Published online Sep 28, 2025. doi: 10.4329/wjr.v17.i9.111215
Revised: July 31, 2025
Accepted: August 14, 2025
Published online: September 28, 2025
Processing time: 84 Days and 1.8 Hours
Endometrial peristalsis (EmP) has been considered as a new indicator for eva
To explore the value of EmP analysis (EmPA) technique in detecting and analy
One hundred and forty-five patients without abnormal findings on conventional transvaginal ultrasound were included in this study. A mid sagittal plane of ute
EmPA was unsuccessful in 9 patients (9/145, 6.21%) due to the interference of respiratory or intestinal movement and was successful in 136 patients (136/145, 93.79%), of whom 21 patients showed no EmP and 115 patients underwent EmPA and obtained quantitative results. The results of EmPA technique and naked eyes analysis about EmP intensity and transfer time had no significant consistence. Menstrual cycle, uterine position and history of induced abortion affected the quantitative results of EmPA.
EmPA technique provides noninvasive, quantitative and accurate detection and analysis of EmP in normal population and can evaluate EmP changes associated with menstrual cycle, uterine position and history of induced abortion.
Core Tip: Endometrial peristalsis analysis (EmPA) is the first automated and quantitative technique applied in the ultrasound system to detect and analyze endometrial peristalsis (EmP) features. In this study, EmPA technique was used to analyze EmP accurately and evaluate the changes of EmP associated with menstrual cycle and other factors such as uterine position and age.
- Citation: Zhang HP, Chen ML, Xue SL, Zou J, Wu JJ, Zhou YQ. Value of endometrial peristalsis analysis technique in detecting and analyzing endometrial peristalsis features. World J Radiol 2025; 17(9): 111215
- URL: https://www.wjgnet.com/1949-8470/full/v17/i9/111215.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i9.111215
Uterine endometrial peristalsis (EmP) refers to the wavelike uterine motion originated from the junctional zone (the layer of myometrium underneath the endometrium). The amplitude, frequency and direction of EmP change with the menstrual cycle due to the fluctuations of ovarian hormonal levels for assisting menstrual blood discharge, sperm transport, embryo implantation and pregnancy maintaining[1-4].
EmP has been considered as a new indicator for evaluating endometrial receptivity and is associated with embryo implantation rate and successful pregnancy rate[5-7]. EmP types could change when complicated with some gynecological diseases, such as uterine leiomyomas, adenomyosis and chronic endometritis[8-11]. So, the study of EmP could provide more information related to the pathogenesis and potential diagnostic methods of these diseases.
The common methods to detect EmP include intra-uterine pressure measurement, hystero-salpingo scintigraphy, transvaginal ultrasound (TVUS) and cine magnetic resonance imaging (MRI)[12-17]. Each method has its advantages and disadvantage for EmP analysis (EmPA) and could not fully satisfy clinical needs. Because of lack of an invasive, con
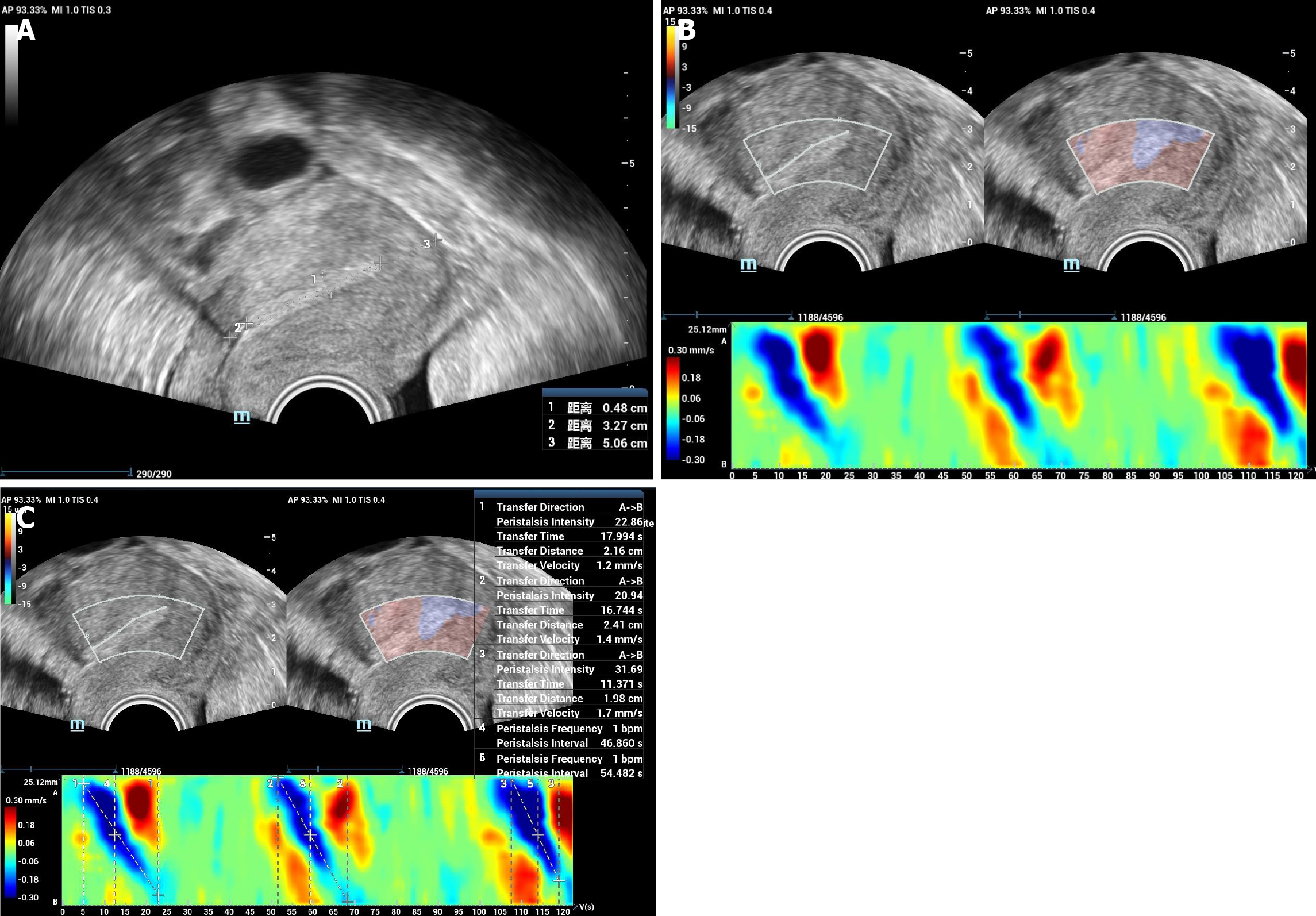
EmPA technique available in a Resona R9 diagnostic ultrasound system (Mindary Medical International, Shenzhen, China) could provide an automatic detection and quantitative analysis of EmP. It continuously sends high frame rate ultrasonic waves to endometrial tissues and tracks endometrial motion accurately based on the original ultrasonic radio frequency signal. The technique displays EmP wave intuitively by color coding, and provides EmP parameters such as transfer direction, peristalsis intensity, transfer distance, transfer time, transfer velocity and peristalsis frequency. To the best of our knowledge, it is the first automated and quantitative technique applied in the ultrasound system for EmPA.
In this study, we aimed to explore the value of EmPA technique in detecting and analyzing EmP features automatically and quantitatively in normal population and to observe EmP changes associated with different menstrual phases and various factors including age, uterine position, induced abortion history.
This was an observational cross-sectional study. The study was approved by our hospital’s Ethics Committee (CNFBLLAR 2023-009) and every patient signed the informed consent before ultrasound examination.
Outpatients in Gynecological Department in our hospital between March and November in 2023 who volunteered to join the study and met the inclusion criteria were included in this study. The inclusion criteria were: (1) ≥ 18 years old; (2) With regular menstrual cycle; and (3) Without contraindications for TVUS. The exclusion criteria were: (1) With a history of pelvic surgery besides induced abortion or cesarean section; (2) With a history of pelvic chemotherapy or pelvic radiotherapy; (3) With a history of endocrine therapy including contraceptive medication in the past one month; and (4) With abnormal findings shown on conventional TVUS, including endometrial, myometrial or cervical lesions and adnexal masses.
Age, body mass index (BMI), menstrual cycle, smoking history and history of induced abortion were recorded for all the patients.
A radiologist with 5 years’ experience in TVUS performed all the conventional ultrasound examinations. A Nuewa A20 diagnostic ultrasound system (Mindray Medical International, Shenzhen, China) with a transvaginal DE 10-3WU probe was used. Before ultrasound examinations, patients emptied the bladder. Conventional TVUS was performed for each patient first for exclusion of abnormal findings in uterus, cervix and adnexa. Uterine position was observed and recorded as anteversion or retroversion. Uterine longitudinal diameter and endometrial thickness were measured and recorded. Uterine endometrial texture was graded as A, B and C according to Smith’s et al study and recorded[20].
The same radiologist performed all the EmPA examinations, who received a strict training about the observation of EmP using ultrasound and the application of EmPA technique. After conventional TVUS examination, a mid sagittal plane of uterus was acquired. The probe frequency was set as 5.0 MHz, depth as 5 cm, dynamic range as 120 db and frame rate as 38 Hz. Then, EmPA function was activated and a region of interest was drawn including the whole endometrium. The color bar was set as -15 μm to 15 μm. The image was observed and recorded for 2 minutes. The 2-minute video was analyzed by drawing a line of interest in the endometrium from uterine fundus to cervix (FC) and then, the color code image of EmP was acquired. The parameters analyzed were as listed: Transfer direction, peristalsis intensity, transfer distance, transfer time, transfer velocity and peristalsis frequency (Figure 1). Transfer direction is as classified as FC wave, from cervix to fundus (CF) wave, opposing (OP) wave and random (R). Peristalsis intensity is a relative intensity with a range of 0-100. Transfer distance (mm) is the distance each peristalsis wave transfers. Transfer time (s) is the time each peristalsis wave transfers. Transfer velocity (mm/second) is calculated as transfer distance/transfer time. Peristalsis frequency is the number of peristalsis wave per minute.
For each patient, usually more than 1 EmP could be observed in 2 minutes, so the above-mentioned parameters of each EmP were averaged and recorded as average parameters for further analysis. If most EmPs had CF transfer direction, then the direction was recorded as CF; if most EmPs had FC transfer direction, the direction was recorded as FC; otherwise, the direction was recorded as OP. And the parameters of the EmP with the highest peristalsis intensity were recorded as highest parameters for further analysis too.
A senior radiologist with 15 years’ experience in TVUS who was blinded to all the patients clinical information and TVUS examination results reviewed the 2 minutes videos of 50 patients selected randomly, and analyzed the endometrial EmP features by naked eyes. The parameters analyzed included transfer direction (FC, CF, CF/FC, OP and No wave)[4], peristalsis frequency, peristalsis intensity (high or low, evaluated objectively by the radiologist) and transfer time (long or short, evaluated objectively by the radiologist).
SPSS version 24.0 software (IBM Corporation, Chicago, IL, United States) was used for statistical analysis. P < 0.05 was considered as statistically significant. Kappa test was used for the consistency analysis between EmPA and naked eyes analysis performed by the senior radiologist. Numerical variables were presented as mean ± SD and compared using independent sample t test, analysis of variance and least significant difference test. Pearson correlation was used for the linear correlation analysis between EmPA quantitative parameters and clinical/ultrasound parameters. The enumerative data were presented as numbers and Pearson’s χ2 test and Fisher’ s exact test were used for statistical analysis.
There were 145 patients included in this study. EmPA was not done successfully in 9 patients (9/145, 6.21%) due to the interference of respiratory or intestinal movement. Twenty-one patients (21/145, 14.48%) showed no EmP according to the result of EmPA. The rest 115 patients (115/145, 79.31%) underwent successful EmPA and quantitative results were obtained.
Only two patients (2/145, 1.40%) had smoking history; and 4 patients (4/145, 2.76%) had BMI larger than 24.0. So these parameters were not compared in different groups. Other basic information of the patients and the comparison among these three groups were shown in Table 1.
| Case numbers | Age (year) | History of induced abortion | Menstrual cycle | Uterine position | Uterine longitudinal diameter (mm) | Endometrial thickness (mm) | Endometrial grade | ||||||||
| 0 | 1 | ≥ 2 | Proliferate phase | Secretory phase | Menopause | Anterior | Posterior | A | B | C | |||||
| EmPA done unsuccessfully | 9 | 35.67 ± 4.69 | 5 | 2 | 2 | 4 | 5 | 0 | 8 | 1 | 58.22 ± 4.09 | 9.41 ± 1.80 | 1 | 2 | 6 |
| With EmP shown on EmPA | 115 | 33.70 ± 7.78 | 54 | 35 | 26 | 68 | 44 | 3 | 70 | 45 | 52.43 ± 7.08 | 6.53 ± 2.52 | 52 | 39 | 24 |
| No EmP shown on EmPA | 21 | 39.71 ± 13.95 | 7 | 3 | 11 | 14 | 1 | 6 | 12 | 9 | 43.38 ± 9.37 | 4.72 ± 2.87 | 9 | 3 | 9 |
| t/χ² | 2.822 | 29.201 | 39.629 | 2.946 | 5.103 | 2.957 | 27.117 | ||||||||
| P value | 0.006 | < 0.001 | < 0.001 | 0.244 | < 0.001 | 0.004 | < 0.001 | ||||||||
Patients with no EmP shown on EmPA tended to be older, be in the proliferate phase, with more induced abortions, with smaller uterine longitudinal diameter and smaller endometrial thickness and with endometrial grade as C, compared with patients with EmP shown on EmPA.
A total of 505 EmPs were detected in the 115 patients with EmP shown by EmPA, among which the average peristalsis intensity was 24.833, average transfer distance was 13.517 mm, average transfer time was 6.503 seconds and average transfer velocity was 2.552 mm/second. The transfer direction of 322 (322/505, 63.76%) EmPs was as CF, 173 (173/505, 34.26%) was as FC and 10 (10/505, 1.98%) was as OP. The transfer number in each patient ranged from 1 to 10.
EmP in 50 patients was analyzed by both EmPA and senior radiologist using naked eyes. The comparison of the results using the two methods was shown in Table 2.
| EmPA (n = 50) | ||||
| No EmP (9) | With EmP (38) | EmPA done unsuccessfully (3) | ||
| Naked eyes analysis of a senior radiologist (n = 50) | No EmP (8) | 4 | 3 | 1 |
| With EmP (42) | 5 | 35 | 2 | |
| χ2 | 7.569 | |||
| P value | 0.016 | |||
Three patients who had successful EmPA quantitative results showed no EmP by naked eyes analysis. Each EmP shown in the 3 patients using EmPA had low peristalsis intensity (lower than the average value of 24.833) and short peristalsis time (shorter than the average value of 6.503 seconds).
Five patients who had EmP by naked eyes analysis showed no EmP by EmPA; two patients who had EmP by naked eyes analysis showed EmPA done unsuccessfully. Each EmP shown in the seven patients by naked eyes had low peristalsis intensity and short peristalsis time.
Thirty-five patients had EmP by both naked eyes analysis and EmPA. The comparison of the results in those 35 patients including EmP intensity, transfer time and direction using the two methods was shown as below.
Eighteen EmP in 35 patients were with low peristalsis intensity evaluated by naked eyes (14 of them were with low intensity and 4 were with high intensity by EmPA) and 17 EmP in 35 patients were with high peristalsis intensity evaluated by naked eyes (9 of them were with high intensity and 8 were with low intensity by EmPA). The results of two methods about EmP intensity had no significant consistence (P as 0.060, Kappa as 0.309).
Eighteen EmP in 35 patients were with short transfer time evaluated by naked eyes (9 of them were with short transfer time and 9 were with long transfer time by EmPA) and 17 EmP in 35 patients were with long transfer time evaluated by naked eyes (9 of them were with long transfer time and 8 were with short transfer time by EmPA). The results of two methods about EmP transfer time had no significant consistence (P as 0.862, Kappa as 0.029).
Twenty two EmP in 35 patients were with CF transfer direction evaluated by naked eyes (21 of them were with CF transfer direction and 1 was with FC transfer direction by EmPA); 6 EmP were with FC transfer direction evaluated by naked eyes (5 of them were with FC transfer direction and 1 was with CF transfer direction by EmPA); 5 EmP were with OP transfer direction evaluated by naked eyes (5 of them were with CF transfer direction and 1 was with FC transfer direction by EmPA); the last 2 EmP were with R transfer direction evaluated by naked eyes (2 of them were with FC transfer direction by EmPA). The results of two methods about EmP transfer direction had moderate consistence (P as 0.000, Kappa as 0.474). Five EmP with OP transfer direction and 2 EmP with R transfer direction were all EmP with high intensity and short transfer time evaluated by naked eyes.
The average parameters of EmPA and their relationship with clinical and ultrasound parameters were shown in Table 3.
| Transfer direction | Peristalsis intensity | Transfer time (sec) | Transfer distance (mm) | Transfer velocity (mm/second) | Peristalsis frequency (/minute) | ||||
| CF | FC | OP | |||||||
| Menstrual cycle | Proliferate phase (n = 68) | 37 | 24 | 7 | 21.842 ± 8.154 | 6.444 ± 4.067 | 13.474 ± 9.033 | 2.494 ± 1.120 | 2.125 ± 0.975 |
| Secretory phase (n = 44) | 30 | 9 | 5 | 26.308 ± 9.866 | 5.804 ± 3.662 | 12.529 ± 6.054 | 2.743 ± 1.205 | 2.330 ± 1.120 | |
| t/χ² | 2.896 | 2.621 | 0.845 | 0.610 | 1.113 | 1.022 | |||
| P value | 0.256 | 0.010 | 0.400 | 0.543 | 0.268 | 0.309 | |||
| Uterine position | Anteverted (70) | 43 | 21 | 6 | 25.280 ± 8.769 | 6.165 ± 3.974 | 11.567 ± 6.124 | 2.404 ± 1.007 | 2.164 ± 0.984 |
| Retroverted (45) | 26 | 13 | 6 | 20.574 ± 8.786 | 6.080 ± 3.790 | 15.263 ± 9.737 | 2.903 ± 1.298 | 2.244 ± 1.161 | |
| t/χ² | 0.740 | 2.807 | 0.115 | 2.501 | 2.311 | 0.397 | |||
| P value | 0.718 | 0.006 | 0.909 | 0.014 | 0.023 | 0.692 | |||
| History of induced abortion | 0 (54) | 30 | 18 | 6 | 23.709 ± 8.808 | 6.234 ± 3.621 | 13.606 ± 8.665 | 2.644 ± 1.202 | 2.472 ± 1.066 |
| 1 (35) | 22 | 9 | 4 | 26.130 ± 7.802 | 5.716 ± 3.598 | 11.689 ± 6.646 | 2.480 ± 1.060 | 2.057 ± 0.914 | |
| ≥ 2 (26) | 17 | 7 | 2 | 19.255 ± 9.828a | 6.480 ± 4.803 | 13.564 ± 7.910 | 2.667 ± 1.192 | 1.808 ± 1.078b | |
| F/χ² | 1.093 | 4.642 | 0.320 | 0.702 | 0.269 | 4.149 | |||
| P value | 0.915 | 0.012 | 0.727 | 0.498 | 0.765 | 0.018 | |||
| Endometrial grade | A (52) | 34 | 12 | 6 | 23.998 ± 9.132 | 6.150 ± 3.767 | 11.886 ± 5.656 | 2.428 ± 0.988 | 2.327 ± 0.949 |
| B (39) | 21 | 15 | 3 | 24.173 ± 9.137 | 6.411 ± 3.907 | 15.030 ± 10.745 | 2.754 ± 1.206 | 2.359 ± 1.063 | |
| C (24) | 14 | 7 | 3 | 21.035 ± 8.615 | 5.637 ± 4.219 | 12.178 ± 6.162 | 2.719 ± 1.370 | 1.646 ± 1.107c | |
| F/χ² | 2.832 | 1.079 | 0.292 | 1.961 | 0.057 | 4.394 | |||
| P value | 0.595 | 0.343 | 0.747 | 0.146 | 0.351 | 0.015 | |||
| Age | ≤ 35 year (80) | 50 | 20 | 10 | 23.364 ± 9.387 | 5.734 ± 3.353 | 1.249 ± 0.795 | 2.583 ± 1.130 | 2.250 ± 1.049 |
| > 35 year (35) | 19 | 14 | 2 | 23.611 ± 8.305 | 7.041 ± 4.826 | 1.422 ± 0.779 | 2.637 ± 1.215 | 2.071 ± 1.065 | |
| t/χ² | 2.996 | 0.134 | 1.455 | 1.079 | 0.233 | 0.836 | |||
| P value | 0.221 | 0.893 | 0.152 | 0.283 | 0.816 | 0.405 | |||
| Age | r | / | 0.068 | 0.079 | 0.043 | 0.008 | 0.136 | ||
| P value | / | 0.471 | 0.400 | 0.652 | 0.932 | 0.146 | |||
| Uterine longitudinal diameter | r | / | 0.042 | 0.036 | 0.096 | 0.100 | 0.216 | ||
| P value | / | 0.659 | 0.703 | 0.309 | 0.286 | 0.020 | |||
| Uterine endometrial thickness | r | / | 0.316 | 0.069 | 0.084 | 0.107 | 0.293 | ||
| P value | / | 0.001 | 0.436 | 0.370 | 0.254 | 0.001 | |||
Uterine position had effect on peristalsis intensity, transfer distance and transfer velocity. Compared with retroversion uterus, EmP in anteversion uterus were tend to be with higher peristalsis intensity, shorter transfer distance and lower transfer velocity.
Compared with proliferate phase uterus, EmP in secretory phase uterus were tend to be with higher peristalsis intensity.
Compared with no history of induced abortion, EmP in uterus with more than 1 induced abortion were tend to be with lower peristalsis intensity and lower peristalsis frequency.
Compared with endometrial grade A or B, EmP in endometrial grade C uterus were tend to be with lower peristalsis frequency.
Uterine longitudinal diameter and uterine endometrial thickness showed linear correlation with EmP peristalsis frequency; and uterine endometrial thickness showed linear correlation with EmP peristalsis intensity, too; but the correlation was moderate.
The highest parameters of EmPA and their relationship with clinical and ultrasound parameters were shown in Table 4. Besides above-mentioned relations with clinical and ultrasound parameters similarly with average parameters of EmPA, EmP in endometrial grade C uterus were tend to be with higher transfer velocity, compared with endometrial grade A uterus.
| Transfer direction | Peristalsis intensity | Transfer time (second) | Transfer distance (mm) | Transfer velocity (mm/second) | |||
| CF | FC | ||||||
| Menstrual cycle | Proliferate phase | 39 | 28 | 25.857 ± 10.307 | 6.255 ± 4.722 | 12.478 ± 7.429 | 2.643 ± 1.790 |
| Secretory phase | 30 | 13 | 32.180 ± 12.162 | 5.793 ± 3.914 | 13.177 ± 6.899 | 3.113 ± 2.213 | |
| t/χ² | 1.497 | 2.953 | 0.539 | 0.500 | 1.235 | ||
| P value | 0.234 | 0.004 | 0.591 | 0.618 | 0.219 | ||
| Uterine position | Anteverted (70) | 43 | 27 | 30.151 ± 11.023 | 6.074 ± 4.363 | 11.447 ± 6.888 | 2.427 ± 1.516 |
| Retroverted (45) | 29 | 14 | 25.043 ± 11.679 | 5.868 ± 4.450 | 14.513 ± 7.210 | 3.462 ± 2.365 | |
| t/χ² | 0.417 | 2.369 | 0.246 | 2.288 | 2.610 | ||
| P value | 0.552 | 0.020 | 0.806 | 0.024 | 0.011 | ||
| History of induced abortion | 0 (54) | 32 | 20 | 28.978 ± 11.596 | 5.816 ± 4.038 | 12.441 ± 6.344 | 2.898 ± 2.111 |
| 1 (35) | 23 | 12 | 31.406 ± 9.595 | 5.755 ± 3.586 | 11.866 ± 6.780 | 2.711 ± 1.909 | |
| ≥ 2 (26) | 17 | 18 | 22.058 ± 11.783a | 6.684 ± 5.894 | 14.127 ± 9.038 | 2.857 ± 1.712 | |
| F/χ² | 2.365 | 5.601 | 0.416 | 0.787 | 0.099 | ||
| P value | 0.321 | 0.005 | 0.661 | 0.458 | 0.906 | ||
| Endometrial grade | A (52) | 34 | 18 | 28.768 ± 11.190 | 6.217 ± 4.455 | 11.746 ± 6.254 | 2.349 ± 1.266 |
| B (39) | 21 | 18 | 29.634 ± 11.939 | 6.195 ± 4.555 | 13.654 ± 8.558 | 2.952 ± 2.058 | |
| C (24) | 17 | 5 | 24.407 ± 11.144 | 5.181 ± 3.984 | 12.963 ± 6.447 | 3.683 ± 2.648b | |
| F/χ² | 3.455 | 1.690 | 0.518 | 0.822 | 4.171 | ||
| P value | 0.185 | 0.189 | 0.597 | 0.442 | 0.018 | ||
| Age, year | ≤ 35 (80) | 52 | 27 | 28.323 ± 11.848 | 5.613 ± 3.752 | 11.800 ± 6.092 | 2.778 ± 1.974 |
| > 35 (35) | 20 | 14 | 27.761 ± 10.851 | 6.862 ± 5.519 | 14.583 ± 8.908 | 2.954 ± 1.920 | |
| t/χ² | 0.504 | 0.240 | 1.221 | 1.684 | 0.442 | ||
| P value | 0.526 | 0.811 | 0.228 | 0.099 | 0.659 | ||
| Age | r | / | 0.139 | 0.056 | 0.063 | 0.066 | |
| P value | / | 0.139 | 0.552 | 0.500 | 0.483 | ||
| Uterine longitudinal diameter | r | / | 0.069 | 0.044 | 0.080 | 0.170 | |
| P value | / | 0.462 | 0.644 | 0.393 | 0.069 | ||
| Uterine endometrial thickness | r | / | 0.317 | 0.083 | 0.152 | 0.035 | |
| P value | / | 0.001 | 0.376 | 0.104 | 0.710 | ||
In this study, we explored the value of EmPA technique in detecting and analyzing EmP features automatically and quantitatively in normal population. And we found EmPA to be a useful tool for detection and analysis of EmP, including the number, direction, intensity, transfer time, distance and velocity.
We examined EmP in 145 normal patients using EmPA technique and the results showed that 115 patients (79.31%) got successful EmPA and quantitative results, while 9 patients (6.21%) did not get successful EmPA due to the interference of respiratory or intestinal movement and 21 patients (14.48%) showed no EmP according to the result of EmPA. Our results indicated that EmPA could analyze EmP, though it could be affected by respiratory or intestinal movement in a few patients. Six of 9 patients in menopause phase showed no EmP; and that may be the reason that patients with no EmP shown on EmPA were tend to be older, with smaller uterine longitudinal diameter and smaller endometrial thickness and with endometrial grade as C, compared with patients with EmP shown on EmPA. Also, induced abortion affected EmP as our results showed that more patients with no EmP had history of induced abortion twice or more. This may be one of the mechanisms of infertility due to induced abortion[21].
A total of 505 EmPs was shown by EmPA in 115 patients. We acquired the transfer frequency of EmP and the transfer direction, time, distance, velocity and peristalsis intensity of each EmP automatically and quantitatively. Most of the EmPs in our study showed a direction of CF (63.76%) or FC (34.26%). Only 10 (1.98%) EmP showed as OP and no EmP showed as R. EmPA technique applied in the ultrasound system could provide a new, automated, objective and quan
We randomly chose 50 patients in this study for the comparison of EmP results by EmPA technique and senior radiologist analysis using naked eyes. Our results showed that the two methods had no significant consistence about the existence of the EmPs (especially for those with lower peristalsis intensity and short transfer time), transfer time and peristalsis intensity of the EmP. The two methods had moderate consistence about the direction of EmP. Compared with analysis by naked eyes, fewer EmPs were evaluated as the direction of OP or R by EmPA. This may indicate that EmPA technique could evaluate the transfer direction more accurately than naked eyes. However, the value of EmPA technique may still need to be confirmed by clinical application and by comparison with other methods such as dynamic MRI.
We acquired both average and highest parameters of EmPA for each patient and analyzed their relationship with clinical and ultrasound parameters. Our results showed that peristalsis intensity of EmP in secretory phase was lower than that in proliferate phase, but peristalsis frequency, transfer direction, transfer time, transfer distance and transfer velocity of EmP had no significant difference in the two different phase. This result was opposite with previous studies, which usually showed that frequency of EmP in secretory phase was much lower than that in proliferate phase and transfer direction in proliferate phase was usually as CF and in secretory phase as OP[22]. The reason for the difference may be that previous studies usually used naked eyes to analyze EmP with inevitable subjectivity and could not be as precise as automatic and quantitative techniques. And this result needed to be confirmed by a further cohort study with more phases enrolled. Our study also found that uterus position affected peristalsis intensity, transfer distance and transfer velocity of EmP. This may be one of the reasons that retroverted uterus is a variable affecting the pregnancy rate of intracervical insemination and frozen embryo transfer[23,24]. Our result showed the effect of induced abortion on EmP with decreased peristalsis intensity and transfer frequency, even no EmP; this could be an evidence of the endometrial injury by uterine endometrial operation which could lead to abnormal EmP and affect conception[21,25]. According to our study, EmP features did not change with age increased, except that more postmenopausal women may have no EmP. This result was similar with the study of Kiguchi et al[26], which explored the correlation of EmP with age using cine MRI. Linear correlation analysis results showed that uterine endometrial thickness was moderately correlated with EmP intensity and transfer frequency; and uterine longitudinal diameter was moderately correlated with EmP transfer frequency. This result emphasized the importance of understanding and analyzing EmP quantitatively as uterine endometrial thickness and uterine longitudinal diameter are important factors that affect successful pregnancy rate[27,28].
Our study had some limitations. First, this was not a cohort study to continuously analyze patients in different menstrual phases. Second, the participants in our study may deviate from the general population and the sample size was not large enough. Further cohort studies with larger sample size are needed to verify our results.
EmPA technique provided a new, noninvasive, convenient, quantitative and accurate method to detect and analyze EmP automatically in normal population and could evaluate the changes of EmP associated with menstrual cycle and other factors such as uterine position and age.
| 1. | Qu M, Lu P, Bellve K, Lifshitz LM, ZhuGe R. Mode Switch of Ca(2 +) Oscillation-Mediated Uterine Peristalsis and Associated Embryo Implantation Impairments in Mouse Adenomyosis. Front Physiol. 2021;12:744745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 2. | Buddhabunyakan N, Sothornwit J, Seejorn K, Buppasiri P, Salang L. Effects of atosiban on uterine peristalsis following frozen embryo transfer: A randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2021;265:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Meirzon D, Jaffa AJ, Gordon Z, Elad D. A new method for analysis of non-pregnant uterine peristalsis using transvaginal ultrasound. Ultrasound Obstet Gynecol. 2011;38:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Ijland MM, Evers JL, Dunselman GA, van Katwijk C, Lo CR, Hoogland HJ. Endometrial wavelike movements during the menstrual cycle. Fertil Steril. 1996;65:746-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 116] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Zhang CH, Chen C, Wang JR, Wang Y, Wen SX, Cao YP, Qian WP. An endometrial receptivity scoring system basing on the endometrial thickness, volume, echo, peristalsis, and blood flow evaluated by ultrasonography. Front Endocrinol (Lausanne). 2022;13:907874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Li X, Peng Y, Mao Y, Li Y, Gong F, Ouyang Y. Endometrial receptivity change: ultrasound evaluation on ovulation day and transplantation day during the natural frozen embryo transfer cycle. Front Endocrinol (Lausanne). 2023;14:1118044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 7. | Cai H, Liu S, Chen L, Xie J, Yang C, Li W, Mol BW, Shi J. Effectiveness of atosiban in women with previous single implantation failure undergoing frozen-thawed blastocyst transfer: study protocol for a randomised controlled trial. BMJ Open. 2023;13:e076390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Kido A, Ascher SM, Hahn W, Kishimoto K, Kashitani N, Jha RC, Togashi K, Spies JB. 3 T MRI uterine peristalsis: comparison of symptomatic fibroid patients versus controls. Clin Radiol. 2014;69:468-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Antero MF, Ayhan A, Segars J, Shih IM. Pathology and Pathogenesis of Adenomyosis. Semin Reprod Med. 2020;38:108-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Arena A, Zanello M, Orsini B, Degli Esposti E, Iodice R, Altieri M, Borgia A, Moro E, Seracchioli R, Casadio P. Uterine peristalsis in women affected by adenomyosis: A step towards functional assessment. Int J Gynaecol Obstet. 2024;165:666-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 11. | Pinto V, Matteo M, Tinelli R, Mitola PC, De Ziegler D, Cicinelli E. Altered uterine contractility in women with chronic endometritis. Fertil Steril. 2015;103:1049-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | van Gestel I, IJland MM, Hoogland HJ, Evers JL. Endometrial wave-like activity in the non-pregnant uterus. Hum Reprod Update. 2003;9:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Kissler S, Zangos S, Wiegratz I, Kohl J, Rody A, Gaetje R, Doebert N, Wildt L, Kunz G, Leyendecker G, Kaufmann M. Utero-tubal sperm transport and its impairment in endometriosis and adenomyosis. Ann N Y Acad Sci. 2007;1101:38-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Soares DM, Junior HW, Bittencourt LK, Lopes FPPL, de Oliveira MAP. The role of cine MR imaging in the assessment of uterine function. Arch Gynecol Obstet. 2019;300:545-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Soares DM, Bittencourt LK, Lopes FPPL, de Oliveira MAP. Deep infiltrating endometriosis: cine magnetic resonance imaging in the evaluation of uterine contractility. Radiol Bras. 2023;56:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 16. | Moliner B, Llacer J, Sellers F, Castillo JC, Fuentes A, Bernabeu A, Bernabeu R. 4D ultrasound as a method to assess uterine peristalsis. Fertil Steril. 2021;116:272-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Javedani Masroor M, Younesi Asl L, Sarchami N. The Effect of Uterine Contractions on Fertility Outcomes in Frozen Embryo Transfer Cycles: A Cohort Study. J Reprod Infertil. 2023;24:132-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Salmeri N, Di Stefano G, Viganò P, Stratton P, Somigliana E, Vercellini P. Functional determinants of uterine contractility in endometriosis and adenomyosis: a systematic review and meta-analysis. Fertil Steril. 2024;122:1063-1078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Kuijsters NPM, Sammali F, Rabotti C, Huang Y, Mischi M, Schoot BC. Visual inspection of transvaginal ultrasound videos to characterize uterine peristalsis: an inter-observer agreement study. J Ultrasound. 2020;23:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Smith B, Porter R, Ahuja K, Craft I. Ultrasonic assessment of endometrial changes in stimulated cycles in an in vitro fertilization and embryo transfer program. J In Vitro Fert Embryo Transf. 1984;1:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 57] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Lin J, Ma H, Li H, Han J, Guo T, Qin Z, Jia L, Zhang Y. The Treatment of Complementary and Alternative Medicine on Female Infertility Caused by Endometrial Factors. Evid Based Complement Alternat Med. 2022;2022:4624311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 22. | Nakashima A, Komesu I, Sakumoto T, Hamakawa H, Terada Y, Takayama H, Kamiyama S, Higashi M, Ishigaki K, Nakaza A, Ushijima K, Tokunaga Y. Study of uterine kinetics in nonpregnant women using cine-mode magnetic resonance imaging. Reprod Med Biol. 2019;18:370-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Hayashi N, Enatsu N, Iwasaki T, Otsuki J, Matsumoto Y, Kokeguchi S, Shiotani M. Predictive factors influencing pregnancy rate in frozen embryo transfer. Reprod Med Biol. 2020;19:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Chen XJ, Wu LP, Lan HL, Zhang L, Zhu YM. Clinical variables affecting the pregnancy rate of intracervical insemination using cryopreserved donor spermatozoa: a retrospective study in china. Int J Fertil Steril. 2012;6:179-184. [PubMed] |
| 25. | Hooker A, Fraenk D, Brölmann H, Huirne J. Prevalence of intrauterine adhesions after termination of pregnancy: a systematic review. Eur J Contracept Reprod Health Care. 2016;21:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Kiguchi K, Kido A, Kataoka M, Shitano F, Fujimoto K, Himoto Y, Moribata Y, Kurata Y, Fushimi Y, Okada T, Togashi K. Uterine peristalsis and junctional zone: correlation with age and postmenopausal status. Acta Radiol. 2017;58:224-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Zhao Y, Huang X, Huang R, Xu R, Xia E, Li TC. A retrospective cohort study to examine factors affecting live birth after hysteroscopic treatment of intrauterine adhesions. Fertil Steril. 2024;121:873-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 28. | Shi J, Dai Y, Zhang J, Li X, Jia S, Leng J. Pregnancy outcomes in women with infertility and coexisting endometriosis and adenomyosis after laparoscopic surgery: a long-term retrospective follow-up study. BMC Pregnancy Childbirth. 2021;21:383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/