Published online Sep 28, 2025. doi: 10.4329/wjr.v17.i9.110267
Revised: June 21, 2025
Accepted: September 9, 2025
Published online: September 28, 2025
Processing time: 115 Days and 16.7 Hours
Spinal cord injury can lead to long-term disability, but current imaging methods are limited in predicting outcomes. Rapid diffusion tensor imaging (DTI) has shown promise, yet its clinical utility remains underexplored.
To evaluate the potential applications of a short DTI sequence, incorporated into a cervical spine magnetic resonance imaging (MRI) protocol, for characterizing a range of symptomatic spinal cord pathologies. We propose that cervical spine tractography can provide essential diagnostic information beyond what is currently available from conventional MRI.
We utilized a quick DTI sequence to create tractography models of the cervical spinal cord in four patients with distinct pathologies of various etiologies: Cord contusion, metastasis, myelopathy, and multiple sclerosis. We used DSI Studio software for post-processing of tractography cases. Fiber tract findings for each pathology case were compared to five control cases from the same scanner by looking for individual differences in white matter tract integrity based on the fractional anisotropy (FA) and mean diffusivity (MD) of the regions of interest from controls. These correlated with clinical presentations and conventional MRI findings.
Control cases showed consistent and intact tract patterns with stable FA and MD values. In pathological cases, abnormalities in fiber orientation and tract continuity correlated with clinical symptoms and lesion locations.
The tractography models can provide additional information on white matter disruption that was not discernible on standard MRI sequences. However, its clinical use remains limited due to the need for specialized imaging protocols and complex post-processing, restricting its use to mostly academic settings.
Core Tip: This study demonstrates the utility of cervical spinal cord MR tractography in characterizing various spinal cord pathologies, including trauma, demyelination, and neoplasms. By analyzing fractional anisotropy and mean diffusivity across lesion sites and adjacent levels, tractography revealed microstructural changes not visible on conventional magnetic resonance imaging. The findings support tractography as a promising adjunct tool for diagnosis, surgical planning, and treatment follow-up, offering unique insights into fiber integrity in spinal cord disease.
- Citation: Supsupin EP, Serrano A, Louviere C, Pearson L, Hernandez M, Sekar V, Amer A, Cikla U, Virarkar M, Gumus KZ. Magnetic resonance tractography of the cervical spine: A rapid diffusion tensor imaging protocol to serve as a clinical evaluation tool. World J Radiol 2025; 17(9): 110267
- URL: https://www.wjgnet.com/1949-8470/full/v17/i9/110267.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i9.110267
Spinal cord injury (SCI) remains one of the most debilitating and financially costly injuries in the United States. It is estimated that around 250000 to 500000 patients each year suffer from SCIs globally, with 17000 new SCI cases occurring in the United States yearly[1]. The most common mechanisms of spinal cord injuries are motor vehicle collisions, con
Clinical imaging is essential for understanding and accurately managing spinal pathologies. Even patients with an overtly unrelated primary injury will often undergo imaging if there is a remote possibility that spinal cord function could be compromised (cite guidelines). Magnetic resonance imaging (MRI) is the gold-standard imaging modality for characterizing and diagnosing spinal cord pathologies in a stabilized spine[1-4]. MRI provides unparalleled soft tissue characterization, facilitating accurate assessment of cord fibers, vertebral bodies, intervertebral spaces, and adjacent anatomical structures such as blood vessels, nerve roots, ligaments, and other para-spinal tissues[1-4].
Diffusion tensor imaging (DTI) is an MRI sequence that indirectly measures the direction of diffusion of water mo
The diffusion directions acquired from the tensors are extracted from the images and are integrated into the tractography workflow. Fibers are traced following the direction of diffusion from voxel to voxel. Fibers are continuously generated until a stopping condition is met, for example, low anisotropy from injury, a sharp angle change, or reaching gray matter. There are two types of tracking algorithms: Deterministic Tractography and Probabilistic Tractography. The former follows a single dominant direction in voxels, this gives cleaner tracts but is limited when discriminating between multiple fibers in one voxel. The latter considers uncertainty in fiber directions, allowing for the mapping of complex regions with crossing fibers, this tracking method includes the possibility of generating false positives.
DTI captures water molecules' Brownian motion, representing their diffusion across three-dimensional space. Tracking their movement allows us to obtain the molecular diffusion rate, also known as the apparent diffusion coefficient[2,4]. Various structures within a cell’s walls act as natural boundaries, restricting water molecules' free movement. Inside an axon, the long and slender portion of a neuron which transmits nerve impulses between synaptic terminals and the cell body, water molecules have greater movement along its longitudinal axis[2,4-6]. This prudential axis for the molecules to diffuse along is “anisotropy”. DTI can also detect the isotropy of water molecules, and the overall directionality of di
Anisotropy, coupled with the rate of water molecule diffusion, can represent neuronal tracts, called “tractography”. The directionality of the tracts is measured in the anteroposterior, transverse, and cranio-caudal axes (CC), with assigned corresponding colors[2,4,5]. Using this technique to assess spinal cord lesions should thus highlight direction interruptions (along with different colors) in the otherwise regular arrangement of nerve impulse tracts in adjacent cord segments.
A DTI sequence typically takes 5-10 minutes to perform as part of clinical imaging, concurrently with conventional MRI sequences. Recent advances in MRI hardware and software can reduce its duration to a few minutes, making it more feasible for large volume clinical imaging. The relatively short time needed to acquire MRI with DTI makes this sequence a feasible tool for the timely clinical evaluation of spinal cord injuries[2,3].
There is a spectrum of applications available for magnetic resonance (MR) tractography[1,4,5]. It is mainly used in the brain due to its ability to non-invasively delineate and visualize white matter tracts, which are critical for understanding brain connectivity and function. For example, connectomes are maps of the brain that reveal the underlying network of connections between major and minor neural structures. A unique insight not possible with conventional imaging. We can study how local damage can affect distant brain areas[6].
Altered connectivity patterns can be seen in diseases like Alzheimer where there is a loss of long-range connections secondary to cell death from Tau deposition. Surgical Planning can benefit by providing information on the location of major tracts to avoid damaging them[4,5,8]. Additionally, it’s possible to quantify the integrity of white matter tracts for neurological diseases like multiple sclerosis (MS), stroke, TBI, and dementia by measuring the FA of the fibers[7]. In cases of SCI, tractography can help visualize the disruption of white matter tracts. For patients that suffered trauma, it may be predictive of neurological recovery by identifying spared tracts. Similarly to how tractography is used in the brain for preoperative planning, in the spinal cord, it is used to highlight healthy boundaries of central nervous system tumors, potentially maximizing the amount of neural tissue spared in tumor excisions by identifying fiber displacement or damage before surgery. It is also useful for understanding how spinal tumors affect surrounding fiber tracts[1,2,4,8].
Several challenges limit the widespread clinical adoption of tractography. Obtaining imaging acquisitions optimized for tractography, and performing the necessary complex post-processing remains a resource-intensive and time-consuming process[3,4]. Even with high-quality data, the maximum resolution attainable is limited due to methodological limitations. Tractography algorithms infer fiber pathways based on local diffusion properties, which can lead to inaccuracies, specifically in regions where fibers cross, merge, or branch[5]. The clinical impact of these inaccuracies will depend on the application. In the case of large tumor lesions, the focus is on preserving the surrounding healthy white matter, and current algorithms create models with enough accuracy to be of use. Additionally, the evaluation of structural integrity using FA and MD yields valuable information about white matter involvement that correlates to damaged white matter fibers[9].
Nevertheless, tractography remains valuable in certain clinical scenarios as it offers a non-invasive method for assessing the extent of white matter injury, evaluating mass effect from tumors on the surrounding tissue, and tracking the progression of lesions in degenerative conditions such as MS[2,9,10]. As acquisition techniques, reconstruction algorithms, and validation approaches continue to advance, the clinical applicability of tractography is expected to expand, further enhancing its role in precision medicine[1,3]. We hypothesize that tractography can synergistically complement the current standard of care workflows for managing SCIs. In this context, we devised a DTI protocol in our institution, using DSI Studio free software (version. 2024.12.28 Hou Release) to create tractography models and assessed the utility of MR tractography for assessing and following up cervical spinal cord lesions.
We obtained Institutional Review Board (IRB-01) approval for this retrospective study. We integrated a DTI sequence based on a spin-echo echo planar imaging technique into our cervical MRI exam protocol on our institution's 3.0 Tesla MRI scanner. The sequence parameters were as follows: Axial plane, repetition time of 9200 ms, echo time of 82 ms, 37 slices, slice thickness of 3 mm, matrix size of 128 × 128, three signal averages, b values of 0 and 1000 s/mm² across six directions in order to improve clinical feasibility with a total scan time of approximately 2 minutes (Table 1).
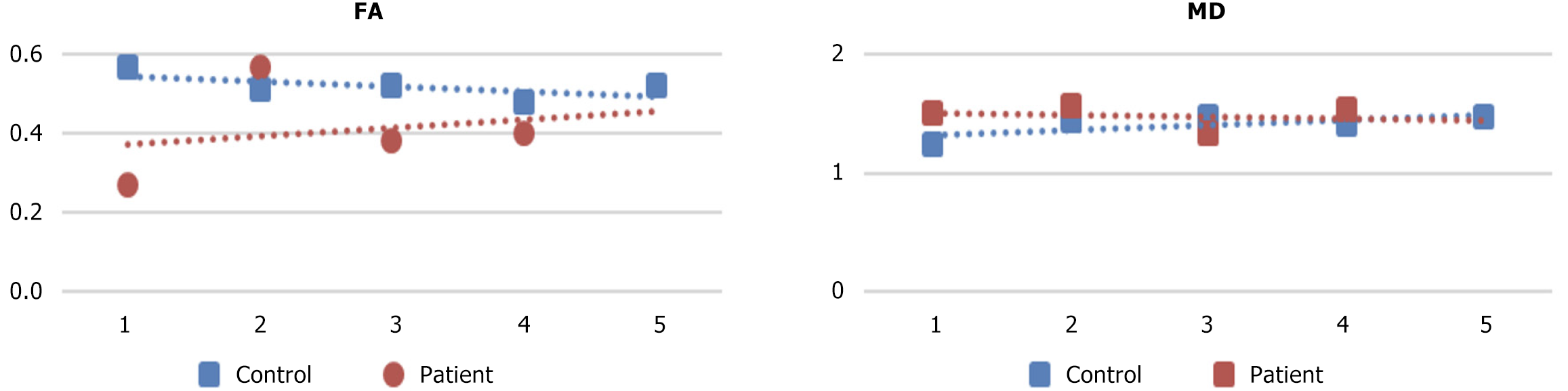
| Case | Cranial FA | z-score | Cranial MD | z-score | Central FA | z-score | Central MD | z-score | Caudal FA | z-score | Caudal MD | z-score |
| Control 1 | 0.57 | 1.97 | 1.35 | -0.78 | 0.57 | 0.86 | 1.24 | -1.91 | 0.53 | 0.97 | 1.49 | -0.03 |
| Control 2 | 0.37 | -0.22 | 0.99 | -1.60 | 0.51 | 0.30 | 1.44 | 0.17 | 0.54 | 1.12 | 1.15 | -1.72 |
| Control 3 | 0.37 | -0.22 | 2.01 | 0.73 | 0.52 | 0.39 | 1.47 | 0.49 | 0.45 | -0.26 | 1.51 | 0.07 |
| Control 4 | 0.31 | -0.88 | 1.71 | 0.04 | 0.48 | 0.02 | 1.41 | -0.14 | 0.47 | 0.05 | 1.78 | 1.41 |
| Control 5 | 0.37 | -0.22 | 2.01 | 0.73 | 0.52 | 0.39 | 1.47 | 0.49 | 0.45 | -0.26 | 1.51 | 0.07 |
| Metastatic | 0.35 | -0.44 | 2.08 | 0.88 | 0.27 | -1.96 | 1.51 | 0.90 | 0.36 | -1.63 | 1.54 | 0.22 |
| Myelopathy | 0.72 | 1.69 | 1.2 | -0.80 | 0.57 | 1.18 | 1.57 | 1.25 | 0.62 | 1.65 | 1.23 | -0.95 |
| Contusion | 0.51 | 0.93 | 1.18 | -0.83 | 0.38 | -1.82 | 1.33 | -0.69 | 0.44 | -0.91 | 1.43 | -0.24 |
| Multiple sclerosis | 0.41 | 0.11 | 2.01 | 0.77 | 0.4 | -1.76 | 1.53 | 1.04 | 0.44 | -0.91 | 1.41 | -0.32 |
We identified cases of spinal cord pathology for visualization with tractography using the EPIC Electronic Medical Record system and PACS at our home institution for patients who underwent MRI-Cervical Spine studies with DTI sequences at our institution. Patients whose reports stated no significant cord changes were identified, and five were selected from PACS to be included as controls. Additionally, we selected four pathological cases representing different etiologies for evaluation against the control group. We present a brief discussion of the four pathological cases below.
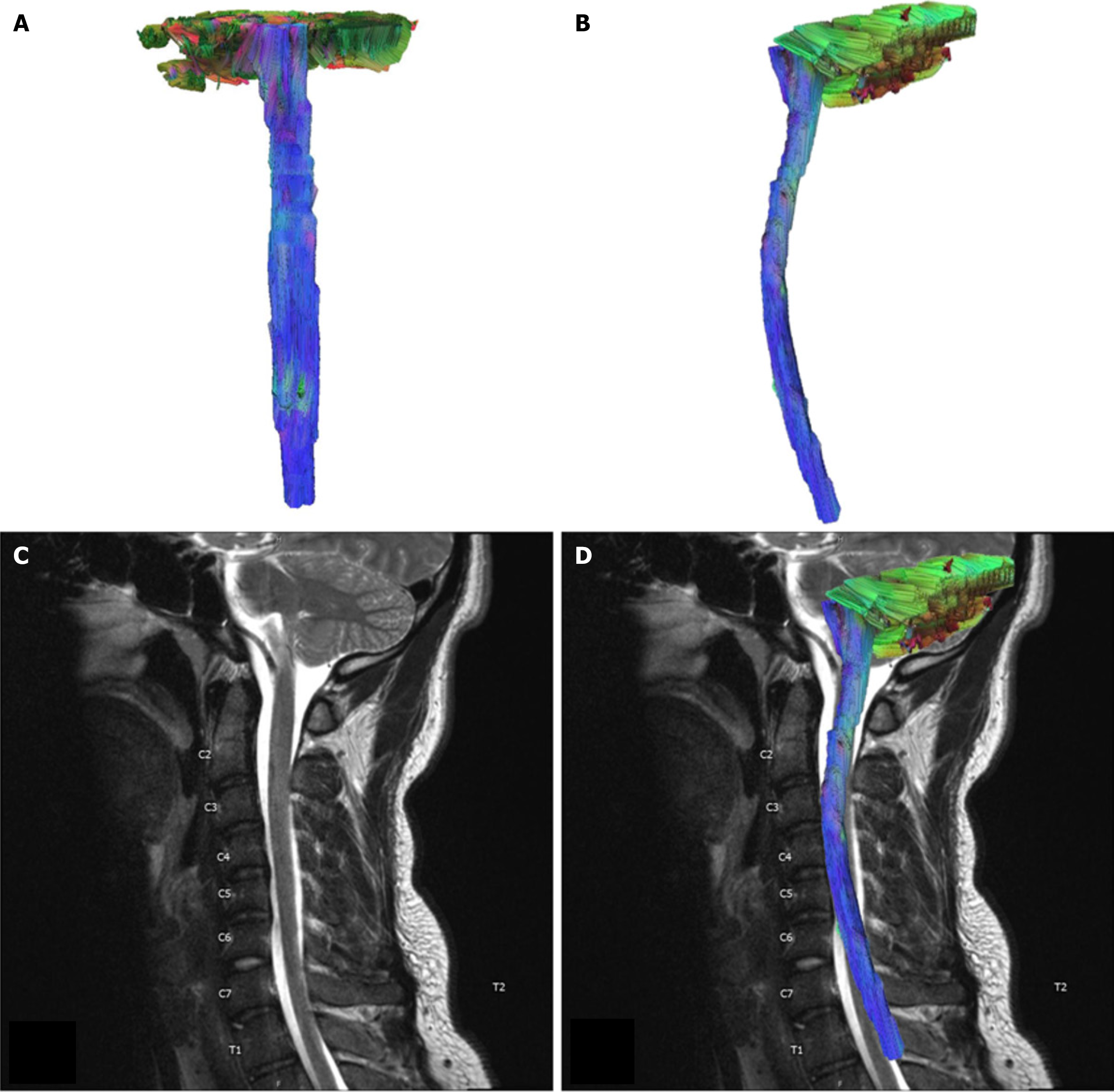
For our control case, we selected imaging from a 22 year-old man undergoing MRI of the cervical spine for trauma, the imaging report showed no cord signal abnormality, neural foraminal narrowing, or spinal cord narrowing. This patient had no significant relevant medical history, such as a history of cancer, traumatic SCI, or neurodegenerative disease (Figure 1).
A case of a 51 year-old male presenting with cervical myelopathy. MRI of the cervical spine without contrast demonstrated disc extrusion at the level of C5-C6 with cord compression and hyper-intense T2 signaling in the spinal cord. The patient reported difficulty walking and balancing and poor motor coordination of his lower extremities. He worked as an automotive mechanic and stated his job performance had worsened because he was repeatedly dropping small objects due to numbness and tingling in his left hand.
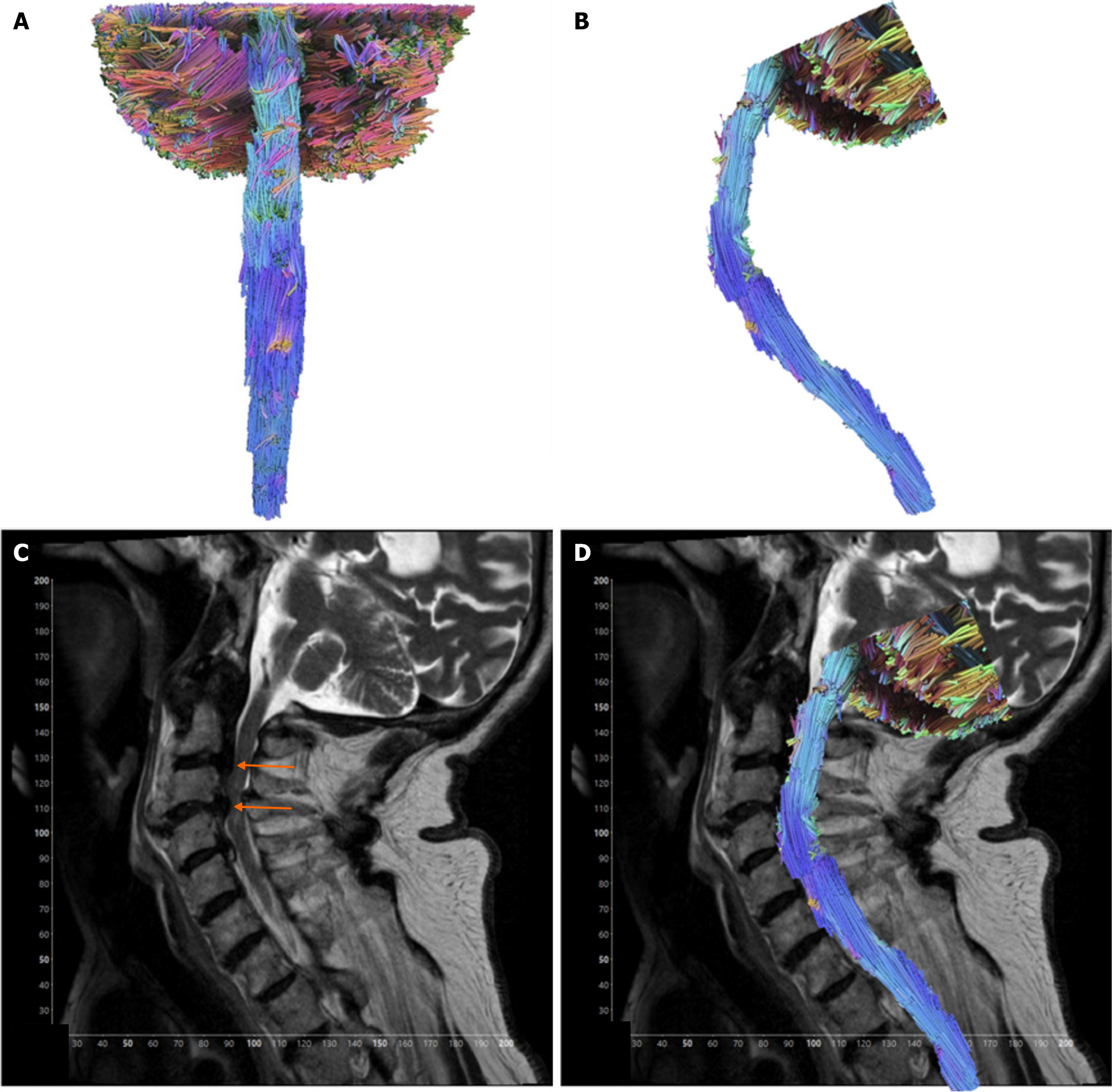
A case of an 82 year-old male presenting with spinal cord contusion. He had a relevant medical history of aortic stenosis, type 2 diabetes mellitus, and carotid stenosis. The patient experienced a traumatic fall after being struck by a vehicle door, causing a temporary loss of consciousness. Subsequent MRI of the cervical spine with contrast illustrated spinal cord acute cord contusion in the setting of severe spinal canal stenosis showing severe cord flattening at C3-C4 and elevated intramedullary signal (Figure 2).
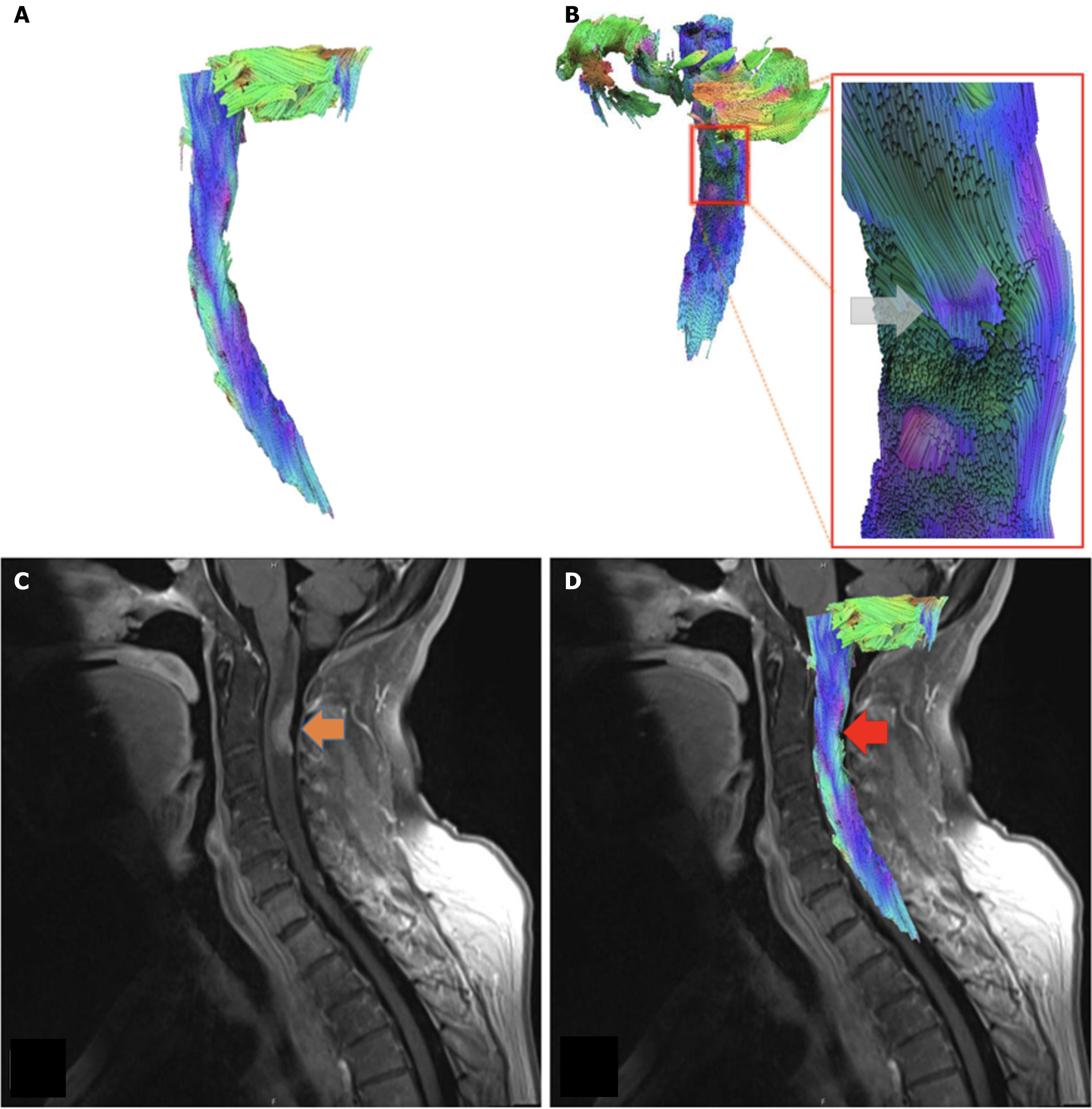
A 62 year-old woman presenting with breast cancer, with metastases to the lungs, spine, and brain. She reported progressive weakness and numbness in her upper and lower extremities. At the time of the imaging, the patient began receiving palliative chemotherapy and radiation therapy. MRI of the cervical spine, with and without contrast, illustrated diffusely abnormal T2 signaling with spinal cord edema and focal abnormal enhancement at the posterior of the cervical spinal cord at the level of C2-C3 where there is a metastatic lesion from breast cancer (Figure 3).
A 35-year-old female who was evaluated for a potential flare-up of MS, with concern for an active demyelinating lesion in the cervical spine. She presented symptoms of numbness and weakness of the lower extremities. We limited our evaluation of this case to obtain measures of white matter integrity, FA, and MD.
We selected the eligible cases based on the imaging report impressions, regardless of the indication for the imaging. Controls the availability of high-quality cervical spine MRI images from our institution’s PACS system (Visage 7). MRI scans from both control and pathology groups were exported and anonymized using the PACS-native anonymization functionality to maintain patient confidentiality. Following anonymization, distortion correction was performed using DSI Studio’s built-in correction tools to mitigate motion artifacts and image distortions inherent to spinal cord imaging. Distortion-corrected DICOM files were subsequently imported into DSI Studio for further processing.
To minimize the inclusion of non-spinal structures, exclusion masks (region of avoidance) were applied. Tracking parameters were customized to optimize visualization and anatomical accuracy, with key parameters set as follows: Tracking index = FA; tracking threshold = variable; minimum fiber length = 10 mm; maximum fiber length = 1000 mm. A comprehensive list of all tracking parameters used can be found in Table 2.
| Tracking parameters | |
| Distortion correction | DSI studio Native Motion Correction tool |
| Software package | DSI studio (version. 2024.12.28 Hou Release) |
| FA threshold | 0.15504-0.23520 |
| Angular threshold | 0-90° |
| Minimum length (mm) | 10 mm |
| Maximum length (mm) | 1000 mm |
| Step size (mm) | 0.1 mm |
| Reconstruction algorithm | Deterministic (Euler) |
| ROA design | One ROA excluding the whole spinal cord |
| Filtering | Topology informed pruning |
Post-processing involved the removal of anatomically implausible fiber tracts and spurious fibers using DSI Studio’s Topology Informed Pruning option[11]. Regions of interest were placed at three distinct spinal levels for each case. In pathology cases, one ROI was centered on the spinal cord lesion, with additional ROIs positioned cranially and caudally relative to the lesion site. In control cases, ROIs were placed at the approximate anatomical levels of C2-C3, C5-C6, and C7-T1. For pathology cases, the C5-C6 ROI was moved as needed to correspond precisely with the lesion location for that subject. Please see Supplementary material containing a detailed technical appendix of tractography post-processing and correction methods.
A quantitative assessment of white matter integrity was performed using FA and MD values from each ROI using DSI Studio. We performed comparative analyses between individual pathology cases and control groups to evaluate differences in spinal cord microstructural integrity. Due to the inherent limitations of the available data: (1) Small overall sample sizes; (2) Differences in clinical etiology of pathological cases; and (3) Lack of standardized FA and MD ranges for different pathologies, only relative differences could be compared between single case measurements and those of the five control values. Therefore, z-scores were calculated for FA and MD values between all controls and single pathology cases across three cervical spinal cord levels: (1) Cranial segment (above the lesion); (2) Central segment (at the level of the lesion); and (3) Cudal (below the lesion). We avoided direct comparisons between pathological cases, or lumping all pathological cases into a group, in order to reduce potential confounding due inter-pathology variability & clinically distinct etiologies. For each lesion, the differences if any, in the z-score of the FA and MD values was quantified for each pathological case relative to the range of the control-derived z-scores, which allowed the team to assess the extent to which spinal cord pathology white matter signal properties varied at level of the lesion as well as across unaffected sections. Lastly, effect sizes (Cohen’s d) could not be calculated for each pathological case comparison, since only one of each case type was available for analysis.
Rendered MR tractography images yielded directional information about the patients' spinal cords and the disruptions in neural fibers resulting from lesions. We visualized the control case of a 22 year-old man undergoing an MRI of the cervical spine for trauma. The imaging report showed no cord signal abnormality, neural foraminal narrowing, or spinal cord narrowing. This patient had no significant relevant medical history, such as a history of cancer, traumatic SCI, or neurodegenerative disease (Figure 1).
Tractography model for the case of the 82-year-old male presenting with spinal cord contusion whose imaging illustrated severe spinal canal stenosis from disc herniation and degenerative spondylosis at the levels of C2-C3 and C3-C4, with severe spinal cord flattening at C3-C4 and reversal of cervical kyphosis. Additionally, there is an elevated intramedullary signal. Tractography model shows a region of fibers in the posterior spinal cord at the lesion level is organized anterior-posteriorly instead of the normal CC (Figure 2). In terms of quantitative measures, the spinal region where the contusion occurred (C3-C4) showed a moderately low FA (0.38, z-score -1.82) (control range 0.48-0.57, control z-score range -0.26-1.14), while MD was within the range of control values (1.33 z-score -0.69) (control range 1.24-1.47, control z-score range -1.68-0.84). Above the contusion, the pathology case’s FA (0.51 z-score 0.93) (control range 0.31-0.57, control z-score range –0.46-1.53) and MD (1.18, z-score -0.83) (control range 1.24-1.47, control z-score range -0.44-1.27) values were within range of the five control values. Below the lesion, the pathological case’s FA value (0.44, z-score -0.91) was slightly lower (control range 0.45-0.53, control z-score range -1.62-1.49), and the MD values (1.43, z-score -0.24) was found to be within the controls’ range (control range 1.15-1.78, control z-score range -1.62-1.49).
Our second case from a 62-year-old woman presenting with breast cancer, with metastases to the lungs, spine, and brain, significant focal abnormal enhancement at the posterior of the cervical spinal cord at the level of C2-C3, due to a metastatic lesion. The tractography model reveals the metastatic lesion left an indentation that is apparent in the posterior-anterior view that can be seen at the level of C2-C3. The fibers anterior to the lesion appear displaced anteriorly, forming a concave groove present at the level of the metastatic lesion in the posterior spinal cord. Fibers in this region curve inwards toward the spinal cord and appear compressed and displaced (Figure 4). FA values at the lesion site (0.27, z-score -1.96) were moderately lower than the control values (control range 0.48-0.57, control z-score range -0.15-0.86), however, MD values were slightly elevated (1.51, z-score 0.90) compared to controls (control range 1.24-1.47, control z-score range -1.91-0.49). Cranial to the lesion, FA values were within normal range (0.35, z-score -0.44) (control range 0.31-0.57, control z-score range -0.88 to -0.22), but MD values were slightly elevated (2.08, z-score 0.88) (control range 0.99-2.01, control z-score range -1.59-0.72). Caudal to the lesion, FA values remained low (0.36, z-score-1.63) (control range 0.45-0.54, control z-score range -0.26-1.12) with MD values (1.54, z-score 0.22) falling within range to the controls (control range 1.15-1.78, control z-score range -1.72-1.40).
The third SCI case was a 51-year-old male presenting with cervical myelopathy. The tractography of this case demonstrates fibers extending from the cerebellum and brainstem to the C7-T1 intervertebral disc. Gross visualization demonstrates spurious fibers throughout the length of the spinal cord. However, the gross structure is visible with an apparent interruption in fiber connections at the level of C5-C6. At this location, the cord extrusion and subsequent compression have narrowed the spinal canal. Fibers crossing this section of the spinal cord area are minimal. Tractography model analysis demonstrated a normal FA (0.57, z-score 1.18) (control range 0.48-0.57, control z-score range -1.36-1.17) and a slightly elevated MD (1.57, z-score 1.25) at the lesion site (control range 1.24-1.47, control z-score range -1.77-0.34). Cranial to the lesion, FA values were slightly elevated (0.72, z-score 1.69) (control range 0.31-0.57, control z-score range -0.89-0.74), and MD (1.2, z-score -0.80) were within normal limits (control range -0.99-2.01, control z-score range -0.45-1.08). Caudal to the lesion, FA values were slightly higher (0.62, z-score 1.65) (control range 0.45-0.54, control z-score range -0.90-0.45), and MD values were within range (1.23, z-score -0.95) (control range 1.15-1.78, control z-score range -1.30-1.48).
The fourth case belongs to a 35-year-old female with a medical history of MS. The patient was admitted to the in
Accurate visualization of the spinal cord is of great interest to the surgical fields for pre-operative planning. Tractography models can demonstrate particularities in the areas of interest where lesions have occurred. Our goal was to determine the feasibility of integrating tractography in clinical scenarios. The imaging necessary for tractography is non-invasive, and acquiring DTI adds negligible increases in scan times. We examined cases with imaging from patients with no reported spinal cord lesions and obtained the key measurements (FA, MD) in three regions for each case. Tractography models were generated using DSI Studio for the pathology and control cases described in the methods.
The ability to characterize nerve fibers with tractography has opened new possibilities for clinical application. After all, the promise of visualizing neural fiber anatomy and function provides additional clinical information that may aid in developing therapeutic plans or surgical planning by assessing severity or improvements on follow-up characterization to assist with evaluating disease progression[2,3]. It has also been routinely used in neurosurgery for surgical purposes. One example is using tractography to determine cranial nerve locations to avoid resecting them during surgery. It has also been used to achieve greater total resection in cases of high-grade gliomas[12]. Despite its inaccuracies, using tractography models for surgical applications remains a viable use case.
In practice, the difficulty in implementing tractography for widespread clinical care stems from the difficulty in obtaining scans optimized for tractography and the following postprocessing. Even under ideal acquisition conditions, tractography is inherently limited by its reliance on modeling algorithms that estimate white matter trajectories. Accurate mapping becomes increasingly unreliable in regions with complex, dense fiber architecture, such as areas with significant crossing, parallel, and branching fibers. What this means for surgical applications will depend on the type of intervention planned. Tumor lesions may be large enough to accurately assess the anatomical location and extent of damage by looking at key measurements like FA and MD values. Still, the risk of false-positive and false-negative fibers being present limits the confidence in tractography models[13,14]. For widespread clinical use, it is essential to consider that imaging protocols vary across institutions. DTI-based acquisitions might not meet the minimum recommended para
This study highlights the utility of tractography in evaluating spinal cord microstructure across various pathologies. By comparing each case to a control subject, we were able to identify patterns of fiber disruption and diffusion. We began with a control case with the case of a 22-year-old male with normal spinal cord imaging and no pathology. The tractography model showed coherent cranio-caudal alignment of fibers, and FA and MD values were within expected ranges, establishing our baseline for healthy subjects[16].
The tractography model from the C3-C4 spinal cord contusion case revealed abnormal fiber orientation in the posterior spinal cord at this level. FA was moderately reduced, consistent with axonal disorganization or injury, while MD re
The second tractography model, involving metastatic breast cancer to the cervical cord at C2-C3 showed anterior displacement and deformation of fibers by the lesion. The lesion FA values decreased, indicating tract disruption, while MD was slightly elevated, suggesting surrounding edema or increased extracellular space from tumor infiltration. This combination reflects a directional disruption (low FA) and mild diffusion facilitation (high MD), a finding consistent with infiltrative masses[19].
The third tractography model involving cervical myelopathy at C5-C6 showed tract disruption on imaging, yet FA was within or above normal, and MD was only mildly elevated. This paradoxical finding likely reflects mechanisms commonly observed in chronic spinal cord compression. In such cases, FA may remain artificially elevated due to selective preservation of organized fiber populations, even as others degenerate[17,18,20]. Slight MD elevation suggests subtle changes without significant tissue loss, additionally, partial volume effects, particularly in regions of cord atrophy or CSF interface, may contribute to misleading scalar values by masking diffusion abnormalities[21]. This case highlights how compression can cause visual tract disruption without clear scalar abnormality, emphasizing the importance of qualitative tractography[19].
The last tractography model, consisting of a MS case at the level of C4-C5, C5 showed that FA values were moderately reduced at the lesion site, while MD was only slightly elevated. This could represent localized demyelination with decreases in (FA), but with limited edema or axonal loss, keeping MD relatively stable. FA was mildly low caudally, which could be explained by the extension of demyelination[22].
This study has several limitations that should be acknowledged. First, our sample size was relatively small, comprising four cases with spinal cord pathology and six control cases. Including a larger set of cases would strengthen the statistical significance of our findings. Another noteworthy limitation involves the diffusion acquisition protocol. The scanners used to obtain diffusion-weighted imaging only included six acquisition directions. The limited number of directions in our protocol may have contributed to variability in key measurements representing white matter integrity, such as FA and MD. Given the lack of reverse-phase encoding sequences for the selected imaging cases, eddy current artifacts could not be corrected.
Although traumatic SCI is a major focus of the field, such cases were excluded from the present study due to practical constraints in the acute setting. Patients with acute traumatic SCI frequently present with hemodynamic instability, with some patients requiring strict immobilization, all of which complicate the implementation of extended or non-standard research imaging protocols[23,24]. As such, we limited inclusion to stable, non-acute cases in which motion constraints and imaging feasibility were manageable. However, we recognize that this is a limitation, and future studies should explore acute trauma in the setting of SCI.
Prior work in literature has emphasized the importance of higher-direction acquisitions; typically, at least 17 directions ensure the accuracy and fidelity in tractography reconstructions[8]. A study by Dauleac et al[8] has demonstrated that high-quality diffusion imaging can be achieved in under 5 minutes through advanced acquisition and reconstruction techniques. However, these protocols often rely on specialized hardware or non-standard pulse sequences, limiting their widespread adoption in routine clinical settings. While higher angular resolution protocols provide richer diffusion information, our approach preserves essential diagnostic metrics such as FA and MD while minimizing scan time and complexity. This positions our protocol as a clinically accessible alternative for spinal cord assessment[25]. Increasing direction acquisitions improves data quality. However, it also significantly increases scan time, which can limit feasibility in acute clinical settings[8]. Note that computed tomography remains the first-line imaging modality for spinal trauma, as outlined in the American College of Radiology appropriateness criteria[9]. Therefore, the current protocol may still be acceptable for certain subacute or chronic spinal cord evaluations. Similar concerns regarding acquisition time and scan times are discussed in the literature[1,4]. Another limitation we recognized was the presence of eddy current artifacts in selected scans; it is likely to have affected tract reconstruction and key measurements. Eddy current artifacts relate inversely to the number of acquisition directions. In the interest of maintaining low scan times, our current protocol did not include reverse phase-encoding sequences, which could have improved distortion correction. We did, however, perform motion correction using DSI Studio’s native motion correction tool. Planned protocol changes will incorporate both increased diffusion directions and reverse phase encoding to enhance image quality while attempting to limit the increase in scan times to maintain clinical practicality.
Fiber resolution methods aim to characterize the orientation and trajectory of white matter tracts by modeling water diffusion patterns within each voxel. These methods differ in how they interpret the diffusion signal and in their ability to resolve complex fiber geometries. DTI models diffusion by capturing only the principal direction of water diffusion, assuming a single dominant fiber direction per voxel. Thus, fiber bundles that cross over or demonstrate sudden changes in direction are challenging to model accurately. Generalized Q-sampling Imaging (GQI) employs a model-free reconstruction method that estimates the spin distribution function from diffusion signals, enabling it to resolve multiple fiber orientations within a single voxel. It can resolve multiple fiber orientations per voxel and provide better reconstructions in regions of complex anatomy. However, this resolution method often yields false-positive fiber bundles[26].
In the current study, we employed deterministic tractography using the Euler algorithm due to its computational efficiency, reproducibility, and ease of clinical interpretation[27]. While deterministic approaches, such as this one, are limited in their ability to model crossing fibers, they are well-suited for routine clinical implementation, especially in settings that require fast turnaround times. Probabilistic methods used with GQI offer clear advantages in resolving complex white matter geometry but generally require longer acquisition times, more advanced post-processing, and specialized expertise[28]. These trade-offs limit their widespread integration into standard clinical workflows.
For future studies we intend to expand the dataset to include a broader spectrum of spinal cord pathologies, including traumatic injuries and progressive neurodegenerative diseases. This will allow us to explore the utility of tractography across a broader clinical spectrum. Several recent studies and systematic reviews in literature support the importance of such methodological advancements for broader clinical applications[2,4,5,9]. Future studies will also benefit from comparing the performance of DTI and GQI in similar datasets.
Planned improvements to our protocol include the implementation of reverse phase-encoding acquisitions and an increase in the number of diffusion directions. Preliminary results using a 15-direction protocol (acquisition time approximately 3.5 minutes) demonstrate improved angular resolution and more robust tract reconstruction without a prohibitive increase in scan duration. Additionally, the incorporation of reverse phase-encoding show improvements in geometric distortion correction, particularly in the lower cervical spine where susceptibility artifacts are most pronounced[29,30].
Diffusion MR tractography offers clinically relevant insights into spinal cord lesions without substantially prolonging scan time. It is essential to understand that tractography models are highly dependent on image acquisition parameters and post-processing. As such, these processes should be performed by trained personnel to ensure the accuracy and reliability of the results. Utility in surgical planning is constrained to procedures that prioritize the preservation of healthy tissue. In clinical scenarios, FA and MD values derived from tractography may correlate with the severity and progression of SCI, offering potential value in the diagnosis and follow-up of patients with spinal cord lesions.
Further research with the goal of refining post-processing protocols is necessary. The inclusion of reverse phase-encoding sequences to address eddy current distortions and the use of a higher number of diffusion directions are expected to address sources of error. Enhancing acquisition fidelity will reduce the need for corrective post-processing and yield models with greater anatomical accuracy. This is essential for tractography to be reliably integrated into the clinical evaluation of spinal cord lesions, particularly in cases where precise anatomical detail is crucial.
| 1. | Nanda G, Jain P, Suman A, Mahajan H. Role of diffusion tensor imaging and tractography in spinal cord injury. J Clin Orthop Trauma. 2022;33:101997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Yamada K, Sakai K, Akazawa K, Yuen S, Nishimura T. MR tractography: a review of its clinical applications. Magn Reson Med Sci. 2009;8:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 223] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 3. | Wu BW, Barr S. Applications of Whole Brain Tractography and Implications for Clinical Practice. Cureus. 2017;9:e1753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Costanzo R, Brunasso L, Paolini F, Benigno UE, Porzio M, Giammalva GR, Gerardi RM, Umana GE, di Bonaventura R, Sturiale CL, Visocchi M, Iacopino DG, Maugeri R. Spinal Tractography as a Potential Prognostic Tool in Spinal Cord Injury: A Systematic Review. World Neurosurg. 2022;164:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Rao JS, Zhao C, Yang ZY, Li SY, Jiang T, Fan YB, Li XG. Diffusion tensor tractography of residual fibers in traumatic spinal cord injury: a pilot study. J Neuroradiol. 2013;40:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Ciccarelli O, Catani M, Johansen-Berg H, Clark C, Thompson A. Diffusion-based tractography in neurological disorders: concepts, applications, and future developments. Lancet Neurol. 2008;7:715-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 283] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 7. | Koskinen E, Brander A, Hakulinen U, Luoto T, Helminen M, Ylinen A, Ohman J. Assessing the state of chronic spinal cord injury using diffusion tensor imaging. J Neurotrauma. 2013;30:1587-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Dauleac C, Frindel C, Mertens P, Jacquesson T, Cotton F. Overcoming challenges of the human spinal cord tractography for routine clinical use: a review. Neuroradiology. 2020;62:1079-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Yeh FC, Irimia A, Bastos DCA, Golby AJ. Tractography methods and findings in brain tumors and traumatic brain injury. Neuroimage. 2021;245:118651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 10. | van Den Hauwe L, Sundgren PC, Flanders AE. Spinal Trauma and Spinal Cord Injury (SCI). 2020 Feb 15. In: Diseases of the Brain, Head and Neck, Spine 2020–2023: Diagnostic Imaging [Internet]. Cham (CH): Springer; 2020. [PubMed] |
| 11. | Yeh FC, Panesar S, Barrios J, Fernandes D, Abhinav K, Meola A, Fernandez-Miranda JC. Automatic Removal of False Connections in Diffusion MRI Tractography Using Topology-Informed Pruning (TIP). Neurotherapeutics. 2019;16:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 158] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 12. | Yang JY, Yeh CH, Poupon C, Calamante F. Diffusion MRI tractography for neurosurgery: the basics, current state, technical reliability and challenges. Phys Med Biol. 2021;66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Irfanoglu MO, Walker L, Sarlls J, Marenco S, Pierpaoli C. Effects of image distortions originating from susceptibility variations and concomitant fields on diffusion MRI tractography results. Neuroimage. 2012;61:275-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 177] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 14. | Thomas C, Ye FQ, Irfanoglu MO, Modi P, Saleem KS, Leopold DA, Pierpaoli C. Anatomical accuracy of brain connections derived from diffusion MRI tractography is inherently limited. Proc Natl Acad Sci U S A. 2014;111:16574-16579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 568] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 15. | Bao J, Tu H, Li Y, Sun J, Hu Z, Zhang F, Li J. Diffusion Tensor Imaging Revealed Microstructural Changes in Normal-Appearing White Matter Regions in Relapsing-Remitting Multiple Sclerosis. Front Neurosci. 2022;16:837452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 16. | Dauleac C, Boukhari A, Jacquesson T, Frindel C, Cotton F. Microstructural Characteristics of Cervical Spinal Cord Using High Angular Resolution Diffusion Imaging (HARDI) and Tractography in Healthy Subjects. Clin Neuroradiol. 2025;35:315-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Ellingson BM, Salamon N, Hardy AJ, Holly LT. Prediction of Neurological Impairment in Cervical Spondylotic Myelopathy using a Combination of Diffusion MRI and Proton MR Spectroscopy. PLoS One. 2015;10:e0139451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative Cervical Myelopathy: Epidemiology, Genetics, and Pathogenesis. Spine (Phila Pa 1976). 2015;40:E675-E693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 689] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 19. | Farquharson S, Tournier JD, Calamante F, Fabinyi G, Schneider-Kolsky M, Jackson GD, Connelly A. White matter fiber tractography: why we need to move beyond DTI. J Neurosurg. 2013;118:1367-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 335] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 20. | Shabani S, Kaushal M, Budde MD, Wang MC, Kurpad SN. Diffusion tensor imaging in cervical spondylotic myelopathy: a review. J Neurosurg Spine. 2020;33:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 21. | Schneider T, Thomas DL, Kachramanoglou C, Ciccarelli O, Alexander DC, Wheeler-Kingshott CA. Fuzzy partial volume correction of spinal cord DTI parameters. 2025. Available from: https://cds.ismrm.org/protected/11MProceedings/PDFfiles/4556.pdf. |
| 22. | Wheeler-Kingshott CA, Stroman PW, Schwab JM, Bacon M, Bosma R, Brooks J, Cadotte DW, Carlstedt T, Ciccarelli O, Cohen-Adad J, Curt A, Evangelou N, Fehlings MG, Filippi M, Kelley BJ, Kollias S, Mackay A, Porro CA, Smith S, Strittmatter SM, Summers P, Thompson AJ, Tracey I. The current state-of-the-art of spinal cord imaging: applications. Neuroimage. 2014;84:1082-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 23. | Chaggar R, Gill R. Evaluating initial screening practices for calcium dysregulation after acute traumatic spinal cord injury: a retrospective review. Spinal Cord Ser Cases. 2024;10:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Korupolu R, Uhlig-Reche H, Achilike EC, Reeh C, Pedroza C, Stampas A. Factors Associated With Ventilator Weaning Success and Failure in People With Spinal Cord Injury in an Acute Inpatient Rehabilitation Setting: A Retrospective Study. Top Spinal Cord Inj Rehabil. 2022;28:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Crombe A, Alberti N, Hiba B, Uettwiller M, Dousset V, Tourdias T. Cervical Spinal Cord DTI Is Improved by Reduced FOV with Specific Balance between the Number of Diffusion Gradient Directions and Averages. AJNR Am J Neuroradiol. 2016;37:2163-2170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Jin Z, Bao Y, Wang Y, Li Z, Zheng X, Long S, Wang Y. Differences between generalized Q-sampling imaging and diffusion tensor imaging in visualization of crossing neural fibers in the brain. Surg Radiol Anat. 2019;41:1019-1028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Petersen MV, Lund TE, Sunde N, Frandsen J, Rosendal F, Juul N, Østergaard K. Probabilistic versus deterministic tractography for delineation of the cortico-subthalamic hyperdirect pathway in patients with Parkinson disease selected for deep brain stimulation. J Neurosurg. 2017;126:1657-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Dauleac C, Frindel C, Pélissou-Guyotat I, Nicolas C, Yeh FC, Fernandez-Miranda J, Cotton F, Jacquesson T. Full cervical cord tractography: A new method for clinical use. Front Neuroanat. 2022;16:993464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 29. | Schilling KG, Combes AJE, Ramadass K, Rheault F, Sweeney G, Prock L, Sriram S, Cohen-Adad J, Gore JC, Landman BA, Smith SA, O'Grady KP. Influence of preprocessing, distortion correction and cardiac triggering on the quality of diffusion MR images of spinal cord. Magn Reson Imaging. 2024;108:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Mohammadi S, Nagy Z, Hutton C, Josephs O, Weiskopf N. Correction of vibration artifacts in DTI using phase-encoding reversal (COVIPER). Magn Reson Med. 2012;68:882-889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/