©The Author(s) 2025.
World J Gastrointest Pharmacol Ther. Dec 5, 2025; 16(4): 110271
Published online Dec 5, 2025. doi: 10.4292/wjgpt.v16.i4.110271
Published online Dec 5, 2025. doi: 10.4292/wjgpt.v16.i4.110271
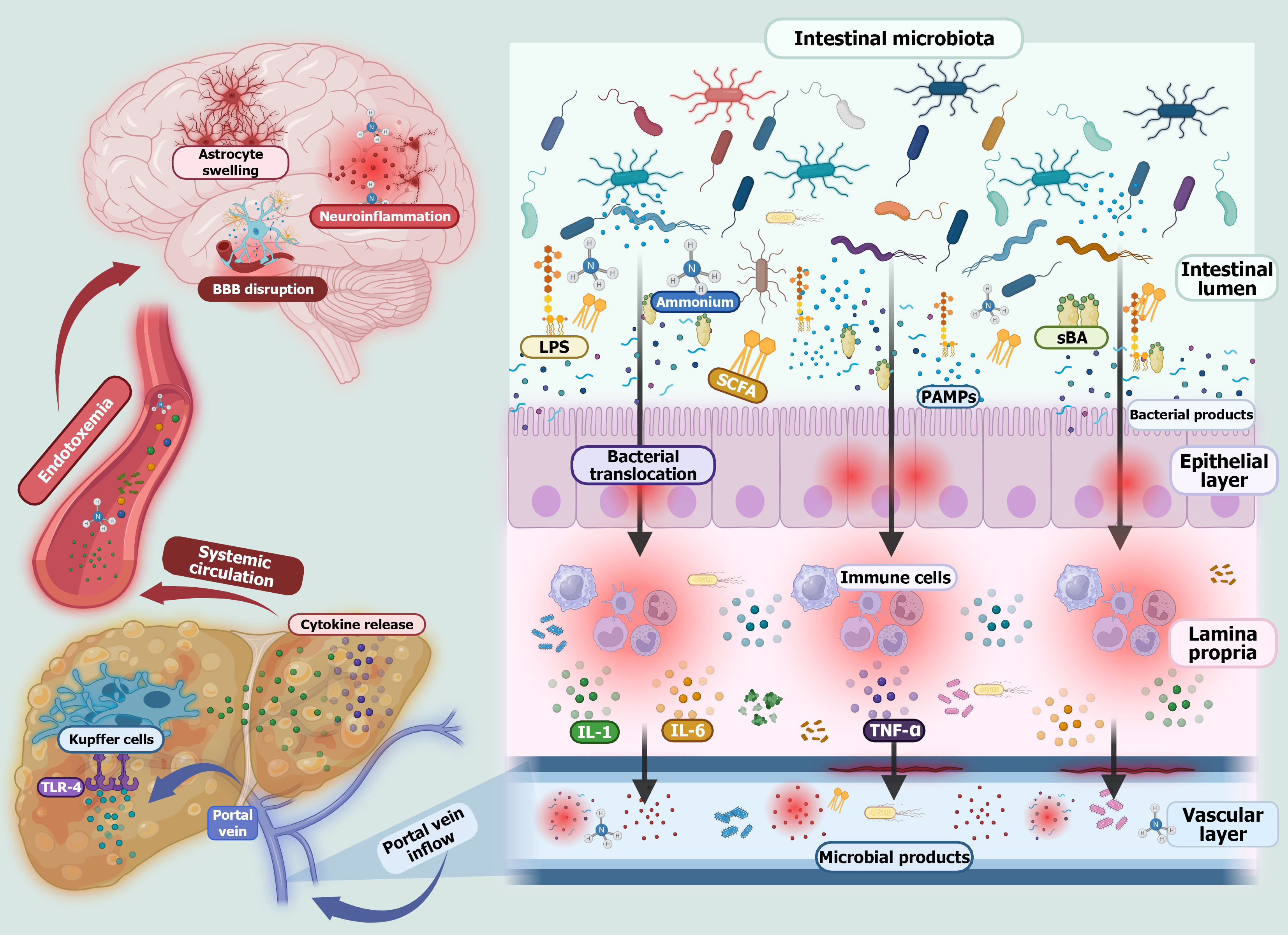
Figure 1 Dysbiosis and its role in hepatic encephalopathy.
During chronic liver disease gut dysbiosis and disruption of the intestinal barrier lead to bacterial overgrowth and a reduction in beneficial commensal organisms. This imbalance results in the release of pathogen-associated molecular patterns such as lipopolysaccharide, flagellin, and peptidoglycan. These molecules along with bacteria translocate across the compromised intestinal barrier, triggering immune cell activation and the production of proinflammatory cytokines. These cytokines travel through the portal vein to the liver. In the context of liver dysfunction, toxins like ammonia are not properly metabolized and can reach the brain, contributing to blood-brain barrier disruption. The combination of systemic inflammation and elevated ammonia levels leads to astrocyte swelling, a hallmark of hepatic encephalopathy. Astrocyte swelling results from ammonia-induced glutamine accumulation within these brain cells, leading to osmotic imbalance and cerebral edema, which contribute to neuroinflammation and the cognitive impairments characteristic of hepatic encephalopathy. PAMPs: Pathogen-associated molecular patterns; sBA: Secondary bile acids; SCFA: Short-chain fatty acids; LPS: Lipopolysaccharide; IL: Interleukin; TNF-α: Tumor necrosis factor-alpha; TLR-4: Toll-like receptor 4.
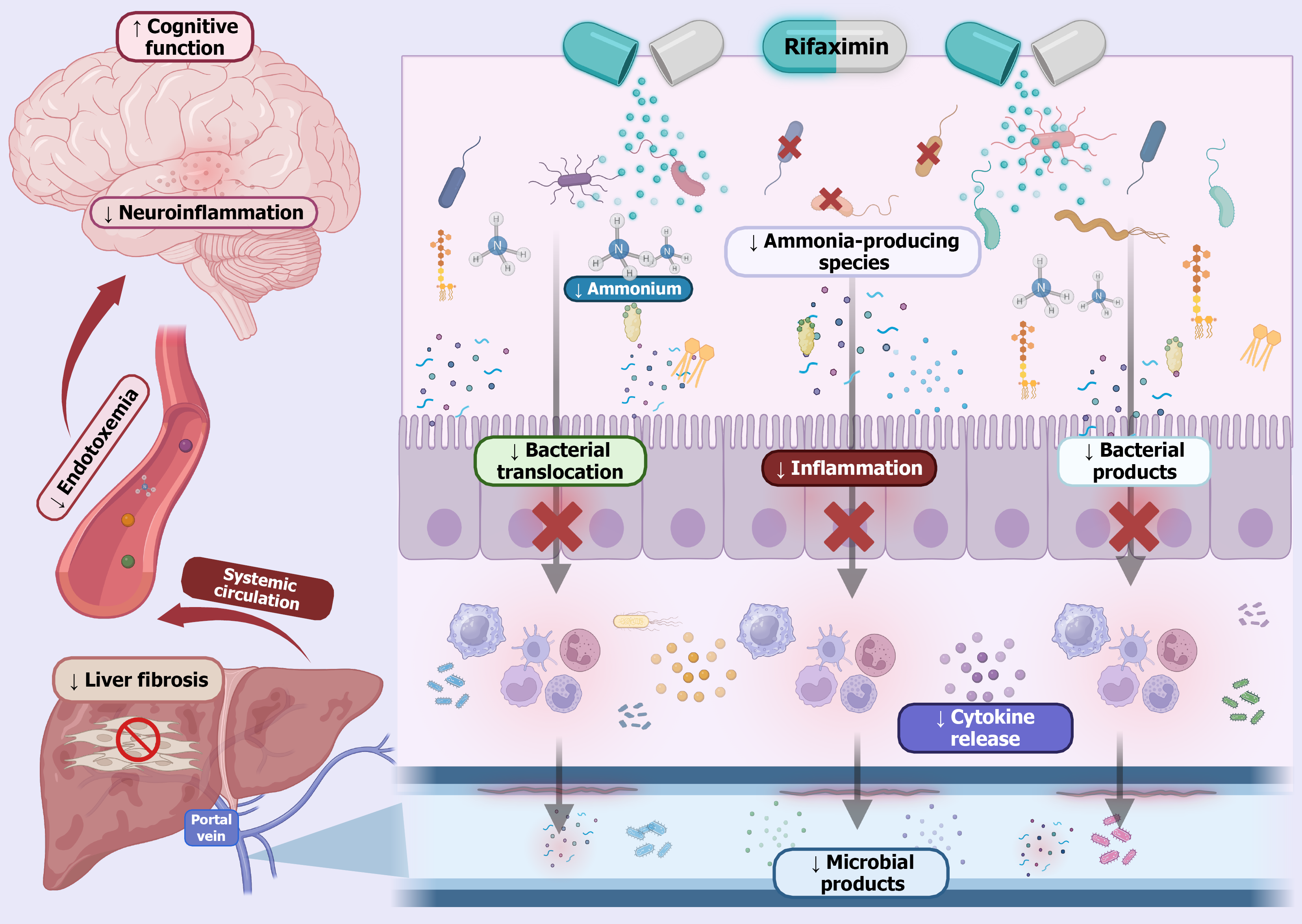
Figure 2 Rifaximin in hepatic encephalopathy.
Rifaximin is a non-absorbable antibiotic that acts locally in the gut to modulate the intestinal microbiota. In patients with hepatic encephalopathy, this antibiotic reduces the abundance of ammonia-producing bacteria, leading to decreased production of ammonia and other gut-derived toxins. This reduction helps limit bacterial translocation and the associated release of proinflammatory cytokines. As a result fewer microbial products enter the portal circulation, contributing to decreased hepatic inflammation and potentially slowing the progression of liver fibrosis. Additionally, by lowering systemic levels of neurotoxic substances, rifaximin helps reduce neuroinflammation and improves cognitive function in patients with hepatic encephalopathy.
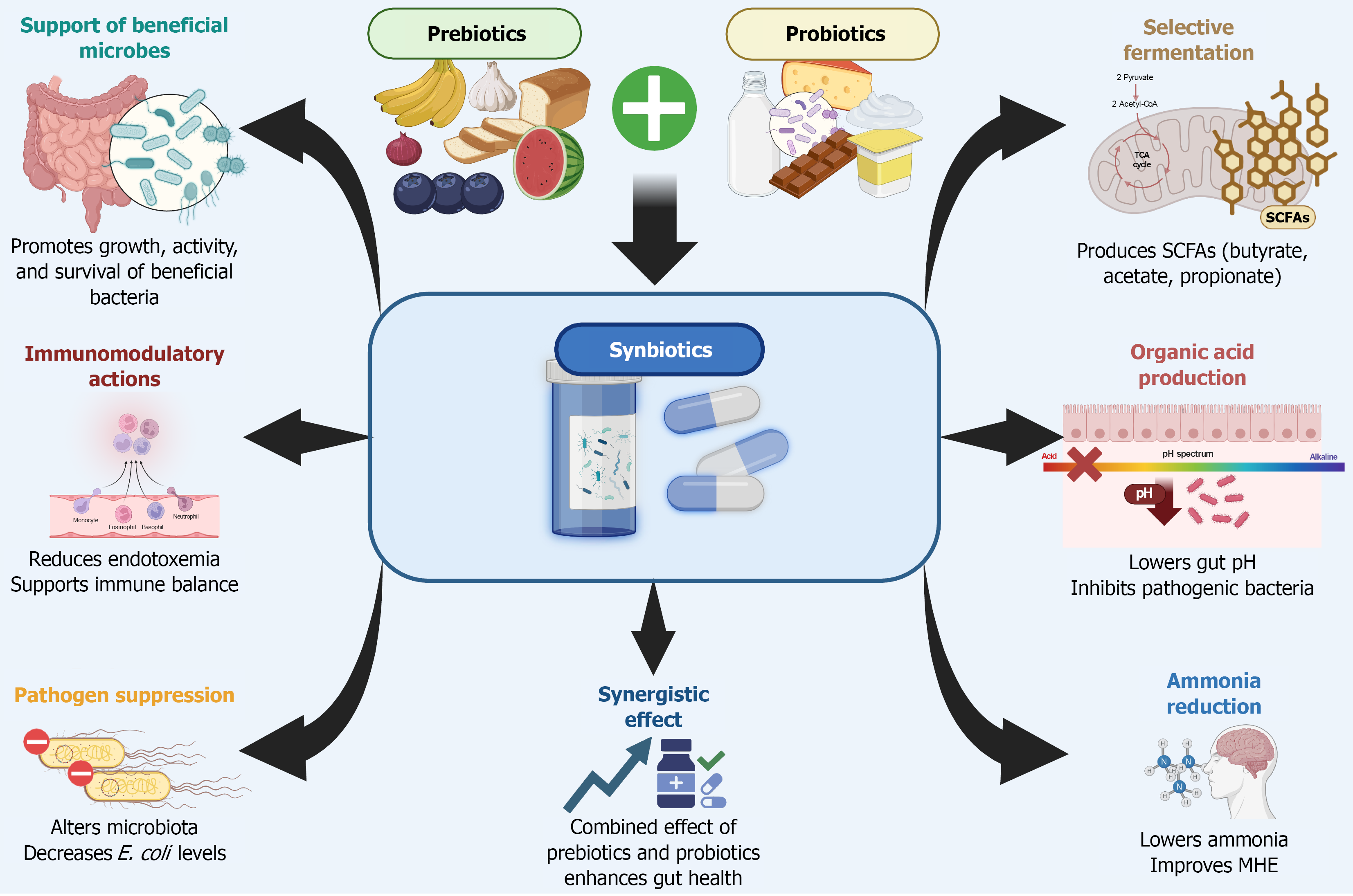
Figure 3 Synbiotic therapy: Microbial and metabolic benefits.
This illustration highlights the key mechanisms through which synbiotic therapy, combining prebiotics and probiotics, exerts beneficial effects on gut microbiota and host metabolism. Synbiotics work synergistically to promote the growth and activity of beneficial bacteria, enhance short-chain fatty acids production through selective fermentation, and lower intestinal pH via organic acid release. These changes suppress pathogenic organisms, reduce systemic endotoxemia, and modulate immune responses. Additionally, synbiotics help decrease intestinal ammonia production and absorption, contributing to improved cognitive outcomes in patients with hepatic encephalopathy. SCFA: Short-chain fatty acids; MHE: Minimal hepatic encephalopathy; E. coli: Escherichia coli.
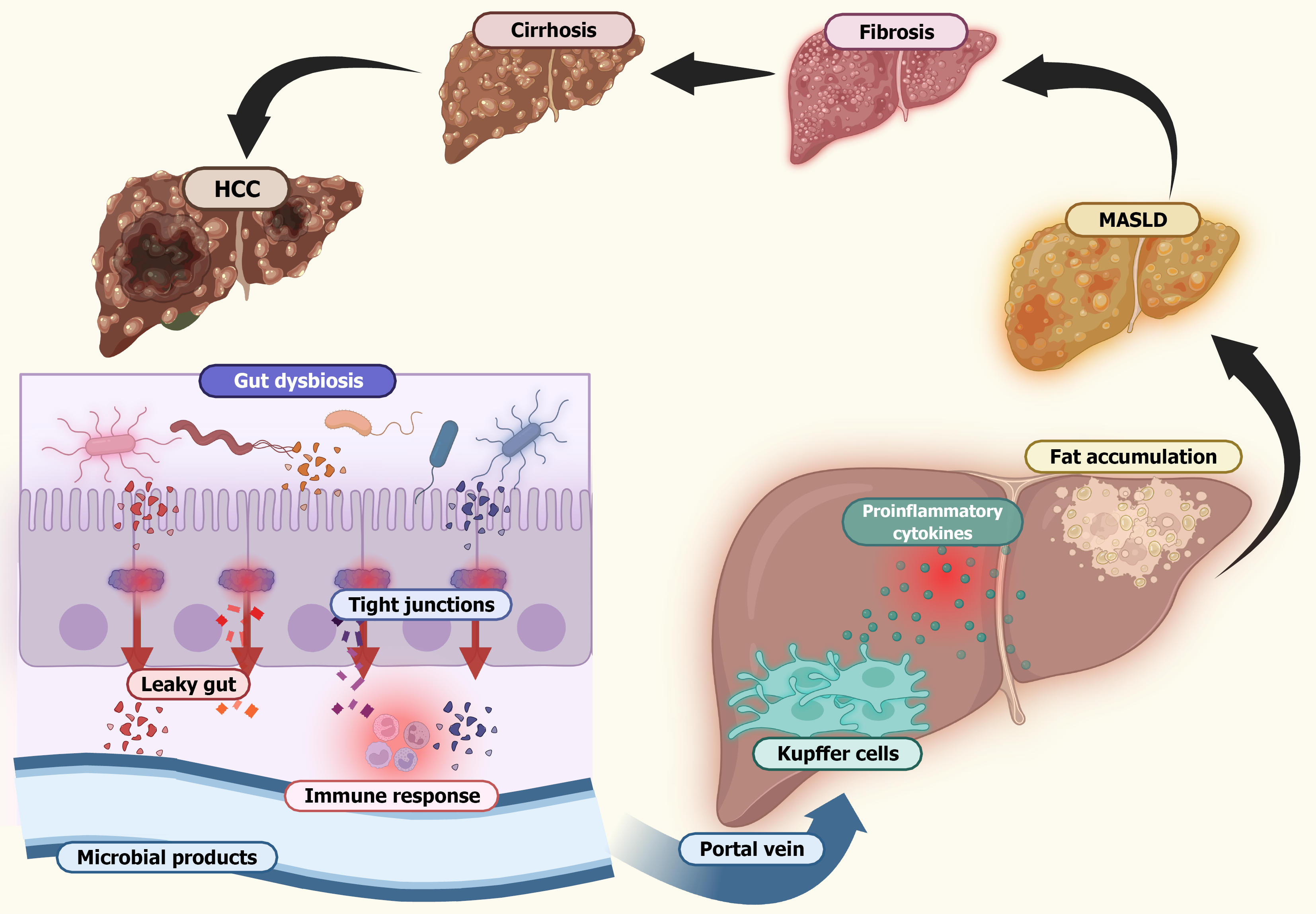
Figure 4 Liver damage progression in gut dysbiosis.
This illustration describes the sequence of events linking gut dysbiosis to progressive liver damage. Disruption of the gut microbiota leads to mucus layer depletion and loss of intestinal barrier integrity, resulting in a leaky gut. This facilitates microbial translocation and increases the production of microbial metabolites such as endotoxins. These microbial products enter the portal circulation and reach the liver where they activate Kupffer cells and stimulate the release of proinflammatory cytokines. The resulting chronic inflammation promotes hepatic fat accumulation and initiates fibrotic processes. Over time this inflammatory cascade drives the progression from metabolic dysfunction-associated steatotic liver disease to more severe forms of liver injury, including fibrosis, cirrhosis, and ultimately hepatocellular carcinoma. MASLD: Metabolic dysfunction-associated steatotic liver disease; HCC: Hepatocellular carcinoma.
- Citation: Vargas-Beltran AM, Mialma-Omana SJ, Vivanco-Tellez DO. Targeting gut microbiota in liver disease: A pharmacological approach for hepatic encephalopathy and beyond. World J Gastrointest Pharmacol Ther 2025; 16(4): 110271
- URL: https://www.wjgnet.com/2150-5349/full/v16/i4/110271.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v16.i4.110271