Published online Sep 28, 2025. doi: 10.4329/wjr.v17.i9.111924
Revised: August 9, 2025
Accepted: September 4, 2025
Published online: September 28, 2025
Processing time: 75 Days and 17.9 Hours
Thermal ablation (TA) has been proved to be effective and safe as minimally invasive treatment method for thyroid nodules. However, patients' experience during the procedures and quality of life varies among operators.
To explore strategy to improve quality of life and subjective experiences during TA for papillary thyroid carcinoma (PTC) based on thermal field management (TFM).
This retrospective propensity-matched cohort study was conducted in a single center. A total of 490 patients with PTC treated with TA from September 2023 to August 2024 were studied and divided into two groups (TFM group and non-TFM group) according to treatment strategies. Propensity score matching (PSM) was used to control for confounding factors. Complications, side effect and com
A total of 113 patients (41.7 ± 10.6; 31 men, 82 women) were assigned to the TFM group, and 377 patients (mean age, 41.1 ± 10.7 year; 116 men, 261 women) were assigned to the non-TFM group. After PSM, a total of 108 patients were included in the TFM group, and 216 patients were included in the non-TFM group. The median follow-up was 10 months (range from 4-15 months). The incidence of voice change in the TFM group was significantly lower than that in the non-TFM group (0.9% vs 6.5%; P = 0.049). Although there was no statistically significant difference in rate of pain between the two groups, the proportion of complaining of pain in the TFM group was numerically lower than that in the non-TFM group (3.7% vs 9.7%, P = 0.090).
TFM, as a novel procedural optimization technique, can effectively improve quality of life and subjective expe
Core Tip: In this study, we introduce the concept of thermal field management (TFM) for the first time as a means to optimize clinical practice in thermal ablation of papillary thyroid carcinoma. This approach does not rely on advanced technical maneuvers but represents a conceptual shift toward more precise, patient-centered ablation. Our results indicate that the TFM strategy effectively enhances quality of life and patient-reported outcomes by reducing specific complications and symptoms.
- Citation: Cai WJ, Li Y, Wei Y, Zhao ZL, Wu J, Cao SL, Peng LL, Li SQ, Yu MA. Thermal field management improves patient-reported outcomes during ablation for papillary thyroid carcinoma: A retrospective cohort study. World J Radiol 2025; 17(9): 111924
- URL: https://www.wjgnet.com/1949-8470/full/v17/i9/111924.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i9.111924
Papillary thyroid carcinoma (PTC) is the most common subtype of thyroid cancer, accounting for approximately 84% of cases. Its management has increasingly become a highlighting common clinical problem[1]. Given the good prognosis of patients with PTC, improving quality of life and achieving better patients' experience should be considered as a high-level priority when treating PTC[2]. As curative surgery is associated with several disadvantages, including postoperative hypothyroidism, the lifelong requirement for hormone replacement therapy, and potential cervical scarring, all of which may substantially impair patients’ quality of life[3], more minimally invasive thermal ablation (TA), including radiofrequency ablation (RFA), and microwave ablation (MWA), was introduced and has been proved to be safe and effective for treating benign thyroid nodule (BTN) and selected low-risk papillary carcinoma of the thyroid[4-6]. Nonetheless, pre
In this context, we propose the concept of thermal field management (TFM) for the first time to optimize clinical practice in thyroid ablation. The central principle of TFM is to achieve complete ablation of thyroid nodules while mini
In the present study, we established and evaluated an improved technique for TA by integrating comprehensive TFM strategies, detailed technical refinements, and practical clinical applications. The aim was to assess the safety and patient experience of this novel TFM-based TA approach in comparison with conventional TA for the treatment of thyroid nodules. We hypothesized that optimized TFM during ablation improves short-term recovery and patient-reported quality of life compared to conventional approaches.
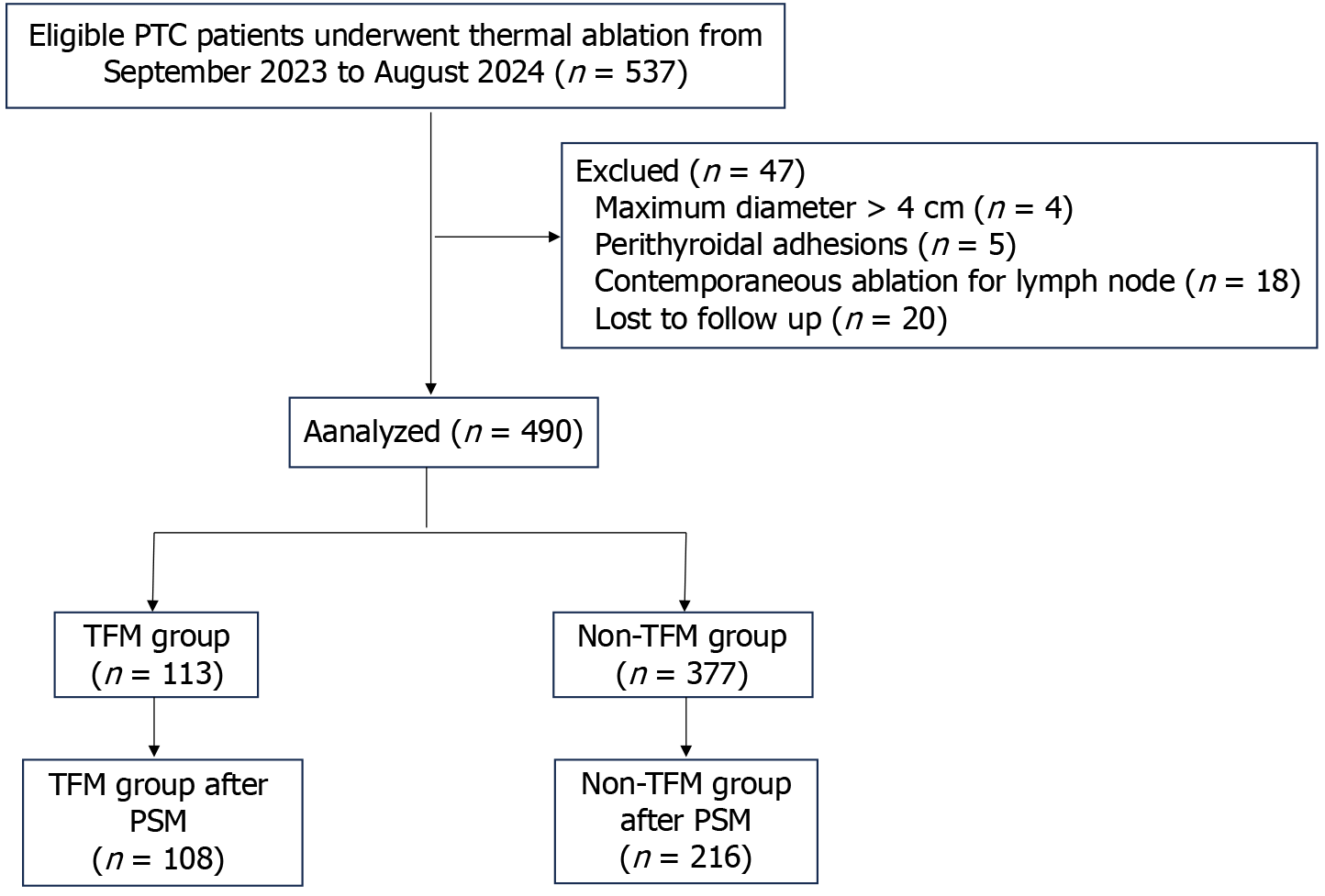
This retrospective study was approved by the Ethics Committees of China–Japan Friendship Hospital (2023-KY-250). Written informed consent for treatment and Quality of life assessment was obtained from each patient. From September 2023 to August 2024, a total of 490 patients underwent TA (MWA or RFA) for PTC in our center were enrolled (Figure 1). The TFM methodology is a comprehensive strategy applied during the TA procedure, involving strict control of the thermal field to ensure thorough energy coverage of the target lesions while minimizing heat leakage as much as possible. For further details, please refer to the following section. An independent experienced radiologist (Cai WJ, 13 years of experience with thyroid US and more than 5 years of experience with TA of thyroid nodules) who was blinded to the final analysis reviewed the stored images and videos of TA procedures. Patients who underwent TA sticked to TFM procedure were allocated to the TFM group, otherwise were allocated to the non-TFM group. The inclusion criteria were as follows: (1) PTC confirmed by US-guided fine-needle aspiration biopsy; (2) Tumor with maximum diameter of 4 cm or less; (3) Patients who were unwilling or ineligible for surgery or refuse to receive iodine 131 treatment and active surveillance; and (4) Follow-up time of at least 3 months. The exclusion criteria were: (1) Perithyroidal adhesions of PTC, unable to definitely separate from surrounding tissues during hydrodissection procedure; (2) Incomplete follow-up data; (3) Patients who had undergone local thyroid treatment before this study (previous thyroidectomy or TA); (4) Age younger than 18 years or pregnancy; and (5) Clinically or pathologically confirmed lymph node metastasis.
Complications and complaints of all patients were reviewed from the medical records presenting to our department and/or collected from telephone interview, and quality of life was assessed by the questionnaires.
Prior to TA, all patients underwent neck ultrasonography to assess the thyroid and cervical lymph nodes. Additional imaging (such as computed tomography or magnetic resonance imaging) and fine-needle aspiration were performed when necessary to exclude lymph node or distant metastases.
For both groups, the general procedures were similar and as follows: Prior to ablation, contrast-enhanced US (CEUS) (SonoVue, Bracco or Sonazoid, GE) was performed to assess the tumor’s enhancement pattern. Patients were positioned supine with the neck extended. After sterilizing the neck, local anesthesia with 1% lidocaine was administered at the designated ablation site and along the thyroid capsule. Patients in non-TFM group received routine TA treatment as previously described[8], while patients in TFM group received novel TA treatment based on TFM strategy.
During TA treatment following the TFM strategy, energy delivery adhered to the principle of “minimum necessary”. In other words, the thermal field was strictly controlled based on the nodule’s size, location, and characteristics to ensure thorough coverage of the lesion while minimizing heat leakage beyond the target area. For PTC, minimal extended ablation (2 mm for non-infiltrative growth) should be achieved for nodules not adjacent to the thyroid capsule, with energy confined within the capsule. An individualized ablation strategy should be followed as outlined below.
Active TFM: The core concept is energy limitation based on tissue properties during TA treatment. Since the thyroid capsule serves as an ideal natural barrier against heat leakage, it is critical to position the active tip of the needle firmly against-but without penetrating-the capsule for nodules adjacent to it. This ensures that heat from the active tip is conducted along the capsule while remaining strictly confined within the thyroid lobe. Additionally, tissue-specific properties significantly influence the effectiveness of ablation in achieving adequate tumor destruction. Factors such as tissue elasticity or fibrosis, water content, and tissue homogeneity can affect thermal conductivity and the distribution of the thermal field within the target tumors[9]. Therefore, the output power and ablation duration must be adjusted in real time according to the extent of thermal field coverage during the procedure. Additionally, optimal equipment selection and personalized needle movement strategies tailored to individual nodules should be employed. Specifically, larger active tips with higher wattage are suited for large thyroid nodules; a fixed-applicator technique is recommended for small malignant nodules, while a multipoint ablation strategy can be applied for larger malignant nodules.
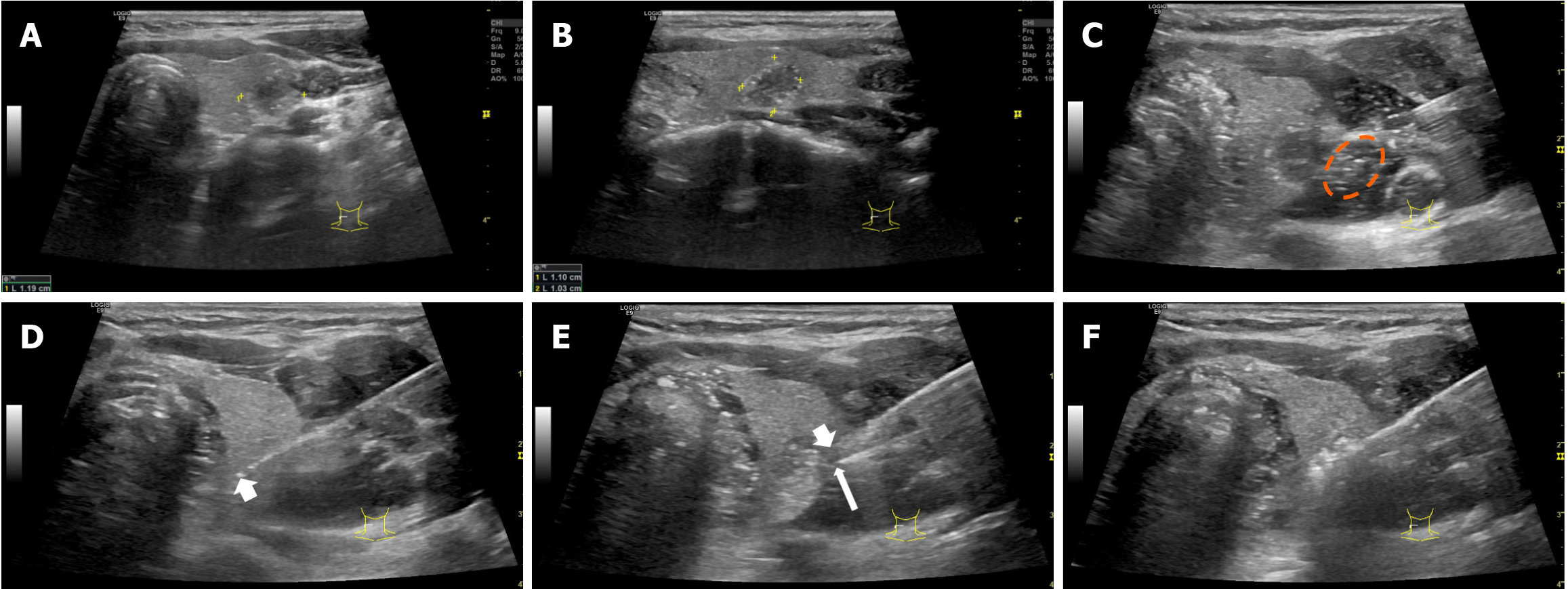
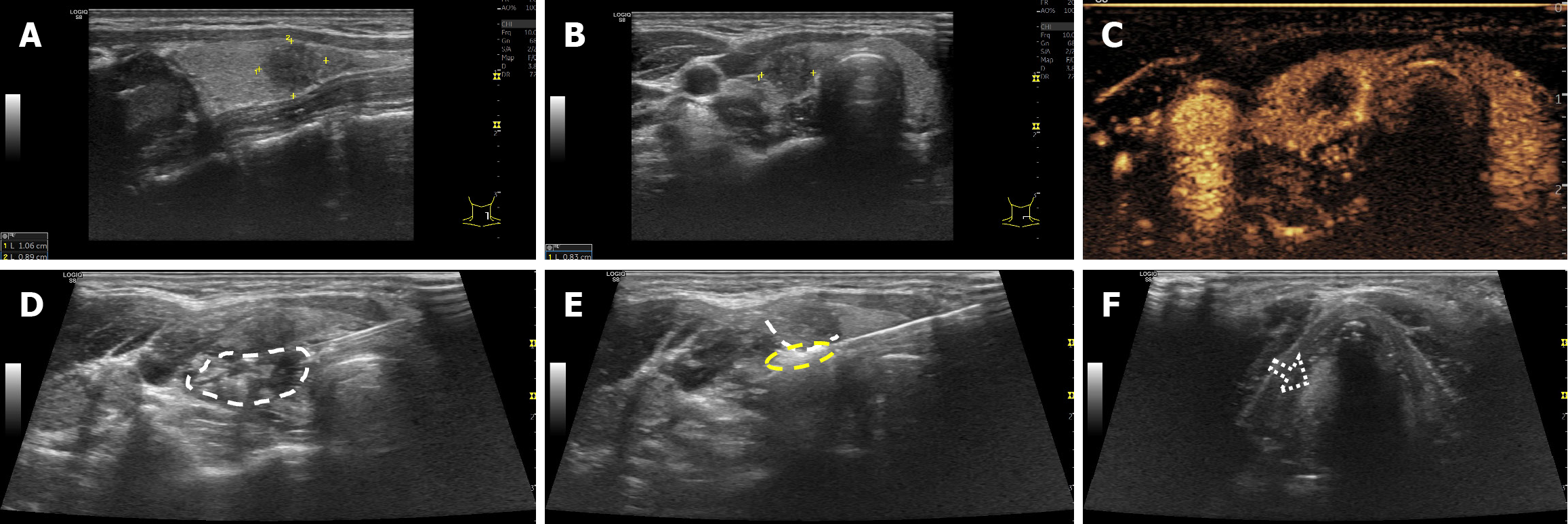
Passive TFM: Improved hydrodissection procedures were performed based on fascial spaces, with continuous injection of a minimal appropriate amount. The injection speed was adjusted according to the characteristics and location of the nodules, as previously described, to avoid thermal injury to perithyroidal soft tissues, including fascial structures, at the same time, without causing discomfort to the patient[10]. The fascial space under successfully improved hydrodissection was characterized by formation of an anechoic, hypoechoic or mixed-echoic isolating band between thyroid and the surrounding critical structures. In addition, if back heating along the shaft of the antenna (or electrode) to the outside of the capsule is detected by real-time US monitoring, the tip of hydrodissection needle should be positioned tightly attached to the thyroid capsule where heat leaks out, and isolating fluid should be injected continuously (Figures 2 and 3) (Videos 1, 2, 3, 4, 5, 6, 7, 8 and 9).
The primary end point was complications, and side effects which would significantly impact quality of life and subjective experiences of patients. The complications and side effects of the patients were observed and recorded during outpatient return visit, through questionnaires of quality of life, or collected through telephone interview. Questionnaires of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) and the Thyroid Cancer-Specific Quality of Life questionnaire (THYCA-QoL) was used to evaluate the quality of life and thyroid-specific symptoms for patients received TA at each follow-up time point. EORTC QLQ-C30 consists of 5 functional scales (physical, role, cognitive, emotional, and social), 3 symptom scales (fatigue, pain, nausea, and vomiting), 6 single-item common symptoms (dyspnea, loss of appetite, insomnia, constipation, diarrhea, and financial difficulties), and global health status subscales, while THYCA-QoL consists of seven symptom scales (neuromuscular, voice, concentration, sympathetic, throat/mouth, psychological and sensory problems) and six single items (problems with scar, feeling chilly, tingling hands/feet, gained weight, headache, less interest in sex). Most items are scored on a four-point response scale ranging from 1, “not at all” to 4, “very much”. After linear transformation, all scales and single-item measures range in score from 0-100. Among them, treatment-related symptoms and subscale scores were extracted for analysis[11,12]. And telephone survey was conducted to assess patient experience prior to the end of data collection for this study. All interviewed patients were asked the following questions: Is there any complications and discomfort related to the procedure after treatment? The initial question was an open question, if the patients responded yes, more details, such as type of complication (including voice change, vomiting) or discomfort (including pain, neck discomfort, coughing, and so on), degree and duration were further collected. The major and minor complications and side effects during or after the procedure are defined by the Society of Interventional Radiology and referred to a multicenter study of complications for thyroid ablation[13,14].
The secondary end points were technical success, which was defined as complete ablation, characterized as the nonenhancement ablation zone completely covering the targeted nodules and extending at least 2 mm from the original margin for PTC on CEUS.
After ablation, patients were followed up every 3 months in the first year, every 6 months for the next years, and annually thereafter. The follow-up included thyroid US, thyroid function tests, and questionnaires of quality of life. If the patient had hoarseness, the movement of the vocal cord was evaluated with US, as well as laryngoscopy, at each follow-up. Any delayed complications and presenting symptoms that occurred during the follow-up period were recorded.
Statistical analysis was performed using IBM SPSS Statistics (version 26.0; IBM), Empower (version 4.1, X&Y Solutions; www.empowerstats.com). Data are presented as the mean ± SD for normal distributions, and the median and range were used if data did not fit a normal distribution. Propensity score matching (PSM) with a 1:2 ratio performed to reduce bias, and the nearest neighbor matching method was used for matching. The caliper for PSM was 0.02. Propensity scores were estimated using a multivariate logistic regression model, by inserting the following variables: Age, sex, FT3, FT4, TSH, maximum diameter and lesion location. The independent two-sided Mann-Whitney U test was used to test the differences between the medians of continuous variables for data that did not fit a normal distribution. All differences were considered significant when P < 0.05.
The demographic and clinical characteristics are described in Table 1. Data from 537 eligible patients who underwent MWA or RFA for PTC from September 2023 to August 2024 were reviewed. After the exclusion of 47 patients who did not meet the inclusion criteria, 490 patients were finally enrolled in this study. The patients were divided into two groups according to treatment strategy: A total of 113 patients (41.7 ± 10.6; 31 men, 82 women) were assigned to the TFM group, and 377 patients (mean age, 41.1 ± 10.7 year; 116 men, 261 women) were assigned to the non-TFM group. After PSM, a total of 108 patients were included in the TFM group, and 216 patients were included in the non-TFM group. The median follow-up time was 10 months (range from 4-15 months).
| Parameter | Before PSM | After PSM | ||||
| TFM (n = 113) | non-TFM (n = 377) | P value | TFM (n = 108) | non-TFM (n = 216) | P value | |
| Sex | ||||||
| Female | 82 (72.6) | 261 (69.2) | 0.497 | 77 (71.3) | 135 (62.5) | 0.148 |
| Male | 31 (27.4) | 116 (30.8) | 31 (28.7) | 81 (37.5) | ||
| Age (year) | 41.7 ± 10.6 | 41.1 ± 10.7 | 0.996 | 41.5 ± 10.6 | 41.2 ± 10.9 | 0.782 |
| FT3 (pg/mL) | 3.1 ± 0.4 | 3.2 ± 0.5 | 0.039 | 3.2 ± 0.4 | 3.2 ± 0.4 | 0.059 |
| FT4 (ng/dL) | 1.3 ± 0.2 | 1.3 ± 0.2 | 0.111 | 1.3 ± 0.2 | 1.3 ± 0.2 | 0.234 |
| TSH (uIU/mL) | 1.9 ± 1.1 | 2.0 ± 1.2 | 0.972 | 2.0 ± 1.1 | 2.1 ± 1.4 | 0.426 |
| Maximum diameter (cm) | ||||||
| ≤ 1 | 75 (66.4) | 293 (77.7) | 0.014 | 75 (69.4) | 159 (73.6) | 0.511 |
| > 1 | 38 (33.6) | 84 (22.3) | 33 (30.6) | 57 (26.4) | ||
| Lesion location | ||||||
| Left lobe | 55 (48.7) | 179 (47.5) | 0.210 | 52 (48.1) | 109 (50.5) | 0.088 |
| Right lobe | 50 (44.2) | 185 (49.1) | 48 (44.4) | 102 (47.2) | ||
| Isthmus | 8 (7.1) | 13 (3.4) | 8 (7.4) | 5 (2.3) | ||
The treatment parameters are summarized in Table 2. Complete absence of enhancement at CEUS examination was observed in all target tumors at the end of ablation. The technical success rate was 100%. And the complete ablation of all target tumors was further verified by follow up imaging, including routine US, color doppler US or CEUS. No disease progression (regrowth for benign nodules; or new tumors or regional lymph node metastasis, or distant metastasis for malignant nodules) were detected during the follow up in this study.
| Variables | Before PSM | After PSM | ||||
| TFM (n = 113) | non-TFM (n = 377) | P value | TFM (n = 108) | non-TFM (n = 216) | P value | |
| Ablation time (second) | 73.0 (18.0-524.0) | 119.0 (17.0-681.0) | < 0.001 | 72 (18-524) | 121 (17-681) | < 0.001 |
| Power (Watt) | 30.0 (25.0-60.0) | 35.0 (15.0-50.0) | < 0.001 | 30 (25-60) | 35 (15-45) | < 0.001 |
| Total energy (J) | 2200.0 (660.0-27540.0) | 4080.0 (570.0-26760.0) | < 0.001 | 2145.0 (660.0-27540.0) | 4112.5 (570.0-26760.0) | < 0.001 |
| Energy/mL (J/mL) | 12019.2 (477.8-84134.6) | 28490.0 (1648.5-338942.3) | < 0.001 | 12497.1 (477.8-84134.6) | 27243.3 (2991.4-288461.5) | < 0.001 |
The complications and side effects are summarized in Table 3. Before PSM, the overall complication in the present study was 5.1% (25 of 490 patients), and there was significant difference in the incidence of voice change between the TFM group and non-TFM group [0.9% (1 of 113 patients) vs 6.1% (23 of 377 patients), respectively; P = 0.024]. After PSM, the incidence of voice change in the TFM group was still lower than that in the non-TFM group [0.9% (1 of 108 patients) vs 6.5% (14 of 216 patients), respectively; P = 0.049]. In addition, the total side effect rate in the TFM group was lower than that in the non-TFM group (14.2% vs 23.3%; P = 0.036) before PSM. Among them, the proportion of complaining of pain in the TFM group was significantly lower than that in the non-TFM group (3.5% vs 9.5%, P = 0.048). However, after PSM, although the proportion of complaining of pain in the TFM group was numerically lower than that in the non-TFM group (3.7% vs 9.7%), there was no statistically significant difference between them (P = 0.090). It is worth noting that all treatment-related symptoms were spontaneously relieved or completely resolved within 3-6 months without any specific intervention, and all patients recovered without any sequelae. No life-threaten complications occurred.
| Variables | Before PSM | After PSM | |||||||
| TFM (n = 113) | non-TFM (n = 377) | HR (95%CI) | P value | TFM (n = 108) | non-TFM (n = 216) | HR (95%CI) | P value | ||
| Complications | Voice change | 1 (0.9) | 23 (6.1) | 0.137 (0.018-0.963) | 0.024 | 1 (0.9) | 14 (6.5) | 0.135 (0.017-0.983) | 0.049 |
| Vomiting | 0 (0.0) | 1 (0.3) | - | 1.000 | 0 (0) | 0 (0) | - | - | |
| Side effect | Pain | 4 (3.5) | 35 (9.3) | 0.359 (0.125-0.987) | 0.048 | 4 (3.7) | 21 (9.7) | 0.357 (0.119-1.068) | 0.090 |
| Neck discomfort | 2 (1.8) | 10 (2.7) | 0.661 (0.143-3.063) | 0.594 | 2 (1.9) | 6 (2.8) | 0.660 (0.131-3.328) | 0.899 | |
| Coughing | 0 (0.0) | 2 (0.5) | - | 1.000 | 0 (0) | 1 (0.5) | - | 1.000 | |
| Dry mouth | 4 (3.5) | 26 (6.9) | 0.495 (0.169-1.451) | 0.192 | 4 (3.7) | 14 (6.5) | 0.555 (0.1789-1.728) | 0.440 | |
| Dysphagia | 0 (0.0) | 3 (0.8) | - | 1.000 | 0 (0) | 3 (1.4) | - | 0.538 | |
| Foreign body sensation | 1 (0.9) | 8 (2.1) | 0.412 (0.051-3.328) | 0.692 | 1 (0.9) | 4 (1.9) | 0.495 (0.055-4.486) | 0.873 | |
| Tachycardiac | 3 (2.7) | 10 (2.7) | 1.001 (0.271-3.701) | 0.999 | 3 (2.8) | 6 (2.8) | 1.000 (0.245-4.078) | 1.000 | |
| Tired | 6 (5.3) | 27 (7.2) | 0.727 (0.292-1.807) | 0.491 | 6 (5.6) | 17 (7.9) | 0.689 (0.263-1.800) | 0.592 | |
As a minimally invasive treatment, TA should prioritize improving health-related quality of life and patient experience when treating thyroid nodules, aiming to reduce treatment-related complications and postoperative discomfort. The concept of TFM represents a crucial advancement for PTC, enabling complete ablation of targeted nodules while maximizing protection of surrounding structures. In this study, we conducted a comprehensive comparison of efficacy, safety, and subjective experiences between groups treated with or without TFM strategies for PTC. The results showed that the incidence of voice changes in the TFM group was significantly lower than in the non-TFM group (0.9% vs 6.5%; P = 0.049). Although the difference in pain rates between the two groups was not statistically significant, the proportion of patients reporting pain was numerically lower in the TFM group compared to the non-TFM group (3.7% vs 9.7%; P = 0.090).
With advances in minimally invasive techniques, TA of thyroid nodules has rapidly progressed due to its proven effectiveness in treating BTNs and differentiated thyroid cancers in selected patient populations[4,5,15]. As demonstrated in this study, complete ablation was achieved in all targeted tumors, with a technical success rate of 100%. No disease progression was observed during the follow-up period. It is worth noting, a small subset of patients with T2-stage PTC (tumor > 2 cm to ≤ 4 cm and limited to the thyroid) who declined surgical resection were also included in this study. Although TA demonstrated promising short-term outcomes in terms of efficacy and safety in treating solitary low-risk T2N0M0 PTC[16,17], it must be emphasized that these patients carry a higher risk of recurrence, and the long-term efficacy still requires further confirmation. Therefore, shared decision-making is recommended, with a thorough discussion of the benefits and risks between clinicians and patients before selecting TA treatment. Moreover, these patients underwent more intensive postoperative surveillance.
Ensuring safety and enhancing patient experience are critical factors in the TA of thyroid nodules. The thyroid gland is located adjacent to vital structures such as the trachea, esophagus, cervical nerves, and carotid artery, and damage to any of these can result in serious complications. Moreover, the fascia surrounding the thyroid contains abundant peripheral nerves, lymphatic vessels, and stem cells, making it particularly sensitive to heat stimulation. Heat leakage during TA can injure the fascia, leading to postoperative pain, neck discomfort, or tissue adhesions, which negatively affect patient experience[7]. Therefore, comprehensive technologies based on the concept of TFM may help reduce complications and side effects through reduced collateral damage, lower nerve irritation, minimized edema. The results of this study suggest that TFM effectively improves quality of life and patient-reported outcomes by reducing specific complications and symptoms. Notably, patients in the TFM group experienced a significantly lower rate of voice changes compared to those in the non-TFM group (0.9% vs 6.5%; P = 0.049).
As pain is the most common complains during and after thyroid TA, reported in up to 24.6% of all studies, which will absolutely affect the health-related quality of life and patients’ experience[7]. To date, prior research has not adequately addressed effective interventions aimed at minimizing complications and improving patient experience, representing an important gap in the literature. The TFM strategy confines heat within the thyroid capsule, reducing damage to sur
Notably, TFM, as a key conceptual framework in TA, is developed upon the principle of utilizing fascial spaces for protection during treatment[10,18]. In current clinical practice, there is often a lack of refined control over thermal energy delivery and insufficient attention to the protection of fascial spaces and surrounding tissues of thyroid. Although ablation may be technically successful, patients are sometimes left with prolonged discomfort or increased risk of complications[19,20]. This is the first clinical report applying TFM method to TA of PTC, the results have demonstrated that the concept of TFM significantly improves patient outcomes and safety. This concept does not require advanced technical maneuvers but rather a conceptual shift toward more precise and patient-centered ablation. The main operational standards were to assure that energy can thoroughly cover the target lesions but minimize heat leakage as much as possible (does not penetrate beyond the thyroid capsule) and continuously effective liquid isolation based on based on fascial space during ablation procedure.
There are several limitations to this study. First, the retrospective nature of this study may have led to an inherent selection bias. Second, the follow-up period was short, meanwhile, considering the previous studies released by our team already confirmed the effectiveness of TA for PTC, the comparison of the treatment efficacy in long term follow up were not performed in the present study. Prospective, multicenter randomized controlled trials with longer follow-up are needed in the future.
This study demonstrates that TFM is an effective strategy for improving quality of life and patient experience during TA of PTC. Compared to patients without TFM, those in the TFM group experienced a significantly lower rate of voice changes and showed a trend toward reduced procedure-related pain. Therefore, the TFM strategy can be recommended as a guiding approach for patients with PTC undergoing TA treatment.
| 1. | Boucai L, Zafereo M, Cabanillas ME. Thyroid Cancer: A Review. JAMA. 2024;331:425-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 346] [Reference Citation Analysis (0)] |
| 2. | Watt T, Christoffersen T, Brogaard MB, Bjorner JB, Bentzen J, Hahn CH, Nygaard B, Feldt-Rasmussen U. Quality of life in thyroid cancer. Best Pract Res Clin Endocrinol Metab. 2023;37:101732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Jin S, Sugitani I. Narrative review of management of thyroid surgery complications. Gland Surg. 2021;10:1135-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | van Dijk SPJ, Coerts HI, Gunput STG, van Velsen EFS, Medici M, Moelker A, Peeters RP, Verhoef C, van Ginhoven TM. Assessment of Radiofrequency Ablation for Papillary Microcarcinoma of the Thyroid: A Systematic Review and Meta-analysis. JAMA Otolaryngol Head Neck Surg. 2022;148:317-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 5. | Gao X, Yang Y, Wang Y, Huang Y. Efficacy and safety of ultrasound-guided radiofrequency, microwave and laser ablation for the treatment of T1N0M0 papillary thyroid carcinoma on a large scale: a systematic review and meta-analysis. Int J Hyperthermia. 2023;40:2244713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 6. | Xu X, Peng Y, Han G. Five-year follow-up results of thermal ablation for benign thyroid nodules: Systematic review and meta-analysis. Am J Otolaryngol. 2024;45:104025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Lim JY, Kuo JH. Thyroid Nodule Radiofrequency Ablation: Complications and Clinical Follow Up. Tech Vasc Interv Radiol. 2022;25:100824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Cao XJ, Wang SR, Che Y, Liu J, Cong ZB, He JF, Wang HL, Liu G, Guo JQ, Hao Y, Wang ZH, Zhou Y, Jian M, Shi LL, Qi L, Zhu YL, Wang X, Yan GZ, Shataer A, Liu XF, Wei Y, Zhao ZL, Peng LL, Li Y, Yu MA. Efficacy and Safety of Thermal Ablation for Treatment of Solitary T1N0M0 Papillary Thyroid Carcinoma: A Multicenter Retrospective Study. Radiology. 2021;300:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 9. | Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, Chen MH, Choi BI, de Baère T, Dodd GD 3rd, Dupuy DE, Gervais DA, Gianfelice D, Gillams AR, Lee FT Jr, Leen E, Lencioni R, Littrup PJ, Livraghi T, Lu DS, McGahan JP, Meloni MF, Nikolic B, Pereira PL, Liang P, Rhim H, Rose SC, Salem R, Sofocleous CT, Solomon SB, Soulen MC, Tanaka M, Vogl TJ, Wood BJ, Goldberg SN; International Working Group on Image-guided Tumor Ablation; Interventional Oncology Sans Frontières Expert Panel; Technology Assessment Committee of the Society of Interventional Radiology,; Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology. 2014;273:241-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 955] [Article Influence: 79.6] [Reference Citation Analysis (0)] |
| 10. | Zhao ZL, Wei Y, Peng LL, Li Y, Lu NC, Wu J, Yu MA. Upgraded hydrodissection and its safety enhancement in microwave ablation of papillary thyroid cancer: a comparative study. Int J Hyperthermia. 2023;40:2202373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 11. | Giesinger JM, Kieffer JM, Fayers PM, Groenvold M, Petersen MA, Scott NW, Sprangers MA, Velikova G, Aaronson NK; EORTC Quality of Life Group. Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J Clin Epidemiol. 2016;69:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 360] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 12. | Husson O, Haak HR, Mols F, Nieuwenhuijzen GA, Nieuwlaat WA, Reemst PH, Huysmans DA, Toorians AW, van de Poll-Franse LV. Development of a disease-specific health-related quality of life questionnaire (THYCA-QoL) for thyroid cancer survivors. Acta Oncol. 2013;52:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Burke DR, Lewis CA, Cardella JF, Citron SJ, Drooz AT, Haskal ZJ, Husted JW, McCowan TC, Van Moore A, Oglevie SB, Sacks D, Spies JB, Towbin RB, Bakal CW; Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for percutaneous transhepatic cholangiography and biliary drainage. J Vasc Interv Radiol. 2003;14:S243-S246. [PubMed] |
| 14. | Baek JH, Lee JH, Sung JY, Bae JI, Kim KT, Sim J, Baek SM, Kim YS, Shin JH, Park JS, Kim DW, Kim JH, Kim EK, Jung SL, Na DG; Korean Society of Thyroid Radiology. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012;262:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 281] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 15. | Trimboli P, Castellana M, Sconfienza LM, Virili C, Pescatori LC, Cesareo R, Giorgino F, Negro R, Giovanella L, Mauri G. Efficacy of thermal ablation in benign non-functioning solid thyroid nodule: A systematic review and meta-analysis. Endocrine. 2020;67:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 16. | Hu Y, Zhou W, Xu S, Jia W, Zhang G, Cao Y, Zhang Q, Zhang L, Zhan W. Thermal ablation for the treatment of malignant thyroid nodules: present and future. Int J Hyperthermia. 2024;41:2379983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Xiao J, Zhang Y, Zhang M, Xie F, Yan L, Luo Y, Tang J. Ultrasonography-guided radiofrequency ablation for the treatment of T2N0M0 papillary thyroid carcinoma: a preliminary study. Int J Hyperthermia. 2021;38:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Wei Y, Zhao ZL, Niu Y, Peng LL, Li Y, Yu MA. Ultrasound imaging of the perithyroid fascial space: a comparative analysis with anatomical correlations. Sci Rep. 2025;15:4503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 19. | Xie T, Fu Y, Jin X, Ren X, Zhang J, Sun Q. Horner syndrome as a complication of ultrasound-guided ablation therapy for thyroid nodules: a scoping review. Front Endocrinol (Lausanne). 2025;16:1607214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Hua Q, Song Y, Zhou W, Liu Z, Zhang L, Zhu Y, Lai L, Yeung YH, Li N, Zhan W, Dong Y, Xia S, Zhou J; Chinese Ablation Alliance for Thyroid and Cervical Lymph Nodes. Recurrent laryngeal nerve thermal injury in radiofrequency ablation for papillary thyroid carcinoma and related risk factors: a prospective large cohort study. Eur Radiol. 2025;35:5804-5816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/