhou Q, Huang B, Thakkar RB, Pangan AL, Lacerda AP, Panes J. Efficacy and Safety of Upadacitinib in a Randomized Trial of Patients With Crohn's Disease. Gastroenterology. 2020;158:2123-2138.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 241] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 33. | Fanizza J, D'Amico F, Lauri G, Martinez-Dominguez SJ, Allocca M, Furfaro F, Zilli A, Fiorino G, Parigi TL, Radice S, Peyrin-Biroulet L, Danese S. The role of filgotinib in ulcerative colitis and Crohn's disease. Immunotherapy. 2024;16:59-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Sandborn WJ, Su C, Sands BE, D'Haens GR, Vermeire S, Schreiber S, Danese S, Feagan BG, Reinisch W, Niezychowski W, Friedman G, Lawendy N, Yu D, Woodworth D, Mukherjee A, Zhang H, Healey P, Panés J; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2017;376:1723-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 1291] [Article Influence: 143.4] [Reference Citation Analysis (0)] |
| 35. | Sandborn WJ, Lawendy N, Danese S, Su C, Loftus EV Jr, Hart A, Dotan I, Damião AOMC, Judd DT, Guo X, Modesto I, Wang W, Panés J. Safety and efficacy of tofacitinib for treatment of ulcerative colitis: final analysis of OCTAVE Open, an open-label, long-term extension study with up to 7.0 years of treatment. Aliment Pharmacol Ther. 2022;55:464-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 124] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 36. | Ollech JE, Eran-Banai H, Goren I, Sharar Fischler T, Avni-Biron I, Snir Y, Broitman Y, Cohen S, Friedenberg A, Pauker MH, Dotan I, Yanai H. Tofacitinib is an effective treatment for moderate to severe ulcerative colitis, and intestinal ultrasound can discriminate response from non-response: a pragmatic prospective real-world study. Ann Med. 2024;56:2358183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 37. | Giri S, Bhrugumalla S, Kamuni A, Mishra D, Pati GK, Agrawal D, Verma G, Wagh R, Chauhan S, Ingle M, Chandnani S, Jain S, Rathi PM, Shukla A, Kale A. Upfront tofacitinib in patients with biological-naïve ulcerative colitis - An Indian multicentric experience. Indian J Gastroenterol. 2024;43:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 38. | Hoffmann P, Globig AM, Thomann AK, Grigorian M, Krisam J, Hasselblatt P, Reindl W, Gauss A. Tofacitinib in Treatment-Refractory Moderate to Severe Ulcerative Colitis: Real-World Experience from a Retrospective Multicenter Observational Study. J Clin Med. 2020;9:2177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 39. | Strauss I, Mundstock A, Hinrichs D, Himstedt R, Knebel A, Reinhardt C, Dorfs D, Caro J. The Interaction of Guest Molecules with Co-MOF-74: A Vis/NIR and Raman Approach. Angew Chem Int Ed Engl. 2018;57:7434-7439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 40. | Singh S, Murad MH, Fumery M, Dulai PS, Sandborn WJ. First- and Second-Line Pharmacotherapies for Patients With Moderate to Severely Active Ulcerative Colitis: An Updated Network Meta-Analysis. Clin Gastroenterol Hepatol. 2020;18:2179-2191.e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 294] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 41. | Paschos P, Katsoula A, Giouleme O, Sarigianni M, Liakos A, Athanasiadou E, Bekiari E, Tsapas A. Tofacitinib for induction of remission in ulcerative colitis: systematic review and meta-analysis. Ann Gastroenterol. 2018;31:572-582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Calméjane L, Laharie D, Kirchgesner J, Uzzan M. Review article: Updated management of acute severe ulcerative colitis: From steroids to novel medical strategies. United European Gastroenterol J. 2023;11:722-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 43. | Gisbert JP, García MJ, Chaparro M. Rescue Therapies for Steroid-refractory Acute Severe Ulcerative Colitis: A Review. J Crohns Colitis. 2023;17:972-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 44. | Berinstein JA, Steiner CA, Regal RE, Allen JI, Kinnucan JAR, Stidham RW, Waljee AK, Bishu S, Aldrich LB, Higgins PDR. Efficacy of Induction Therapy With High-Intensity Tofacitinib in 4 Patients With Acute Severe Ulcerative Colitis. Clin Gastroenterol Hepatol. 2019;17:988-990.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 45. | Berinstein JA, Sheehan JL, Dias M, Berinstein EM, Steiner CA, Johnson LA, Regal RE, Allen JI, Cushing KC, Stidham RW, Bishu S, Kinnucan JAR, Cohen-Mekelburg SA, Waljee AK, Higgins PDR. Tofacitinib for Biologic-Experienced Hospitalized Patients With Acute Severe Ulcerative Colitis: A Retrospective Case-Control Study. Clin Gastroenterol Hepatol. 2021;19:2112-2120.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 46. | Jena A, Mishra S, Sachan A, Singh H, Singh AK, Sharma V. Tofacitinib in Acute Severe Ulcerative Colitis: Case Series and a Systematic Review. Inflamm Bowel Dis. 2021;27:e101-e103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 47. | Uzzan M, Bresteau C, Laharie D, Stefanescu C, Bellanger C, Carbonnel F, Serrero M, Viennot S, Nachury M, Amiot A, Altwegg R, Picon L, Nahon S, Vuitton L, Ah Soune P, Kirchgesner J, Peyrin-Biroulet L, Bouhnik Y; GETAID-TALC Study Group. Tofacitinib as salvage therapy for 55 patients hospitalised with refractory severe ulcerative colitis: A GETAID cohort. Aliment Pharmacol Ther. 2021;54:312-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 48. | Singh A, Goyal MK, Midha V, Mahajan R, Kaur K, Gupta YK, Singh D, Bansal N, Kaur R, Kalra S, Goyal O, Mehta V, Sood A. Tofacitinib in Acute Severe Ulcerative Colitis (TACOS): A Randomized Controlled Trial. Am J Gastroenterol. 2024;119:1365-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 49. | Honap S, Pavlidis P, Ray S, Sharma E, Anderson S, Sanderson JD, Mawdsley J, Samaan MA, Irving PM. Tofacitinib in Acute Severe Ulcerative Colitis-A Real-World Tertiary Center Experience. Inflamm Bowel Dis. 2020;26:e147-e149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 50. | Sedano R, Jairath V. High-Dose Rescue Tofacitinib Prevented Inpatient Colectomy in Acute Severe Ulcerative Colitis Refractory to Anti-TNF. Inflamm Bowel Dis. 2021;27:e59-e60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Kotwani P, Terdiman J, Lewin S. Tofacitinib for Rescue Therapy in Acute Severe Ulcerative Colitis: A Real-world Experience. J Crohns Colitis. 2020;14:1026-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 52. | Malakar S, Kothalkar S, Shamsul Hoda U, Ghoshal UC. Tofacitinib in Steroid-Refractory Acute Severe Ulcerative Colitis: A Retrospective Analysis. Cureus. 2023;15:e45416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 53. | Bercier B, Niland B. S2282Use of Tofacitinib as Rescue Therapy in a Biologic-Experienced Patient Hospitalized With Acute Severe Ulcerative Colitis. Am J Gastroenterol. 2020;115:S1207-S1207. [DOI] [Full Text] |
| 54. | Narula N, Pray C, Hamam H, Peerani F, Hansen T, Bessissow T, Bressler B, Arun A, Schmit M, Castelli J, Marshall JK. Tofacitinib for Hospitalized Acute Severe Ulcerative Colitis Management (The TRIUMPH Study). Crohns Colitis 360. 2025;7:otaf013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 55. | Panés J, Sandborn WJ, Schreiber S, Sands BE, Vermeire S, D'Haens G, Panaccione R, Higgins PDR, Colombel JF, Feagan BG, Chan G, Moscariello M, Wang W, Niezychowski W, Marren A, Healey P, Maller E. Tofacitinib for induction and maintenance therapy of Crohn's disease: results of two phase IIb randomised placebo-controlled trials. Gut. 2017;66:1049-1059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 300] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 56. | Danese S, Vermeire S, Zhou W, Pangan AL, Siffledeen J, Greenbloom S, Hébuterne X, D'Haens G, Nakase H, Panés J, Higgins PDR, Juillerat P, Lindsay JO, Loftus EV Jr, Sandborn WJ, Reinisch W, Chen MH, Sanchez Gonzalez Y, Huang B, Xie W, Liu J, Weinreich MA, Panaccione R. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399:2113-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 405] [Article Influence: 101.3] [Reference Citation Analysis (0)] |
| 57. | Sandborn WJ, Ghosh S, Panes J, Schreiber S, D'Haens G, Tanida S, Siffledeen J, Enejosa J, Zhou W, Othman AA, Huang B, Higgins PDR. Efficacy of Upadacitinib in a Randomized Trial of Patients With Active Ulcerative Colitis. Gastroenterology. 2020;158:2139-2149.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 198] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 58. | Friedberg S, Choi D, Hunold T, Choi NK, Garcia NM, Picker EA, Cohen NA, Cohen RD, Dalal SR, Pekow J, Sakuraba A, Krugliak Cleveland N, Rubin DT. Upadacitinib Is Effective and Safe in Both Ulcerative Colitis and Crohn's Disease: Prospective Real-World Experience. Clin Gastroenterol Hepatol. 2023;21:1913-1923.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 95] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 59. | Panaccione R, Vermeire S, Danese S, Higgins PDR, Lichtenstein GR, Nakase H, Glover S, Colombel JF, Eccleston J, Kujawski M, Remple V, Yao X, Geng Z, Palac H, Sharma D, Suravaram S, Schreiber S. Long-term efficacy and safety of upadacitinib in patients with moderately to severely active ulcerative colitis: an interim analysis of the phase 3 U-ACTIVATE long-term extension study. Lancet Gastroenterol Hepatol. 2025;10:507-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 60. | Loftus EV Jr, Panés J, Lacerda AP, Peyrin-Biroulet L, D'Haens G, Panaccione R, Reinisch W, Louis E, Chen M, Nakase H, Begun J, Boland BS, Phillips C, Mohamed MF, Liu J, Geng Z, Feng T, Dubcenco E, Colombel JF. Upadacitinib Induction and Maintenance Therapy for Crohn's Disease. N Engl J Med. 2023;388:1966-1980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 293] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 61. | D'Haens G, Panés J, Louis E, Lacerda A, Zhou Q, Liu J, Loftus EV Jr. Upadacitinib Was Efficacious and Well-tolerated Over 30 Months in Patients With Crohn's Disease in the CELEST Extension Study. Clin Gastroenterol Hepatol. 2022;20:2337-2346.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 62. | Dalal RS, Kallumkal G, Cabral HJ, Barnes EL, Allegretti JR. One-Year Comparative Effectiveness of Upadacitinib vs Tofacitinib for Ulcerative Colitis: A Multicenter Cohort Study. Am J Gastroenterol. 2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 63. | Shehab M, Alrashed F, Alsayegh A, Aldallal U, Ma C, Narula N, Jairath V, Singh S, Bessissow T. Comparative Efficacy of Biologics and Small Molecule in Ulcerative Colitis: A Systematic Review and Network Meta-analysis. Clin Gastroenterol Hepatol. 2025;23:250-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 64. | Reinisch W, Melmed GY, Nakase H, Seidelin J, Ma C, Xuan S, Tran J, Remple V, Wegrzyn L, Levy G, Sanchez Gonzalez Y, Panaccione R. Comparative Efficacy and Safety of Upadacitinib vs. Vedolizumab, Ustekinumab, and Tofacitinib After Induction and Maintenance for Ulcerative Colitis: Three Matching-Adjusted Indirect Comparisons. Adv Ther. 2024;41:3832-3849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 65. | Feagan BG, Danese S, Loftus EV Jr, Vermeire S, Schreiber S, Ritter T, Fogel R, Mehta R, Nijhawan S, Kempiński R, Filip R, Hospodarskyy I, Seidler U, Seibold F, Beales ILP, Kim HJ, McNally J, Yun C, Zhao S, Liu X, Hsueh CH, Tasset C, Besuyen R, Watanabe M, Sandborn WJ, Rogler G, Hibi T, Peyrin-Biroulet L. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): a phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet. 2021;397:2372-2384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 324] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 66. | Vermeire S, Schreiber S, Petryka R, Kuehbacher T, Hebuterne X, Roblin X, Klopocka M, Goldis A, Wisniewska-Jarosinska M, Baranovsky A, Sike R, Stoyanova K, Tasset C, Van der Aa A, Harrison P. Clinical remission in patients with moderate-to-severe Crohn's disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet. 2017;389:266-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 350] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 67. | Liu E, Aslam N, Nigam G, Limdi JK. Tofacitinib and newer JAK inhibitors in inflammatory bowel disease-where we are and where we are going. Drugs Context. 2022;11:2021-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 68. | Lasa JS, Olivera PA, Danese S, Peyrin-Biroulet L. Efficacy and safety of biologics and small molecule drugs for patients with moderate-to-severe ulcerative colitis: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 269] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 69. | Shivaji UN, Nardone OM, Cannatelli R, Smith SC, Ghosh S, Iacucci M. Small molecule oral targeted therapies in ulcerative colitis. Lancet Gastroenterol Hepatol. 2020;5:850-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 70. | Wang Z, Sun L, Xu Y, Liang P, Xu K, Huang J. Discovery of novel JAK1 inhibitors through combining machine learning, structure-based pharmacophore modeling and bio-evaluation. J Transl Med. 2023;21:579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 71. | Sands BE, Sandborn WJ, Feagan BG, Lichtenstein GR, Zhang H, Strauss R, Szapary P, Johanns J, Panes J, Vermeire S, O'Brien CD, Yang Z, Bertelsen K, Marano C; Peficitinib-UC Study Group. Peficitinib, an Oral Janus Kinase Inhibitor, in Moderate-to-severe Ulcerative Colitis: Results From a Randomised, Phase 2 Study. J Crohns Colitis. 2018;12:1158-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 72. | Chen B, Zhong J, Li X, Pan F, Ding Y, Zhang Y, Chen H, Liu F, Zhang Z, Zhang L, Drozda R, Oliinyk O, Goh AH, Chen X, Sun X, Rubin DT, Sandborn WJ, Chen M. Efficacy and Safety of Ivarmacitinib in Patients With Moderate-to-Severe, Active, Ulcerative Colitis: A Phase II Study. Gastroenterology. 2022;163:1555-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 73. | Nielsen OH, Boye TL, Chakravarti D, Gubatan J. Selective tyrosine kinase 2 inhibitors in inflammatory bowel disease. Trends Pharmacol Sci. 2022;43:424-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 74. | Sandborn WJ, Nguyen DD, Beattie DT, Brassil P, Krey W, Woo J, Situ E, Sana R, Sandvik E, Pulido-Rios MT, Bhandari R, Leighton JA, Ganeshappa R, Boyle DL, Abhyankar B, Kleinschek MA, Graham RA, Panes J. Development of Gut-Selective Pan-Janus Kinase Inhibitor TD-1473 for Ulcerative Colitis: A Translational Medicine Programme. J Crohns Colitis. 2020;14:1202-1213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 75. | Singh S, Loftus EV Jr, Limketkai BN, Haydek JP, Agrawal M, Scott FI, Ananthakrishnan AN; AGA Clinical Guidelines Committee. AGA Living Clinical Practice Guideline on Pharmacological Management of Moderate-to-Severe Ulcerative Colitis. Gastroenterology. 2024;167:1307-1343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 76. | Banerjee R, Sharma V, Patel R, Jena A, Pal P, Raghunathan N, Kumar A, Sood A, Puri AS, Goswami B, Desai D, Mekala D, Ramesh GN, Rao GV, Peddi K, Philip M, Tandon M, Bhatia S, Godbole S, Bhatia S, Ghoshal UC, Dutta U, Midha V, Prasad VGM, Reddy DN. Tofacitinib use in ulcerative colitis: An expert consensus for day-to-day clinical practice. Indian J Gastroenterol. 2024;43:22-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 77. | Darie AM, Sinopoulou V, Ajay V, Bel Kok K, Patel KV, Limdi J, Arebi N, Smith P, Din S, Din S, Shale M, Subramanian S, Pavlidis P, Cooney R, McGonagle D, A C S Wong N, Moran GW, Gordon M. BSG 2024 IBD guidelines protocol (standard operating procedures). BMJ Open Gastroenterol. 2023;10:e001067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 78. | Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, Hayee B, Lomer MCE, Parkes GC, Selinger C, Barrett KJ, Davies RJ, Bennett C, Gittens S, Dunlop MG, Faiz O, Fraser A, Garrick V, Johnston PD, Parkes M, Sanderson J, Terry H; IBD guidelines eDelphi consensus group, Gaya DR, Iqbal TH, Taylor SA, Smith M, Brookes M, Hansen R, Hawthorne AB. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1-s106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1929] [Cited by in RCA: 1718] [Article Influence: 245.4] [Reference Citation Analysis (0)] |
| 79. | Patel PV, Rigmaiden M, Goyal A, Bensen R, Bass D, Moses J, Rosen MJ, Colman RJ. Intensified Upadacitinib Dosing for Adolescent Patients with Acute Severe Ulcerative Colitis. Children (Basel). 2025;12:401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 80. | Damianos JA, Osikoya O, Brennan G. Upadacitinib for Acute Severe Ulcerative Colitis: A Systematic Review. Inflamm Bowel Dis. 2025;31:1145-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 81. | Turner D, Ruemmele FM, Orlanski-Meyer E, Griffiths AM, de Carpi JM, Bronsky J, Veres G, Aloi M, Strisciuglio C, Braegger CP, Assa A, Romano C, Hussey S, Stanton M, Pakarinen M, de Ridder L, Katsanos K, Croft N, Navas-López V, Wilson DC, Lawrence S, Russell RK. Management of Paediatric Ulcerative Colitis, Part 1: Ambulatory Care-An Evidence-based Guideline From European Crohn's and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018;67:257-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 360] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 82. | Miller M, Patel AS, Pasternak B. Rescue therapy with upadacitinib in medically refractory pediatric ulcerative colitis. JPGN Rep. 2024;5:197-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 83. | Cohen S, Spencer EA, Dolinger MT, Suskind DL, Mitrova K, Hradsky O, Conrad MA, Kelsen JR, Uhlig HH, Tzivinikos C, Henderson P, Wlazlo M, Hackl L, Shouval DS, Bramuzzo M, Urlep D, Olbjørn C, D'Arcangelo G, Pujol-Muncunill G, Yogev D, Kang B, Gasparetto M, Rungoe C, Kolho KL, Hojsak I, Norsa L, Rinawi F, Sansotta N, Rimon RM, Granot M, Scarallo L, Trindade E, Rodríguez-Belvís MV, Turner D, Yerushalmy-Feler A. Upadacitinib for Induction of Remission in Paediatric Crohn's Disease: An International Multicentre Retrospective Study. Aliment Pharmacol Ther. 2025;61:1372-1380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 84. | Bernstein CN, Blanchard JF, Rawsthorne P, Yu N. The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2001;96:1116-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 520] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 85. | Vavricka SR, Schoepfer A, Scharl M, Lakatos PL, Navarini A, Rogler G. Extraintestinal Manifestations of Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21:1982-1992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 546] [Cited by in RCA: 500] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 86. | Brooklyn TN, Dunnill MG, Shetty A, Bowden JJ, Williams JD, Griffiths CE, Forbes A, Greenwood R, Probert CS. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006;55:505-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 434] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 87. | Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley JD, Gruben D, Koncz T, Krishnaswami S, Wallenstein GV, Zang C, Zwillich SH, van Vollenhoven RF; ORAL Start Investigators. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370:2377-2386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 613] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 88. | Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, Gruben D, Wallenstein GV, Zwillich SH, Kanik KS; ORAL Solo Investigators. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367:495-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 788] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 89. | Kremer J, Li ZG, Hall S, Fleischmann R, Genovese M, Martin-Mola E, Isaacs JD, Gruben D, Wallenstein G, Krishnaswami S, Zwillich SH, Koncz T, Riese R, Bradley J. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2013;159:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 373] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 90. | van der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, Cardiel MH, Cohen S, Nash P, Song YW, Tegzová D, Wyman BT, Gruben D, Benda B, Wallenstein G, Krishnaswami S, Zwillich SH, Bradley JD, Connell CA; ORAL Scan Investigators. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 2013;65:559-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 463] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 91. | Burmester GR, Blanco R, Charles-Schoeman C, Wollenhaupt J, Zerbini C, Benda B, Gruben D, Wallenstein G, Krishnaswami S, Zwillich SH, Koncz T, Soma K, Bradley J, Mebus C; ORAL Step investigators. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381:451-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 571] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 92. | Rubin DT, Reinisch W, Greuter T, Kotze PG, Pinheiro M, Mundayat R, Maller E, Fellmann M, Lawendy N, Modesto I, Vavricka SR, Lichtenstein GR. Extraintestinal manifestations at baseline, and the effect of tofacitinib, in patients with moderate to severe ulcerative colitis. Therap Adv Gastroenterol. 2021;14:17562848211005708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 93. | Gregory MH, Ciorba MA, Deepak P, Christophi GP. Successful Treatment of Pyoderma Gangrenosum with Concomitant Tofacitinib and Infliximab. Inflamm Bowel Dis. 2019;25:e87-e88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 94. | Westhovens R, Taylor PC, Alten R, Pavlova D, Enríquez-Sosa F, Mazur M, Greenwald M, Van der Aa A, Vanhoutte F, Tasset C, Harrison P. Filgotinib (GLPG0634/GS-6034), an oral JAK1 selective inhibitor, is effective in combination with methotrexate (MTX) in patients with active rheumatoid arthritis and insufficient response to MTX: results from a randomised, dose-finding study (DARWIN 1). Ann Rheum Dis. 2017;76:998-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 192] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 95. | Kavanaugh A, Kremer J, Ponce L, Cseuz R, Reshetko OV, Stanislavchuk M, Greenwald M, Van der Aa A, Vanhoutte F, Tasset C, Harrison P. Filgotinib (GLPG0634/GS-6034), an oral selective JAK1 inhibitor, is effective as monotherapy in patients with active rheumatoid arthritis: results from a randomised, dose-finding study (DARWIN 2). Ann Rheum Dis. 2017;76:1009-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 186] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 96. | Mease P, Coates LC, Helliwell PS, Stanislavchuk M, Rychlewska-Hanczewska A, Dudek A, Abi-Saab W, Tasset C, Meuleners L, Harrison P, Besuyen R, Van der Aa A, Mozaffarian N, Greer JM, Kunder R, Van den Bosch F, Gladman DD. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active psoriatic arthritis (EQUATOR): results from a randomised, placebo-controlled, phase 2 trial. Lancet. 2018;392:2367-2377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 162] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 97. | van der Heijde D, Baraliakos X, Gensler LS, Maksymowych WP, Tseluyko V, Nadashkevich O, Abi-Saab W, Tasset C, Meuleners L, Besuyen R, Hendrikx T, Mozaffarian N, Liu K, Greer JM, Deodhar A, Landewé R. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active ankylosing spondylitis (TORTUGA): results from a randomised, placebo-controlled, phase 2 trial. Lancet. 2018;392:2378-2387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 211] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 98. | Greuter T, Rieder F, Kucharzik T, Peyrin-Biroulet L, Schoepfer AM, Rubin DT, Vavricka SR. Emerging treatment options for extraintestinal manifestations in IBD. Gut. 2021;70:796-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 99. | D'Amico F, Danese S, Fiorino G. Could small molecules be used in combination with biologics for inflammatory bowel disease? Expert Rev Clin Immunol. 2022;18:991-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 100. | Llano EM, Shrestha S, Burstein E, Boktor M, Fudman DI. Favorable Outcomes Combining Vedolizumab With Other Biologics or Tofacitinib for Treatment of Inflammatory Bowel Disease. Crohns Colitis 360. 2021;3:otab030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 101. | Miyatani Y, Choi D, Choi NK, Rubin DT. Dual-Targeted Therapy with Upadacitinib and Ustekinumab in Medically Complex Crohn's Disease. Dig Dis Sci. 2024;69:355-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (3)] |
| 102. | Triantafillidis JK, Zografos CG, Konstadoulakis MM, Papalois AE. Combination treatment of inflammatory bowel disease: Present status and future perspectives. World J Gastroenterol. 2024;30:2068-2080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 40] [Article Influence: 20.0] [Reference Citation Analysis (3)] |
| 103. | Hilley P, Gilmore R, Srinivasan A, Choy M, De Cruz P. Combined Targeted Treatment Using Biologic-Tofacitinib Co-Therapy in Chronic Active Ulcerative Colitis. Inflamm Bowel Dis. 2021;27:e105-e106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 104. | Perner F, Perner C, Ernst T, Heidel FH. Roles of JAK2 in Aging, Inflammation, Hematopoiesis and Malignant Transformation. Cells. 2019;8:854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 185] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 105. | Núñez P, Quera R, Yarur AJ. Safety of Janus Kinase Inhibitors in Inflammatory Bowel Diseases. Drugs. 2023;83:299-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 106. | Sedano R, Ma C, Jairath V, Feagan BG. Janus Kinase Inhibitors for the Management of Patients With Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y). 2022;18:14-27. [PubMed] |
| 107. | Sánchez González CO, Nieto González JC. JAK kinase inhibitors and varicella zoster virus infection in patients with rheumatoid arthritis. Systematic review of the literature. Reumatol Clin (Engl Ed). 2022;18:453-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 108. | Verweij MC, Wellish M, Whitmer T, Malouli D, Lapel M, Jonjić S, Haas JG, DeFilippis VR, Mahalingam R, Früh K. Varicella Viruses Inhibit Interferon-Stimulated JAK-STAT Signaling through Multiple Mechanisms. PLoS Pathog. 2015;11:e1004901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 109. | Baskurt D, Vural S. JAK inhibitors: Unveiling varicella and herpes risks in atopic dermatitis. Int J Dermatol. 2025;64:9-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 110. | Fenster M, Alayo QA, Khatiwada A, Wang W, Dimopoulos C, Gutierrez A, Ciorba MA, Christophi GP, Hirten RP, Ha C, Beniwal-Patel P, Cohen BL, Syal G, Yarur A, Patel A, Colombel JF, Pekow J, Ungaro RC, Rubin DT, Deepak P. Real-World Effectiveness and Safety of Tofacitinib in Crohn's Disease and IBD-U: A Multicenter Study From the TROPIC Consortium. Clin Gastroenterol Hepatol. 2021;19:2207-2209.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 111. | Honap S, Temido MJ, Shakweh E, Badrulhisham F, Shields N, Mehta S, McBride J, Uzzan M, Lukáš M, Fumery M, Nogami A, Kobayashi T, Nancey S, Neves JC, Mendes JM, Wegener BA, Bergereau E, Fernandes R, Kuzhiyanjal AJK, Vieujean S, Spencer A, Baillie S, Estevinho MM, Simão I, O'Neill C, Gututui M, Thompson E, Jama A, Chai N, Pavlidis P, D'Amico F, Limdi J, Magro F, Sebastian S, Digby-Bell J, Parkes GC, Din S, Hart A, Peyrin-Biroulet L; JAKne International Study Group. Janus Kinase (JAK) Inhibitor-Induced Acne in Inflammatory Bowel Disease: An International, Multicenter, Retrospective Cohort Study. Clin Gastroenterol Hepatol. 2025;S1542-3565(25)00465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 112. | Burmester GR, Cohen SB, Winthrop KL, Nash P, Irvine AD, Deodhar A, Mysler E, Tanaka Y, Liu J, Lacerda AP, Palac H, Shaw T, Mease PJ, Guttman-Yassky E. Safety profile of upadacitinib over 15 000 patient-years across rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and atopic dermatitis. RMD Open. 2023;9:e002735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 153] [Reference Citation Analysis (0)] |
| 113. | Wang S, Wang X, Ding J, Zhang X, Zhu H, Fan Y, Sun C. Disproportionality analysis of upadacitinib-related adverse events in inflammatory bowel disease using the FDA adverse event reporting system. Front Pharmacol. 2025;16:1436183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 114. | Wu Y, Wei M, Zhang J. A real-world pharmacovigilance analysis of FDA adverse event reporting system database for upadacitinib. Front Pharmacol. 2023;14:1200254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 115. | Zhang C, Fu Z, Liu J, Li S, Chen X, Zhang Y, Xie J. Safety profile and dose-dependent adverse events of upadacitinib in randomized clinical trials: a systematic review and meta-analysis. Front Pharmacol. 2025;16:1598972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 116. | Olivera PA, Lasa JS, Bonovas S, Danese S, Peyrin-Biroulet L. Safety of Janus Kinase Inhibitors in Patients With Inflammatory Bowel Diseases or Other Immune-mediated Diseases: A Systematic Review and Meta-Analysis. Gastroenterology. 2020;158:1554-1573.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 251] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 117. | Winthrop KL, Park SH, Gul A, Cardiel MH, Gomez-Reino JJ, Tanaka Y, Kwok K, Lukic T, Mortensen E, Ponce de Leon D, Riese R, Valdez H. Tuberculosis and other opportunistic infections in tofacitinib-treated patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1133-1138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 183] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 118. | Mease P, Charles-Schoeman C, Cohen S, Fallon L, Woolcott J, Yun H, Kremer J, Greenberg J, Malley W, Onofrei A, Kanik KS, Graham D, Wang C, Connell C, Valdez H, Hauben M, Hung E, Madsen A, Jones TV, Curtis JR. Incidence of venous and arterial thromboembolic events reported in the tofacitinib rheumatoid arthritis, psoriasis and psoriatic arthritis development programmes and from real-world data. Ann Rheum Dis. 2020;79:1400-1413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 175] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 119. | Xie W, Xiao S, Huang Y, Sun X, Zhang Z. Effect of tofacitinib on cardiovascular events and all-cause mortality in patients with immune-mediated inflammatory diseases: a systematic review and meta-analysis of randomized controlled trials. Ther Adv Musculoskelet Dis. 2019;11:1759720X19895492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 120. | Curtis JR, Lee EB, Kaplan IV, Kwok K, Geier J, Benda B, Soma K, Wang L, Riese R. Tofacitinib, an oral Janus kinase inhibitor: analysis of malignancies across the rheumatoid arthritis clinical development programme. Ann Rheum Dis. 2016;75:831-841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 121. | Clowse ME, Feldman SR, Isaacs JD, Kimball AB, Strand V, Warren RB, Xibillé D, Chen Y, Frazier D, Geier J, Proulx J, Marren A. Pregnancy Outcomes in the Tofacitinib Safety Databases for Rheumatoid Arthritis and Psoriasis. Drug Saf. 2016;39:755-762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 122. | Mahadevan U, Dubinsky MC, Su C, Lawendy N, Jones TV, Marren A, Zhang H, Graham D, Clowse MEB, Feldman SR, Baumgart DC. Outcomes of Pregnancies With Maternal/Paternal Exposure in the Tofacitinib Safety Databases for Ulcerative Colitis. Inflamm Bowel Dis. 2018;24:2494-2500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 123. | Arzivian A, Zhang E, Laube R, Leong R. First-trimester exposure to tofacitinib in ulcerative colitis: A case report of a healthy newborn and literature review. Clin Case Rep. 2024;12:e8764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 124. | Duricova D, Sonnenberg E, Augustijns P, Mitrova K, Anderson PO; Upadacitinib Pregnancy-Lactation Study Group, Julsgaard M. Upadacitinib in Mothers With Inflammatory Bowel Disease: Placental Transfer and Breast Milk Excretion. Clin Gastroenterol Hepatol. 2025;S1542-3565(25)00526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 125. | van Staa TP, Card T, Logan RF, Leufkens HG. 5-Aminosalicylate use and colorectal cancer risk in inflammatory bowel disease: a large epidemiological study. Gut. 2005;54:1573-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 208] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 126. | Koelink PJ, Mieremet-Ooms MA, Corver WE, Wolanin K, Hommes DW, Lamers CB, Verspaget HW. 5-aminosalicylic acid interferes in the cell cycle of colorectal cancer cells and induces cell death modes. Inflamm Bowel Dis. 2010;16:379-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 127. | Bao MM, Kennedy JM, Dolinger MT, Dunkin D, Lai J, Dubinsky MC. Cytomegalovirus Colitis in a Patient with Severe Treatment Refractory Ulcerative Colitis. Crohns Colitis 360. 2024;6:otae014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 128. | Valenzuela F, Papp KA, Pariser D, Tyring SK, Wolk R, Buonanno M, Wang J, Tan H, Valdez H. Effects of tofacitinib on lymphocyte sub-populations, CMV and EBV viral load in patients with plaque psoriasis. BMC Dermatol. 2015;15:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 129. | Adas MA, Alveyn E, Cook E, Dey M, Galloway JB, Bechman K. The infection risks of JAK inhibition. Expert Rev Clin Immunol. 2022;18:253-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/