©The Author(s) 2025.
World J Gastrointest Pharmacol Ther. Dec 5, 2025; 16(4): 110642
Published online Dec 5, 2025. doi: 10.4292/wjgpt.v16.i4.110642
Published online Dec 5, 2025. doi: 10.4292/wjgpt.v16.i4.110642
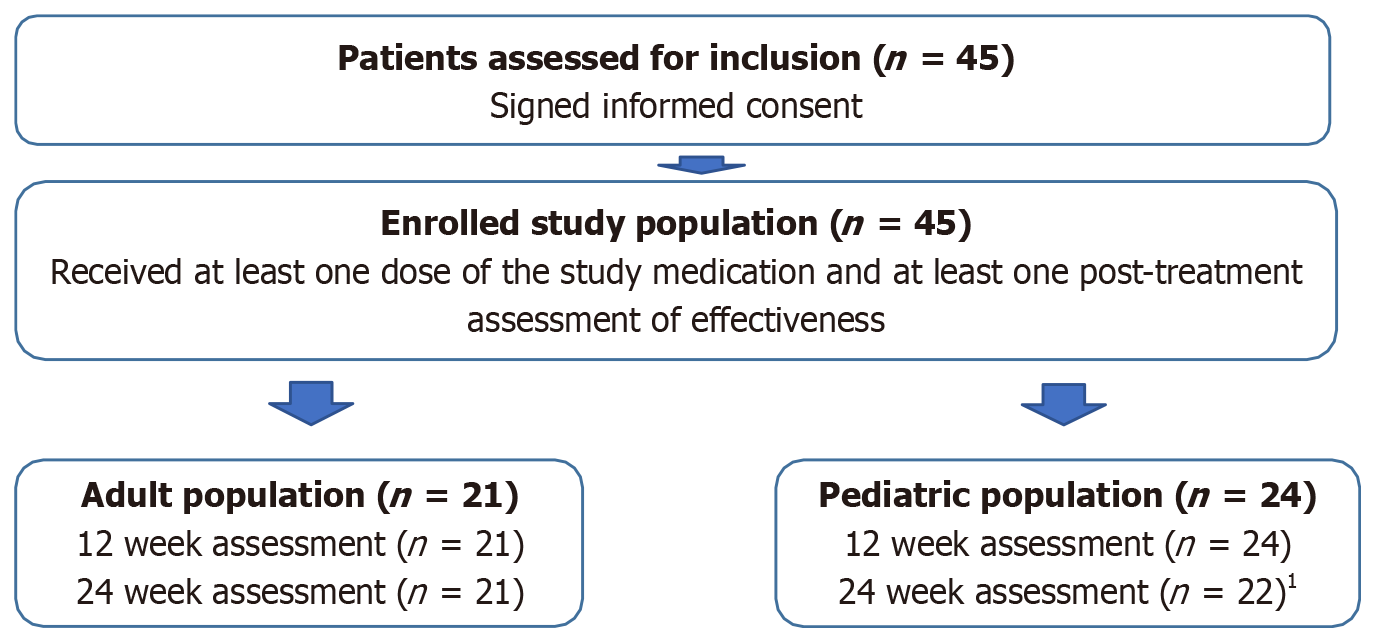
Figure 1 Patient flowchart and patient disposition.
1Two patients did not reach the administrative 24-week assessment because of late enrollment.
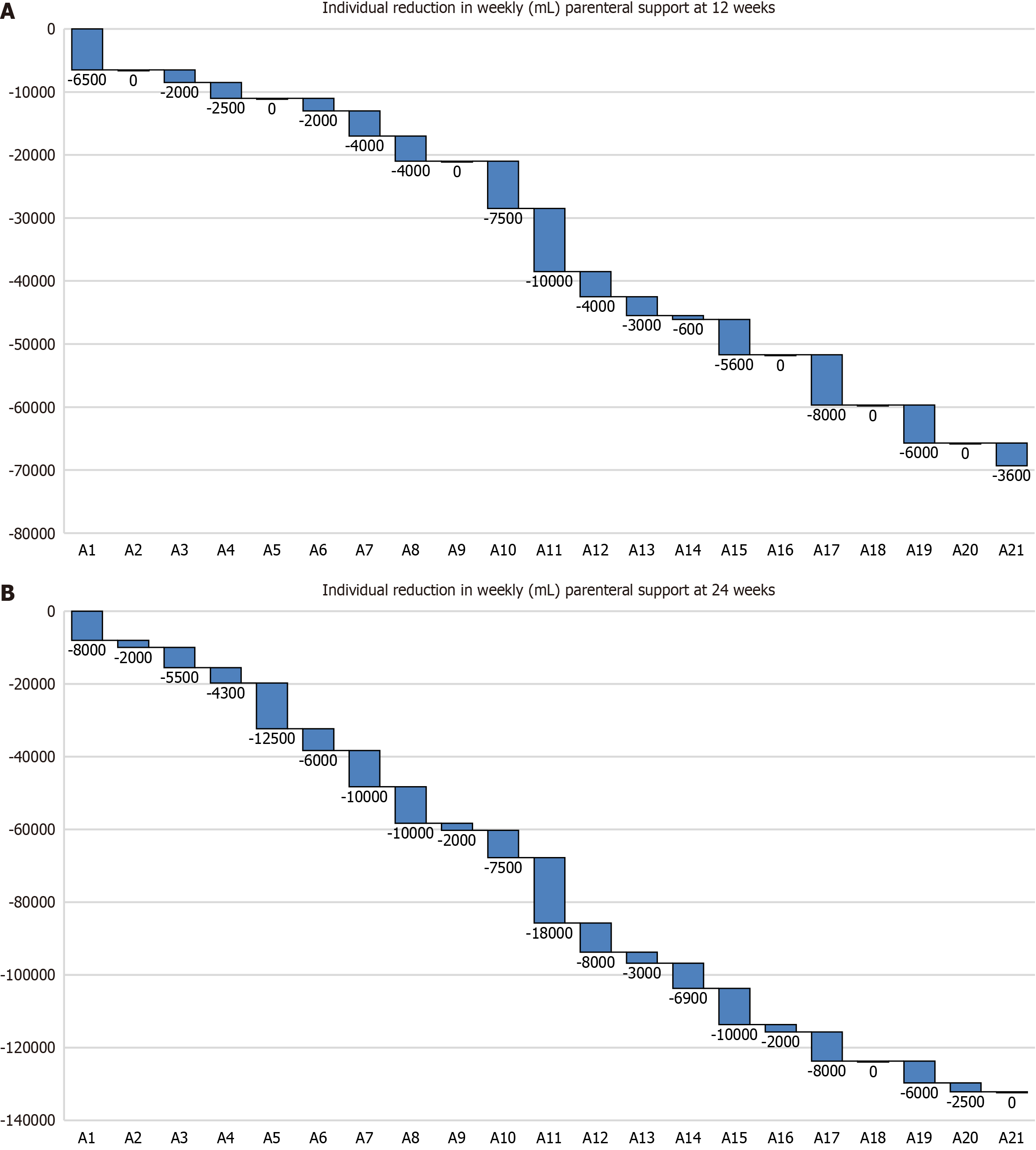
Figure 2 Reduction in weekly parenteral support in the adult cohort.
A: Individual reduction in weekly (mL) parenteral support (PS) at 12 weeks; B: Individual reduction in weekly (mL) PS at 24 weeks. Waterfall plots illustrating reductions in weekly PS volume among the adult cohort. Each individual bar depicts the change in weekly PS volume (in milliliters) for a single patient, with reductions plotted as negative values, increases as positive values, and no change as zero. The Y axis represents the cumulative effect of these sequential volume changes across the cohort, relative to the baseline volume (designated as zero).
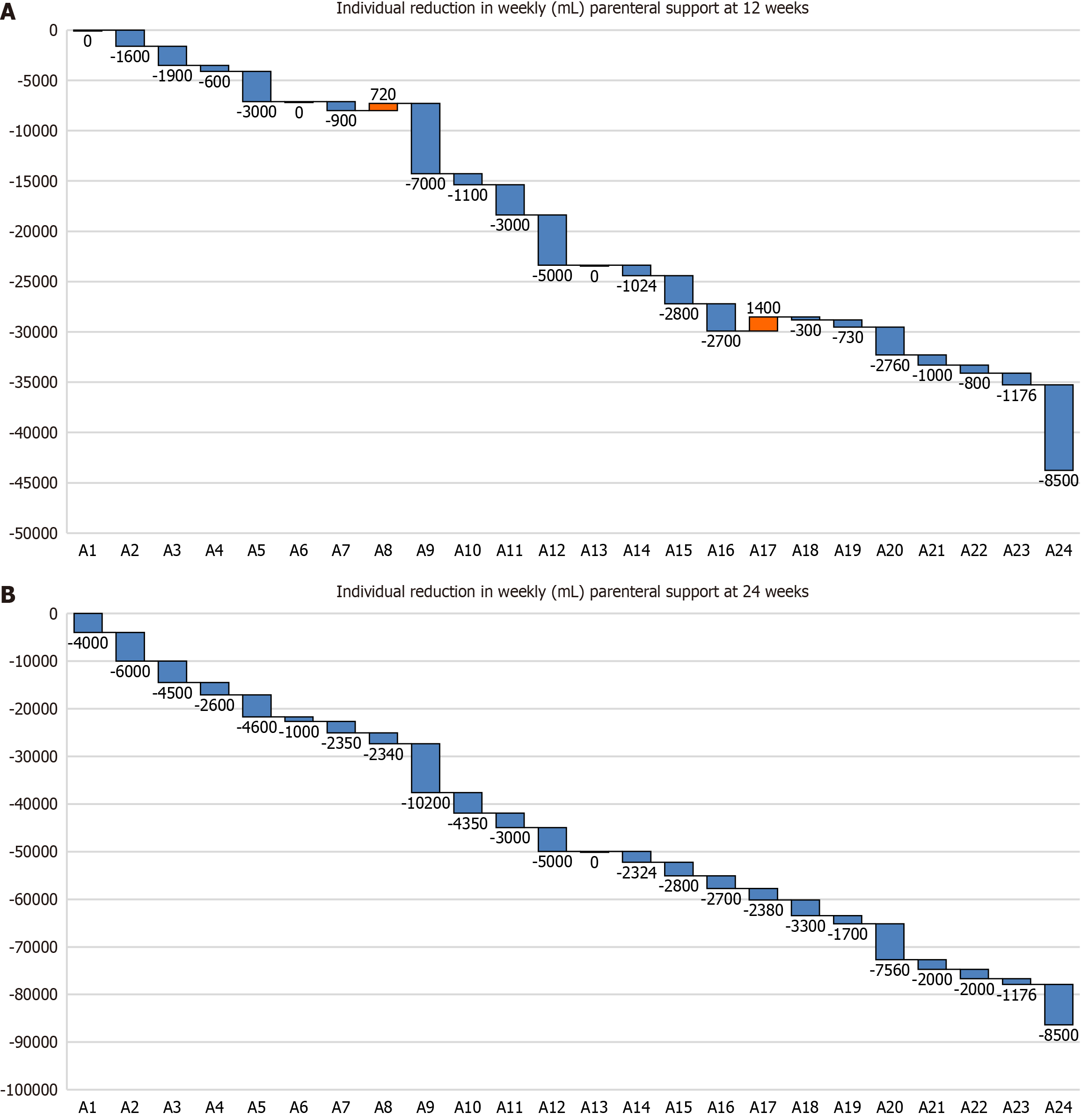
Figure 3 Reduction in weekly parenteral support in the pediatric cohort.
A: Individual reduction in weekly (mL) parenteral support (PS) at 12 weeks; B: Individual reduction in weekly (mL) PS at 24 weeks. Waterfall plots illustrating reductions in weekly PS volume among the pediatric cohort. Each individual bar depicts the change in weekly PS volume (in milliliters) for a single patient, with reductions plotted as negative values, increases as positive values, and no change as zero. The Y axis represents the cumulative effect of these sequential volume changes across the cohort, relative to the baseline volume (designated as zero).
- Citation: Solar Muñiz H, Fernández A, Busoni V, Martínez MI, Rumbo C, De Barrio S, Saure C, Balacco M, Buncuga MG, Dlugoszweski C, Manzur A, Rudi L, Matoso MD, Cosentino S, Ussher F, Manzur F, Demarchi J, Malaver E, Brion L, Ungar L. Real-world effectiveness and safety of teduglutide in adult and pediatric patients with short bowel syndrome in Argentina. World J Gastrointest Pharmacol Ther 2025; 16(4): 110642
- URL: https://www.wjgnet.com/2150-5349/full/v16/i4/110642.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v16.i4.110642