Published online Dec 5, 2025. doi: 10.4292/wjgpt.v16.i4.110642
Revised: August 14, 2025
Accepted: November 17, 2025
Published online: December 5, 2025
Processing time: 177 Days and 12.9 Hours
Approval of teduglutide is an important addition to the limited treatment options for short bowel syndrome (SBS). However, real-world evidence on teduglutide therapy for SBS in Latin America is scarce.
To investigate the effectiveness and safety of teduglutide in clinical practice in Argentina with a 24-week follow-up.
This non-interventional multicentre cohort study included consecutive patients (aged ≥ 1 years) with SBS who were dependent on parenteral support (PS) and received ≥ 1 dose of teduglutide according to currently approved indi
The study population (n = 45) included 21 adult and 24 pediatric patients. The proportion of adult and pediatric patients who showed clinical response (defined as a ≥ 20% reduction in weekly PS volume) after 24 weeks of treatment was 90.4% [95% confidence interval (CI): 69.6%-98.8%] and 83.3% (95%CI: 62.6%-95.2%), respectively. Overall, 12 patients (26.6%; 95%CI: 14.6-41.9) were weaned from PS support at the 24-week assessment, 6 (28.5%; 95%CI: 11.5-52.1) in the adult cohort, and 6 (25%; 95%CI: 9.7-46.7) in the pediatric cohort. Only baseline PS requirement was inversely associated with weaning from PS (P = 0.025). The most frequently reported treatment-emergent adverse events (TEAEs) were mild to moderate abdominal pain and abdominal distension (16.6%; and 9.5%, respectively). None of the reported TEAEs led to treatment discontinuation.
This prospective real-world study demonstrated the effectiveness and safety of teduglutide in adult and pediatric patients with SBS in Argentina. The clinical response observed in both adults and pediatric patients was greater than that reported in phase 3 trials and was consistent with the results of other real-world studies.
Core Tip: This multicenter real-world evidence study demonstrates the effectiveness and safety of teduglutide in adult and pediatric patients with short bowel syndrome-IF in the Latin American population. The results showed that teduglutide induced significant reductions in parenteral support (PS) in both adult and pediatric populations, consistent with pivotal studies, with a low incidence of mild to moderate adverse events. The findings highlight the importance of early identification of eligible patients for teduglutide (patients with reduced baseline PS volume dependence and/or favorable bowel anatomy), which may increase the likelihood of achieving PS independence. This approach could potentially reduce the morbidity associated with intestinal failure, ultimately improving long-term patient outcomes in Latin America.
- Citation: Solar Muñiz H, Fernández A, Busoni V, Martínez MI, Rumbo C, De Barrio S, Saure C, Balacco M, Buncuga MG, Dlugoszweski C, Manzur A, Rudi L, Matoso MD, Cosentino S, Ussher F, Manzur F, Demarchi J, Malaver E, Brion L, Ungar L. Real-world effectiveness and safety of teduglutide in adult and pediatric patients with short bowel syndrome in Argentina. World J Gastrointest Pharmacol Ther 2025; 16(4): 110642
- URL: https://www.wjgnet.com/2150-5349/full/v16/i4/110642.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v16.i4.110642
Short bowel syndrome (SBS) is usually a postsurgical condition with a multifactorial etiology that occurs after a large resection of the small bowel[1]. The prevalence has been estimated to be 4-20 per 1000000 inhabitants, derived from secondary data from the United States and Europe[1-3]. Chronic intestinal failure (IF) secondary to SBS is associated with significant morbidity and mortality in both adult and pediatric populations[3-5]. Historically, therapy with parenteral support (PS), intestinal rehabilitation, and intestinal transplantation have been the main treatment options for patients with SBS-IF.
Teduglutide is a recombinant, degradation-resistant, longer-acting glucagon-like peptide 2 (GLP-2) analog. GLP-2 is a peptide secreted primarily in the distal intestine that increases intestinal and portal blood flow, increasing absorptive capacity, and decreasing gut motility and secretion[4,6,7].
The Food and Drug Administration approved teduglutide in 2012 for adult patients with SBS-IF depending on home PS, and pediatric use was approved in 2019.
Adult approval was based on two randomized, double-blind, placebo-controlled studies (NCT 00081458 and NCT 00798967) that enrolled subjects with SBS who were dependent on PS at least three times per week for at least 12 months[8,9].
In NCT 00081458, patients were randomized to receive 24 weeks of one of the following treatment regimens: (1) Teduglutide 0.05 mg/kg/day (n = 35); (2) Teduglutide 0.1 mg/kg/day (twice the recommended dose) (n = 33); and (3) Placebo (n = 16). The primary efficacy endpoint was a graded categorical score that did not achieve statistical significance for high-dose therapy. Further evaluation of PS volume reduction using the endpoint of response (defined as at least 20% reduction in PS fluid from baseline to weeks 20 and 24) showed that 46% of patients on teduglutide 0.05 mg/kg/day responded vs 6% on placebo[7,8]. In NCT 00798967, patients were randomized 1:1 to placebo (n = 43) or teduglutide 0.05 mg/kg/day (n = 43). The treatment was administered subcutaneously once daily for 24 weeks. The primary efficacy endpoint was based on clinical response, defined as a patient achieving at least a 20% reduction in weekly PS volume from baseline to weeks 20 and 24. Sixty-three percent (27/43) of teduglutide-treated patients vs 30% (13/43) of placebo-treated patients were considered responders (P = 0.002)[7,9].
Two long-term extension studies (NCT 00930644 and NCT 00172185) showed that teduglutide benefits were main
Pediatric approval was based on a 24-week multicenter trial (NCT 02682381), conducted in 59 pediatric patients with SBS aged 1-17 years who were dependent on PS[12]. Patients chose whether to receive teduglutide or standard of care (SOC). Patients who opted for teduglutide treatment were subsequently randomized in a double-blind manner to 0.025 mg/kg/day (n = 24) or 0.05 mg/kg/day (n = 26), while 9 patients enrolled in the SOC arm. At the end of the 24-week study, 69% of the patients treated with 0.05 mg/kg teduglutide administered subcutaneously once daily showed a reduction in weekly PS volume of 20% or more[12].
Similar short-term and long-term results have been observed in randomized studies conducted in other regions[13,14].
Real-world evidence on the use of teduglutide in adult and pediatric patients with SBS-IF is of great importance to validate the data of pivotal studies in populations that may be different due to health-care infrastructure, including SOC and surgical practices, patient characteristics, wider spectrum of disease severity and ethnical differences. However, this type of evidence is limited in Latin America. As part of new drug application commitments, local health authorities in Argentina requested a post-authorization study to monitor the real-world effectiveness and safety of teduglutide. Thus, the aim of this study was to investigate the effectiveness and safety of teduglutide in two cohorts of adult and pediatric patients with SBS-IF who were dependent on PS support in routine clinical practice in Argentina.
This was a mandatory post-authorization non-interventional multicenter cohort study. The study population included all consecutive patients with SBS who were dependent on PS, received at least one dose of teduglutide according to app
Patients were treated, followed, and monitored by physicians according to local clinical practice. Data collection was prospective and retrospective, depending on the start of treatment with teduglutide. Data were collected at the time of treatment initiation and at 12 weeks and 24 weeks from the start of teduglutide treatment.
The main objective was to determine the effectiveness and safety of teduglutide in real-world clinical practice in Argentina. The main effectiveness objective was to assess the proportion of patients in both study cohorts (adult and pediatric) achieving clinical response after 12 weeks and 24 weeks of treatment. Clinical response was defined as at least 20% or more reductions in weekly PS volume. The main safety objective was the incidence of treatment-emergent adverse events (TEAEs). Additional study objectives were variation in weekly PS volume and change in the number of days per week requiring PS after 12 weeks and 24 weeks of treatment with teduglutide. The exploratory post hoc objective of this study was to analyze potential variables associated with weaning from PS. Weaning from PS was defined as those patients requiring 0 mL/week of PS within the last 12 weeks of study follow-up. SBS anatomy was categorized into three types: (1) Type 1: End-ostomy, and no colon in continuity; (2) Type 2: Jejuno-colic anastomosis and part of the colon in continuity; and (3) Type 3: Jejuno-ileal anastomosis with the entire colon (100%) in continuity.
TEAEs were defined as undesirable events that were not experienced before medical treatment or an event that had already been experienced by the patient and worsened either in intensity or frequency following the treatment with teduglutide. The intensity of TEAEs was categorized as mild, moderate, or severe based on investigator assessment. TEAEs of special interest were defined as serious or non-serious TEAEs of scientific and medical concern that require ongoing monitoring and rapid communication.
The study protocol (TAK-633-4003) was approved by an Independent Ethics Committee (Fundación de Estudios Farmacológicos y Medicamentos) on September 25, 2020. All patients were required to sign the applicable mandatory written informed consent/assent. Good pharmacoepidemiology practice guidelines, applicable local regulations, and the declaration of Helsinki were followed. A local clinical research organization managed the study. The study was incorporated in the clinical study registry of the city of Buenos Aires, Argentina and in clinicaltrials.gov (NCT04877431).
As the local health authority mandated this study, the sample size was based on all consecutive patients who received teduglutide in Argentina during the accrual period of both legacy (expanded access or compassionate use) and de novo patients (after marketing authorization) in Argentina. The proportion of patients with clinical response was expressed with a 95% confidence interval (CI) calculation (using the exact Clopper Pearson method). Weekly volume was expressed as mean (SD); num
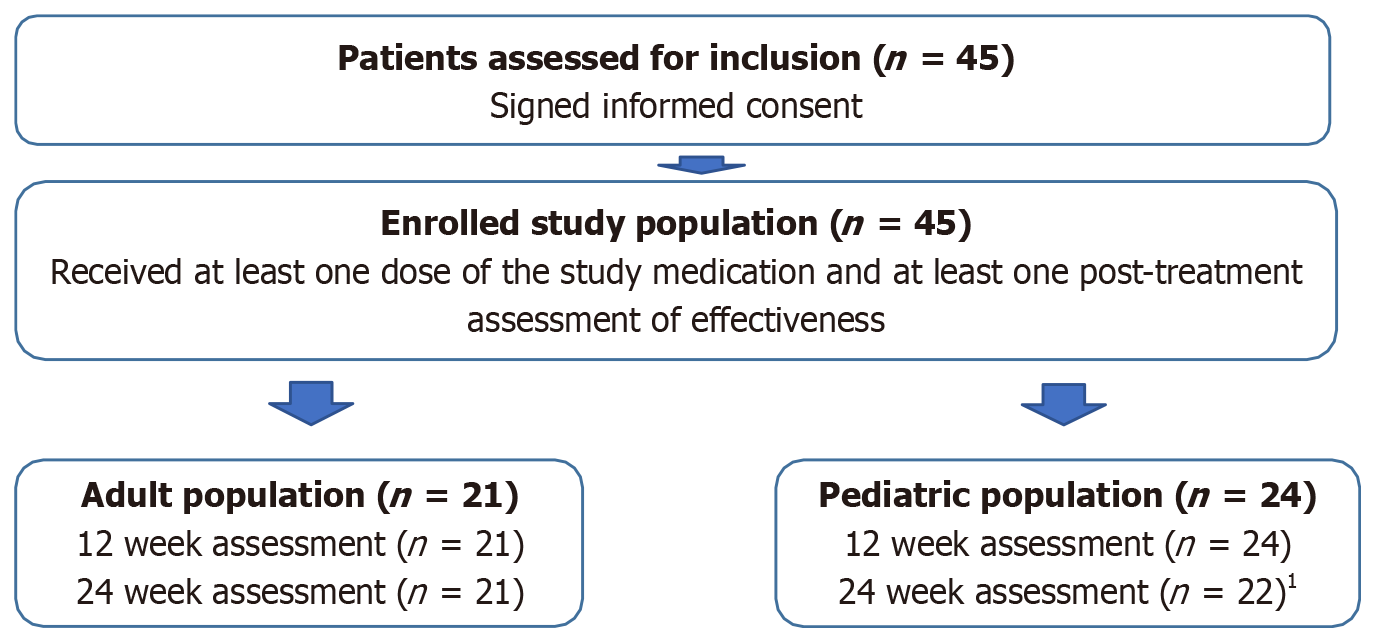
From June 5, 2014 (date of first patient treated) until June 5, 2023 (end of data collection), 45 patients were assessed for inclusion and enrolled in this real-world study by 14 specialized physicians in 10 sites who were invited and agreed to participate. Patients were followed for 24 weeks. The patient flow chart and final disposition at week 24 are shown in Figure 1.
The overall study population (n = 45) included 21 adult patients with a mean age (mean ± SD) of 42.9 ± 17.6 years and 24 pediatric patients with a mean age of 9.7 ± 4.6 years, respectively. The most frequent cause of SBS was vascular disease in the adult population (42.9%), and volvulus (33.3%), intestinal atresia (29.2%), or gastroschisis (20.8%) in the pediatric population. The most frequent anatomy type in the adult and pediatric cohorts was type 2 (jejuno-colic anastomosis and part of the colon in continuity) at 61.9% and 66.7%, respectively, with a low proportion of patients with type 1 anatomy (end-ostomy and no colon in continuity) at 9.5% and 4.2%, respectively. Table 1 summarizes the baseline characteristics of the included patients.
| Adults (n = 21) | Pediatric (n = 24) | |
| Age (years) | ||
| mean (SD) | 42.9 (17.6) | 9.7 (4.6) |
| Range (minimum-maximum) | 19-75 | 3-17 |
| Sex | ||
| Female | 11 (52.4) | 5 (20.8) |
| Male | 10 (47.6) | 19 (79.2) |
| Weight and BMI | ||
| Weight (kg), mean (SD) | 57.5 (12.8) | 27.3 (12.7) |
| BMI, mean (SD) | 21.9 (3.4) | 16.4 (1.9) |
| Time of parenteral support (years), mean (SD) | 7 (5.9) | 7.8 (4.7) |
| Cause of major intestinal resection | ||
| Vascular disease | 9 (42.9) | 0 (0) |
| Postoperative complication | 5 (23.8) | 0 (0) |
| Volvulus | 3 (14.3) | 8 (33.3) |
| Intestinal atresia | 2 (9.5) | 7 (29.2) |
| Gastroschisis | 0 (0) | 5 (20.8) |
| Injury/traumatic | 2 (9.5) | 1 (5.6) |
| Necrotizing enterocolitis | 0 (0) | 1 (4.2) |
| Other cause | 0 (0) | 2 (8.4) |
| Anatomy type | ||
| Type 1 | 2 (9.5) | 1 (4.2) |
| Type 2 | 13 (61.9) | 16 (66.7) |
| Type 3 | 6 (28.9) | 7 (29.2) |
| Colon in continuity | ||
| Yes | 19 (90.5) | 22 (91.7) |
| No | 2 (9.5) | 2 (8.3) |
| Remanent colon | ||
| > 0%-25% | 2 (9.5) | 2 (8.3) |
| > 25%-50% | 13 (61.9) | 6 (25) |
| > 50%-75% | 0 (0) | 6 (25) |
| > 75%-100% | 6 (28.6) | 9 (37.5) |
| Other or not available | 0 (0) | 1 (4.2) |
| Remanent small bowel length (cm), mean (SD) | 48.2 (40.6) | 43 (39.8) |
| Ostomy | ||
| Colostomy | 0 (0) | 2 (8.3) |
| Jejunostomy | 1 (4.8) | 1 (4.2) |
| Ileostomy | 1 (4.8) | 0 (0) |
Duration of PS before initiation of treatment with teduglutide was similar in both the adult and pediatric cohorts, 7.03 ± 5.94 years, and 7.84 ± 4.70 years respectively (P = 0.97).
The treatment dose of teduglutide was 0.05 mg/kg/day subcutaneously in 95.2% of adult patients and all pediatric patients (one adult patient received 0.025 mg/kg/day, due to a glomerulopathy). The initial number of treatment days per week was 7 in all adult and pediatric patients.
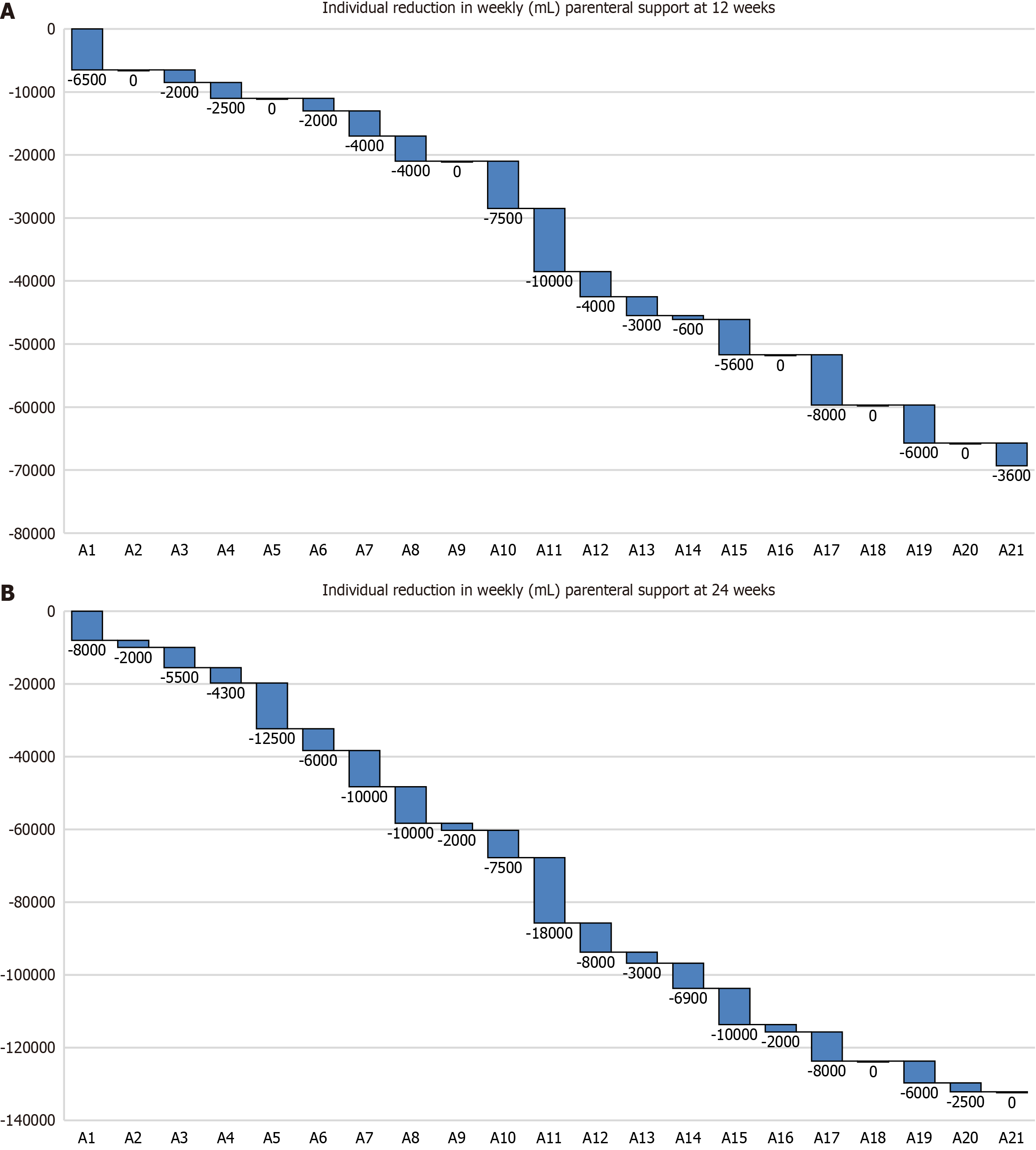
The proportion of adult patients showing clinical response (defined as a ≥ 20% reduction in weekly PS volume) after 12 weeks and 24 weeks of treatment was 57.1% (95%CI: 34%-78.1%) and 90.4% (95%CI: 69.6%-98.8%), respectively.
Adult patients showed a significant reduction in weekly PS volume after 12 weeks (3.30 L/week; P = 0.00071) and 24 weeks (6.29 L/week; P = 0.00014) of treatment compared with baseline (Table 2 and Figure 2). In addition, there were significant reductions in the number of days per week requiring PS after 12 weeks (P = 0.00482) and 24 weeks (P = 0.00019) of treatment compared with baseline.
| Weekly PS volume (mL) | P value | Number of days per week with PS | P value | |
| Adult cohort | ||||
| Baseline | 12000 (4663) | NA | 4 (4-7) | NA |
| Week 12 | 8700 (3884) | 0.00071 | 4 (4-5) | 0.00482 |
| Week 24 | 5705 (4514) | 0.00014 | 3 (0-4) | 0.00019 |
| Pediatric cohort | ||||
| Baseline | 9090 (4954) | NA | 6 (5-7) | NA |
| Week 12 | 7266 (5082) | 0.00044 | 6 (4-6) | 0.00143 |
| Week 24 | 5491 (4433) | 0.00002 | 5 (2-5) | 0.00008 |
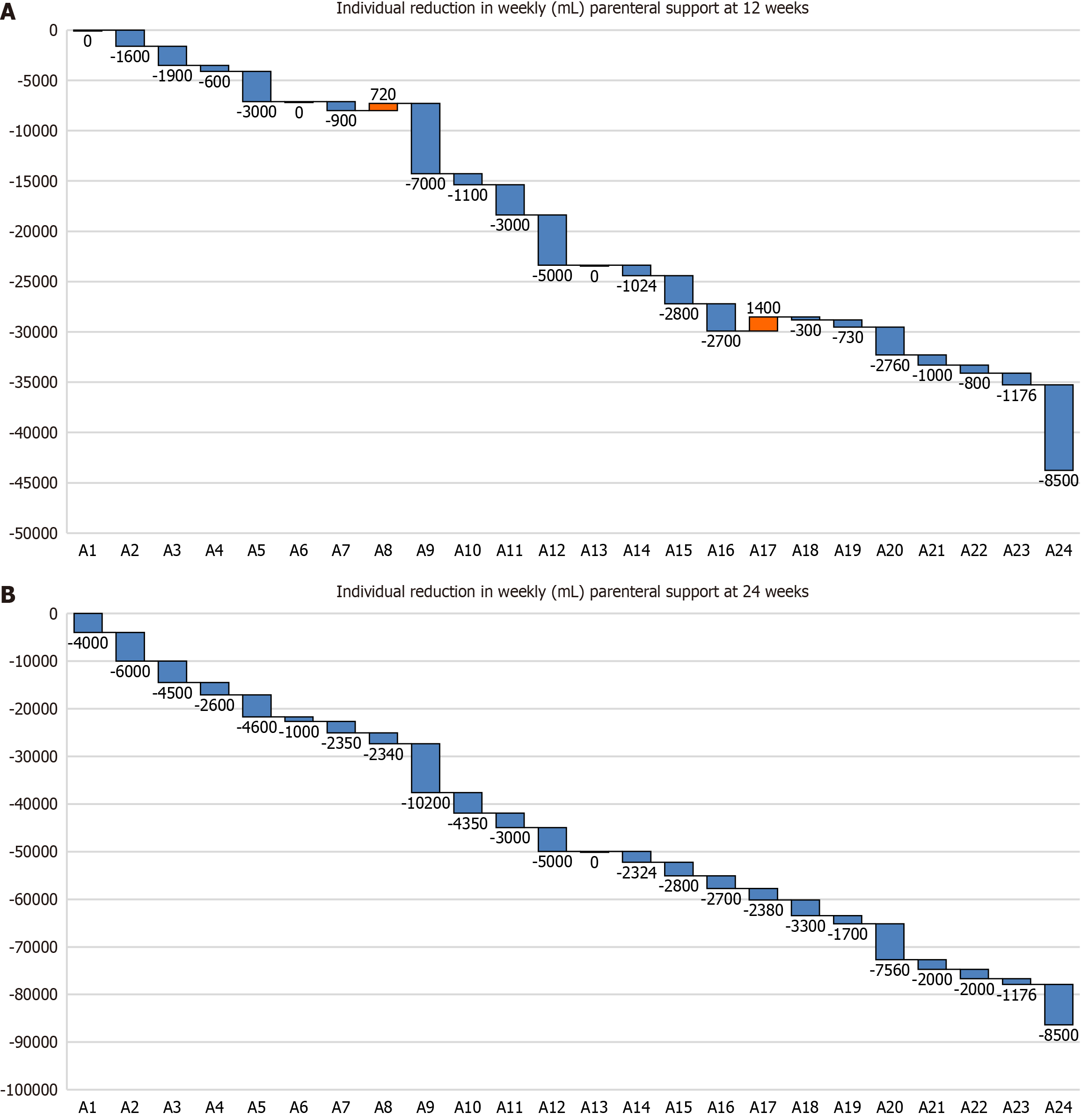
The proportion of pediatric patients showing clinical response after 12 weeks and 24 weeks of treatment was 37.5% (95%CI: 18.7%-59.4%) and 83.3% (95%CI: 62.6%-95.2%), respectively.
Pediatric patients showed significant reductions in weekly PS volume after 12 weeks (1.82 L/week; P = 0.00044) and 24 weeks (3.59 L/week; P = 0.00002) of treatment compared with baseline (Table 2 and Figure 3). In addition, there were significant reductions in the number of days per week requiring PS after 12 weeks (P = 0.00143) and 24 weeks (P = 0.00008) of treatment compared with baseline.
Overall, 12 patients (26.6%; 95%CI: 14.6-41.9) were weaned from PS support at the 24-week assessment, 6 (28.5%; 95%CI: 11.5-52.1) in the adult cohort, and 6 (25%; 95%CI: 9.7-46.7) in the pediatric cohort (Table 3).
| Not weaned (n = 33) | Weaned (n = 12) | P value1 | |
| Study cohort | |||
| Adult | 15 (45.5) | 6 (50) | |
| Pediatric | 18 (54.5) | 6 (50) | 0.19 |
| Anatomy type | |||
| Type 1 | 2 (6.1) | 1 (8.3) | |
| Type 2 | 23 (69.7) | 6 (50) | 0.12 |
| Type 3 | 8 (24.2) | 5 (41.7) | |
| Time from PS to treatment, mean (SD) | 7.0 (5.9) | 7.5 (6.4) | 0.76 |
| Remanent small bowel length (cm), mean (SD) | 40.9 (40) | 57.9 (38) | 0.11 |
| Weekly baseline PS volume (mL), mean (SD) | 11541 (4634) | 7441 (4850) | 0.02 |
As an exploratory post hoc analysis, we analyzed whether selected variables (study cohort, anatomy type, small bowel length, time from PS to start of treatment, and baseline PS requirement) were associated with weaning from PS. Of these selected variables, only baseline PS requirement was significantly and inversely associated with weaning from PS after 24 weeks of treatment (P = 0.025; Table 3).
Overall, 19 patients (42.2%) in the study population experienced at least 1 TEAE. Five patients (23.8%) in the adult popu
The most frequently reported TEAEs were mild to moderate abdominal pain and abdominal distension (3 in the adult population, 14.2%; and 4 in the pediatric population, 16.6%) (Table 4). None of the reported TEAEs led to treatment dis
| Adults (n = 21) | Pediatric (n = 24) | |||
| All TEAE | Severe TEAE | All TEAE | Severe TEAE | |
| Infections and infestations | ||||
| Device related infection | 0 (0) | 0 (0) | 2 (8.3) | 1 (4.1) |
| Upper respiratory tract infection | 0 (0) | 0 (0) | 2 (8.3) | 0 (0) |
| Gastrointestinal disorders | ||||
| Abdominal pain | 1 (4.7) | 0 (0) | 4 (16.6) | 0 (0) |
| Abdominal distension | 2 (9.5) | 0 (0) | 0 (0) | 0 (0) |
| General disorders and administration site conditions | ||||
| Injection site pain or erythema1 | 0 (0) | 0 (0) | 2 (8.3) | 0 (0) |
| Injury, poisoning and procedural complications | ||||
| Stoma complications | 1 (4.7) | 0 (0) | 2 (8.3) | 1 (4.1) |
A total of 17 TEAEs were considered serious (Table 5). The most frequently reported serious TEAE was gastrointestinal stoma complications (1 adult, 4.7%; and 2 pediatric patients, 8.3%). None of the serious TEAEs led to treatment discontinuation. There were no deaths during the observational period.
| Adults (n = 21) | Pediatric (n = 24) | |||
| All TEAE | Severe TEAE | All TEAE | Severe TEAE | |
| Infections and infestations | ||||
| Catheter site infection | 0 (0) | 0 (0) | 1 (4.1) | 0 (0) |
| Device related infection | 0 (0) | 0 (0) | 2 (8.3) | 1 (4.1) |
| Influenza | 0 (0) | 0 (0) | 1 (4.1) | 0 (0) |
| Rhinovirus infection | 0 (0) | 0 (0) | 1 (4.1) | 0 (0) |
| Vascular device infection | 0 (0) | 0 (0) | 1 (4.1) | 0 (0) |
| General disorders and administration site conditions | ||||
| Vascular device occlusion | 0 (0) | 0 (0) | 1 (4.1) | 0 (0) |
| Metabolism and nutrition disorders | ||||
| Hypolabuminaemia | 0 (0) | 0 (0) | 1 (4.1) | 0 (0) |
| Lactic acidosis | 0 (0) | 0 (0) | 1 (4.1) | 0 (0) |
| Injury, poisoning and procedural complications | ||||
| Stoma complications1 | 1 (4.7) | 0 (0) | 2 (8.3) | 1 (4.1) |
| Hepatobiliary disorders | ||||
| Cholestasis | 0 (0) | 0 (0) | 1 (4.1) | 0 (0) |
| Hyperbilirubinemia | 0 (0) | 0 (0) | 1 (4.1) | 0 (0) |
| Blood and lymphatic system disorders | ||||
| Anaemia | 0 (0) | 0 (0) | 1 (4.1) | 0 (0) |
| Investigations | ||||
| Liver function test abnormal | 0 (0) | 0 (0) | 1 (4.1) | 0 (0) |
| Nervous system disorders | ||||
| Seizure | 0 (0) | 0 (0) | 1 (4.1) | 0 (0) |
The most commonly reported TEAE of special interest (adverse event of special interest) were injection site reactions (none in adults; and 2 in pediatrics 8.3%) and gastrointestinal stoma complications (1 adult, 4.7%; and 1 pediatric patient, 4.7%), representing overall 40% of patients with a stoma (2 out of 5), followed by cholestasis (none in adults; and 1 in pe
In addition, there was 1 reported pregnancy in the adult cohort that resulted in a full-term live birth without any com
SBS is the leading cause of IF in both adult and pediatric populations worldwide. Since 2012, the introduction of teduglutide for the management of SBS-IF has significantly improved treatment outcomes, reducing reliance on PS and enabling intestinal sufficiency recovery, even in patients with an unfavorable anatomy. The efficacy and safety of tedu
In Argentina, teduglutide was first used under an expanded access program in 2014 and 2017 in adult and pediatric patients, respectively, and became commercially available in November 2020. In this study, we report the effectiveness and safety of teduglutide in a cohort of adult and pediatric patients with SBS-IF across 10 specialized centers in Argentina.
Teduglutide showed a significant clinical response (defined as a ≥ 20% reduction in weekly PS volume) after 12 weeks and 24 weeks of treatment in both the adult and pediatric cohorts. Both patient cohorts showed significant reductions in the number of days per week requiring PS support after 12 weeks and 24 weeks of treatment compared with baseline. The clinical response observed in both the adult and pediatric cohorts (90.4% and 83.3% respectively) was above what has been reported in the respective phase 3 trials (63% in study NCT 00798967, and 69% in study NCT 02682381) after 24 weeks of therapy[9,12]. However, our results are consistent with the response rates observed in other real-world studies after 24 weeks and 48 weeks of treatment[15-18].
The differences observed in comparison with the clinical trials may be explained by the small sample sizes in both this study and the pivotal studies, by differences in the baseline populations, and the PS reduction protocol used. The adult patients in our cohort were characterized by a younger age and higher prevalence of vascular disease as the cause of SBS. Additionally, both the adult and pediatric cohorts had a lower prevalence of type 1 anatomy (9.5% and 4.2%, respec
Response to treatment was previously correlated with baseline PS volume requirements[19]. Factors associated with treatment response, and more importantly, predictors of successful weaning from PS in patients with SBS treated with teduglutide, are an area of active investigation[20]. Various recent studies[15,17] have provided relevant insights into this matter[19-23].
Overall, in the present study, 26.6% of the SBS patients were weaned from PS after 24 weeks of treatment (6 adults, 28.6% and 6 pediatrics, 25.0%). Baseline PS volume was inversely associated with weaning from PS after 24 weeks of treatment, and only baseline PS requirement was associated with weaning from PS after logistic regression analysis. Importantly, we did not find any correlation between the presence of colon in continuity and PS autonomy, as observed in seven studies[15,17,19-23]. However, the majority of our patients had a colon in continuity, unlike most patient cohorts in the United States and Europe, where type 1 anatomy is predominant (usually associated with Crohn’s disease).
In a recent revision, the real-life weaning-off rate in adults was estimated to be 11% at 6 months and 21% after 2 or more years of treatment[20]. The use of teduglutide, post-surgical intestinal length, presence of ileocecal valve, and colon in continuity have been associated with weaning from PS in previous studies involving patients with type 3 IF undergoing autologous gastrointestinal reconstruction surgery[20-22]. A pooled analysis of adult pivotal phase 3 trials and their respective extension studies evaluated the weaning from PS during teduglutide treatment[23]. This pooled analysis included 134 patients, of whom 16 (11.9%) achieved complete independence from PS after 89 weeks of treatment. Most patients (75%) who discontinued PS had at least partial colon in continuity, although the difference with those who did not achieve independence from PS was not statistically significant[23]. Moreover, a real-word multicentre French study investigated the response to treatment (reduction of ≥ 20% in daily PS volume) and the rate of PS discontinuation after 24 weeks of treatment with teduglutide in 54 consecutive adult patients from 15 specialized centers[15]. Overall, 85% of the population showed a response to teduglutide treatment, and 24% were weaned from PS support. After adjustment for selected covariates (age, bowel length, anatomical classification, and etiology), increased basal oral intake was the only factor significantly associated with 24-week response. Weaning from PS was significantly associated with colon in conti
A systematic review focused on pediatric patients with chronic IF included 223 patients treated with teduglutide in 14 studies, of whom 16% achieved enteral autonomy after 24 weeks of treatment (interquartile range: 24-48 weeks)[16]. A single-center real-world study included 25 pediatric patients with SBS who had two or more years of PS and small bowel length < 80 cm who received subcutaneous teduglutide (0.05 mg/kg/day) with a longer 48-week follow-up. A total of 8 patients (32%) were weaned-off PS[17]. Weaning from PS was significantly associated with oral intake and inversely associated with PS dependence (volume, parenteral nutrition index, and number of weekly infusions). There were no dif
It is important to note that these studies, including ours, featured a small number of patients with different baseline characteristics (e.g., etiology and anatomic type), definitions of successful weaning, length of follow up, and patient mana
Interestingly, other real-world studies with SBS-IF patients in Argentina with longer follow-up times showed sustained enteral autonomy in selected patients after PS weaning with teduglutide every other day dosing or even after its discontinuation[22,24]. This topic was addressed in the clinical recommendations of local experts on SBS.
The management of these patients in specialized centers must be well-standardized, with surgery considered for all patients to optimize anatomy before initiating medical rehabilitation. Medical treatment should follow standardized pro
In this study, teduglutide treatment was tolerated well in both adult and pediatric cohorts with a low incidence of mild to moderate TEAEs. Moreover, none of the TEAE led to treatment discontinuation. The incidence of TEAEs[8-10] in our study is below what has been reported in pivotal trials with both adult and pediatric patients and their respective follow-up studies[11-14]. These differences may be related to the small number of patients in both cohorts and potentially to the underreporting of adverse events in real-world settings.
A pooled analysis of adult pivotal phase 3 trials and their respective extension studies examined the safety of tedu
TEAEs with higher incidence in the teduglutide randomized arm compared with the placebo arm were abdominal pain (38.5% vs 27.1%), gastrointestinal stoma complications (37.8% vs 13.6% in patients with stoma), upper respiratory tract infections (27.5% vs 13.6%), and abdominal distension (16.5% vs 1.7%)[22]. TEAEs led to study discontinuation in 9.2% of patients in the teduglutide arm of randomized studies and 19.7% in the extension studies compared with 6.8% in the placebo arm of randomized studies[25]. Gastrointestinal disorders such as abdominal pain and distension, stoma com
The present study has several limitations related to study design in the context of routine clinical practice (real-world data) and the small number of patients included in each cohort with a limited follow-up period (24 weeks). Moreover, we did not systematically collect detailed information on intake or the type of oral or enteral nutrition that patients were receiving before starting teduglutide (such as whether they used elemental formulas, polymeric diets, or other specific nutritional strategies). Potential associations between nutritional intake characteristics and treatment outcomes were not evaluated and should be explored in future studies. However, the inclusion of all consecutive adult and pediatric patients with SBS-IF treated with teduglutide from 10 specialized centers in Argentina has the value of high external validity, reflecting real-world outcomes. Our study provides additional and valuable evidence related to the treatment of SBS-IF with teduglutide in a real-word setting in Argentina. However, regional multicentre registries are needed to better understand the long-term effectiveness and safety of teduglutide in the diverse Latin American patient population.
This study aimed to demonstrate the effectiveness and safety of teduglutide in adult and pediatric patients with SBS in Argentina in routine clinical practice. The results of this study showed that teduglutide induced significant reductions in PS in both adult and pediatric populations, consistent with pivotal studies, with a low incidence of mild to moderate adverse events. These results support the use of teduglutide for the treatment of SBS in a Latin American population of adults and pediatric patients. The findings of our study highlight the importance of early identification of eligible patients for teduglutide (patients with reduced baseline PS volume dependence and/or favorable bowel anatomy), which may increase the likelihood of achieving PS independence[21,23]. This approach could potentially reduce the morbidity associated with IF, ultimately improving long-term patient outcomes in Latin America.
| 1. | DiBaise JK, Young RJ, Vanderhoof JA. Intestinal rehabilitation and the short bowel syndrome: part 1. Am J Gastroenterol. 2004;99:1386-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 2. | Bakker H, Bozzetti F, Staun M, Leon-Sanz M, Hebuterne X, Pertkiewicz M, Shaffer J, Thul P. Home parenteral nutrition in adults: a european multicentre survey in 1997. ESPEN-Home Artificial Nutrition Working Group. Clin Nutr. 1999;18:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 3. | Wales PW, de Silva N, Kim J, Lecce L, To T, Moore A. Neonatal short bowel syndrome: population-based estimates of incidence and mortality rates. J Pediatr Surg. 2004;39:690-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 256] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 4. | Parrish CR, DiBaise JK. Managing the Adult Patient With Short Bowel Syndrome. Gastroenterol Hepatol (N Y). 2017;13:600-608. [PubMed] |
| 5. | Mutanen A, Engstrand Lilja H, Wester T, Norrby H, Borg H, Persson S, Bjornland K, Brun AC, Telborn L, Stenström P, Pakarinen MP. A nordic multicenter study on contemporary outcomes of pediatric short bowel syndrome in 208 patients. Clin Nutr. 2023;42:1095-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 6. | Kim ES, Keam SJ. Teduglutide: A Review in Short Bowel Syndrome. Drugs. 2017;77:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | GATTEX (teduglutide). Full prescribing information. 2024. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/203441s021lbl.pdf. |
| 8. | Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, O'Keefe SJ. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011;60:902-914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 322] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 9. | Jeppesen PB, Pertkiewicz M, Messing B, Iyer K, Seidner DL, O'keefe SJ, Forbes A, Heinze H, Joelsson B. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology. 2012;143:1473-1481.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 352] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 10. | Schwartz LK, O'Keefe SJ, Fujioka K, Gabe SM, Lamprecht G, Pape UF, Li B, Youssef NN, Jeppesen PB. Long-Term Teduglutide for the Treatment of Patients With Intestinal Failure Associated With Short Bowel Syndrome. Clin Transl Gastroenterol. 2016;7:e142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 159] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 11. | O'Keefe SJ, Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B. Safety and efficacy of teduglutide after 52 weeks of treatment in patients with short bowel intestinal failure. Clin Gastroenterol Hepatol. 2013;11:815-23.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Kocoshis SA, Merritt RJ, Hill S, Protheroe S, Carter BA, Horslen S, Hu S, Kaufman SS, Mercer DF, Pakarinen MP, Venick RS, Wales PW, Grimm AA. Safety and Efficacy of Teduglutide in Pediatric Patients With Intestinal Failure due to Short Bowel Syndrome: A 24-Week, Phase III Study. JPEN J Parenter Enteral Nutr. 2020;44:621-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 13. | Nakamura S, Wada M, Mizushima T, Sugita A, Tazuke Y, Ohge H, Udagawa E, Suzuki RK, Yoon M, Grimm A, Chen ST, Ikeuchi H. Efficacy, safety, and pharmacokinetics of teduglutide in adult Japanese patients with short bowel syndrome and intestinal failure: two phase III studies with an extension. Surg Today. 2023;53:347-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 14. | Chiba M, Masumoto K, Kaji T, Matsuura T, Morii M, Fagbemi A, Hill S, Pakarinen MP, Protheroe S, Urs A, Chen ST, Sakui S, Udagawa E, Wada M. Efficacy and Safety of Teduglutide in Infants and Children With Short Bowel Syndrome Dependent on Parenteral Support. J Pediatr Gastroenterol Nutr. 2023;77:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Joly F, Seguy D, Nuzzo A, Chambrier C, Beau P, Poullenot F, Thibault R, Armengol Debeir L, Layec S, Boehm V, Lallemand J, Quilliot D, Schneider SM. Six-month outcomes of teduglutide treatment in adult patients with short bowel syndrome with chronic intestinal failure: A real-world French observational cohort study. Clin Nutr. 2020;39:2856-2862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 16. | Gigola F, Cianci MC, Cirocchi R, Ranucci MC, Del Riccio M, Coletta R, Morabito A. Use of Teduglutide in Children With Intestinal Failure: A Systematic Review. Front Nutr. 2022;9:866518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Lambe C, Talbotec C, Kapel N, Barbot-Trystram L, Brabant S, Nader EA, Pigneur B, Payen E, Goulet O. Long-term treatment with teduglutide: a 48-week open-label single-center clinical trial in children with short bowel syndrome. Am J Clin Nutr. 2023;117:1152-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Ramos Boluda E, Redecillas Ferreiro S, Manrique Moral O, García Romero R, Irastorza Terradillos I, Nuñez Ramos R, Germán Díaz M, Polo Miquel B, Vives Piñera I, Alcolea Sánchez A, González Sacristán R, Bautista Barea M, Moreno Villares JM. Experience With Teduglutide in Pediatric Short Bowel Syndrome: First Real-life Data. J Pediatr Gastroenterol Nutr. 2020;71:734-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Jeppesen PB, Gabe SM, Seidner DL, Lee HM, Olivier C. Factors Associated With Response to Teduglutide in Patients With Short-Bowel Syndrome and Intestinal Failure. Gastroenterology. 2018;154:874-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 20. | Bioletto F, D'Eusebio C, Merlo FD, Aimasso U, Ossola M, Pellegrini M, Ponzo V, Chiarotto A, De Francesco A, Ghigo E, Bo S. Efficacy of Teduglutide for Parenteral Support Reduction in Patients with Short Bowel Syndrome: A Systematic Review and Meta-Analysis. Nutrients. 2022;14:796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 21. | Mazzuoli S, Regano N, Lamacchia S, Silvestri A, Guglielmi FW. Forty-eight months outcomes of teduglutide treatment in adult stable patients with short bowel syndrome and home parenteral nutrition dependence: A real-world Italian single-center observational cohort study. Nutrition. 2025;131:112640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 22. | Solar H, Doeyo M, Ortega M, De Barrio S, Olano E, Moreira E, Buncuga M, Manzur A, Crivelli A, Gondolesi G. Postsurgical Intestinal Rehabilitation Using Semisynthetic Glucagon-Like Peptide-2 Analogue (sGLP-2) at a Referral Center: Can Patients Achieve Parenteral Nutrition and sGLP-2 Independency? JPEN J Parenter Enteral Nutr. 2021;45:1072-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Iyer KR, Kunecki M, Boullata JI, Fujioka K, Joly F, Gabe S, Pape UF, Schneider SM, Virgili Casas MN, Ziegler TR, Li B, Youssef NN, Jeppesen PB. Independence From Parenteral Nutrition and Intravenous Fluid Support During Treatment With Teduglutide Among Patients With Intestinal Failure Associated With Short Bowel Syndrome. JPEN J Parenter Enteral Nutr. 2017;41:946-951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | Martínez MI, Busoni V, Saure C, Dlugosewsky C, Dalieri M, Cosentino S, Balacco M, Rudi L, Fernandez A, Rumbo C. Real-life experience of teduglutide use in pediatric patients with short bowel syndrome in Argentina. A multicenter study. Intest Fail. 2024;3:100027. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Pape UF, Iyer KR, Jeppesen PB, Kunecki M, Pironi L, Schneider SM, Seidner DL, Lee HM, Caminis J. Teduglutide for the treatment of adults with intestinal failure associated with short bowel syndrome: pooled safety data from four clinical trials. Therap Adv Gastroenterol. 2020;13:1756284820905766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/