Published online Sep 28, 2025. doi: 10.4329/wjr.v17.i9.110447
Revised: August 2, 2025
Accepted: August 27, 2025
Published online: September 28, 2025
Processing time: 112 Days and 6.7 Hours
The application of artificial intelligence (AI) in carotid atherosclerotic plaque detection via computed tomography angiography (CTA) has significantly ad
Core Tip: This is the first mini-review to comprehensively analyze artificial intelligence (AI)-driven advancements in carotid computed tomography angiography plaque detection over ten years. We provide novel insights into hybrid deep learning architectures, domain adaptation techniques, and their clinical translation. The work establishes quantitative benchmarks for diagnostic performance and highlights future directions for explainable AI systems in vascular imaging.
- Citation: Wang DY, Yang T, Zhang CT, Zhan PC, Miao ZX, Li BL, Yang H. Artificial intelligence in carotid computed tomography angiography plaque detection: Decade of progress and future perspectives. World J Radiol 2025; 17(9): 110447
- URL: https://www.wjgnet.com/1949-8470/full/v17/i9/110447.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i9.110447
Carotid atherosclerosis is a major contributor to ischemic stroke, a leading cause of global morbidity and mortality. In 2021, ischemic stroke accounted for approximately 3.59 million deaths worldwide, and the lifetime risk of any stroke approaches 1 in 4 adults globally, underscoring its enormous public-health impact[1,2]. Carotid artery disease contributes roughly 10%–20% of ischemic strokes, with degree of stenosis and plaque vulnerability driving ipsilateral risk[3,4]. Non-invasive imaging techniques such as computed tomography angiography (CTA) have played a crucial role in visualizing carotid plaques and assessing vascular risk, and are recommended in contemporary stroke work-ups to define vascular etiology[5]. However, traditional plaque evaluation relies heavily on radiologist expertise, which is subject to variability and limited by time-intensive interpretation.
In recent years, artificial intelligence (AI)-particularly deep learning (DL)-has emerged as a transformative tool in medical imaging, offering automated segmentation, feature extraction, and risk stratification. A 2024 systematic review and meta-analysis (11 studies, 1484 patients) reported pooled areas under the receiver operating characteristic curve of 0.96 (95%CI: 0.94–0.97), sensitivity of 0.90 and specificity of 0.93 for AI-assisted CTA in atherosclerotic plaque assessment, with strong performance for detecting ≥ 70% stenosis[6]. On carotid CTA specifically, interpretable/clinical machine learning (ML) and radiomics models have distinguished symptomatic from asymptomatic plaques and associated radiomic signatures with ipsilateral ischemic events[7,8]. A recent two-step clinical validation showed a carotid-CTA deep-learning model achieving patient-level sensitivity approximately 92% and diagnosis time approximately 6 seconds, outperforming several radiologists and improving readers’ sensitivity in a simulated clinical setting[9]. Methodological advances also include dual-energy CTA virtual monoenergetic image radiomics to detect symptomatic plaques, sug
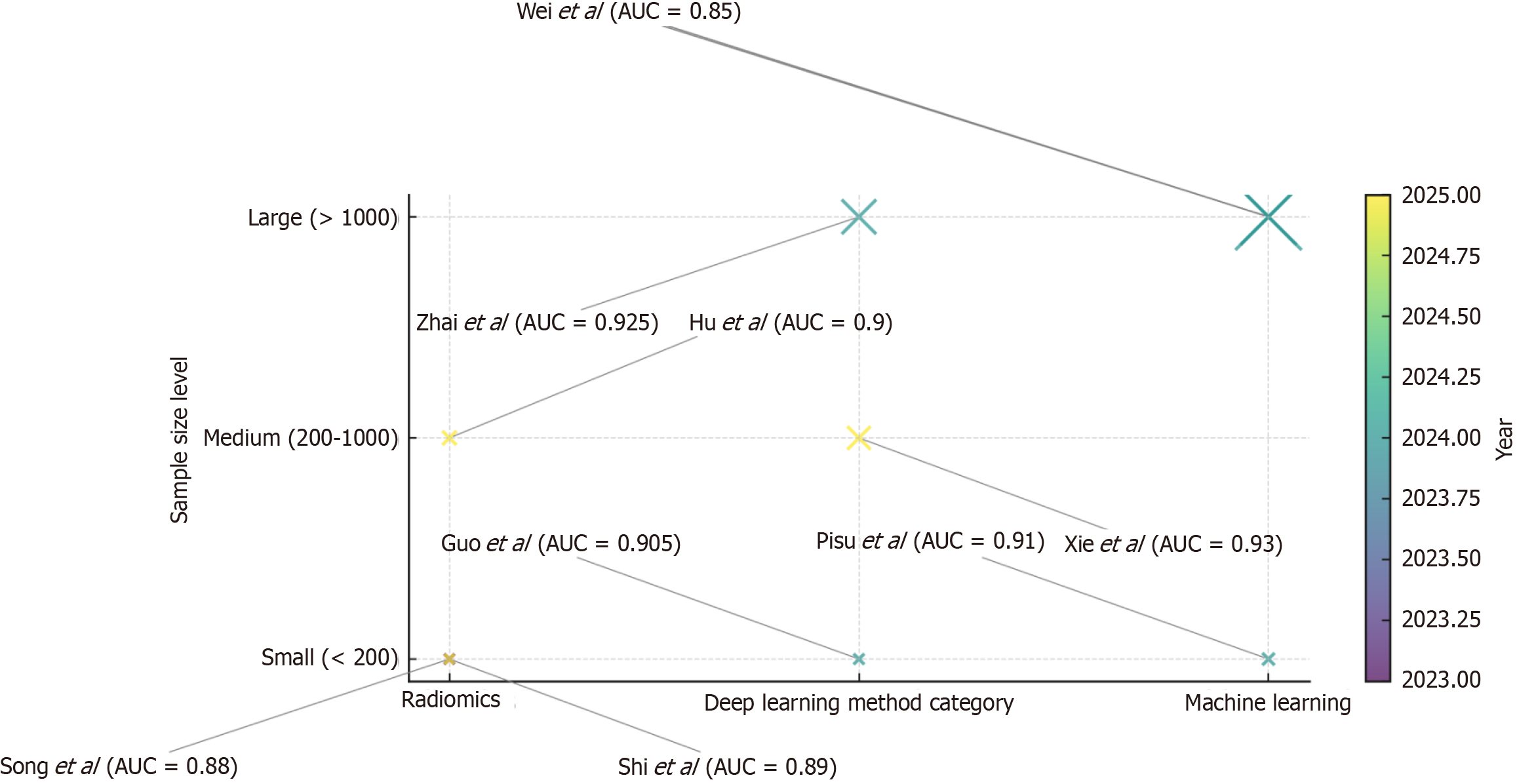
Over the past decade, AI applications in carotid CTA have progressed from preliminary segmentation tasks to sophisticated systems capable of identifying high-risk plaque features, predicting clinical outcomes, and enhancing diagnostic consistency across diverse imaging datasets. A synthesis of representative studies is summarized in Table 1[6-14], which outlines sample sizes, AI model types, data sources, diagnostic performance, and clinical relevance. Furthermore, the bubble matrix in Figure 1 visualizes these studies by method category, sample size, and publication year, highlighting the transition from small single-center radiomics studies to large-scale multicenter DL applications[7-14]. Together, these data illustrate the methodological and clinical trajectory of AI-based carotid CTA plaque detection over the last decade.
| Ref. | Sample size | Model type | Dataset source | Results | Clinical impact |
| Jie et al[6] | 3245 (meta-analysis, 17 studies) | Mixed AI models | Multicenter/multi-country | Sensitivity of 0.91, specificity of 0.88, AUC of 0.94 | Improved CTA plaque detection accuracy |
| Pisu et al[7] | 156 | ML | Single-center CTA | AUC of 0.91, sensitivity of 87%, specificity of 85% | Early identification of high-risk symptomatic plaques |
| Shi et al[8] | 112 | Radiomics + logistic regression | Single-center CTA | AUC of 0.89 | Differentiates plaque stability, optimizes treatment planning |
| Zhai et al[9] | 1234 | Convolutional neural network (fully automatic detection) | Multicenter CTA | Sensitivity of 0.93, specificity of 0.92 | Rapid automated plaque screening |
| Hu et al[10] | 205 | Radiomics + dual-energy CTA | Multicenter CTA | AUC of 0.90, accuracy of 88% | Improved symptomatic plaque recognition |
| Guo et al[11] | 120 | Two-stage deep learning | Single-center CTA | Sensitivity of 0.92, specificity of 0.89 | Good performance in early-stage validation |
| Xie et al[12] | 560 | Swin-UNet + multi-scale supervision | Multicenter CTA | Dice coefficients of 0.93 | High-precision segmentation for quantitative analysis |
| Song et al[13] | 98 | AI segmentation + ultrasound radiomics | Single-center ultrasound | AUC of 0.88 | Ultrasound-based method applicable to CTA risk assessment |
| Wei et al[14] | 4562 | ML (random forest, etc.) | Single-center health check-up | AUC of 0.85 | Early population risk prediction tool |
This mini-review aims to systematically summarize and evaluate the evolution, current state, and future prospects of AI in carotid CTA plaque detection. We focus on technical innovations, clinical applicability, and challenges-notably data heterogeneity, model robustness/quality, interpretability, and regulatory/translation issues highlighted by recent quality assessments of carotid-plaque radiomics studies-and emphasize AI’s role as a catalyst for precision cerebrovascular medicine[15].
AI in carotid CTA has undergone significant evolution over the past decade, transitioning from basic ML models to advanced DL architectures capable of precise plaque detection and classification. Early implementations were limited in scope and interpretability, but recent advances in computational power, algorithmic design, and annotated datasets have enabled the development of more robust and clinically relevant tools.
One notable advancement is the integration of hybrid DL networks. For example, a recent study proposed a model combining residual U-Net (ResUNet) with the Pyramid Scene Parsing Network (PSPNet) to enhance plaque detection in CTA images[11]. The model was trained on data from 647 patients with carotid atherosclerosis who underwent CTA between October 2020 and October 2022. It demonstrated strong diagnostic performance, achieving a precision of 80.49% on the validation set and 78.37% on the test set. Subgroup analysis showed reliable detection across diverse anatomical locations and plaque morphologies, with recall values ranging from 76.32% to 89.25% (by location) and 79.17% to 86.03% (by morphology). In a comparative clinical evaluation, the model outperformed 4 out of 6 experienced radiologists in diagnostic accuracy (P < 0.001) and significantly reduced interpretation time (6 seconds vs several minutes, P < 0.001), underscoring its potential for real-time clinical deployment.
Another key area of algorithmic progression lies in domain adaptation, which addresses the generalizability of AI models across datasets acquired from heterogeneous imaging systems. The Lesion Assessment through Tracklet Evaluation (LATTE) framework exemplifies this approach. Based on convolutional neural networks (CNNs) and domain-adaptive learning strategies, LATTE was developed to assess carotid plaque burden on three-dimensional magnetic resonance (MR) vessel wall images[16]. Trained on data from 279 patients and evaluated across four external datasets comprising 271 patients from eight institutions with varying imaging parameters, the model achieved area under the curves (AUCs) exceeding 0.88 for advanced lesion classification and 0.83 for all lesion types-an improvement over non-adapted model. These findings highlight the clinical importance of domain-adaptive algorithms in managing data variability and ensuring consistent diagnostic performance across institutions.
The refinement of carotid plaque detection models remains a central research focus, with the goal of achieving high diagnostic accuracy, automation, and clinical interpretability. A variety of models have been proposed, ranging from segmentation-based approaches to integrated end-to-end systems.
The aforementioned ResUNet–PSPNet hybrid model exemplifies a segmentation-focused architecture designed to delineate atherosclerotic plaques in CTA. Trained on a cohort of 647 patients, the model achieved a precision of 80.49%, sensitivity of 90.70%, and recall of 84.62% on the validation set; corresponding metrics on the test set were 78.37%, 91.86%, and 84.58%, respectively. Performance remained consistent across diverse plaque morphologies and anatomical regions[11].
Beyond segmentation, end-to-end frameworks that combine image analysis and classification are gaining traction. One such approach integrated a U-Net–based semantic segmentation model with Efficient-Net as the backbone to extract intima-media masks, followed by a Bayesian-optimized CNN for plaque quantification and classification[17]. Evaluated on the REGICOR database, comprising over 8000 carotid ultrasound images, this framework achieved correlation coefficients of 0.89 and 0.74 in the common carotid artery and bulb regions, respectively. The corresponding F1 scores for plaque detection were 0.60 and 0.59, reflecting competitive performance in real-world vascular imaging scenarios.
Additionally, models tailored to detect specific high-risk plaque characteristics have been explored. For instance, a study investigated abnormal plaque motion on carotid sonography as a marker of vulnerable plaques[18]. Among 49 patients (50 carotid arteries) undergoing endarterectomy, sonographic ulceration yielded a sensitivity of 48% and specificity of 90%, whereas fine trembling motion inside plaques achieved higher sensitivity (93%) but lower specificity (60%). Notably, plaques exhibiting systolic retractive motion of the plaque surface were significantly more likely to harbor both necrotic cores and intraplaque hemorrhage (80% vs 30%, P = 0.0005), suggesting that dynamic plaque motion on ultrasound may serve as a non-invasive biomarker of instability.
The AI with carotid CTA has led to substantial advancements in the diagnostic evaluation of carotid artery disease. By enabling automated image interpretation and advanced quantitative analysis, AI enhances both the precision and efficiency of vascular imaging workflows.
One prominent application is the automated segmentation of carotid arteries from CTA datasets. A notable example is the Multi-Flux Swin-Deepsup-U-Net (MFSD-UNet) model, which was specifically developed for three-dimensional carotid artery segmentation[12]. Utilizing a dataset of 214 patient CTA scans, the model incorporated multi-scale deep supervision and a multi-flux fusion architecture. It achieved an average Dice similarity coefficient of 0.9119 and an accuracy of 0.9819, outperforming two state-of-the-art benchmark models (with Dice coefficients of 0.8770 and 0.8910, respectively). Ablation experiments confirmed the critical role of the Swin Transformer and deep supervision com
Beyond anatomical segmentation, AI has also demonstrated notable utility in enhancing plaque characterization and cardiovascular risk assessment from CTA imaging. For instance, in the context of coronary artery calcium (CAC) evaluation, an AI-enabled framework (AI-CAC) was developed to automatically quantify cardiac chamber volumes and calcified plaque burden[19]. In a large-scale, multi-ethnic cohort study involving 5830 asymptomatic individuals with longitudinal follow-up over 15 years, AI-CAC consistently outperformed the traditional Agatston scoring method. The AUC for AI-CAC vs Agatston score at 1-year, 5-year, 10-year, and 15-year follow-up intervals was 0.784 vs 0.701, 0.771 vs 0.709, 0.789 vs 0.712, and 0.816 vs 0.729, respectively (all P < 0.0001). Importantly, AI-CAC–derived plaque features-such as plaque count, distribution, density, and the number of affected vessels-significantly improved the prediction of coronary heart disease (CHD) in patients with intermediate CAC scores (1–100), highlighting its added value in risk stratification beyond conventional metrics.
He et al[19] employed optical coherence tomography (OCT) to quantitatively analyze carotid plaques obtained from 31 ex vivo samples, aiming to assess plaque vulnerability. Using a random forest classifier, the study extracted both texture and pixel-level features to effectively differentiate among fibrous tissue, calcified regions, and lipid cores. The classification achieved accuracies of 80%, 62%, and 83.1% for the three components, respectively, with corresponding sensitivity and specificity pairs of (80.5%, 91.2%), (64.7%, 90.7%), and (87.5%, 80.2%), demonstrating high reliability in tissue characterization.
In the context of AI-assisted analysis, Acharya et al[20] investigated carotid CTA images from 20 patients by extracting local binary pattern and wavelet features, followed by classification using a support vector machine (SVM) model. Their method achieved an overall classification accuracy of 88% in distinguishing symptomatic from asymptomatic individuals. Additionally, they employed the Atherosclerosis Index developed by AtheroPoint Inc. (California, United States) to further quantify plaque morphology, providing a novel metric for risk stratification.
Focusing on the impact of imaging range on stroke prediction, Arora et al[21] found that extending the scan coverage 20 mm proximally and distally from the carotid bifurcation yielded optimal differentiation between stroke and non-stroke cases. Furthermore, they utilized an automated classification algorithm to analyze plaque components in CTA images from 136 patients, conducting both univariate and multivariate analyses to explore the clinical significance of different plaque compositions.
Collectively, these findings illustrate that AI-powered CTA analysis not only enhances anatomical delineation and diagnostic accuracy but also enables more nuanced and individualized risk assessment. As algorithmic sophistication and data integration capabilities continue to improve, AI is poised to play a central role in the next generation of precision cerebrovascular and cardiovascular imaging.
AI recent advancements in AI have significantly enhanced the diagnostic potential of carotid CTA, particularly in the domains of vascular segmentation and plaque characterization. These AI-driven techniques offer not only improved diagnostic accuracy but also streamlined image analysis workflows, addressing longstanding challenges in the as
A prominent innovation in this area is the application of DL models to carotid artery segmentation. Among these, the MFSD-UNet model represents a significant leap forward in automated three-dimensional segmentation of carotid vasculature[12]. Incorporating multi-scale deep supervision and a multi-flux fusion architecture, the model was evaluated on a dataset of 214 CTA scans and demonstrated superior performance, achieving a mean Dice similarity coefficient of 0.9119 and an overall accuracy of 0.9819. These results outperformed several state-of-the-art models used as benchmarks. Furthermore, ablation studies confirmed the critical contribution of the Swin Transformer and deep supervision components, as their exclusion led to marked declines in segmentation performance (Dice scores reduced to 0.8630 and 0.8371, respectively), highlighting the necessity of advanced architectural components for optimal accuracy.
Beyond anatomical segmentation, AI has also been instrumental in enhancing plaque detection and cardiovascular risk stratification using CTA imaging. For example, in a study focused on CAC, an AI-based framework-referred to as AI-CAC-was employed for the automated quantification of cardiac chamber volumes and the characterization of calcified plaques from CTA scans[22]. This system was tested in a longitudinal, multi-ethnic cohort of 5830 asymptomatic individuals followed over a 15-year period. The AI-CAC model consistently demonstrated higher predictive performance compared to the conventional Agatston score, with AUCs at 1-year, 5-year, 10-year, and 15-year follow-ups recorded at 0.784 vs 0.701, 0.771 vs 0.709, 0.789 vs 0.712, and 0.816 vs 0.729, respectively (all P < 0.0001). Importantly, AI-derived plaque characteristics-including plaque count, spatial distribution, tissue density, and number of affected vessels-significantly improved the prediction of CHD within the intermediate-risk group (CAC score 1–100), indicating the added value of AI in refining risk models.
Collectively, these diagnostic advances demonstrate that AI-enhanced carotid CTA analysis not only offers robust tools for anatomical delineation but also introduces more sophisticated approaches for early detection and prognostic evaluation of atherosclerotic disease. As AI models become increasingly adept at integrating complex imaging features with clinical outcomes, their role in guiding individualized cerebrovascular and cardiovascular care is poised to expand substantially.
The accuracy and reliability of AI–driven approaches in detecting carotid atherosclerotic plaques have garnered considerable attention in the past 3 years, with numerous studies yielding encouraging results.
A representative example involves the development of a DL model that integrates ResUNet with the PSPNet for plaque identification based on carotid CTA images[11]. Utilizing a dataset comprising 647 patients with confirmed carotid atherosclerotic plaques, the model achieved robust diagnostic performance. In the validation cohort, the model yielded a precision of 80.49%, a sensitivity of 90.70%, and a recall of 84.62%. Comparable results were observed in the test set, with precision at 78.37%, sensitivity at 91.86%, and recall at 84.58%. Subgroup analyses further demonstrated consistent accuracy across diverse anatomical locations (recall ranging from 76.32% to 89.25%) and plaque morphologies (recall ranging from 79.17% to 86.03%). In a simulated clinical deployment scenario, the model outperformed four out of six experienced radiologists in diagnostic accuracy (P < 0.001) and exhibited a significantly shorter diagnostic time for plaque detection (6 seconds vs considerably longer by radiologists, P < 0.001), underscoring its potential to augment clinical workflows.
In the domain of carotid ultrasound imaging, AI models have likewise demonstrated substantial promise. A comparative study involving four DL architectures-including YOLOv7 and Faster Region-Based Convolutional Neural Network (Faster R-CNN)-was conducted using 5611 ultrasound images from 3683 patients[23]. Among the evaluated models, Faster R-CNN with ResNet-50 backbone achieved the most favorable classification performance, reporting an accuracy of 0.88, sensitivity of 0.94, specificity of 0.71, and an area under the AUC of 0.91. These findings indicate that AI-based methods not only exhibit high diagnostic precision but, in some instances, may approach or even surpass the performance of intermediate-level physicians in plaque detection tasks.
Taken together, these studies suggest that AI technologies-whether applied to CTA or ultrasound imaging-possess significant diagnostic utility for carotid plaque detection. As these models continue to evolve and undergo clinical validation, they hold the potential to enhance diagnostic consistency, reduce interpretation time, and improve early identification of high-risk atherosclerotic lesions in both routine and high-throughput clinical environments.
Comparative evaluations between AI–based models and conventional diagnostic approaches in carotid plaque detection have been instrumental in assessing the clinical utility and added value of AI-assisted diagnostics.
Traditional imaging modalities, including carotid ultrasound and CTA, have long been the cornerstone of plaque assessment. However, these techniques are not without limitations. Carotid ultrasound, for instance, is inherently operator-dependent, and diagnostic outcomes may vary significantly due to inter-observer variability and subjective interpretation. In contrast, AI-based systems offer the potential to deliver standardized, reproducible, and objective assessments by minimizing human bias and enhancing diagnostic consistency.
Evidence supporting the comparative effectiveness of AI is increasingly emerging from diverse clinical domains. For example, a study evaluating an AI model for detecting periapical periodontitis from radiographic images reported promising diagnostic metrics, including an accuracy of 89.6%, sensitivity of 86.5%, and specificity of 88.1%[24]. Among two experienced radiologists assessed in parallel, one achieved superior performance (accuracy of 98.5%, sensitivity of 93.8%, specificity of 96.7%), whereas the other performed slightly below the AI model (accuracy of 81.7%, sensitivity of 83.3%, specificity of 80.0%). Notably, the AI model achieved the highest mean confidence level (86.5% ± 9.18), under
In the specific context of carotid plaque detection, AI models are capable of rapidly processing large volumes of imaging data and identifying subtle radiographic features that may elude human observers-such as early morphological changes or microcalcifications. Nevertheless, traditional diagnostic methods retain irreplaceable advantages, including the integrative clinical reasoning and contextual judgment of experienced radiologists. Rather than viewing AI and traditional methods as mutually exclusive, their integration may yield the most effective diagnostic strategy. AI systems can function as preliminary screening tools, offering quantitative assessments and highlighting regions of interest, while radiologists provide nuanced interpretation based on the patient's comprehensive clinical profile.
This collaborative paradigm-merging algorithmic precision with clinical acumen-has the potential to significantly improve the diagnostic accuracy, efficiency, and overall quality of carotid atherosclerosis evaluation. As AI technologies continue to mature and integrate into clinical practice, their role in complementing rather than replacing traditional expertise will likely define the next generation of precision cerebrovascular imaging.
AI–driven personalized treatment strategies are rapidly emerging as a transformative approach in the management of carotid atherosclerotic plaques. By harnessing the computational power of AI to analyze complex and large-scale biomedical data, these strategies enable the customization of therapeutic interventions tailored to individual patient profiles.
In the realm of drug discovery and development, AI plays an increasingly pivotal role, particularly in virtual screening and drug repurposing. Through the integration and analysis of high-throughput genomic, proteomic, metabolomic, and clinical datasets, AI algorithms can identify novel therapeutic targets and predict drug efficacy on a patient-specific basis. For example, in managing comorbid conditions associated with carotid plaque, such as systemic atherosclerosis, AI-based predictive models have been utilized to detect candidate drug targets by correlating molecular signatures with disease phenotypes[25]. This approach not only streamlines the drug development pipeline but also reduces the time and cost traditionally required for clinical trials by identifying promising therapeutic agents with a higher probability of efficacy.
Beyond pharmacologic discovery, AI also facilitates the refinement of individualized therapeutic regimens. Recent developments, such as the Immuno-Net framework-a DL–based system originally designed for autoimmune disease management-illustrate the potential of AI to synthesize genetic, molecular, and clinical variables for precision diagnosis and treatment planning[26]. Translating similar methodologies into the domain of carotid plaque care, AI algorithms can process multifactorial data inputs-ranging from plaque morphology (e.g., size, calcification, lipid core content) and hemodynamic factors to patient-specific variables such as age, sex, genetic predisposition, and comorbid conditions (e.g., hypertension, diabetes, hyperlipidemia). Based on these analyses, AI can generate predictive models to forecast patient response to different interventions, including lifestyle modification, pharmacotherapy (e.g., statins, antiplatelets), or revascularization procedures such as carotid endarterectomy and stenting.
The clinical value of such AI-guided precision medicine lies in its potential to optimize treatment efficacy while minimizing adverse outcomes. By identifying patients most likely to benefit from aggressive intervention and those who may respond well to conservative management, healthcare providers can implement more nuanced, cost-effective, and patient-centric therapeutic strategies. Moreover, the integration of continuous learning from real-world data can further refine AI models, enabling iterative improvement of clinical decision-making over time.
AI plays an increasingly pivotal role in the dynamic monitoring of carotid plaque progression, offering the potential for earlier detection, personalized risk stratification, and timely clinical intervention.
A primary avenue through which AI contributes to plaque monitoring is the quantitative analysis of imaging data. ML and DL algorithms have demonstrated considerable promise in evaluating serial changes in carotid intima-media thickness (cIMT) and plaque area-both of which are established surrogate markers of atherosclerotic burden. A recent review on joint assessment of cIMT and plaque area via carotid ultrasonography for cardiovascular and stroke risk prediction emphasized the utility of AI-enabled image analysis tools in this domain[27]. Automated algorithms can extract and quantify cIMT and plaque features with high precision and reproducibility, thus facilitating longitudinal assessment of plaque progression or regression. These measurements offer clinicians objective indicators to evaluate therapeutic efficacy and modify treatment strategies accordingly.
Beyond morphological analysis, AI algorithms are also being leveraged for predictive modeling of plaque evolution. In preclinical research, for instance, ML techniques have been applied to identify key determinants of plaque progression in murine models. One such study employed SVM and decision tree (DT) classifiers to analyze multifactorial data in apolipoprotein E-deficient (apoE-/-) mice, a widely used model of atherosclerosis[28]. The study revealed that the dia
The integration of AI into plaque monitoring paradigms not only enhances diagnostic precision but also streamlines clinical workflows. By automating repetitive and time-consuming image interpretation tasks, AI systems can alleviate clinician burden, reduce interobserver variability, and ensure more consistent follow-up assessments. Furthermore, as these models continue to learn from expanding datasets, their predictive accuracy and clinical utility are expected to improve over time.
Recent innovations in carotid plaque intervention techniques are aimed at enhancing the precision, safety, and efficacy of therapeutic strategies for carotid artery disease. One notable advancement is the application of ultrafast ultrasound imaging to assess plaque vulnerability. This technique enables the extraction of novel hemodynamic and mechanical parameters, including plaque stiffness heterogeneity, wall shear stress (WSS), and intraplaque microflows, which are critical for evaluating plaque stability[29]. In a representative case study, elastography was combined with WSS quantification and ultrafast Doppler (UFD) imaging to assess the biomechanical profile of a carotid plaque. Elastographic analysis identified regions of increased stiffness associated with calcification and a softer area suggestive of intraplaque hemorrhage. UFD imaging revealed elevated WSS along the plaque surface and detected microvascular flows indicative of plaque rupture-findings later confirmed via histopathological analysis. These results underscore the clinical utility of ultrafast ultrasound as a non-invasive, real-time modality for identifying high-risk plaques and stratifying patients who may benefit from carotid endarterectomy or intensified pharmacologic intervention.
Another frontier in plaque intervention is the development of imaging-derived biomarkers to detect and characterize plaque vulnerability. Non-invasive modalities-such as computed tomography (CT), MR imaging (MRI), and carotid duplex ultrasonography-alongside intravascular techniques, including intravascular ultrasound and OCT, are being actively utilized to visualize plaque morphology and composition[30]. Among the most clinically significant features is intraplaque hemorrhage, a well-established marker of plaque instability. These imaging biomarkers allow for refined risk stratification and can inform individualized treatment decisions, ultimately contributing to the prevention of ischemic strokes and other atherosclerotic cardiovascular events.
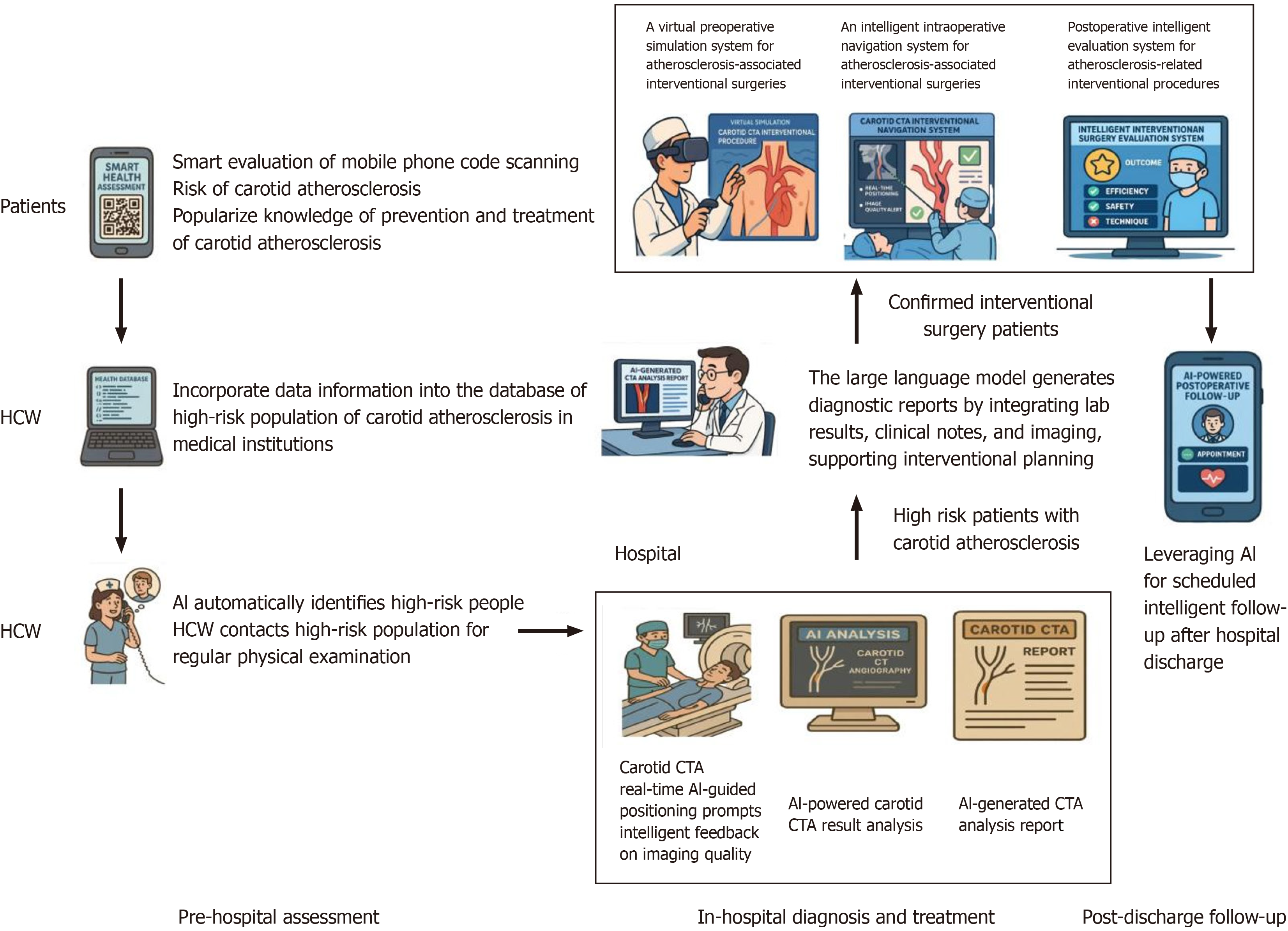
Collectively, these technological advances are reshaping the landscape of carotid intervention by enabling earlier detection of high-risk lesions and guiding more precise, evidence-based clinical management (Figure 2).
As AI becomes increasingly integrated into carotid plaque detection and clinical decision-making, ethical considerations surrounding its use have garnered critical attention. Ensuring the responsible and equitable deployment of AI technologies in healthcare demands rigorous attention to issues such as data privacy, algorithmic transparency, and informed consent.
A primary ethical concern is the protection of patient data privacy. AI algorithms depend on the aggregation and analysis of extensive datasets, including imaging data, electronic health records, and, in some cases, genomic information. Safeguarding this sensitive information is paramount. Studies involving AI-based diagnostic tools must implement stringent data governance frameworks to ensure anonymization, secure encryption, and restricted usage in accordance with the stated research or clinical objectives. For instance, in investigations of AI-driven prediction models for Alzheimer’s disease, patient privacy was highlighted as a foundational ethical requirement[31]. Analogously, in the context of carotid plaque detection, it is imperative that all patient data be handled with the highest level of confidentiality to mitigate risks of unauthorized access, data breaches, or secondary misuse.
Another pressing ethical issue is algorithmic transparency. Given that AI models used in plaque detection may directly influence diagnostic conclusions and treatment pathways, the underlying decision-making processes must be interpretable to clinicians and, where appropriate, to patients. The so-called “black-box” nature of some DL models can pose challenges to clinical trust, particularly when AI-generated outputs diverge from traditional diagnostic interpretations. For example, if an AI system recommends a more aggressive intervention based on image analysis, it is essential that the rationale behind such recommendations is explainable and justifiable. Enhancing model interpretability not only fosters greater accountability but also strengthens the clinician–patient–technology relationship by promoting informed decision-making.
The integration of AI into carotid plaque diagnostics holds transformative potential, but its ethical deployment must be guided by principles of privacy protection, transparency, equity, and trustworthiness. Proactive governance, robust validation, and multidisciplinary collaboration are essential to ensure that AI technologies serve as trustworthy tools that enhance rather than compromise the quality of care.
The integration of AI into carotid CTA for plaque detection presents a number of pressing regulatory challenges that must be resolved to facilitate its safe, effective, and widespread clinical application.
One of the foremost barriers is the absence of clearly defined and universally accepted regulatory frameworks. The pace of AI technological advancement has significantly outstripped the development of corresponding legal and regulatory structures. In the United States, for instance, the Food and Drug Administration (FDA) is still in the process of establishing robust guidelines for the approval, oversight, and post-market surveillance of AI-based medical devices[32]. This regulatory ambiguity poses substantial difficulties for developers and clinicians alike. Specifically, uncertainty persists regarding the appropriate classification of AI-powered carotid CTA analysis tools-whether they should fall under low-risk software as a medical device or be treated as high-risk diagnostic systems. This distinction has direct implications for the evidentiary standards, clinical trial design, and data quality requirements necessary for regulatory approval, thereby impacting the speed and trajectory of product development and market entry.
A second major challenge concerns the validation and verification of AI algorithms. Regulatory authorities universally demand that medical tools demonstrate consistent accuracy, reliability, and safety. However, the inherently complex, non-deterministic nature of many AI algorithms-particularly those based on DL-makes conventional validation processes more difficult. In the context of carotid plaque detection, algorithms must be rigorously tested across diverse populations, varying imaging protocols, and different clinical environments to ensure generalizability and prevent algorithmic bias. Furthermore, AI models are often designed to improve iteratively through updates and retraining, necessitating a continuous oversight mechanism. Regulators must determine how to evaluate and approve post-deployment algorithm modifications without compromising safety, a paradigm shift from traditional “locked” software to “adaptive” learning systems.
In light of these challenges, there is a growing consensus on the need for adaptive regulatory frameworks that balance innovation with patient safety. Initiatives such as the FDA’s proposed “Predetermined Change Control Plan” and the European Union’s AI Act represent preliminary steps in this direction. However, ongoing international collaboration and stakeholder engagement will be essential to develop a regulatory infrastructure that supports transparent, accountable, and evidence-based AI integration in clinical practice.
Bias and variability in AI algorithms present critical challenges that may compromise the accuracy, generalizability, and clinical reliability of carotid plaque detection. Addressing these issues is essential to ensure the safe and equitable deployment of AI in diagnostic applications.
Data bias is one of the most pervasive and consequential limitations in current AI development. As ML models are inherently dependent on the quality and representativeness of their training datasets, the use of homogenous or unbalanced data can result in systematic biases. For instance, a review of AI-based skin disease diagnostic tools revealed that many training datasets lacked adequate representation of diverse ethnic groups and were deficient in metadata regarding patient race or skin type[33]. In the context of carotid plaque detection, an algorithm trained predominantly on data from a specific demographic subgroup-such as elderly male patients or individuals from a single ethnic background-may exhibit reduced diagnostic accuracy when applied to underrepresented populations. To mitigate such bias, it is imperative to construct inclusive, demographically diverse, and well-annotated datasets. This includes proactive efforts to recruit data from varied age groups, sexes, ethnicities, and comorbidity profiles, as well as employing stratified sampling and augmentation techniques to maintain class balance.
Variability in algorithm performance constitutes another significant barrier to reproducibility and clinical translation. Divergences in algorithm design, data preprocessing techniques, annotation standards, and training strategies across research groups can lead to inconsistent performance metrics and limited comparability between models. A scoping review of AI applications during the coronavirus disease 2019 pandemic highlighted this issue, noting wide discrepancies in algorithm efficacy attributable to heterogeneous data pipelines and non-standardized training procedures[34]. Within carotid plaque detection, this problem is exacerbated by differences in imaging protocols, plaque labeling criteria, and the use of proprietary data.
To address this challenge, there is a pressing need to establish standardized frameworks for AI algorithm development and evaluation. This includes the adoption of benchmark datasets, harmonized performance metrics (e.g., AUC, Dice coefficient, sensitivity/specificity), and reproducible training-validation-testing protocols. Additionally, transparent reporting following guidelines such as CONSORT-AI and TRIPOD-AI should be encouraged to facilitate external validation and regulatory review.
AI has shown significant promise in carotid plaque detection, yet its application in routine ultrasound imaging faces persistent challenges. A fundamental limitation lies in the operator-dependent nature of carotid ultrasound. Unlike CT or MRI, ultrasound image quality is highly variable, influenced by probe angle, patient anatomy, and technician expertise. This variability introduces inconsistencies that compromise the robustness and reproducibility of AI algorithms trained on curated datasets. In real-world settings, AI models frequently underperform when applied to low-quality or non-standardized ultrasound images collected in diverse clinical environments[23]. Moreover, the absence of universally accepted protocols for plaque annotation-including definitions of plaque echogenicity, ulceration, and motion artifacts-further limits model generalization and impedes external validation efforts[13].
Another challenge is the limited availability of large-scale, high-quality annotated ultrasound datasets. Manual labeling of plaque features is time-consuming and requires expert consensus, leading to bottlenecks in training data preparation. Although recent efforts have applied transfer learning or self-supervised learning to mitigate data scarcity[35], these methods still rely on a base of reasonably consistent inputs, which remains difficult to achieve in ultrasound practice. In addition, real-time AI implementation at the point of care remains immature. Most AI systems operate offline and are not yet optimized for deployment on portable ultrasound devices, where hardware constraints limit computational performance. Attempts to integrate lightweight AI models into handheld systems are ongoing, but latency, limited feedback, and model interpretability remain major obstacles to clinical adoption[36].
Clinical trust and regulatory readiness also present substantial barriers. Many DL models, especially CNNs, function as “black boxes” with limited interpretability. Inconsistencies between AI-generated assessments and radiologist interpretations raise concerns regarding accountability, particularly in high-stakes diagnostic decisions[37]. To address this, explainable AI frameworks are being developed to provide visual or textual rationale for predictions, yet their use in ultrasound remains rare and under-validated. Furthermore, studies show that AI models trained in one population often fail to perform reliably across others, especially when applied to underrepresented demographic groups, contributing to algorithmic bias[33]. These issues highlight the need for diverse training data, standardized reporting guidelines, and collaborative model refinement involving clinicians, engineers, and regulatory stakeholders.
Recent advancements in AI technologies are poised to significantly transform carotid imaging, introducing novel approaches that promise enhanced precision, efficiency, and diagnostic insight in plaque detection.
A prominent innovation lies in DL–based image reconstruction techniques, which have demonstrated the ability to markedly improve image quality in CTA. By reducing image noise and suppressing artifacts, these algorithms enable clearer visualization of anatomical structures and plaque characteristics. For example, deep CNNs have been successfully employed in cardiovascular imaging to reconstruct high-fidelity images from low-dose or motion-degraded scans, thereby facilitating more reliable clinical interpretation[38]. When applied to carotid CTA, such technologies can enhance the delineation of plaque morphology, size, and distribution. Moreover, their capacity to learn intricate spatial patterns from large-scale datasets holds potential for detecting subtle imaging biomarkers of plaque vulnerability that may elude conventional image processing methods.
Another emerging frontier is the integration of AI with multimodal imaging for comprehensive carotid plaque assessment. The combination of multiple imaging modalities-such as ultrasonography, CTA, and MRI-can provide complementary information on plaque dynamics, structure, and composition. AI algorithms, particularly those based on data fusion and ensemble learning, can effectively integrate heterogeneous data streams to construct a unified, high-resolution representation of the carotid artery. For instance, real-time hemodynamic data from Doppler ultrasound can be synchronized with structural information from CTA and compositional insights from MRI, allowing for a more nuanced evaluation of plaque vulnerability. Such multimodal AI-assisted imaging frameworks have the potential to enhance diagnostic accuracy, facilitate risk stratification, and guide personalized therapeutic strategies for patients with carotid atherosclerotic disease.
The convergence of DL–driven image enhancement and AI-enabled multimodal data integration represents a promising direction for the next generation of carotid imaging. These technologies may not only augment the diagnostic capabilities of clinicians but also pave the way for earlier detection and intervention in cerebrovascular disease.
AI exhibits substantial potential in predictive modeling for carotid plaque, offering new avenues for early risk stratification, personalized treatment planning, and proactive disease management.
ML algorithms are capable of synthesizing data from diverse sources-such as patient demographics, clinical histories, imaging parameters, and genomic profiles-to model the risk of plaque development, progression, and vulnerability. In one study, predictive models were constructed using routine health metrics and blood biomarkers to assess the likelihood of carotid plaque formation[14]. Among the seven ML algorithms evaluated, the Light Gradient Boosting Machine demonstrated the highest discriminative performance, achieving an area under the AUC of 85.4%. The top five predictors identified were age, systolic blood pressure, gender, low-density lipoprotein cholesterol, and total cholesterol, un
Beyond risk prediction, AI algorithms also offer promise in forecasting therapeutic response. By learning from retrospective datasets involving patients treated with pharmacologic or interventional therapies, AI models can estimate the likely trajectory of plaque morphology and stability under different clinical regimens. For instance, an AI-based model employing ultrasound image segmentation and radiomics-derived features was developed to predict plaque stability[13]. The model achieved robust performance, with AUC of 89.42% in the training cohort and 82.73% in the validation cohort, highlighting its utility in guiding individualized treatment decisions. Such predictive frameworks can assist clinicians in selecting the most appropriate therapeutic strategies based on a patient’s unique plaque characteristics and comorbidity profile, ultimately improving clinical outcomes and minimizing the risk of adverse cardiovascular events.
The long-term integration of AI into carotid plaque management strategies is poised to significantly reshape the landscape of diagnosis, treatment, and disease monitoring, ultimately advancing the paradigm of precision vascular medicine.
In the realm of diagnosis, AI-powered tools are anticipated to enhance both accuracy and efficiency by reducing reliance on time-consuming, operator-dependent processes inherent to conventional imaging interpretation. AI-augmented CTA has shown promise in providing rapid, reproducible, and objective assessments of plaque morphology and vulnerability, thereby facilitating earlier and more precise identification of high-risk lesions. For instance, recent studies have demonstrated that AI systems trained on large-scale ultrasound datasets can effectively recognize features of vulnerable atherosclerotic plaques-such as intraplaque hemorrhage and lipid-rich necrotic core-surpassing the diagnostic performance of traditional ultrasound techniques[35]. This evolution in diagnostic capability has the potential to streamline clinical workflows and improve detection rates of clinically significant carotid lesions.
In the domain of treatment, AI-driven personalized therapeutic strategies are expected to gain prominence. By synthesizing multimodal data-including genetic profiles, clinical histories, and imaging phenotypes-AI models can inform individualized treatment recommendations, optimizing the selection among pharmacologic, lifestyle, and surgical interventions. This level of personalization not only enhances treatment efficacy and minimizes adverse events but also holds the potential to improve cost-efficiency in healthcare delivery. Furthermore, in terms of disease monitoring, AI-enabled systems can facilitate longitudinal surveillance of plaque evolution through continuous, automated analysis of serial imaging studies. Such real-time monitoring allows for timely adjustments in therapeutic regimens based on dynamic changes in plaque characteristics, thereby supporting proactive and adaptive disease management.
The application of AI in carotid CTA plaque detection has evolved significantly, enhancing diagnostic accuracy, processing efficiency, and individualized risk assessment. AI models now rival or surpass experienced radiologists in plaque identification and classification, while also showing great promise in disease prediction, treatment guidance, and longitudinal monitoring. Despite ongoing challenges related to ethics, bias, and regulatory oversight, continued algorithmic refinement and clinical validation position AI as a transformative force in the future of precision ce
| 1. | Hou S, Zhang Y, Xia Y, Liu Y, Deng X, Wang W, Wang Y, Wang C, Wang G. Global, regional, and national epidemiology of ischemic stroke from 1990 to 2021. Eur J Neurol. 2024;31:e16481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 37] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 2. | Feigin VL, Brainin M, Norrving B, Martins SO, Pandian J, Lindsay P, F Grupper M, Rautalin I. World Stroke Organization: Global Stroke Fact Sheet 2025. Int J Stroke. 2025;20:132-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 351] [Article Influence: 351.0] [Reference Citation Analysis (0)] |
| 3. | Fandler-Höfler S, Stauber RE, Kneihsl M, Wünsch G, Haidegger M, Poltrum B, Pichler A, Deutschmann H, Enzinger C, Fickert P, Gattringer T. Non-invasive markers of liver fibrosis and outcome in large vessel occlusion stroke. Ther Adv Neurol Disord. 2021;14:17562864211037239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Miceli G, Basso MG, Pintus C, Pennacchio AR, Cocciola E, Cuffaro M, Profita M, Rizzo G, Tuttolomondo A. Molecular Pathways of Vulnerable Carotid Plaques at Risk of Ischemic Stroke: A Narrative Review. Int J Mol Sci. 2024;25:4351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 5. | Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, Kamel H, Kernan WN, Kittner SJ, Leira EC, Lennon O, Meschia JF, Nguyen TN, Pollak PM, Santangeli P, Sharrief AZ, Smith SC Jr, Turan TN, Williams LS. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2021;52:e364-e467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 1981] [Article Influence: 396.2] [Reference Citation Analysis (0)] |
| 6. | Jie P, Fan M, Zhang H, Wang O, Lv J, Liu Y, Zhang C, Liu Y, Zhao J. Diagnostic value of artificial intelligence-assisted CTA for the assessment of atherosclerosis plaque: a systematic review and meta-analysis. Front Cardiovasc Med. 2024;11:1398963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 7. | Pisu F, Williamson BJ, Nardi V, Paraskevas KI, Puig J, Vagal A, de Rubeis G, Porcu M, Cau R, Benson JC, Balestrieri A, Lanzino G, Suri JS, Mahammedi A, Saba L. Machine Learning Detects Symptomatic Plaques in Patients With Carotid Atherosclerosis on CT Angiography. Circ Cardiovasc Imaging. 2024;17:e016274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 8. | Shi J, Sun Y, Hou J, Li X, Fan J, Zhang L, Zhang R, You H, Wang Z, Zhang A, Zhang J, Jin Q, Zhao L, Yang B. Radiomics Signatures of Carotid Plaque on Computed Tomography Angiography : An Approach to Identify Symptomatic Plaques. Clin Neuroradiol. 2023;33:931-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 9. | Zhai D, Liu R, Liu Y, Yin H, Tang W, Yang J, Liu K, Fan G, Ju S, Cai W. Deep learning-based fully automatic screening of carotid artery plaques in computed tomography angiography: a multicenter study. Clin Radiol. 2024;79:e994-e1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Hu W, Lin G, Chen W, Wu J, Zhao T, Xu L, Qian X, Shen L, Yan Z, Chen M, Xia S, Lu C, Yang J, Xu M, Chen W, Ji J. Radiomics based on dual-energy CT virtual monoenergetic images to identify symptomatic carotid plaques: a multicenter study. Sci Rep. 2025;15:10415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Guo Z, Liu Y, Xu J, Huang C, Zhang F, Miao C, Zhang Y, Li M, Shan H, Gu Y. A deep learning model for carotid plaques detection based on CTA images: a two stepwise early-stage clinical validation study. Front Neurol. 2024;15:1480792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Xie H, Gu H, Li M, Zhu L, Wang T, Li Z, Wu H. Carotid artery segmentation in computed tomography angiography (CTA) using multi-scale deep supervision with Swin-UNet and advanced data augmentation. Quant Imaging Med Surg. 2025;15:3161-3175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Song J, Zou L, Li Y, Wang X, Qiu J, Gong K. Combining artificial intelligence assisted image segmentation and ultrasound based radiomics for the prediction of carotid plaque stability. BMC Med Imaging. 2025;25:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Wei Y, Tao J, Geng Y, Ning Y, Li W, Bi B. Application of machine learning algorithms in predicting carotid artery plaques using routine health assessments. Front Cardiovasc Med. 2024;11:1454642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 15. | Hou C, Li S, Zheng S, Liu LP, Nie F, Zhang W, He W. Quality assessment of radiomics models in carotid plaque: a systematic review. Quant Imaging Med Surg. 2024;14:1141-1154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 16. | Chen L, Zhao H, Jiang H, Balu N, Geleri DB, Chu B, Watase H, Zhao X, Li R, Xu J, Hatsukami TS, Xu D, Hwang JN, Yuan C. Domain adaptive and fully automated carotid artery atherosclerotic lesion detection using an artificial intelligence approach (LATTE) on 3D MRI. Magn Reson Med. 2021;86:1662-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Gago L, Vila MDM, Grau M, Remeseiro B, Igual L. An end-to-end framework for intima media measurement and atherosclerotic plaque detection in the carotid artery. Comput Methods Programs Biomed. 2022;223:106954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Muraki M, Mikami T, Yoshimoto T, Fujimoto S, Kitaguchi M, Kaga S, Sugawara T, Tokuda K, Kaneko S, Kashiwaba T. Sonographic Detection of Abnormal Plaque Motion of the Carotid Artery: Its Usefulness in Diagnosing High-Risk Lesions Ranging from Plaque Rupture to Ulcer Formation. Ultrasound Med Biol. 2016;42:358-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | He C, Li Z, Wang J, Huang Y, Yin Y, Li Z. Atherosclerotic Plaque Tissue Characterization: An OCT-Based Machine Learning Algorithm With ex vivo Validation. Front Bioeng Biotechnol. 2020;8:749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Acharya UR, Sree SV, Mookiah MR, Saba L, Gao H, Mallarini G, Suri JS. Computed tomography carotid wall plaque characterization using a combination of discrete wavelet transform and texture features: A pilot study. Proc Inst Mech Eng H. 2013;227:643-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Arora S, Chien JD, Cheng SC, Chun KA, Wintermark M. Optimal carotid artery coverage for carotid plaque CT-imaging in predicting ischemic stroke. J Neuroradiol. 2010;37:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Naghavi M, Reeves AP, Atlas K, Zhang C, Atlas T, Henschke CI, Yankelevitz DF, Budoff MJ, Li D, Roy SK, Nasir K, Molloi S, Fayad Z, McConnell MV, Kakadiaris I, Maron DJ, Narula J, Williams K, Shah PK, Levy D, Wong ND. Artificial intelligence applied to coronary artery calcium scans (AI-CAC) significantly improves cardiovascular events prediction. NPJ Digit Med. 2024;7:309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 23. | Zhang H, Zhao F. Deep Learning-Based Carotid Plaque Ultrasound Image Detection and Classification Study. Rev Cardiovasc Med. 2024;25:454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Nagareddy B, Vadlamani R, Venkannagari NR, Jain S, Basheer SN, Murugesan S. Comparison of the Artificial Intelligence Versus Traditional Radiographic Interpretation in Detecting Periapical Periodontitis: A Diagnostic Accuracy Study. J Pharm Bioallied Sci. 2024;16:S3676-S3678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 25. | Kale M, Wankhede N, Pawar R, Ballal S, Kumawat R, Goswami M, Khalid M, Taksande B, Upaganlawar A, Umekar M, Kopalli SR, Koppula S. AI-driven innovations in Alzheimer's disease: Integrating early diagnosis, personalized treatment, and prognostic modelling. Ageing Res Rev. 2024;101:102497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 64] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 26. | Ullah R, Sarwar N, Alatawi MN, Alsadhan AA, Salamah Alwageed H, Khan M, Ali A. Advancing personalized diagnosis and treatment using deep learning architecture. Front Med (Lausanne). 2025;12:1545528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 27. | Biswas M, Saba L, Omerzu T, Johri AM, Khanna NN, Viskovic K, Mavrogeni S, Laird JR, Pareek G, Miner M, Balestrieri A, Sfikakis PP, Protogerou A, Misra DP, Agarwal V, Kitas GD, Kolluri R, Sharma A, Viswanathan V, Ruzsa Z, Nicolaides A, Suri JS. A Review on Joint Carotid Intima-Media Thickness and Plaque Area Measurement in Ultrasound for Cardiovascular/Stroke Risk Monitoring: Artificial Intelligence Framework. J Digit Imaging. 2021;34:581-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 28. | Li B, Jiao Y, Fu C, Xie B, Ma G, Teng G, Yao Y. Contralateral artery enlargement predicts carotid plaque progression based on machine learning algorithm models in apoE(-/-) mice. Biomed Eng Online. 2016;15:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Goudot G, Khider L, Pedreira O, Poree J, Julia P, Alsac JM, Amemiya K, Bruneval P, Messas E, Pernot M, Mirault T. Innovative Multiparametric Characterization of Carotid Plaque Vulnerability by Ultrasound. Front Physiol. 2020;11:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Fernández-Alvarez V, Linares-Sánchez M, Suárez C, López F, Guntinas-Lichius O, Mäkitie AA, Bradley PJ, Ferlito A. Novel Imaging-Based Biomarkers for Identifying Carotid Plaque Vulnerability. Biomolecules. 2023;13:1236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 31. | Ursin F, Timmermann C, Steger F. Ethical Implications of Alzheimer's Disease Prediction in Asymptomatic Individuals through Artificial Intelligence. Diagnostics (Basel). 2021;11:440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Harvey HB, Gowda V. Regulatory Issues and Challenges to Artificial Intelligence Adoption. Radiol Clin North Am. 2021;59:1075-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Daneshjou R, Smith MP, Sun MD, Rotemberg V, Zou J. Lack of Transparency and Potential Bias in Artificial Intelligence Data Sets and Algorithms: A Scoping Review. JAMA Dermatol. 2021;157:1362-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 179] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 34. | Delgado J, de Manuel A, Parra I, Moyano C, Rueda J, Guersenzvaig A, Ausin T, Cruz M, Casacuberta D, Puyol A. Bias in algorithms of AI systems developed for COVID-19: A scoping review. J Bioeth Inq. 2022;19:407-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 35. | Wang DD, Lin S, Lyu GR. Advances in the Application of Artificial Intelligence in the Ultrasound Diagnosis of Vulnerable Carotid Atherosclerotic Plaque. Ultrasound Med Biol. 2025;51:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 36. | Ouyang D, He B, Ghorbani A, Yuan N, Ebinger J, Langlotz CP, Heidenreich PA, Harrington RA, Liang DH, Ashley EA, Zou JY. Video-based AI for beat-to-beat assessment of cardiac function. Nature. 2020;580:252-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 509] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 37. | Masoumi N, Rivaz H, Hacihaliloglu I, Ahmad MO, Reinertsen I, Xiao Y. The Big Bang of Deep Learning in Ultrasound-Guided Surgery: A Review. IEEE Trans Ultrason Ferroelectr Freq Control. 2023;70:909-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 38. | Fortuni F, Ciliberti G, De Chiara B, Conte E, Franchin L, Musella F, Vitale E, Piroli F, Cangemi S, Cornara S, Magnesa M, Spinelli A, Geraci G, Nardi F, Gabrielli D, Colivicchi F, Grimaldi M, Oliva F. Advancements and applications of artificial intelligence in cardiovascular imaging: a comprehensive review. Eur Heart J Imaging Methods Pract. 2024;2:qyae136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/