Published online Sep 28, 2025. doi: 10.4329/wjr.v17.i9.111493
Revised: July 28, 2025
Accepted: August 20, 2025
Published online: September 28, 2025
Processing time: 87 Days and 18.2 Hours
Endometrial cancer (EC) is the most common gynecological malignancy in high-income countries, with incidence rates rising globally. Early and accurate diag
To systematically review recent advances in US-based imaging techniques for the diagnosis and staging of EC, and to compare their performance with magnetic resonance imaging (MRI).
A systematic search of PubMed, Scopus, Web of Science, and Google Scholar was performed to identify studies published between January 2010 and March 2025. Eligible studies evaluated TVUS, 3D-US, CEUS, elastography, or AI-enhanced US in EC diagnosis and staging. Methodological quality was assessed using the QUADAS-2 tool. Sensitivity, specificity, and area under the curve (AUC) were extracted where available, with narrative synthesis due to heterogeneity.
Forty-one studies met the inclusion criteria. TVUS demonstrated high sensitivity (76%–96%) but moderate specificity (61%–86%), while MRI achieved higher spe
TVUS remains a highly sensitive initial screening tool, with MRI preferred for definitive staging. 3D-US, CEUS, elastography, and AI-enhanced techniques show promise as complementary or alternative approaches, particularly in low-resource settings. Standardization, multicenter validation, and integration of multi-modal imaging are needed to optimize diagnostic pathways for EC.
Core Tip: This study provides a comprehensive review of evolving ultrasound (US)-based imaging techniques in the dia
- Citation: Tlais M, Hamze H, Hteit A, Haddad K, El Fassih I, Zalzali I, Mahmoud S, Karaki S, Jabbour D. Advances in ultrasound-based imaging for diagnosis of endometrial cancer. World J Radiol 2025; 17(9): 111493
- URL: https://www.wjgnet.com/1949-8470/full/v17/i9/111493.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i9.111493
Endometrial cancer (EC), which affects the inner lining of the uterus, is seeing a global rise in both incidence and mortality rates. It currently stands as the most prevalent gynecological malignancy in high-income nations[1]. Over 90% of EC diagnoses occur in women over the age of 50, with the average age at diagnosis being 63. Nevertheless, about 4% of cases are found in women under 40, many of whom are still considering future fertility[2].
Fortunately, most EC cases are detected at an early stage—approximately 80% are diagnosed at stage I—where the prognosis is excellent, with 5-year survival rates exceeding 95%. In contrast, the outlook worsens significantly when the disease progresses beyond the uterus, with survival dropping to 68% in cases of regional spread and just 17% when distant metastases are present[3].
In 2020 alone, 417367 new cases of EC were reported worldwide, with North America and Western Europe bearing the highest burden[4]. This regional disparity is likely influenced by modifiable risk factors—particularly obesity, linked to around half of all EC cases[5]. If current trends persist, annual EC diagnoses in the United States will double by 2030, reaching approximately 122000 new cases each year[6]. In Europe, uterine cancer ranked as the fourth most common cancer among women as of 2020, with incidence rates ranging from 12.9 to 20.2 per 100000 women and mortality rates between 2.0 and 3.7 per 100000[4].
The incidence and outcomes of EC show significant geographic, socioeconomic, and racial disparities. The disease is notably more common in high-income nations, potentially due to varying healthcare access and differences in specialist availability[7]. Socioeconomic status (SES) also critically influences diagnosis and outcomes. Women with higher SES are typically diagnosed earlier and experience better prognoses due to better healthcare access. Conversely, lower SES often correlates with advanced disease and reduced survival rates[8]. Racial disparities are pronounced; in the United States, Black women are more frequently diagnosed with aggressive EC subtypes and advanced stages compared to White women, consistently leading to poorer outcomes[9].
Genetic variations in tumors also significantly influence EC outcomes. Type II EC in Black women commonly features genetic mutations in TP53 and PIK3R1 genes, along with increased human epidermal growth factor receptor 2 (HER2) expression, potentially explaining the more aggressive nature of their disease[10].
Ethnic disparities are evident among Asian women, who are diagnosed at a younger average age (around 58.4 years) compared to White women (approximately 65.1 years). Asian women are also more likely to present with advanced-stage disease. Among Asian Americans, United States-born women have higher rates of type I EC compared to immigrant counterparts[11].
Numerous risk factors influence the development of EC. These include increasing age, higher body mass index (BMI), certain ethnic backgrounds, prolonged exposure to estrogen (either endogenous or through hormone therapies), tamoxifen use, early onset of menstruation, late menopause, low parity, metabolic syndrome, and a family history of cancer or known genetic predispositions. Protective factors include maintaining a normal BMI, having more children, and using oral contraceptives[12]. A particularly high-risk group includes individuals with hereditary non-polyposis co
Endometrial carcinoma is primarily categorized into two main subtypes: Type I and type II, with the vast majority being adenocarcinomas[13,14]. Type I endometrial carcinoma, accounting for approximately 80% of cases, develops in the context of unopposed estrogen exposure and is often associated with endometrial hyperplasia. This subtype typically affects obese women aged 55-65, tends to be well-differentiated, and follows a slower progression, which contributes to its generally favorable prognosis. Genetic mutations, particularly in the PTEN gene, are observed in 30%-80% of these cases[13,14]. In contrast, type II endometrial carcinoma represents around 20% of cases and typically arises in older women (65-75 years), usually in a background of endometrial atrophy or endometrial intraepithelial carcinoma. These tumors are often poorly differentiated, more aggressive, and prone to early lymphatic spread or dissemination into the peritoneal cavity via the fallopian tubes. Up to 50% harbor mutations in the p53 gene, and the prognosis is generally poorer than that of type I cancers[13,14].
Type I tumors are most commonly represented by endometrioid carcinoma, which accounts for approximately 85% of cases[13]. Type II tumors include less common but more aggressive forms such as serous carcinoma (5%–10%), clear cell carcinoma (1%–5.5%), as well as undifferentiated and dedifferentiated carcinomas[13].
Postmenopausal bleeding (PMB) is the most frequent symptom, prompting about two-thirds of gynecological con
Timely and accurate diagnosis is vital in improving survival outcomes for individuals with EC. The five-year survival rate exceeds 95% when the disease is caught early but plummets to around 15% once it has spread beyond the uterus. This stark contrast underscores the critical need for public awareness of early symptoms, accessible and equitable healthcare, and systematic early screening and diagnosis, especially in high-risk populations. Early intervention not only improves outcomes but also significantly reduces the physical and emotional burden of advanced disease[3].
Imaging is a cornerstone in the diagnosis, staging, and management of EC. Among the available modalities, transvaginal ultrasound (TVUS) is frequently used due to its accessibility, affordability, and non-invasive nature, particularly as an initial diagnostic step.
TVUS is typically the first-line imaging method, especially in women presenting with abnormal uterine bleeding, a hallmark symptom of EC. It provides valuable information on endometrial thickness (a key diagnostic marker), echo
In postmenopausal women, an endometrial thickness over 5 mm is often considered abnormal when measured in the sagittal plane. This threshold yields a sensitivity of approximately 96% and specificity of about 61% for detecting EC[16]. For women on hormone replacement therapy or tamoxifen, the threshold may be raised to 8 mm due to baseline thi
Contrast-enhanced ultrasound (CEUS) enhances visualization of tumor vascularity, helping detect features like deep myometrial invasion and cervical stromal involvement, both critical for accurate staging[18].
When standard TVUS is inconclusive, sono-hysterography may be performed. This involves instilling sterile saline into the uterine cavity to expand and better visualize the endometrial lining, offering improved detail with the ultrasound (US) probe[18].
Magnetic resonance imaging (MRI) is considered the most accurate tool for local staging of EC due to its superior soft tissue contrast. It plays a pivotal role in assessing depth of myometrial invasion, cervical stromal involvement, and tumor extension into surrounding tissues[19-21]. Dynamic contrast-enhanced MRI and T2-weighted imaging (T2WI) are especially valuable for staging. For patients unable to receive contrast agents, diffusion-weighted imaging (DWI) serves as a useful alternative. Studies have shown that combining T2WI with DWI can provide even greater diagnostic accuracy than using either technique alone[21].
While computed tomography (CT) is not ideal for initial diagnosis or precise local staging, it is useful for detecting distant metastases, including to the lungs or abdomen. On contrast-enhanced CT, EC may appear as diffuse thickening of the endometrium and a hypoenhancing mass within the uterine cavity. However, CT struggles to differentiate early-stage EC from normal tissue, particularly on non-contrast scans[22].
Positron emission tomography (PET)-CT has limited utility in evaluating primary tumors due to physiological uptake in the uterus, particularly in premenopausal women. It is less effective than MRI or US for primary diagnosis but may assist in evaluating metastatic spread in advanced or recurrent cases[23].
In recent years, technological innovations—including three-dimensional (3D) US, CEUS, and artificial intelligence (AI)-driven diagnostic tools—have expanded the capabilities of imaging. The integration of AI into radiology is rapidly reshaping the field by enhancing diagnostic accuracy, streamlining workflows, and improving patient care outcomes. AI, particularly through machine learning (ML) and deep learning techniques, is being applied to a wide range of imaging tasks, including lesion detection, image segmentation, and pattern recognition. These technologies enable radiologists to interpret images more efficiently, reduce diagnostic errors, and prioritize critical cases for immediate review. AI algorithms can analyze complex imaging features beyond human perception, allowing for earlier identification of subtle pathological changes. In addition to diagnostic applications, AI contributes significantly to predictive analytics, using imaging and clinical data to forecast disease progression and treatment response[24].
The aim of this study is to explore and critically review the development of US imaging techniques, from basic two-dimensional (2D) scans to more recent AI-assisted approaches, in the diagnosis of EC.
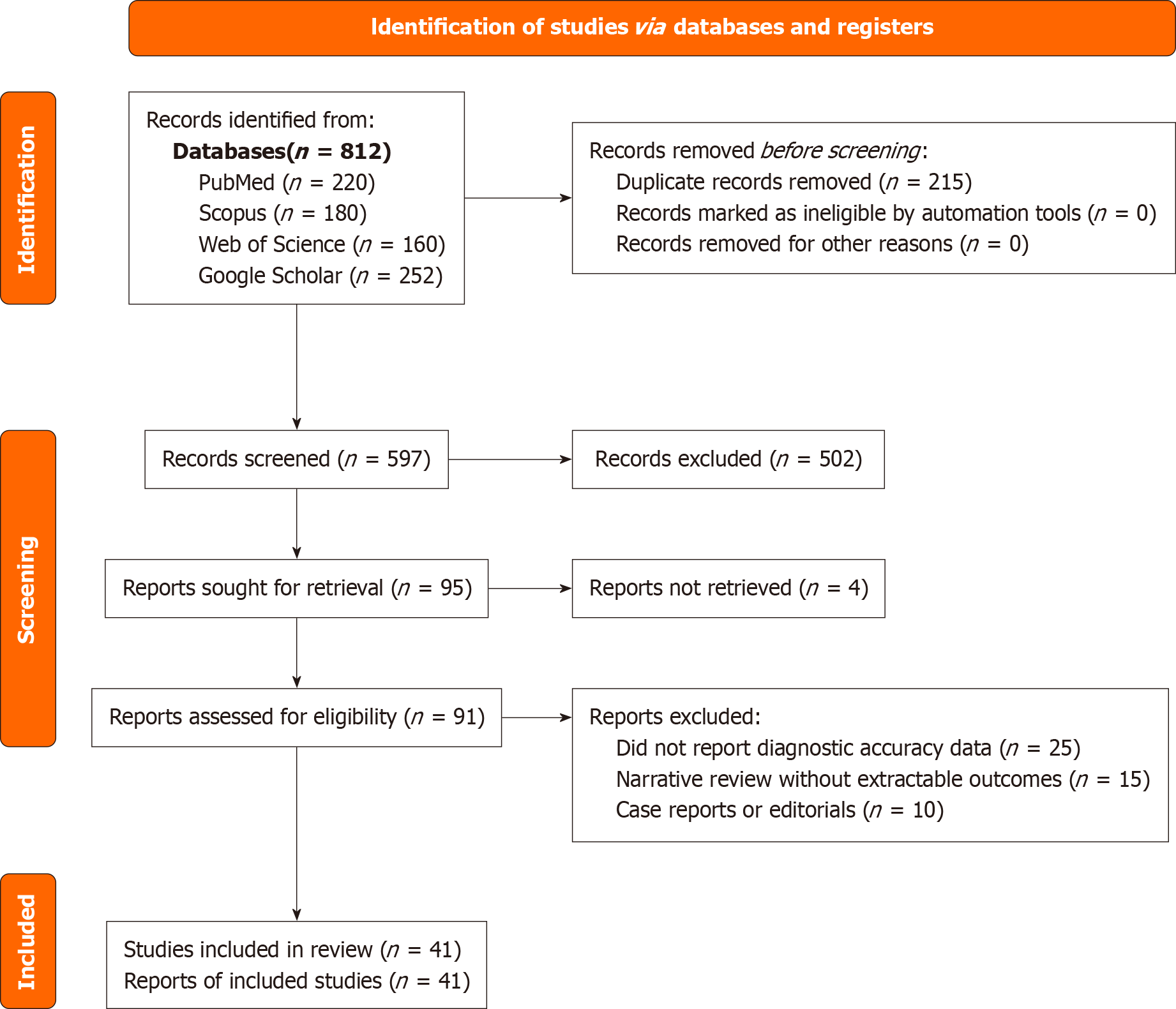
To ensure methodological rigor and transparency, this review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. A systematic literature search was conducted using four electronic databases: (1) PubMed; (2) Scopus; (3) Web of Science; and (4) Google Scholar. The search covered studies published between January 2010 and March 2025. Search terms included combinations of "endometrial cancer", "transvaginal ultrasound", "3D ultrasound", "contrast-enhanced ultrasound", "elastography", "artificial intelligence", "radiomics", and "deep learning". Studies were included if they involved original research evaluating US or AI-based imaging for the diagnosis of EC, were published in English, and involved human subjects. Studies such as editorials, letters, animal studies, and case reports with fewer than five participants were excluded. The study selection process is illustrated in a PRISMA flow diagram (Figure 1).
In order to appraise the methodological quality and risk of bias in the included studies, the QUADAS-2 tool was applied to each imaging modality, including TVUS, 3D-US, CEUS, elastography, and AI-based methods. The assessment covered domains such as patient selection, index test quality, reference standard, and timing of assessments. The results of this evaluation are summarized in the Results section and detailed further in a supplementary appendix.
Where data allowed, we extracted and synthesized sensitivity, specificity, and area under the curve (AUC) metrics. Although a formal meta-analysis was not feasible due to heterogeneity in study designs, sample populations, and imaging protocols, we provide a quantitative synthesis where appropriate. A comparative summary table was developed to display pooled or median diagnostic performance for each modality, along with their primary indications, clinical strengths, and limitations. Variability in results arising from factors such as patient demographics, imaging equipment, and operator experience is discussed to contextualize the findings.
A total of 41 studies were included in this systematic review after screening and eligibility assessment across the four databases. We conducted a quality appraisal of these studies using the QUADAS-2 tool. The majority demonstrated low risk of bias across key domains including patient selection, index test conduct, reference standard quality, and timing. However, studies focusing on emerging modalities, particularly elastography and AI-enhanced US, showed variable methodological rigor. Specific concerns included incomplete reference standard description and lack of external validation. The full assessment is presented in Table 1[24-38].
| Ref. | Imaging modality | Patient selection | Index test | Reference standard | Flow and timing | Overall risk of bias |
| Tameish et al[24], 2023 | TVUS vs MRI | Low | Low | Low | Low | Low risk |
| Jacobs et al[25], 2011 | TVUS | Low | Low | Unclear | Low | Some concerns |
| Bian et al[26], 2023 | Elastography | Low | Low | Low | Low | Low risk |
| Ziogas et al[27], 2022 | 3D-US | Low | Low | Low | Low | Low risk |
| Xydias et al[28], 2022 | 3D-US | Low | Low | Unclear | Low | Some concerns |
| Stoelinga et al[29], 2021 | Contrast-enhanced US | Low | Low | Low | Low | Low risk |
| Guler et al[30], 2024 | Elastography | Unclear | Low | Low | Unclear | Some concerns |
| Moro et al[38], 2022 | AI | Low | Low | Unclear | Low | Some concerns |
| Jin and Zhou[37], 2025 | TVUS vs MRI | Low | Low | Low | Low | Low risk |
| Tameish et al[24], 2023 | Two-dimensional-US | Unclear | Low | Unclear | Unclear | High risk |
| Jacobs et al[25], 2011 | TVUS | Low | Low | Low | Low | Low risk |
| Wu et al[34], 2020 | AI (breast US) | Low | Low | Low | Low | Low risk |
| Song et al[33], 2022 | AI (renal US) | Low | Low | Low | Low | Low risk |
| Zheng et al[31], 2024 | AI (thyroid US) | Low | Low | Unclear | Low | Some concerns |
| Mohit et al[32], 2023 | AI review | N/A | N/A | N/A | N/A | Not applicable |
| Nathani et al[35], 2024 | AI (pulmonology US) | Unclear | Low | Low | Unclear | Some concerns |
| Bajaj et al[36], 2021 | AI (intravascular US) | Low | Low | Low | Low | Low risk |
The diagnostic performance of each imaging modality was synthesized from eligible studies. TVUS exhibited high sensitivity (76%–96%) for initial screening but showed moderate to variable specificity (61%–86%), particularly in postmenopausal and obese populations. MRI provided superior specificity (84%–95%) and was particularly effective in assessing myometrial invasion and cervical stromal extension, and staging of type II tumors. Its diagnostic accuracy was consistently higher for deep invasion and advanced-stage disease.
3D-US demonstrated diagnostic accuracy approaching that of MRI in carefully selected early-stage patients, especially when combined with volumetric assessments. CEUS and shear wave elastography (SWE) provided additional tissue-level insights, particularly for vascularity and stiffness, with AUC values ranging between 0.85 and 0.88. AI-enhanced US models, incorporating radiomics or deep learning, demonstrated promising results with pooled AUCs as high as 0.91, particularly in risk prediction and lesion segmentation tasks.
Table 2 summarizes these pooled performance metrics alongside the respective strengths and limitations of each modality.
| Modality | Sensitivity | Specificity | Area under the curve | Primary indication | Strengths | Weaknesses |
| Transvaginal US | 76%–96% | 61%–86% | 0.88 | Initial screening; endometrial thickness measurement | Widely available; cost-effective; high sensitivity | Operator-dependent; reduced accuracy in obesity/retroversion |
| MRI | 79%–92% | 84%–95% | 0.89–0.91 | Staging (myometrial/cervical invasion, lymph nodes) | High soft tissue contrast; reliable staging | Expensive; limited availability |
| Three-dimensional US | 75%–88% | 75%–91% | 0.86–0.90 | Volumetric analysis; preoperative planning | Similar accuracy to MRI; enhanced resolution | Requires advanced equipment and training |
| Contrast-enhanced US | 82%–90% | 78%–89% | 0.85–0.88 | Vascular characterization; benign vs malignant differentiation | Real-time microvascular imaging | Contrast contraindications; standardization needed |
| Elastography | 78%–85% | 70%–88% | 0.83–0.87 | Tissue stiffness evaluation; adjunct diagnostic tool | Non-invasive characterization | Limited operator experience; fewer studies |
| Artificial intelligence (radiomics/machine learning) | 80%–90% | 78%–92% | 0.88–0.91 | Risk prediction; segmentation; decision support | High potential for automation; promising accuracy | Data bias; hardware demands; limited external validation |
Substantial heterogeneity was observed across the studies. Variations in patient demographics (e.g., menopausal status and BMI), imaging equipment, operator expertise, and diagnostic thresholds all contributed to inconsistencies in reported performance. Moreover, differences in reference standards, such as histopathological confirmation vs MRI-only staging, impacted comparability. These factors underscore the need for standardized imaging protocols, operator training, and multicenter external validation, especially for AI models intended for broader clinical adoption.
Basic principles and usage: 2D-US serves as a fundamental imaging modality in the assessment of endometrial pa
Limitations in EC diagnosis: Despite its widespread use, 2D-US has limitations in the accurate staging of EC. The modality's inability to assess myometrial invasion and cervical involvement restricts its utility in determining the extent of the disease. Additionally, factors such as obesity, uterine fibroids, and fixed uterine positions can hinder optimal visualization, leading to potential misinterpretations. Tameish et al[24] noted that while 2D-US is effective in detecting endometrial abnormalities, its diagnostic accuracy can be compromised by these factors[25].
Diagnostic value and sensitivity: TVUS enhances the diagnostic capability of 2D-US by providing higher resolution images of the endometrium and surrounding structures. A prospective study within the United Kingdom Collaborative Trial of Ovarian Cancer Screening cohort found that an endometrial thickness cutoff of 5.15 mm yielded a sensitivity of 80.5% and specificity of 86.2% for detecting EC or atypical endometrial hyperplasia[26].
Standard measurement parameters: TVUS is particularly effective in measuring endometrial thickness and volume, which are pivotal in assessing the risk of malignancy. A systematic review comparing 3D-US, 2D-US, and 3D Doppler in the diagnosis of endometrial carcinoma found that endometrial volume had higher specificity (75%) compared to endometrial thickness (69%), suggesting that volumetric assessment may offer more reliable measurements[39].
Assessment of vascularity: Doppler US assesses the vascular characteristics of uterine arteries, providing insights into the hemodynamic changes associated with EC. A prospective study involving 74 women with confirmed EC revealed that uterine artery Doppler indices did not show statistically significant differences between histologic subtypes, tumor grade, or other prognostic factors[40].
Clinical significance of resistance and pulsatility indices: The resistance index (RI) and pulsatility index (PI) are utilized to evaluate blood flow patterns. A study on postmenopausal women with abnormal uterine bleeding found that spiral artery PI ≤ 0.33 and RI ≤ 0.5 helped in differentiating malignant from benign pathology. Power Doppler flow mapping also demonstrated a better diagnostic ability in detecting EC[27].
With its superior spatial resolution and accurate volumetric and morphological evaluations over 2D-US, 3D-TVUS is becoming increasingly useful in gynecologic oncology. According to Xydias et al[28], 3D-TVUS is more affordable and accessible than 2D-TVUS and can identify deep myometrial invasion in EC with sensitivity and specificity comparable to MRI.
In diagnosing endometrial carcinoma, Xydias et al[28] demonstrated that 3D-US's measurement of endometrial volume has similar sensitivity but better specificity compared to 2D-US 's measurement of endometrial thickness, potentially minimizing the need for unnecessary biopsies[29].
With instruments like VOCAL, 3D-US enables comprehensive volumetric analysis, increasing measurement con
Overall, 3D-US is a viable first-line method for evaluating EC, as it improves diagnostic accuracy and is non-invasive, repeatable, and economical. Its clinical application needs further standardization through additional research[28,29].
By employing microbubble contrast agents, CEUS enhances the visibility of uterine vascular systems, providing real-time microvasculature imaging often undetectable by traditional ultrasonography.
CEUS improves blood flow evaluation in uterine tissues. Normal enhancement progresses sequentially from the uterine artery to the myometrium and endometrium. Conversely, malignant lesions exhibit uneven, disordered, and hypervascular patterns, whereas benign lesions like fibroids present distinct vascular rims or "pseudocapsules".
Benign lesions usually have clear borders and a gradual, homogeneous enhancement. Malignant tumors, characterized by aberrant angiogenesis, develop rapidly and unevenly. Compared to traditional US, CEUS demonstrates superior diagnostic accuracy, particularly in detecting leiomyosarcomas and staging EC[30].
Thus, CEUS is promising for assessing uterine vascularity and differentiating between benign and malignant diseases, warranting further clinical integration[30].
Elastography, an advanced US technique, evaluates tissue stiffness via shear wave propagation. Guler et al[30] de
ML significantly impacts US-based illness identification. Zheng et al[31] trained ML algorithms, including XGBoost, logistic regression, decision trees, random forest, support vector machine, k-nearest neighbors, and LightGBM, on clinical and US features to differentiate follicular thyroid carcinoma from adenoma. XGBoost achieved an impressive AUC of 0.969, demonstrating potential clinical applicability[32].
However, ML faces challenges. Mohit et al[32] noted that noise/artifacts, inconsistent feature extraction, and image variability hinder model reproducibility and accuracy. Equipment variability, operator experience, and dataset heterogeneity further limit generalization, underscoring the need to address these barriers before clinical adoption[33].
Overall, ML models exhibit potential for distinguishing benign and malignant tumors, contingent on standardizing methods and ensuring robust feature extraction[32,33].
Deep learning demonstrates significant potential in US imaging for segmentation and diagnosis automation. Song et al[33] employed models like DeepLabV3+ and UNet++ for renal US segmentation to determine hydronephrosis severity, achieving high accuracy [Dice similarity coefficient (DSC) of 0.91] and reducing operator variability[34].
Wu et al[34] evaluated a convolutional neural network (CNN) for breast cancer screening, achieving an AUC of 0.895, matching radiologist performance. A hybrid approach incorporating radiologists' ratings with CNN predictions further enhanced diagnostic accuracy, emphasizing deep learning's potential clinical role[35].
Thus, deep learning models exhibit high performance in segmentation and classification tasks, enhancing diagnostic precision and reducing human error in clinical practice[34,35].
AI integration into real-time US holds significant bedside diagnostic potential. Nathani et al[35] emphasized AI-enhanced US in interventional pulmonology, improving procedural guidance and reducing complications, especially with hand
Bajaj et al[36] presented a deep learning model for real-time intravascular US image segmentation, demonstrating high accuracy (DSC ≥ 0.96) even in complex scenarios. This facilitates immediate clinical decisions, supporting AI integration into clinical workflows[37].
Integrating AI into real-time US enhances diagnostic accuracy, efficiency, and operator confidence, requiring further validation across diverse scenarios for widespread adoption[36,37].
Diagnostic accuracy across modalities: While both TVUS and MRI have demonstrated high diagnostic value in EC assessment, their performance should be contextualized rather than generalized. Pooled analyses show that TVUS can approach MRI in diagnostic accuracy for evaluating myometrial invasion, with reported sensitivities of 76%–96% for TVUS and 79%–92% for MRI[38]. However, this apparent equivalence holds primarily for early-stage, low-risk disease[38].
MRI remains unequivocally superior in several critical contexts. It provides more reliable assessment of cervical stromal invasion, which is essential for accurate FIGO staging and surgical planning[19,20]. Furthermore, MRI offers better sensitivity for lymph node evaluation and is crucial for detecting type II EC subtypes, such as serous or clear cell carcinomas, which are more likely to exhibit deep invasion and extrapelvic spread[21,22]. These distinctions are particularly relevant when determining the extent of surgical staging or the need for adjuvant therapy.
Although AI-enhanced US shows promise in expanding the diagnostic capabilities of TVUS through automation and radiomic pattern recognition, it cannot yet match MRI’s multi-parametric imaging or soft-tissue contrast resolution. That said, in low-resource settings, AI-augmented US represents a compelling alternative where MRI is not accessible. US units are more affordable, portable, and logistically feasible in rural or underserved healthcare systems. The combination of 3D-US, CEUS, and ML algorithms could offer a cost-effective staging tool in these environments, provided that its implementation is standardized and validated[30,38,41].
In summary, while TVUS and AI-enhanced US are invaluable for initial screening and triage, MRI remains irre
TVUS is commended for being easily accessible, inexpensive, and non-ionizing, which makes it ideal for preliminary testing, especially in resource-limited environments. However, inter-observer variability might affect its dependability, and its accuracy is greatly dependent on the operator[38]. MRI, while offering better soft tissue contrast and greater consistency, is limited by higher costs, restricted availability, and longer acquisition times.
Radiomics extracts hidden information from US images to provide a quantitative and repeatable assessment of ima
It is crucial to highlight the critical role of imaging in guiding surgical planning. Accurate assessment of MI determines the need for procedures like lymphadenectomy, directly influencing morbidity and survival outcomes. A sequential diagnostic strategy is emphasized using TVUS initially and reserving MRI for ambiguous or high-risk cases[38]. Ra
The distinction between type I and type II EC subtypes has important implications for imaging strategy and clinical management. Type I EC, typically endometrioid in histology, is generally well-differentiated and estrogen-dependent, with a favorable prognosis and 5-year survival rates exceeding 85%–90% when diagnosed early. In contrast, type II EC, which includes serous, clear cell, and other high-grade subtypes, is associated with aggressive behavior, deep myometrial invasion, and a 5-year survival rate often below 50%. These tumors are frequently p53-mutated and may also exhibit HER2 overexpression, making them biologically and radiologically distinct.
From an imaging standpoint, MRI is the modality of choice for assessing type II EC, as it provides superior soft-tissue resolution critical for evaluating deep myometrial and cervical stromal invasion, lymphovascular space involvement, and adnexal spread. While US techniques, including 3D and Doppler-enhanced TVUS, can detect endometrial abnormalities, their accuracy diminishes in advanced or atypical histologies. Type II tumors often present with a flat or indistinct morphology and minimal endometrial thickening, making them more challenging to detect with US alone.
Furthermore, the integration of radiomic and AI tools holds promise in identifying high-risk molecular features, such as p53 mutation status or non-endometrioid histology, through advanced pattern recognition in imaging data. This could support non-invasive risk stratification and guide the selection of appropriate surgical or adjuvant therapies.
Personalized medicine and predictive modeling: The combination of ML and radiomics has advanced personalized imaging. The models' high specificity and modest sensitivity in identifying high-risk EC may help physicians customize treatment regimens. Predictive analytics based on preoperative biopsy, US, and radiomics data offers a non-invasive approach to personalized care.
Multi-modal imaging integration (MRI/CT + US + AI): Future clinical protocols may integrate multi-modal imaging systems combining radiomics' computational power, US’s real-time imaging, and MRI’s structural detail. This approach could enhance treatment planning and staging accuracy, particularly for unclear or borderline cases.
Regulatory, ethical, and implementation challenges in AI: AI and ML models present challenges, including data privacy management, standardized image acquisition, and model generalizability across populations. Clinical training, re
Clinical translation and real-world implementation: The integration of AI into US imaging holds substantial promise for enhancing diagnostic workflows in EC, but its clinical adoption faces several real-world challenges. One major hurdle is the lack of standardized image acquisition protocols, which complicates consistent AI model training and validation across different institutions. Real-time AI applications, such as lesion segmentation or myometrial thickness mea
In addition, the regulatory landscape for medical AI remains in flux. AI tools intended for diagnostic support must undergo rigorous evaluation and approval processes by bodies such as the United States Food and Drug Administration or European CE marking authorities. These evaluations require evidence of safety, reproducibility, and clinical utility, which many academic or prototype models still lack. Moreover, data privacy regulations (e.g., Health Insurance Por
Nonetheless, several AI-assisted US features are approaching clinical readiness. These include automated mea
Importantly, addressing health equity is vital to ensuring that these innovations do not widen disparities. MRI remains inaccessible in many low-resource settings due to high costs and logistical limitations. AI-enhanced, portable US platforms offer a feasible and scalable alternative, especially if embedded with cloud-based decision support. Ensuring diverse, representative training datasets and involving local clinical stakeholders in tool design will be essential for closing diagnostic gaps across populations.
Optimizing multi-modal imaging integration: While the integration of MRI, CT, US, and AI technologies is often conceptually referenced, its implementation in clinical workflows requires a stepwise and function-specific approach. Each imaging modality offers distinct advantages and limitations that should inform their sequencing and combination in diagnostic and staging algorithms for EC.
TVUS remains the ideal first-line modality for initial evaluation, especially in patients presenting with abnormal uterine bleeding. Its affordability, accessibility, and real-time imaging make it particularly suitable for early triage and screening, especially in primary care or low-resource environments. 3D-US and Doppler imaging can further characterize endometrial morphology and vascularity, enhancing diagnostic confidence when available.
MRI should be reserved for intermediate- or high-risk cases, particularly when TVUS suggests structural distortion, myometrial invasion, or possible type II histology. MRI’s superior tissue contrast allows for precise evaluation of depth of invasion, cervical stromal involvement, and nodal architecture, all of which critically influence surgical planning.
CT, although limited by poor soft tissue resolution, retains value in detecting distant metastases, particularly to the lungs or omentum. When MRI is contraindicated or unavailable, CT may serve as a fallback for broad staging—though its limitations in differentiating between uterine layers must be acknowledged. These limitations could be mitigated by combining CT with radiomics or AI-based enhancement algorithms that can improve contrast differentiation in post-processing phases.
An effective hybrid diagnostic pathway could thus proceed as follows: (1) TVUS for initial screening; (2) MRI for local staging and surgical planning; and (3) CT or PET-CT for distant metastasis evaluation if advanced disease is suspected. In resource-limited settings, this sequence may be adapted by using AI-enhanced US as a surrogate for MRI, provided that validation standards are met.
The integration of AI models across this diagnostic chain could offer real-time risk stratification, lesion segmentation, and clinical decision support, helping to streamline referrals and reduce diagnostic delays. Ultimately, building algo
This review acknowledges several limitations across technological, algorithmic, and methodological domains. Technological limitations are evident in US-based approaches. TVUS and elastography techniques are highly operator-dependent, with inter-observer variability in endometrial thickness measurements reported to reach up to 20%. Visualization challenges also exist in patients with obesity, where abdominal wall fat reduces image quality, and in those with a retroverted uterus, fibroids, or adenomyosis, all of which can obscure or distort anatomical structures. For instance, fibroids are present in up to 70% of women by age 50, potentially leading to false negatives or positives. Furthermore, CEUS is limited by contraindications such as renal impairment and known hypersensitivity to contrast agents, which are often underreported in current literature.
AI introduces its own set of constraints. Deep learning models, particularly CNNs, are often criticized for their lack of interpretability, the so-called "black box" problem, which can hinder clinical trust and adoption. Additionally, many AI algorithms have been trained on datasets from high-income regions, raising concerns about generalizability to LMICs where demographics, disease patterns, and imaging equipment may differ. Real-time AI applications further require high-performance US hardware, posing barriers to implementation in resource-limited settings. Most AI models cited in this review also lack robust external validation across diverse clinical environments.
Methodologically, this review may be affected by potential publication bias, as studies with positive findings are more likely to be published and included. There is also a lack of standardization in study protocols across the literature, including differences in imaging acquisition, interpretation criteria, and outcome definitions. Despite adherence to the PRISMA guidelines, limitations in database coverage and language restriction to English may have excluded relevant studies, potentially narrowing the comprehensiveness of the evidence base.
Leveraging advanced US techniques and computational models to enhance diagnostic precision in EC is becoming pivotal in gynecologic oncology. TVUS and MRI show comparable diagnostic value, while radiomics-enhanced US models provide new avenues for risk prediction and personalized care. Future directions will likely focus on integrating AI and multi-modal imaging while addressing ethical and implementation challenges to ensure equitable and effective patient care.
| 1. | Lee NK, Cheung MK, Shin JY, Husain A, Teng NN, Berek JS, Kapp DS, Osann K, Chan JK. Prognostic factors for uterine cancer in reproductive-aged women. Obstet Gynecol. 2007;109:655-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 2. | Braun MM, Overbeek-Wager EA, Grumbo RJ. Diagnosis and Management of Endometrial Cancer. Am Fam Physician. 2016;93:468-474. [PubMed] |
| 3. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68637] [Article Influence: 13727.4] [Reference Citation Analysis (201)] |
| 4. | Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5430] [Cited by in RCA: 5359] [Article Influence: 233.0] [Reference Citation Analysis (2)] |
| 5. | Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5379] [Cited by in RCA: 5365] [Article Influence: 447.1] [Reference Citation Analysis (0)] |
| 6. | Chatterjee S, Gupta D, Caputo TA, Holcomb K. Disparities in Gynecological Malignancies. Front Oncol. 2016;6:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Svanvik T, Marcickiewicz J, Sundfeldt K, Holmberg E, Strömberg U. Sociodemographic disparities in stage-specific incidences of endometrial cancer: a registry-based study in West Sweden, 1995-2016. Acta Oncol. 2019;58:845-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Felix AS, Weissfeld JL, Stone RA, Bowser R, Chivukula M, Edwards RP, Linkov F. Factors associated with Type I and Type II endometrial cancer. Cancer Causes Control. 2010;21:1851-1856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 9. | Feinberg J, Albright B, Black J, Lu L, Passarelli R, Gysler S, Whicker M, Altwerger G, Menderes G, Hui P, Santin AD, Azodi M, Silasi DA, Ratner ES, Litkouhi B, Schwartz PE. Ten-Year Comparison Study of Type 1 and 2 Endometrial Cancers: Risk Factors and Outcomes. Gynecol Obstet Invest. 2019;84:290-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Simons E, Blansit K, Tsuei T, Brooks R, Ueda S, Kapp DS, Chan JK. Foreign- vs US-born Asians and the association of type I uterine cancer. Am J Obstet Gynecol. 2015;212:43.e1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, Marth C, Nout R, Querleu D, Mirza MR, Sessa C; ESMO-ESGO-ESTRO Endometrial Consensus Conference Working Group. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27:16-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 837] [Article Influence: 76.1] [Reference Citation Analysis (0)] |
| 12. | Sorosky JI. Endometrial cancer. Obstet Gynecol. 2008;111:436-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | Clarke MA, Long BJ, Del Mar Morillo A, Arbyn M, Bakkum-Gamez JN, Wentzensen N. Association of Endometrial Cancer Risk With Postmenopausal Bleeding in Women: A Systematic Review and Meta-analysis. JAMA Intern Med. 2018;178:1210-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 284] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 14. | Fischerova D, Smet C, Scovazzi U, Sousa DN, Hundarova K, Haldorsen IS. Staging by imaging in gynecologic cancer and the role of ultrasound: an update of European joint consensus statements. Int J Gynecol Cancer. 2024;34:363-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Gull B, Karlsson B, Milsom I, Wikland M, Granberg S. Transvaginal sonography of the endometrium in a representative sample of postmenopausal women. Ultrasound Obstet Gynecol. 1996;7:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Gupta A, Desai A, Bhatt S. Imaging of the Endometrium: Physiologic Changes and Diseases: Women's Imaging. Radiographics. 2017;37:2206-2207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Daoud T, Sardana S, Stanietzky N, Klekers AR, Bhosale P, Morani AC. Recent Imaging Updates and Advances in Gynecologic Malignancies. Cancers (Basel). 2022;14:5528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 18. | Viswanathan AN, Buttin BM, Kennedy AM. Oncodiagnosis Panel: 2006. Ovarian, cervical, and endometrial cancer. Radiographics. 2008;28:289-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Sala E, Wakely S, Senior E, Lomas D. MRI of malignant neoplasms of the uterine corpus and cervix. AJR Am J Roentgenol. 2007;188:1577-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 148] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 20. | Peungjesada S, Bhosale PR, Balachandran A, Iyer RB. Magnetic resonance imaging of endometrial carcinoma. J Comput Assist Tomogr. 2009;33:601-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Nogami Y, Iida M, Banno K, Kisu I, Adachi M, Nakamura K, Umene K, Masuda K, Tominaga E, Tanaka K, Aoki D. Application of FDG-PET in cervical cancer and endometrial cancer: utility and future prospects. Anticancer Res. 2014;34:585-592. [PubMed] |
| 22. | Faria SC, Sagebiel T, Balachandran A, Devine C, Lal C, Bhosale PR. Imaging in endometrial carcinoma. Indian J Radiol Imaging. 2015;25:137-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Syed AB, Zoga AC. Artificial Intelligence in Radiology: Current Technology and Future Directions. Semin Musculoskelet Radiol. 2018;22:540-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 24. | Tameish S, Florez N, Vidal JRP, Chen H, Vara J, Alcázar JL. Transvaginal ultrasound versus magnetic resonance imaging for preoperative assessment of myometrial infiltration in patients with low-grade endometrioid endometrial cancer: A systematic review and head-to-head meta-analysis. J Clin Ultrasound. 2023;51:1188-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Jacobs I, Gentry-Maharaj A, Burnell M, Manchanda R, Singh N, Sharma A, Ryan A, Seif MW, Amso NN, Turner G, Brunell C, Fletcher G, Rangar R, Ford K, Godfrey K, Lopes A, Oram D, Herod J, Williamson K, Scott I, Jenkins H, Mould T, Woolas R, Murdoch J, Dobbs S, Leeson S, Cruickshank D, Skates SJ, Fallowfield L, Parmar M, Campbell S, Menon U. Sensitivity of transvaginal ultrasound screening for endometrial cancer in postmenopausal women: a case-control study within the UKCTOCS cohort. Lancet Oncol. 2011;12:38-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 26. | Bian J, Li J, Liu Y. Diagnostic accuracy of shear wave elastography for endometrial cancer: A meta-analysis. Medicine (Baltimore). 2023;102:e32700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 27. | Ziogas A, Xydias E, Kalantzi S, Papageorgouli D, Liasidi PN, Lamari I, Daponte A. The diagnostic accuracy of 3D ultrasound compared to 2D ultrasound and MRI in the assessment of deep myometrial invasion in endometrial cancer patients: A systematic review. Taiwan J Obstet Gynecol. 2022;61:746-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 28. | Xydias EM, Kalantzi S, Tsakos E, Ntanika A, Beis N, Prior M, Daponte A, Ziogas AC. Comparison of 3D ultrasound, 2D ultrasound and 3D Doppler in the diagnosis of endometrial carcinoma in patients with uterine bleeding: A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2022;277:42-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 29. | Stoelinga B, Juffermans L, Dooper A, de Lange M, Hehenkamp W, Van den Bosch T, Huirne J. Contrast-Enhanced Ultrasound Imaging of Uterine Disorders: A Systematic Review. Ultrason Imaging. 2021;43:239-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Guler AH, Ates MC, Avcı F, Seher N, Cintesun E, Bilgi A, Korez MK, Koplay M, Celik C. The role of shear wave elastography in predicting endometrial cancer in patients presenting with abnormal uterine bleeding. Eur Rev Med Pharmacol Sci. 2024;28:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Zheng Y, Zhang Y, Lu K, Wang J, Li L, Xu D, Liu J, Lou J. Diagnostic value of an interpretable machine learning model based on clinical ultrasound features for follicular thyroid carcinoma. Quant Imaging Med Surg. 2024;14:6311-6324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 32. | Mohit K, Gupta R, Kumar B. A Survey on the Machine Learning Techniques for Automated Diagnosis from Ultrasound Images. Curr Med Imaging. 2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 33. | Song SH, Han JH, Kim KS, Cho YA, Youn HJ, Kim YI, Kweon J. Deep-learning segmentation of ultrasound images for automated calculation of the hydronephrosis area to renal parenchyma ratio. Investig Clin Urol. 2022;63:455-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 34. | Wu N, Phang J, Park J, Shen Y, Huang Z, Zorin M, Jastrzebski S, Fevry T, Katsnelson J, Kim E, Wolfson S, Parikh U, Gaddam S, Lin LLY, Ho K, Weinstein JD, Reig B, Gao Y, Toth H, Pysarenko K, Lewin A, Lee J, Airola K, Mema E, Chung S, Hwang E, Samreen N, Kim SG, Heacock L, Moy L, Cho K, Geras KJ. Deep Neural Networks Improve Radiologists' Performance in Breast Cancer Screening. IEEE Trans Med Imaging. 2020;39:1184-1194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 277] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 35. | Nathani A, Keshishyan S, Cho RJ. Advancements in Interventional Pulmonology: Harnessing Ultrasound Techniques for Precision Diagnosis and Treatment. Diagnostics (Basel). 2024;14:1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 36. | Bajaj R, Huang X, Kilic Y, Ramasamy A, Jain A, Ozkor M, Tufaro V, Safi H, Erdogan E, Serruys PW, Moon J, Pugliese F, Mathur A, Torii R, Baumbach A, Dijkstra J, Zhang Q, Bourantas CV. Advanced deep learning methodology for accurate, real-time segmentation of high-resolution intravascular ultrasound images. Int J Cardiol. 2021;339:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 37. | Jin Y, Zhou C. Diagnostic accuracy of TVUS and MRI in the preoperative evaluation of myometrial infiltration in patients with endometrial cancer: A meta-analysis. Clin Radiol. 2025;85:106868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 38. | Moro F, Albanese M, Boldrini L, Chiappa V, Lenkowicz J, Bertolina F, Mascilini F, Moroni R, Gambacorta MA, Raspagliesi F, Scambia G, Testa AC, Fanfani F. Developing and validating ultrasound-based radiomics models for predicting high-risk endometrial cancer. Ultrasound Obstet Gynecol. 2022;60:256-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 39. | Faria SC, Devine CE, Rao B, Sagebiel T, Bhosale P. Imaging and Staging of Endometrial Cancer. Semin Ultrasound CT MR. 2019;40:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 40. | Luna C, Balcacer P, Castillo P, Huang M, Alessandrino F. Endometrial cancer from early to advanced-stage disease: an update for radiologists. Abdom Radiol (NY). 2021;46:5325-5336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 41. | Capozzi VA, Rosati A, Rumolo V, Ferrari F, Gullo G, Karaman E, Karaaslan O, HacioĞlu L. Novelties of ultrasound imaging for endometrial cancer preoperative workup. Minerva Med. 2021;112:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/