©The Author(s) 2025.
World J Gastrointest Pharmacol Ther. Dec 5, 2025; 16(4): 110827
Published online Dec 5, 2025. doi: 10.4292/wjgpt.v16.i4.110827
Published online Dec 5, 2025. doi: 10.4292/wjgpt.v16.i4.110827
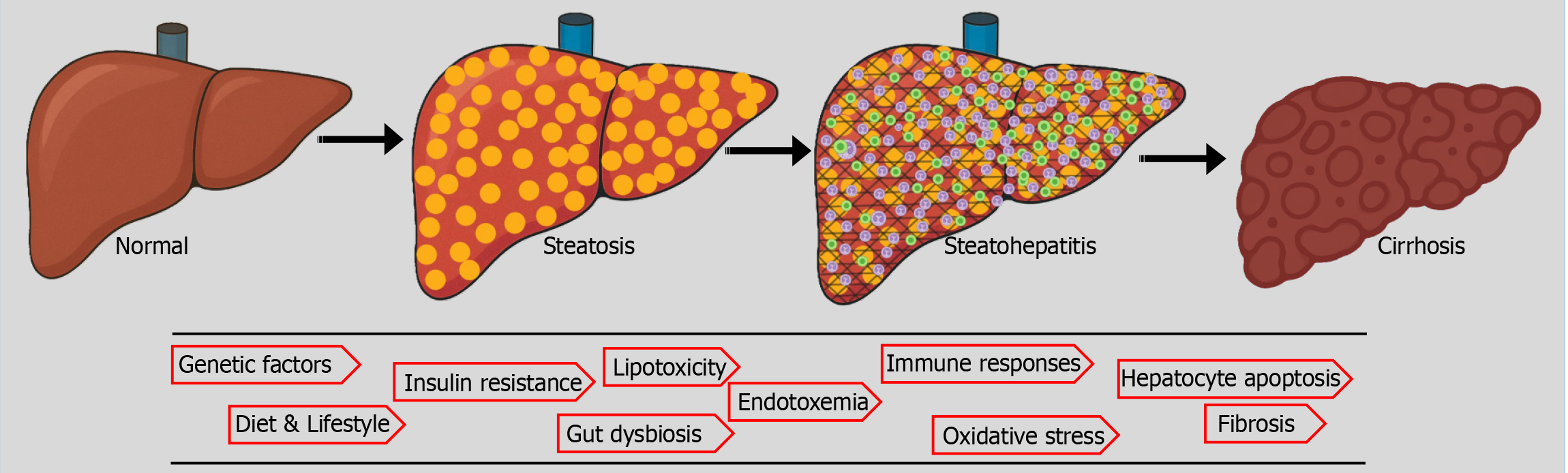
Figure 1 Natural history and pathogenesis of metabolic dysfunction-associated steatotic liver disease/metabolic dysfunction-associated steatohepatitis.
The metabolic dysfunction-associated steatotic liver disease spectrum extends from simple steatosis to steatohepatitis to fibrosis and cirrhosis and could predispose to hepatocellular carcinoma. The "multiple hit" hypothesis best explains the pathogenesis, although the sequence or influence of each hit is unclear and may vary between individual patients.
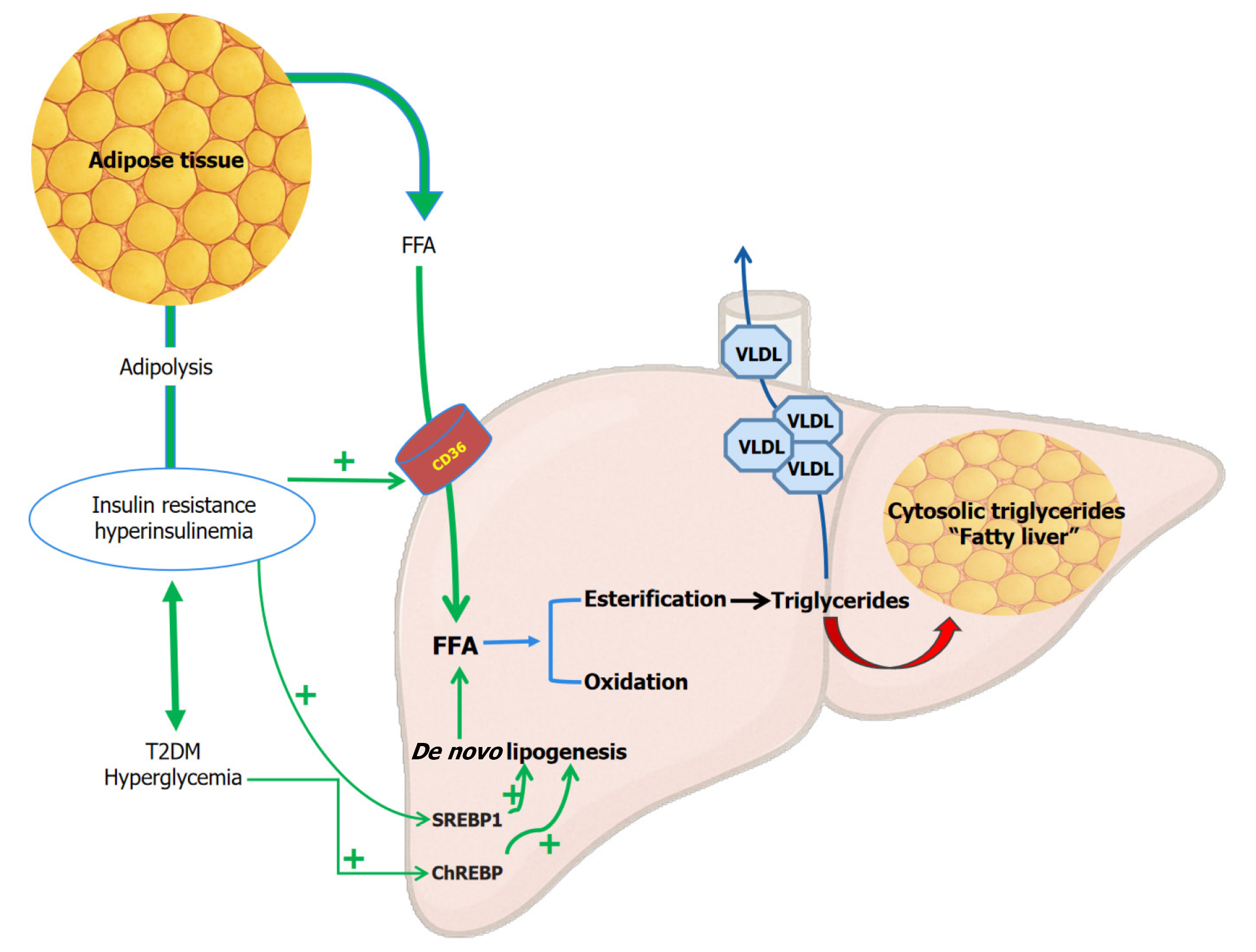
Figure 2 Insulin resistance and metabolic dysfunction-associated steatotic liver disease/metabolic dysfunction-associated stea
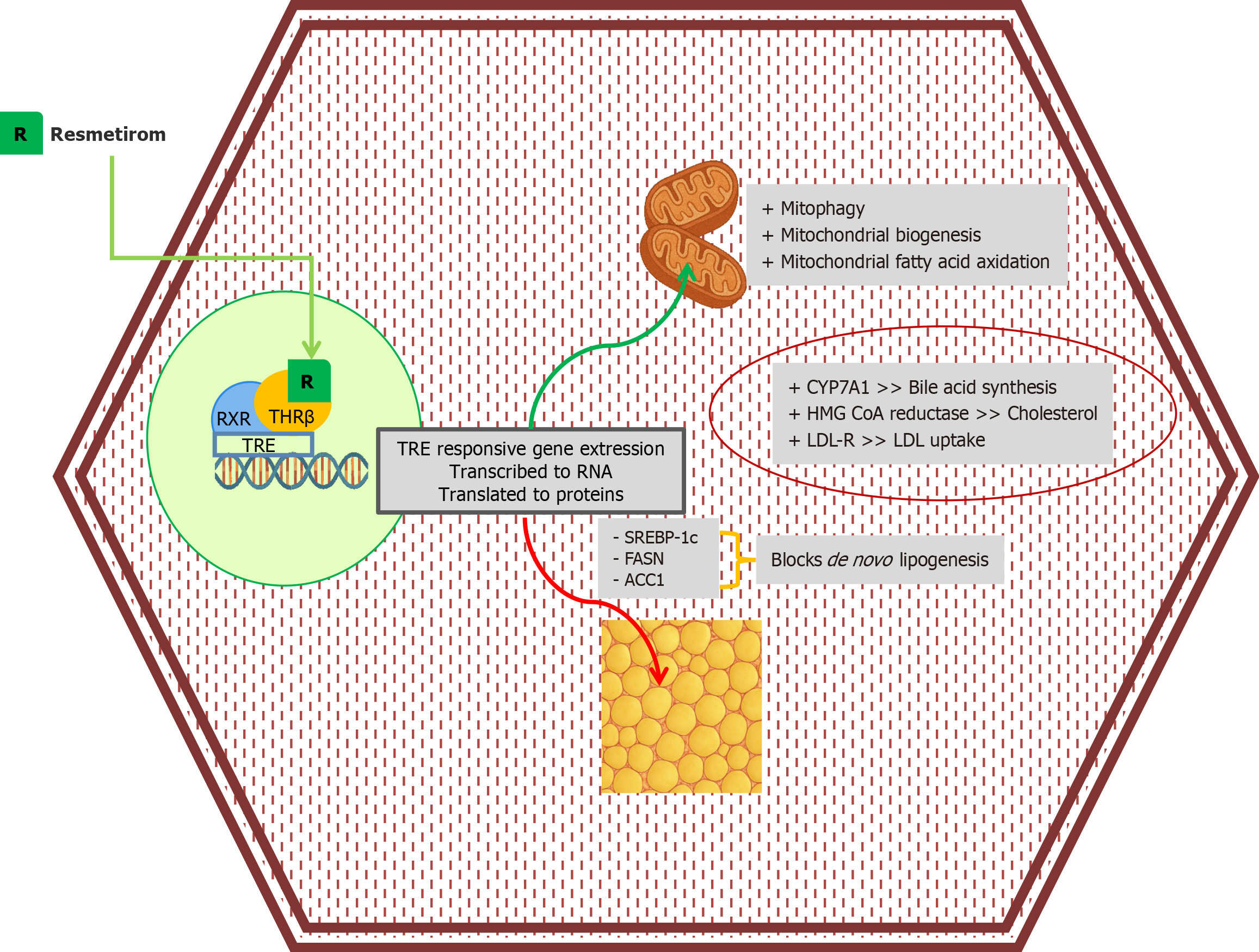
Figure 3 The nuclear thyroid hormone receptor β - retinoid X receptor heterodimer is physiologically activated by triiodothyronine (T3).
Resmetirom, a liver-directed agonist of thyroid hormone receptor β (THRβ), interacts with the THRβ - retinoid X receptor heterodimer to activate the thyroid hormone response elements, resulting in selective messenger ribonucleoprotein (mRNA) transcription and protein translation. The resultant proteins promote mitophagy (the removal of damaged mitochondria), mitochondrial biogenesis, and mitochondrial fatty acid oxidation, allowing for improved clearance of free fatty acids. It suppresses hepatic de novo lipogenesis by downregulating sterol regulatory element-binding protein 1c, fatty acid synthase, and acetyl-CoA carboxylase 1. Additional proteins: Cytochrome P450, family 7, subfamily A, polypeptide 1 increases bile acid synthesis; hydroxymethylglutaryl-CoA reductase increases cholesterol synthesis; the low-density lipoprotein receptor increases hepatic low-density lipoproteins uptake. RXR: Retinoid X receptor; TRE: Thyroid hormone response elements; LDL: Low-density lipoproteins; LDL-R: Low-density lipoprotein receptor; THRβ: Thyroid hormone receptor β.
- Citation: Zacharia GS, Ashraf MH, Sosa F, Jacob A, Patel H. Quick glance at 'metabolic dysfunction associated steatotic liver disease' therapeutics: Targets, trials, and trends. World J Gastrointest Pharmacol Ther 2025; 16(4): 110827
- URL: https://www.wjgnet.com/2150-5349/full/v16/i4/110827.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v16.i4.110827