©The Author(s) 2026.
World J Gastrointest Pharmacol Ther. Mar 5, 2026; 17(1): 112640
Published online Mar 5, 2026. doi: 10.4292/wjgpt.v17.i1.112640
Published online Mar 5, 2026. doi: 10.4292/wjgpt.v17.i1.112640
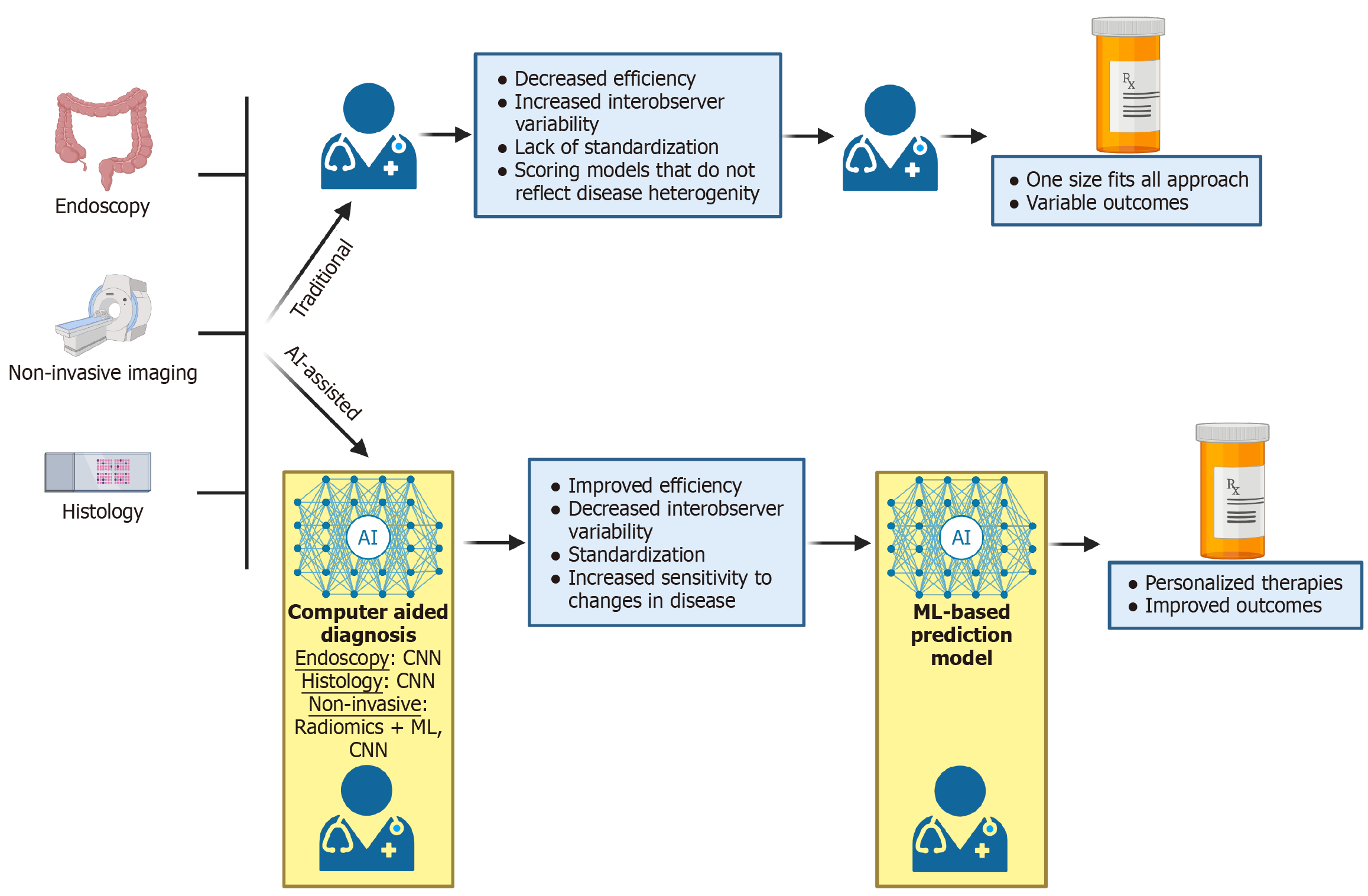
Figure 1 Limitations of traditional management of inflammatory bowel disease and potential improvements with the use of artificial intelligence.
Figure created with BioRender.com (https://BioRender.com/29up61d). The traditional management of inflammatory bowel disease relies on endoscopy, non-invasive imaging, and histology, interpreted by clinicians with variable experience in inflammatory bowel disease, who then make treatment decisions based upon these results and clinical symptoms. This often leads to a one size fits all approach. In an artificial intelligence-assisted model, clinicians are assisted by artificial intelligence, which may improve analysis in endoscopy, non-invasive imaging, and histology. With these results, clinicians may then use prediction models to determine the best therapeutic regiment tailored to the patient’s unique disease phenotype. AI: Artificial intelligence; CNN: Convolutional neural networks; ML: Machine learning.
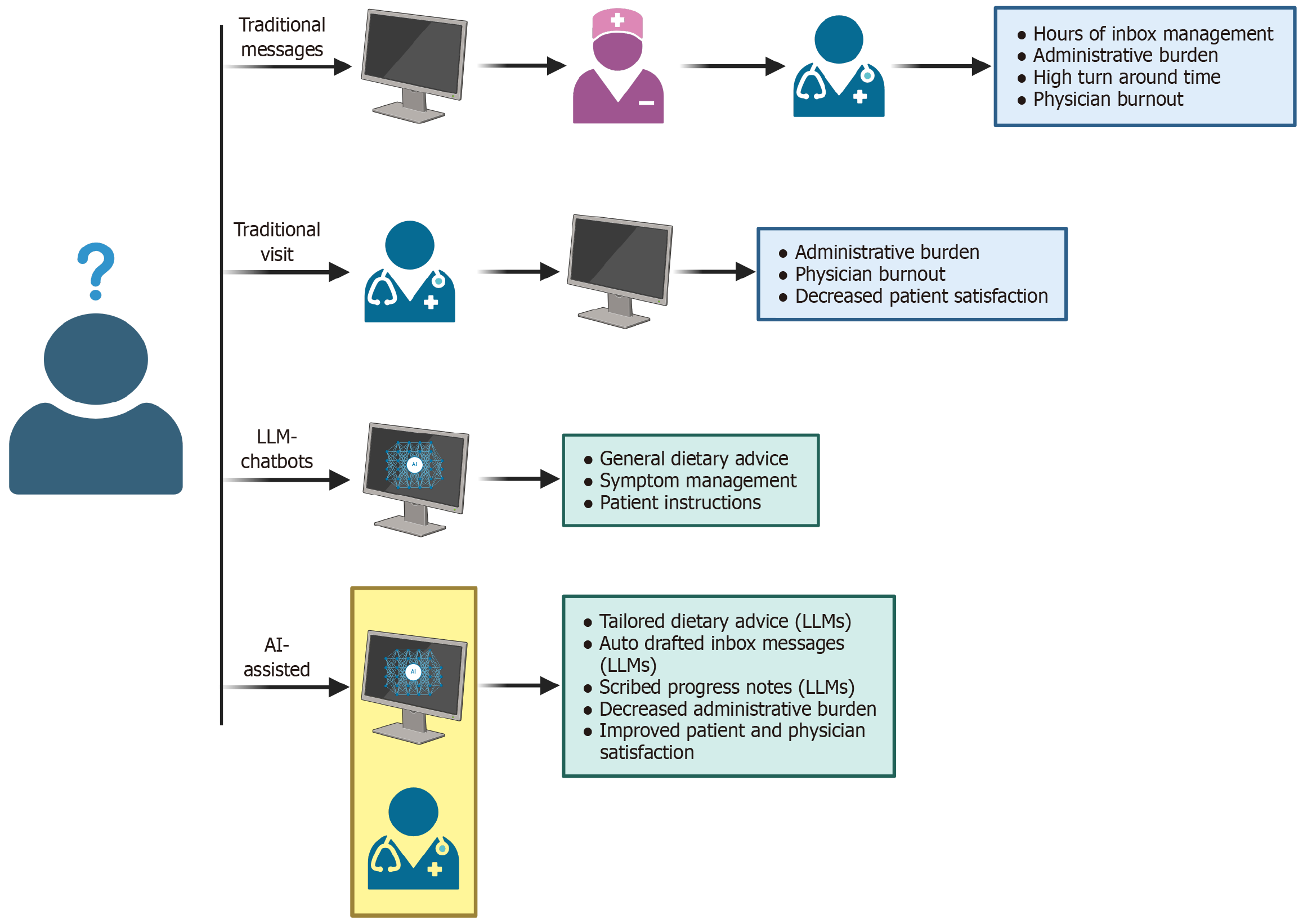
Figure 2 Limitations of traditional management of administrative duties and potential improvements with applications of artificial intelligence.
Figure created with BioRender.com (https://BioRender.com/ygwivmn). In traditional inflammatory bowel disease management, patients may send clinicians messages, that are normally screened by ancillary staff, prior to a response from a clinician. During clinic visits, clinicians are often multi-tasking to improve efficiency. This often leads to increased staff burnout, patient dissatisfaction, and high administrative burden. In an artificial intelligence (AI)-assisted model, patients may be able to use chatbots to answer basic questions about their disease. If they require more specific advice, patients may send clinicians messages, with responses being AI-generated with clinician oversight for accuracy. During AI-assisted appointments, notes are written by AI-assisted technology, decreasing administrative burden. This may lead to improvements in clinician and patient satisfaction. AI: Artificial intelligence; LLM: Large language model.
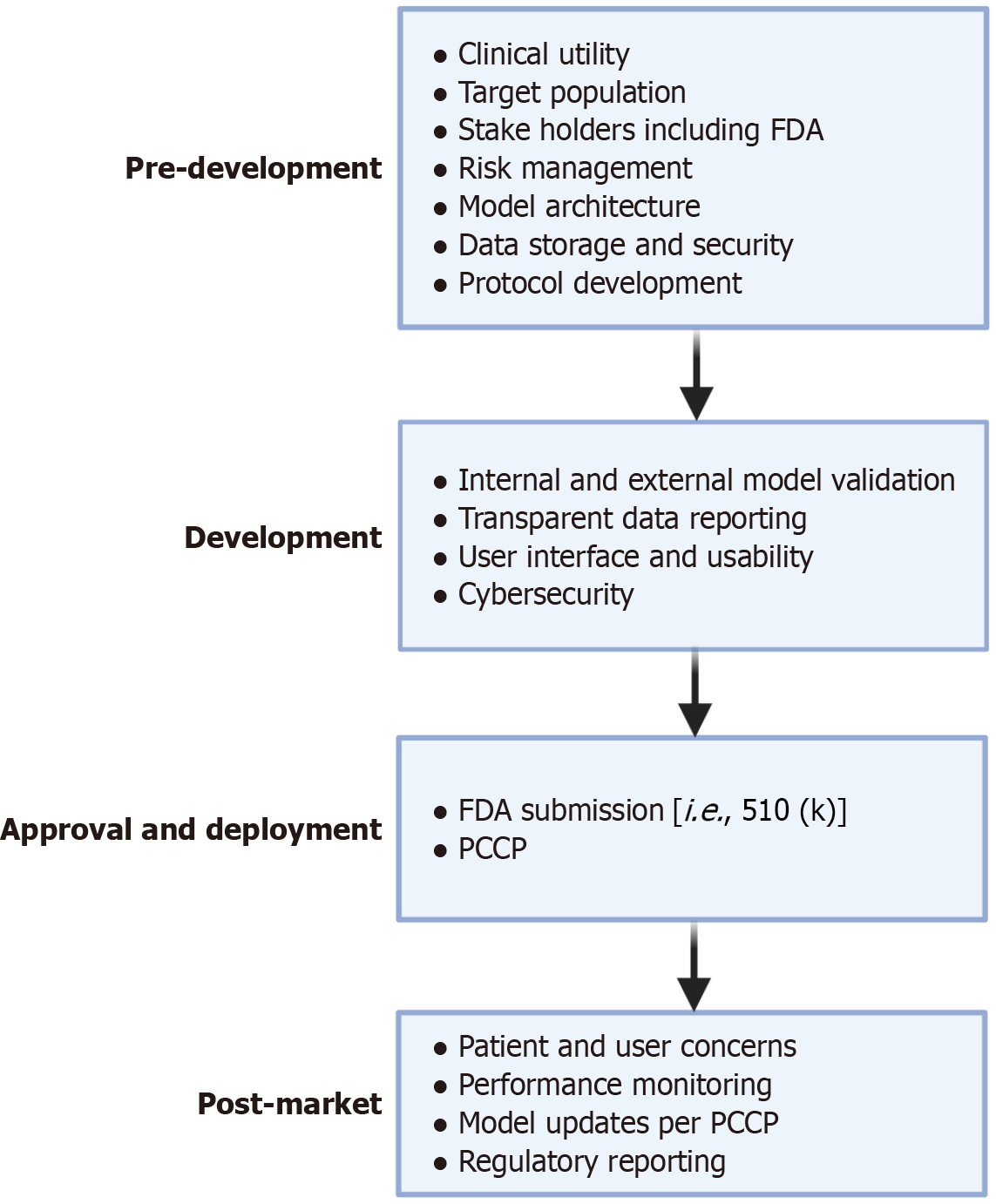
Figure 3 Food and Drug Administration roadmap for approval of artificial intelligence as software as a medical device: There are four major areas of software development for software as a medical device.
Figure created with BioRender.com (https://BioRender.com/t8xxcz4). In pre-development, the architecture and infrastructure behind the model is discussed, and involves key stake holders such as the Food and Drug Administration. In development, focus shifts towards model validation while ensuring usability of the software and refining the user interface. During approval and deployment, models may be submitted to the Food and Drug Administration through different regulatory pathways such as 501 (k). During this time, a predetermined change control plan may be submitted, which allows for premarket approval of future modifications to a device. In post-marketing, feedback is gained on the model in the real-world setting with periodic model refinements per predetermined change control plan. FDA: Food and Drug Administration; PCCP: Predetermined change control plan.
- Citation: Bilotta AJ, Trebilcock JA, Hebda NJ, Sasan CK, Cooper KM, Rupawala AH. Artificial intelligence in the management of inflammatory bowel disease: What’s next? World J Gastrointest Pharmacol Ther 2026; 17(1): 112640
- URL: https://www.wjgnet.com/2150-5349/full/v17/i1/112640.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v17.i1.112640