INTRODUCTION
Cardiac arrest is considered one of the most critical emergencies in clinical medicine, with survival frequently depends on rapid intervention and post-resuscitation management. Although advances in cardiopulmonary resuscitation (CPR) and intensive care have increased the rates of return of spontaneous circulation (ROSC), overall patient outcomes remain poor[1,2]. Survivors often experience high mortality rates and substantial long-term neurological impairment, underlining the need for accurate post-arrest evaluation to guide treatment strategies.
Imaging has become a vital element in the management of patients following cardiac arrest. Indeed, it serves multiple roles such as identifying the underlying cause, evaluating the severity of organ damage, particularly hypoxic-ischemic brain injury and guiding immediate clinical decisions and long-term prognostication[3]. Neuroimaging, including computed tomography (CT) and magnetic resonance imaging (MRI), is particularly valuable for evaluating brain injury and predicting neurological outcomes. Early CT scan might contribute to rule out acute intracranial events, while advanced MRI techniques provide greater sensitivity for detecting anoxic brain injury[4]. In parallel, cardiac imaging plays a key role in diagnosis, with echocardiography and invasive coronary angiography commonly used to identify structural heart diseases, wall motion defects or acute coronary syndromes as potential causes of cardiac arrest[5,6]. Last but not least, whole body imaging including CT of the chest, abdomen and pelvis could reveal extracardiac or systemic conditions such as pulmonary embolism, aortic dissection and intra-abdominal pathologies, that might otherwise remain unrecognized[7].
Given the diverse potential causes of cardiac arrest, a structured imaging strategy tailored to the patient's specific clinical context is essential to optimize outcomes. Such an approach allows rapid identification of reversible etiologies, guides targeted interventions, and minimizes unnecessary delays in critical decision-making. Embedding these choices within clear clinical decision-making pathways helps clinicians determine when each imaging modality should be prioritized in real-world scenarios - based on factors such as hemodynamic stability and suspected underlying cause.
This review aims to provide an overview of current evidence regarding imaging in the post-cardiac arrest setting and explore the role of neurological, cardiac and whole-body imaging modalities, with a particular focus on when to use each modality and how imaging aids in diagnostic clarity and outcome prediction.
CARDIAC IMAGING
Cardiac imaging plays a central role in determining the etiology of cardiac arrest, particularly in cases where acute coronary syndrome, structural heart disease or arrhythmogenic conditions are suspected. Post-resuscitation cardiac imaging helps identify reversible causes and guide immediate management (e.g., coronary intervention).
Echocardiography
Echocardiography serves as a useful tool in cardiac imaging following cardiac arrest, owing to its bedside accessibility, rapid execution and capacity to deliver real-time functional assessments and should be performed in all patients “as soon as possible” after ROSC[8-10]. This modality facilitates early diagnosis by enabling the detection of global or regional wall motion abnormalities indicative of ischemia or infarction, left or right ventricular dysfunction, pericardial effusion and cardiac tamponade, as well as signs suggestive of massive pulmonary embolism, valvular pathology or congenital disorders[10].
It is well established that the presence of regional wall motion abnormalities in the immediate period following ROSC is not exclusively indicative of an acute coronary syndrome. Distinguishing new wall motion abnormalities caused by acute coronary syndrome from pre-existing abnormalities due to prior coronary events or underlying cardiomyopathies can be challenging[8]. A retrospective, single-center observational study involving 617 adults, admitted following resuscitated cardiac arrest and underwent cardiologist-performed ultrasound within 60 minutes of admission, found that regional wall motion abnormalities were present in 37.6% of patients. Nonetheless, among those who subsequently underwent coronary angiography, only half were found to have a culprit coronary lesion[11].
Moreover, a condition that is usually seen even in the absence of coronary artery disease (CAD) and present as global or regional wall motion abnormalities is post-resuscitation myocardial dysfunction. This condition affects up to 60% of patients following cardiac arrest and usually resolves within 24 to 48 hours[9]. A prospective study of 280 patients who survived non-traumatic out-of-hospital cardiac arrest (OHCA) without prior cardiac disease found that approximately one-third of them developed echocardiographic post-resuscitation myocardial dysfunction. The predominating pattern was global hypokinesis (20%), followed by regional wall motion abnormalities (7%) and a Takotsubo-like pattern (5%). There were no differences in outcome measures, including in-hospital mortality, across the dysfunction subtypes. Serial imaging demonstrated significant recovery of left ventricular systolic function by 72 hours in almost all patients, with the rare exception of a persistent Takotsubo-pattern case two weeks post-arrest[5].
The true value of echocardiographic assessment following cardiac arrest might reside in its capacity to facilitate serial evaluations, thereby enabling the detection of evolving cardiac function and the assessment of progressive improvement over time. Comparing sequential studies also allows clinicians to identify persistent dysfunction that might point to underlying structural disease, evolving ischemia or new complications. In the absence of advanced imaging such as cardiac MRI, this capacity for safe, bedside, repeatable evaluation makes echocardiography a uniquely valuable tool in the continuum of post-cardiac arrest care[12].
Echocardiography in post-resuscitated patients might also reveal features suggestive of cardiomyopathy or myocarditis. While pronounced asymmetrical myocardial hypertrophy readily points toward hypertrophic cardiomyopathy, identifying characteristic features of other cardiomyopathies during the immediate post-resuscitation period remains challenging. This difficulty arises from the overlap between these pathologies and the biventricular systolic and diastolic dysfunction commonly associated with post-resuscitation myocardial dysfunction[8,12].
Echocardiography serves as a valuable tool for identifying secondary signs of massive pulmonary embolism. Notable sonographic findings include right ventricular enlargement due to elevated pressure from pulmonary artery obstruction, the presence of the D sign characterized by flattening of the interventricular septum toward the left ventricle as a result of right ventricular pressure overload, visualization of thrombi within the right atrium or right ventricle and a dilated, congested inferior vena cava[13]. Echocardiography is also the primary imaging modality for the identification of cardiac tamponade, typically performed during CPR. It plays a crucial role in guiding pericardiocentesis and, in the post-resuscitation era, facilitates monitoring of effective drainage and cardiac decompression. Additionally, pericardial effusion and tamponade might represent extremely rare, but serious, complications of CPR, which, if unrecognized, can hinder hemodynamic stabilization[14].
Lastly, early echocardiographic evaluation provides valuable prognostic insight, particularly in cases of cardiac-origin arrest. A retrospective cohort study of 1050 patients, who experienced in-hospital cardiac arrest (IHCA) and received CPR, examined the effect of transthoracic echocardiography within 24 hours of ROSC on outcomes. After propensity score matching, in-hospital survival was significantly higher in the echocardiography group (49.3%) compared to the control group (29.2%), with an odds ratio of 2.35. Multivariable analysis confirmed this association (odds ratio 2.26). The survival benefit was most notable in cardiac-origin arrests (49.3% vs 26%), while no significant benefit was seen in non-cardiac cases[15].
Invasive coronary angiography
CAD is implicated in approximately 60% to 80% of OHCA and might arise from acute coronary syndrome or stable CAD characterized by prior myocardial injury and scarring[16]. In a cohort study of OHCA, acute coronary syndrome was identified as the underlying cause in 59% of cases, with ST-elevation myocardial infarction (STEMI) accounting for 85% and non-STEMI (NSTEMI) for 15% of these events[17]. On the other hand, acute coronary syndrome is a less frequent etiology of cardiac arrest in the in-hospital setting[18]. A recent systematic review and meta-analysis involving 27102 patients with IHCA reported that acute coronary syndrome was present in 18.2% of cases[19].
Due to the high prevalence of obstructive CAD especially in the out-of-hospital setting, coronary angiography and percutaneous coronary intervention represent vital components of post-resuscitation management. Indeed, previous observation studies[20,21] reported a survival benefit of early coronary catheterization in comatose survivors of cardiac arrest with NSTEMI on the post-resuscitation electrocardiogram. However, more recent trials[22-24] have reported conflicting results, failing to support this statement. Specifically, they demonstrated that patients with NSTEMI do not appear to benefit from early coronary catheterization. The COACT trial was the first large randomized controlled study to evaluate the impact of immediate coronary angiography in successfully resuscitated OHCA patients without evidence of STEMI. In this trial, 552 patients were randomly assigned to either immediate or delayed coronary angiography and no significant difference in 90-day survival was observed between the two groups[24]. Similarly, the EMERGE trial evaluated whether immediate angiography improved outcomes compared to delayed angiography (performed 48-96 hours after ROSC) in 279 comatose OHCA survivors. The study showed that immediate coronary angiography did not outperform delayed angiography, regarding both neurological outcomes and survival at 180 days[23]. In line with the abovementioned results, the TOMAHAWK trial demonstrated that routine immediate coronary angiography was not superior to a delayed/selective approach in patients with OHCA and NSTEMI, with regard to 30-day all-cause mortality[22].
These findings have been reflected in recent international guidelines, including those from the European Resuscitation Council (ERC), the European Society of Cardiology (ESC) and the American Heart Association (AHA), which provide updated recommendations regarding the role and timing of coronary angiography in post-cardiac arrest care. The 2021 ERC guidelines advocate for immediate coronary angiography in adult survivors of cardiac arrest presenting with STEMI. In the absence of ST-elevation, immediate angiography is recommended only when there is clinical evidence of ongoing myocardial ischemia or the patient exhibits hemodynamic or electrical instability[25]. Similarly, the ESC guidelines advise immediate coronary angiography for patients with STEMI, while for hemodynamically stable patients without ST-elevation, a delayed or selective approach is considered reasonable[26]. The 2023 AHA Focused Update on Adult Advanced Cardiovascular Life Support reinforces these principles, emphasizing that emergent coronary angiography should be performed in patients with suspected cardiac cause of arrest and ST-segment elevation on electrocardiogram. For comatose patients with NSTEMI, routine immediate angiography is not suggested, but early angiography is considered reasonable in those with shock, electrical instability, signs of significant ongoing myocardial damage or ongoing ischemia[27]. For patients receiving Extracorporeal Membrane Oxygenation (ECMO), the Extracorporeal Life Support Organization guidelines recommend emergent coronary angiography for all individuals without an obvious non-cardiac cause, regardless of their age or presenting cardiac rhythm[28].
Cardiac MRI
Excluding CAD as the cause of cardiac arrest is a crucial first step in diagnosis; nonetheless, identifying nonischemic causes afterward remains a complex challenge. Cardiac MRI provides a noninvasive approach to comprehensively assess myocardial structure and function, along with tissue characterization techniques such as late gadolinium enhancement (LGE) for detecting replacement fibrosis, T1 mapping for evaluating interstitial fibrosis and T2 mapping for identifying myocardial edema and inflammation. It has emerged as a valuable diagnostic tool in the evaluation of patients achieving ROSC after cardiac arrest, particularly when the initial workup fails to identify a clear etiology. In patients with non-obstructive coronary arteries on angiography, cardiac MRI can uncover important non-ischemic pathologies such as myocarditis, infiltrative diseases (e.g., sarcoidosis, amyloidosis) and various cardiomyopathies including hypertrophic, dilated or arrhythmogenic types[29,30].
Several studies have demonstrated the diagnostic utility of cardiac MRI in this setting. A retrospective multicenter registry assessed the role of this imaging modality in 104 survivors of OHCA with inconclusive coronary angiograms. Cardiac MRI successfully identified an underlying diagnosis, revealing ischemic heart disease in 41%, non-ischemic heart disease in 28% and non-specific findings in 8% of patients. Importantly, cardiac MRI had a significant clinical impact in 77 out of 110 patients (70%), contributing to a change in diagnosis in 25%, a change in management in 29% and both diagnosis and management in 16% of cases[31]. Researchers from the London Chest and University College London Hospitals NHS Trusts conducted a review of cardiac investigations and clinical outcomes in 164 consecutive survivors of cardiac arrest in whom CAD had been excluded. Cardiac MRI revealed an underlying diagnosis in approximately 49% of cases and was deemed decisive in 50 patients (30%). The most frequently identified conditions included dilated cardiomyopathy, myocarditis or sarcoidosis, occult myocardial infarction and hypertrophic cardiomyopathy while no definitive cause was determined in 36% of the cohort[29]. Another retrospective study of 65 survivors of sudden cardiac arrest that underwent cardiac MRI between 2007 and 2022 revealed that this imaging modality was diagnostic in 57 patients (88%), most commonly identifying ischemic cardiomyopathy in 28.1%, dilated cardiomyopathy in 17.5% and structurally normal hearts in 14%. Remarkably, among 10 patients assessed for myocardial edema, extracellular volume (ECV) was elevated in 80% whereas T2 mapping was elevated in only 50%, indicating superior sensitivity of ECV in detecting edema post-resuscitation[32]. These findings highlight the diagnostic value of cardiac MRI in uncovering underlying structural or inflammatory myocardial disease not evident on echocardiography or angiography. Cardiac MRI provides highly detailed anatomical assessment, precise evaluation of ventricular volumes and function, and advanced tissue characterization - such as fibrosis, edema, or infiltration-enabling more accurate detection, differentiation of disease etiology, and risk stratification.
The 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death recommend the use of cardiac MRI with LGE in all survivors of cardiac arrest when no clear underlying cause has been identified. However, the guidelines do not provide specific recommendations regarding the optimal timing for performing the examination[10]. Furthermore, factors such as patient instability and the presence of non-MRI-compatible devices may limit the feasibility of conducting MRI in the immediate post-arrest period.
NEUROLOGICAL IMAGING
Neurological injury is a major determinant of outcomes following cardiac arrest and primary cause of death in patients that achieve ROSC. Neuroimaging provides critical information on the presence and severity of hypoxic-ischemic brain injury and plays an important role in prognostication, particularly when neurological examination is limited due to sedation or targeted temperature management. In these patients, the optimal timing of imaging for neuroprognostication is typically delayed-often to at least 72 hours after return to normothermia-because sedation and hypothermia can transiently suppress neurological function and alter imaging findings, potentially leading to inaccurate prognostic assessment if performed too early. Waiting allows sedative agents to clear, the effects of temperature modulation to resolve, and imaging changes related to irreversible injury to become more clearly detectable[9,33,34].
CT brain
CT brain is the most commonly utilized initial neuroimaging modality, primarily due to its rapid accessibility and effectiveness in detecting acute structural abnormalities. It is typically performed in the immediate period following ROSC after cardiac arrest to evaluate for potential neurological causes, such as intracranial hemorrhage or large ischemic stroke, as well as to identify features suggestive of severe primary anoxic brain injury. Cardiac arrests of neurological origin account for approximately 2.3% to 16% of all OHCA cases, but are associated with particularly poor neurological outcomes and high mortality, emphasizing the need for early identification and tailored post-resuscitation care in these patients[35-37]. Current guidelines advocate for the prompt identification of a neurological etiology by conducting a brain CT scan upon hospital admission, either prior to or following coronary angiography[9,10].
It is estimated that about 50% of cardiac arrest survivors remain comatose 72 hours following ROSC[38]. Imaging plays a critical role in neurological prognostication after cardiac arrest by identifying the extent of hypoxic-ischemic brain injury and helping predict long-term outcomes and make therapeutic decisions. Early non-contrast CT might reveal findings, such as the loss of boundary sign, the sulcal effacement sign, the pseudo subarachnoid haemorrhage (p-SAH) sign and an increased optic nerve sheath diameter (ONSD), that are associated with poor prognosis. The loss of boundary sign refers to the diminished differentiation between gray and white matter, typically quantified using the gray-white matter ratio (GWR). The GWR, calculated as the ratio of Hounsfield Units between gray and white matter regions, is the most extensively studied CT-based parameter for evaluating post-arrest brain injury. The sulcal effacement sign refers to the loss of the normal grooves (sulci) on the surface of the brain and this finding is suggestive of cerebral edema and increased intracranial pressure. The p-SAH sign mimics true SAH, but results from distension of superficial cerebral veins and appears in case of cerebral edema. These imaging changes are time-dependent, with the loss of the boundary sign observable as early as one hour following cardiac arrest while findings such as increased ONSD and the p-SAH sign typically emerge within a few hours post-arrest[39].
A single-center retrospective cohort study involving 2204 patients resuscitated from cardiac arrest underlined the time-dependent prognostic value of the GWR on early CT brain. GWR values lower than 1.10 or 1.20 were highly specific predictors of in-hospital mortality and neurologic death, with false-positive rates remaining below 5% across all time intervals. Notably, the sensitivity of these GWR thresholds increased steadily during the first few hours following cardiac arrest, peaking around 4-5 hours, suggesting that the reliability of CT-based prognostication improves with time. These findings highlight that while early CT can identify severe hypoxic-ischemic brain injury, its prognostic accuracy depends on the timing of imaging, supporting recommendations to delay neuroimaging beyond the immediate post-arrest period for more reliable outcome prediction[40].
Therefore, early CT brain after cardiac arrest is valuable for acute diagnostic and management purposes since this imaging modality can exclude alternative causes of coma such as intracranial hemorrhage, large territorial infarcts or mass lesions and identify severe cerebral edema that may require urgent intervention. Even certain early imaging features might suggest extensive hypoxic-ischemic injury, subtle ischemic changes often evolve over hours to days, limiting the sensitivity of early CT for accurate prognostication. On the other hand, delayed imaging allows for the full evolution of cytotoxic edema and other radiographic markers of irreversible injury, providing a more reliable basis for neuroprognostication[41]. Indeed, the Neurocritical Care Society guidelines characterize neuroimaging as a "moderately reliable" tool for prognostication, emphasizing that brain CT scans should ideally be performed at least 48 hours following cardiac arrest[38].
Brain MRI
Brain MRI is increasingly being recognized as a valuable prognostic tool for assessing neurological outcomes after cardiac arrest. Following ROSC, hypoxic-ischemic brain injury leads to cytotoxic edema, where water enters and accumulates inside the cells, restricting diffusion. This appears as increased signal on diffusion weighted imaging (DWI) and decreased apparent diffusion coefficient (ADC) values, making them reliable markers of early brain injury and poor neurological prognosis[42]. A multicenter study evaluated DWI MRI in 125 comatose patients after cardiac arrest and found that the volume of brain tissue with ADC ≤ 650 × 10-6 mm²/s was strongly predictive of outcome. Patients with ≥ 10% of brain volume below this threshold had a 72% sensitivity and 91% specificity for poor neurological outcome. Notably, involvement of > 22% of brain volume was 100% specific for predicting failure to regain consciousness[43].
Moreover, fluid-attenuated inversion recovery (FLAIR) MRI, a specialized imaging sequence, can detect vasogenic edema, that typically becomes apparent 1-2 days following cardiac arrest and remains visible after DWI appears to normalize. When combined with DWI and ADC imaging, FLAIR findings are associated with poor neurological outcomes[44]. A systematic review and meta-analysis that analyzed 21 MRI-based prognostication studies (4 included both DWI and FLAIR) in over 4000 post–cardiac arrest patients found that combining DWI and FLAIR yielded a pooled sensitivity of 70% and specificity of 95% for predicting poor neurological outcomes[45]. In addition, a recently published prospective multicenter cohort study found that combining electroencephalography (EEG) with MRI (both DWI and FLAIR conducted around 3 ± 1 days post-cardiac arrest) significantly improved prognostic accuracy in 50 comatose survivors. While visual EEG alone identified only 15% of poor outcomes, adding MRI visual grading detected 65% and quantitative ADC analysis captured 55%. Crucially, integrating EEG and MRI allowed prediction of poor outcome in 80% of cases with 100% specificity and identification of good outcome in 80% with 63% specificity[46].
International guidelines for post-cardiac arrest care advise postponing neurological prognostication until at least 72 hours after ROSC[9,38]. Nevertheless, several studies attempted to predict neurological outcome during the early stage following cardiac arrest. A retrospective observational study investigated ultraearly (within 6 hours postROSC) DWI MRI in 110 comatose survivors of OHCA and found that 46 patients (42%) showed high-signal intensity (HSI) on DWI with matching ADC reduction; none of these patients had a favorable neurological outcome at six months. This imaging finding predicted poor outcome with 100% specificity and 74% sensitivity. When combined with neuron-specific enolase, a biomarker released into cerebrospinal fluid when neurons are damaged, the sensitivity increased to almost 90% while maintaining perfect specificity[47]. A retrospective cohort study evaluated the optimal timing for ultra-early DWI MRI by examining factors associated with the presence of HSI in 206 cardiac arrest survivors. Multivariate regression identified longer low-flow time, non-shockable initial rhythm and the interval from ROSC to imaging as significant predictors of MRI findings. Performing the MRI at least 2.2 hours after ROSC, particularly in patients with shorter low-flow durations or shockable rhythms, enhanced the detection of brain injury and minimized false-negative results[48].
CHEST CT
Following cardiac arrest, a thorax CT scan, including CT pulmonary angiography (CTPA) and a CT angiogram as well, serves as a key diagnostic tool to evaluate both vascular and parenchymal causes of cardiac arrest. Chest CT provides detailed views of the lung parenchyma, allowing identification of conditions such as acute respiratory distress syndrome, pneumonia and pneumothorax. CTPA is primarily used to rule out pulmonary embolism, a reversible cause of cardiac arrest. Additionally, the presence and extent of pleural effusions can be visualized, which might contribute to impaired oxygenation or indicate underlying pathology such trauma. Importantly, this imaging approach also offers insight into secondary findings, such as rib fractures or sternal injuries related to CPR. The CT angiogram allows assessment of the thoracic aorta and major branches, helping to identify life-threatening conditions such as aortic dissection or traumatic aortic injury. In summary, post-cardiac arrest chest CT with angiographic protocols offers a rapid, high-yield investigation to identify reversible causes of arrest, guide supportive care and anticipate complications in critically ill patients[3].
A study assessed the diagnostic yield of thorax CT scans performed within the first four hours of hospital admission in patients experienced non-traumatic OHCA. Thirty-one patients underwent CT imaging, with 22 receiving contrast enhancement. Key findings included pulmonary embolism in three patients, hemothorax in two, tracheal rupture in one and pneumonia in eleven. Overall, CT scans revealed clinically significant abnormalities in approximately 55% of cases, highlighting the importance of early chest CT in detecting potential causes following cardiac arrest[49]. CT imaging can also identify complications related to resuscitation efforts. A retrospective single-center study involving 137 with non-traumatic OHCA that achieved ROSC revealed skeletal fractures, including rib and sternal fractures in 40 patients (29.2%). Pneumothorax was also detected in 12 patients (8.8%). These findings are often significantly underreported on portable chest X-rays compared to CT imaging[50].
WHOLE BODY CT
In patients with cardiac arrest of unclear etiology, whole body imaging combining brain, thorax and abdominopelvic CT might uncover life-threatening, potentially reversible extracardiac conditions. This approach should also be considered in patients receiving ECMO[28,51]. In the PROCAT registry study, 1274 patients were admitted to the intensive care unit after OHCA, with 896 (70%) undergoing early imaging within 24 hours. Among these, 355 had CT scans (non-contrast brain CT and/or contrast chest CT angiogram), which identified a clear non-cardiac cause in 72 cases (20%), including stroke (38 cases) and pulmonary embolism (19 cases). Overall, early imaging helped establish a diagnosis in 59% of patients, supporting its value in guiding targeted post-arrest management[52]. The CT FIRST cohort study evaluated the impact of incorporating a head-to-pelvis CT scan within six hours of hospital arrival into the standard post-resuscitation care for patients following OHCA. The study compared 104 patients in the intervention group (post-cohort) with 143 controls who received standard care alone (pre-cohort). In the standard-care group, only 74 patients (52%) underwent CT imaging (head, chest and/or abdomen). The addition of systematic head-to-pelvis CT significantly increased the diagnostic yield from 75% to 92%. Notably, the comprehensive CT protocol reduced the median time to diagnosis by 78%, from 14.1 hours to just 3.1 hours. It also led to an 81% reduction in the delayed (> 6 hours) recognition of time-sensitive diagnoses. These improvements were achieved without an increase in adverse events, including contrast-associated acute kidney injury. While survival to hospital discharge did not differ significantly between groups, the study demonstrates that early, systematic whole-body CT is a safe and highly effective strategy for rapidly identifying critical etiologies in post-cardiac arrest patients[53].
However, while promising, this approach is not yet universally adopted. Logistical barriers such as limited scanner availability, the need for rapid patient transport and coordination with ongoing critical care interventions can delay imaging. Additionally, safety concerns, including hemodynamic instability during transport, contrast-induced nephropathy, and radiation exposure, pose significant challenges in critically ill post-cardiac arrest patients[54,55].
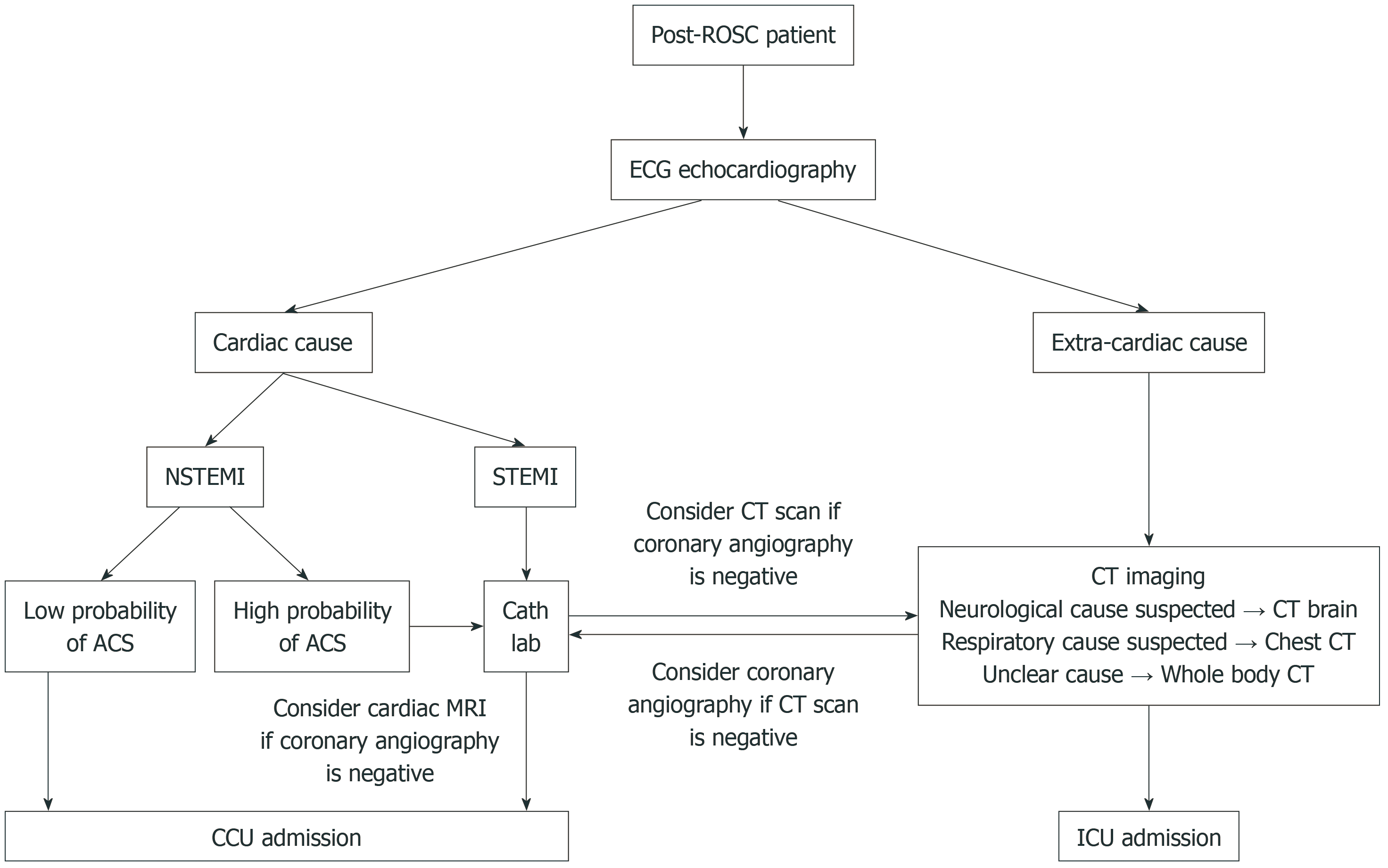
Figure 1 illustrates a clinical decision-making algorithm that incorporates imaging modalities for patients achieved ROSC following cardiac arrest. The algorithm guides clinicians through a step-by-step process to optimize post-resuscitation care by integrating imaging modalities to identify potential causes of arrest and tailor therapeutic interventions accordingly. Table outlines imaging priorities after ROSC according to clinical context.
Figure 1 Imaging clinical decision algorithm in the post-return of spontaneous circulation setting.
ACS: Acute coronary syndrome; Cath lab: Cardiac catheterization laboratory; CCU: Coronary care unit; CT: Computed tomography; ECG: Electrocardiogram; ICU: Intensive care unit; NSTEMI: Non-ST-elevation myocardial infarction; ROSC: Return of spontaneous circulation; STEMI: ST-elevation myocardial infarction.
CONCLUSION
Imaging including cardiac, chest and abdominopelvic scans are integral to the evaluation and management of patients after cardiac arrest. Cardiac imaging, including, echocardiography, invasive coronary angiography and cardiac MRI, plays a key role in identifying acute coronary syndromes and structural cardiac causes of arrest. Brain CT and MRI provide critical information on the presence and extent of hypoxic-ischemic injury, aiding in prognostication. Chest CT with angiography is essential for detecting reversible causes such as pulmonary embolism, pneumothorax and injuries related to resuscitation efforts. Abdominopelvic CT helps identify intra-abdominal pathologies that might contribute to or result from cardiac arrest. Whole body CT protocol enables rapid and comprehensive assessment of potentially reversible extracardiac and cardiac conditions, increasing diagnostic yield and expediting management decisions without increased risk. The integration of these imaging modalities supports timely diagnosis, tailored therapy and improved prognostic accuracy in the post-cardiac arrest setting.
The studies included in this review vary considerably in design, patient populations, and clinical settings, and the possibility of bias should be taken into account when interpreting the available evidence. Further high-quality investigations, particularly randomized controlled trials, are needed to evaluate the clinical efficacy of imaging in improving outcomes following cardiac arrest.