Published online May 28, 2025. doi: 10.4329/wjr.v17.i5.106102
Revised: April 20, 2025
Accepted: May 8, 2025
Published online: May 28, 2025
Processing time: 99 Days and 17 Hours
Obstructed defecation syndrome (ODS) is a subtype of constipation that is consi
To study the distribution of causes of ODS in patients with chronic constipation by magnetic resonance defecography (MRD).
This observational study evaluated the causes of ODS in 57 patients with chronic constipation who presented to Bangabandhu Sheikh Mujib Medical University between July 2020 and June 2021. After obtaining institutional review board approval and informed consent, patients underwent history taking, physical exams, and relevant investigations. ODS was diagnosed using Rome III criteria, with colonoscopy ruling out organic causes. Standard MRD was performed in different phases, and images were analyzed by expert radiologists and reported in a stan
Pelvic floor descent and anorectal junction descent were the most frequent findings, each present in 94.7% of cases. Rectocele was observed in 78.9% of patients, while vaginal or uterine prolapse was seen in 59.4% of females. Less common abnormalities included paradoxical contraction (7%), and there were no cases of sigmoidocele. Functional measurements showed significant differences in pelvic floor dynamics between rest and defecation, particularly in the H-line, M-line, and descent of pelvic organs (P < 0.05).
Pelvic floor descent and anorectal descent were the most common findings in patients suffering from ODS, fo
Core Tip: Obstructed defecation syndrome (ODS), is a common and complex digestive condition with multiple causes. Patients with ODS are usually characterized by constipation and a frequent urge to defecate but difficulty in passing stool. This study aimed to identify the types, causes, and severity of ODS in constipated patients using magnetic resonance defecography. The predominant findings were descent of the pelvic floor and anorectal region, with ODS being more common in females, particularly those < 30 years-old. It is recommended that young female patients be prioritized in ODS assessments, and that health policies be developed to improve management of ODS.
- Citation: Or-Rashid MH, Sultana A, Khanduker N, Ony TA, Hossain MM, Rahman J, Chowdhury MZ, Ahmed WU, Uddin MN, Uzzaman MS. Magnetic resonance defecography assessment of obstructed defecation syndrome in patients with chronic constipation in a tertiary care hospital. World J Radiol 2025; 17(5): 106102
- URL: https://www.wjgnet.com/1949-8470/full/v17/i5/106102.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i5.106102
Chronic constipation is a common gastrointestinal disorder which significantly impacts many people worldwide, leading to a reduced the quality of life. Obstructed defecation (OD) causes a range of frustrating symptoms in most patients with severe constipation. OD syndrome (ODS) is a complex and multifaceted condition characterized by its distinct presenta
Normal defecation is a coordinated process involving several pelvic floor muscles, including the puborectalis muscle, the external anal sphincter, as well as both somatic and autonomic reflexes[2].
Normal evacuation takes approximately 30 seconds and involves the pelvic floor descending, the anal canal opening, and the anorectal angle (ARA) widening. Usually, the alteration in ARA between rest and defecation phase is about 15-20 degrees. When there is infrequent defecation, difficulty during stool passage, and frequent sensation of incomplete rectal evacuation, the condition is referred to as constipation[3]. Patients with ODS typically present with complaints of diffi
Constipation caused by OD is classified into two types: (1) Functional; and (2) Mechanical. Functional causes include idiopathic megarectum, anismus (pelvic floor dyssynergy), and descending perineal syndrome; mechanical causes include rectocele (herniation of the rectum into the vagina), enterocele, intussusception (internal rectal prolapse), and overt rectal prolapse[4-6]. It is important to note that although functional and mechanical variants of ODS are described, they are actually part of the same condition, with functional ODS usually caused by pelvic floor dysfunction and mechanical ODS caused by structural problems. The worsening of the functional form can cause structural changes in pelvic floor dynamics, resulting in a blockage of fecal passage, thereby worsening ODS[1,4,6].
The predisposing factors of ODS are multifactorial, involving elements such as hysterectomy, low estrogen, advanced age, high body mass index, and excessive straining during defecation. Vaginal childbirth and pelvic surgery can damage pelvic innervation and soft tissues, leading to endopelvic fascial and pelvic support defects, while progressive nerve damage and chronic intra-abdominal pressure from obesity or chronic cough may predispose individuals to symptomatic defects[7-9].
ODS diagnosis often involves a combination of physiological tests, including the balloon expulsion test, anorectal manometry, electromyography, and evacuation proctography. Imaging techniques such as transperineal and endoanal ultrasonography are useful for detecting pelvic floor abnormalities, with magnetic resonance (MR) defecography (MRD) considered the gold standard[1-4]. MRD evaluates pelvic floor anatomy, function, and abnormalities, aiding in the precise diagnosis and treatment planning of pelvic floor disorders. For females, it investigates the pelvic floor in three compar
Treatment for ODS varies depending on the severity and underlying causes of the condition. Conservative approaches, such as biofeedback therapy, are effective in many patients, helping retrain the pelvic floor muscles for coordinated defecation. However, for those with significant structural defects or when conservative methods fail, surgery may be necessary[10,11]. Surgical options include procedures to repair pelvic floor damage, such as rectopexy for rectal prolapse or surgical correction of rectocele[10]. Since MRD allows physicians to evaluate all three pelvic compartments in females, it aids in the decision of whether surgery or conservative treatments are needed. It also facilitates precise preoperative planning, ensuring that patients receive tailored care according to their specific condition and needs[10-13].
Currently, there are limited data on the causes of ODS in Bangladesh. This study detected the types, etiology and extent of ODS among patients with constipation by using MRD, which provides detailed anatomical and functional evaluation of the pelvic floor, including identifying abnormalities, aiding in diagnosis and treatment planning.
This observational study was conducted in the Department of Colorectal Surgery at Bangabandhu Sheikh Mujib Medical University (BSMMU) (Dhaka, Bangladesh) from July 2020 to June 2021, with data collection taking place between November 2020 and March 2021. Ethical clearance was obtained from the institutional review board of BSMMU.
A total of 57 patients presenting with ODS in the colorectal outpatient department were included following purposive sampling. Informed written consent was obtained from each patient before enrollment into the study.
Patients eligible for inclusion in the study were those diagnosed with ODS according to the Rome III criteria, irrespective of sex, and aged 18 years or older. The Rome III criteria for constipation require that symptoms be present for the last 3 months, with onset at least 6 months before diagnosis. To meet the criteria, a patient must experience at least two of the following symptoms in at least 25% of defecations: (1) Straining; (2) Lumpy or hard stools; (3) A sensation of incomplete evacuation; (4) A feeling of anorectal blockage; (5) The need for manual maneuvers to facilitate defecation; and (6) Fewer than three bowel movements per week[4]. ODS symptoms must not fulfill the criteria for irritable bowel syndrome, and loose stools should rarely occur without the use of laxatives. Patients were excluded if they presented with any of the following: (1) Pregnancy or lactation; (2) Had a pacemaker, metallic implant, prosthesis, or any metallic foreign body; (3) Had colonoscopy findings such as neoplasms or polyps; and (4) Claustrophobia. Patients who refused to provide consent for participation were also excluded. All patients had undergone colonoscopy prior to MRD, to rule out the excluding factor of other colonic pathologies like colon neoplasms and polyps.
MRD was performed on a closed tunnel configuration MR imaging (MRI) system using a standardized protocol. Pre-procedural colon preparation was not required. The procedure was explained in detail for optimal patient cooperation. A moderately filled bladder and use of a disposable diaper was recommended. Following a detailed explanation of the procedure and obtainment of informed consent, up to 120 mL ultrasound jelly was instilled rectally, and 30 mL was administered vaginally to females.
The patients were placed in the supine position on the MR machine gantry, and static imaging was performed including high-resolution axial T1-weighted images, and high-resolution axial, coronal, and sagittal T2-weighted images (T2WI) at rest for anatomical evaluation. Subsequently, dynamic imaging was performed in the midsagittal plane through the anal canal using a T2-weighted (T2W) sequence. This sequence was run for almost 2 minutes, while the patient was asked to perform the following maneuvers: (1) Short transient downward straining effort followed by immediate relaxation; (2) Sustained downward straining to pass the ultrasound jelly (defecate) with maximum straining efforts; and (3) Post defecation resting stage. The dynamic scan was repeated if necessary. The study was considered to be of non-diagnostic quality if the patient was unable to effectively strain in the supine position, and those patients were excluded from the study.
Imaging included high-resolution static axial, coronal, and sagittal scans and dynamic midsagittal T2W sequences during specific maneuvers. Non-diagnostic scans due to ineffective straining were excluded. MRI findings were validated by consultant radiologists, with consensus in uncertain cases, and correlated with clinical findings (Tables 1 and 2, Figures 1, 2 and 3)[10,14,15].
| Terminology | Definition |
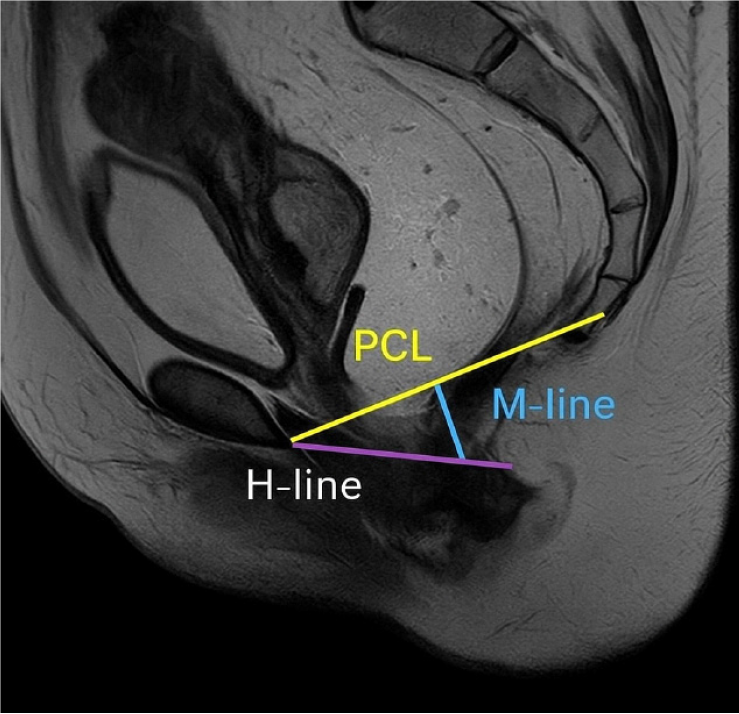
| Pubococcygeal line | Line between the inferior margin of the symphysis pubis and the tip of the coccyx (Figure 1) |
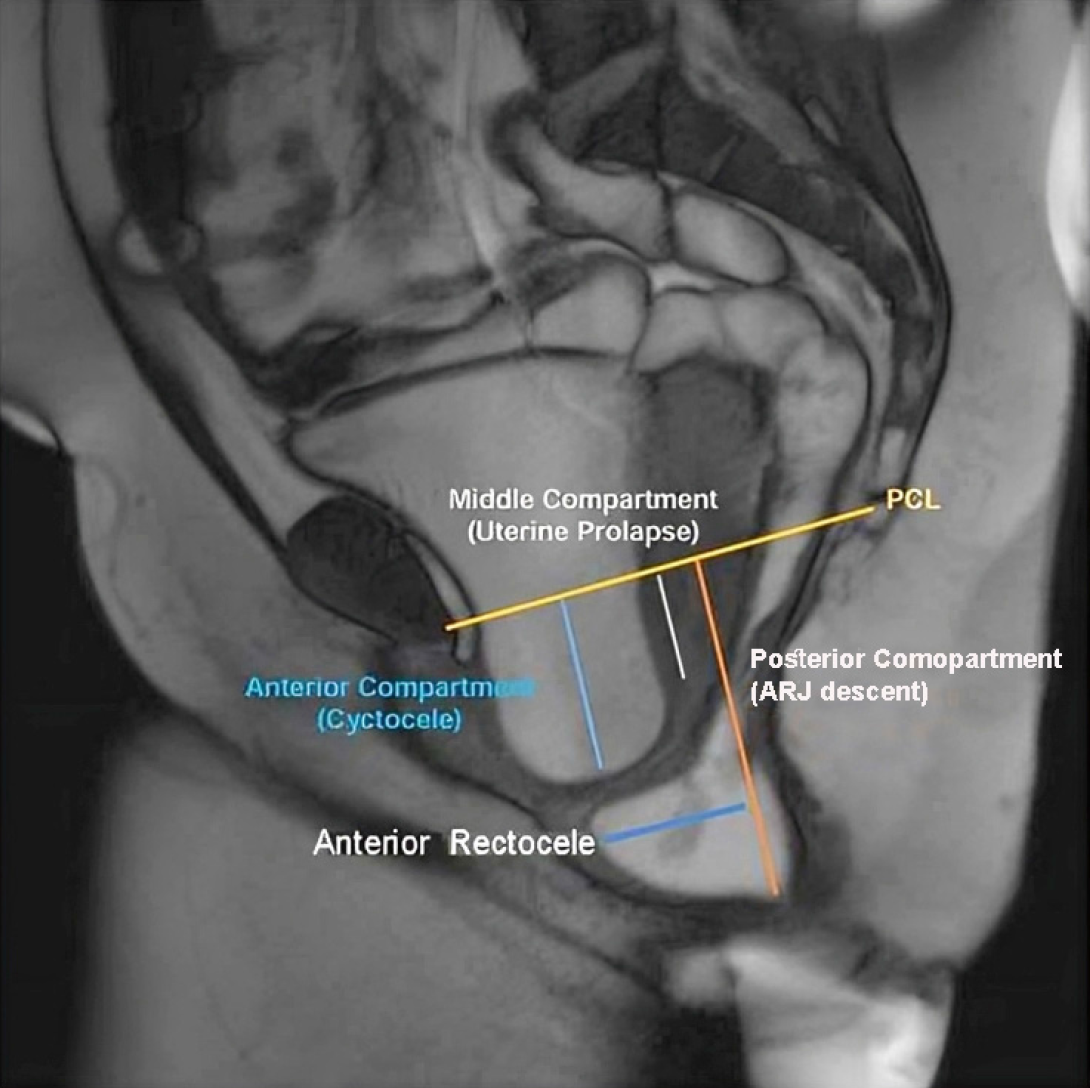
| Anterior compartment | The posterior and most inferior part of the bladder base is the reference point |
| Middle compartment | The most anterior and inferior aspect of the cervix or posterosuperior vaginal apex in patients who have undergone hysterectomy is the reference point |
| Posterior compartment | The anterior aspect of the anorectal junction is the reference point |
| H-line | Distance between the inferior border of the pubic symphysis and the posterior wall of the rectum at the level of the anorectal junction. It indicates the width of the levator hiatus (Figure 1) |
| M-line | Vertical line drawn perpendicularly from the PCL to the posterior end of the H-line. It indicates the degree of decent of the levator hiatus or the degree of pelvic floor laxity (Figure 1) |
| Cystocele | Abnormal descent of urinary bladder at rest/straining using the PCL as the reference line (Figure 2) |
| Urethral hypermobility | Urethra rotation of > 30° from rest, from the vertical to horizontal axis |
| Prolapse (uterine/vaginal/cervical) | Abnormal descent of the anteroinferior aspect of the cervix/posterosuperior vaginal apex from the PCL (Figure 2) |
| Peritoneocele and enterocele | Inferior herniation of the peritoneal pouch along the anterior rectal wall with an increased distance between the vagina and rectum and wide rectovaginal fossa. Enterocele-abnormal descent of small bowel loops below the PCL (Figure 2) |
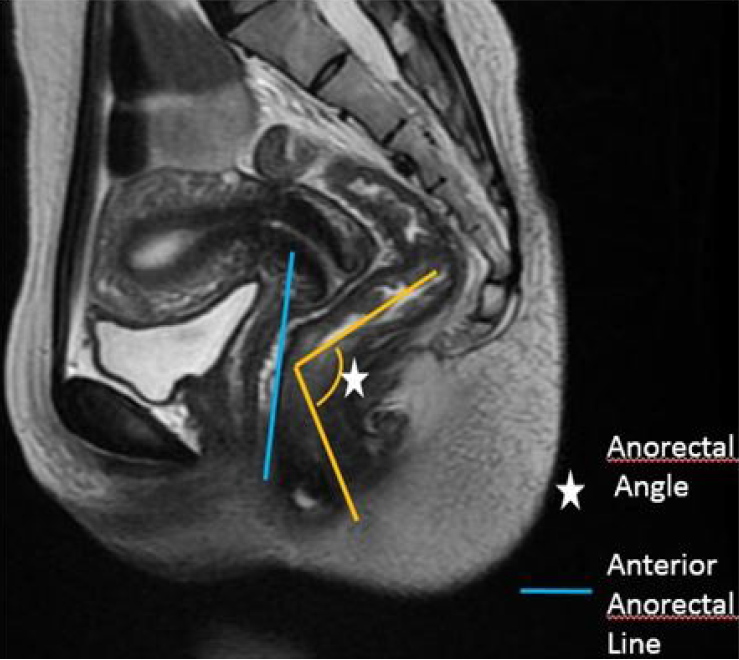
| ARA | Angle between the midline of the anal canal and a line tangent to the posterior rectal wall. At rest the normal angle measures approximately 70°-134° (Figure 3) |
| Rectocele | Abnormal bulge of the anterior rectal wall into the posterior vaginal wall (Figure 2) |
| Rectal intussusception | Infoldings of the full thickness of the rectal wall into the rectum (intrarectal/recto-rectal) or into the anal canal (intra- anal/anorectal) or beyond (extra-anal/rectal prolapse) |
| Pelvic floor descent | Excessive descent of the pelvic floor at rest or during defecation |
| Anismus | Lack of pelvic floor descent/prominent puborectalis impression/failure of opening of the ARA |
| Condition | Grading for each condition |
| Cystocele | |
| Prolapse (uterine/vaginal/cervical) | Grade 0: Absent |
| Enterocele | Grade 1: Mild/small (< 3 cm) |
| Peritoneocele | Grade 2: Moderate/medium (3-6 cm) |
| Pelvic floor widening | Grade 3: Severe/large (> 6 cm) |
| Pelvic floor descends | |
| Rectocele | Grade 0: Absent |
| Grade 1: Mild/small (< 2 cm) | |
| Grade 2: Moderate/medium (2-4 cm) | |
| Grade 3: Severe/large (> 4 cm) |
Data were collected through demographic records, clinical history, examination findings, investigation reports, and standardized questionnaires. All eligible patients followed MRD using a standardized MRI protocol.
Statistical analyses were performed using Statistical Package for the Social Sciences version 24 (IBM SPSS Statistics, Armonk, NY, United States), employing χ² tests for categorical data. P < 0.05was considered statistically significant.
The mean age of the patients was 36.1 years ± 13.3 years (range: 17-73 years). Most patients (23, 40.4%) were below 30 years of age. Seventeen (29.8%) patients were between 31 years and 40 years of age, followed by 8 (14.0%) patients aged 41-50 years, 6 (10.5%) patients aged 51-60 years, and 3 (5.3%) patients aged over 61 years. Approximately two-thirds of the patients were female (Table 3).
| Characteristics | Frequency | % | mean ± SD | Range |
| Age in years | ||||
| ≤ 30 | 23 | 40.4 | 36.1 ± 13.3 | 17-73 |
| 31-40 | 17 | 29.8 | ||
| 41-50 | 8 | 14.0 | ||
| 51-60 | 6 | 10.5 | ||
| ≥ 60 | 3 | 5.3 | ||
| Sex | ||||
| Male | 20 | 35.1 | ||
| Female | 37 | 64.9 | N/A | N/A |
Table 4 summarizes the MRD findings among the study participants. The most common abnormalities were pelvic organ prolapse and anorectal junction descent, which were seen in 94.7% of cases each. Rectocele was observed in 78.9% of patients, with vaginal/uterine prolapse noted in 59.4% of females. Paradoxical contraction was seen in 7.0% of patients, and no cases of sigmoidocele were reported.
| Magnetic resonance defecography findings | Number | % |
| Pelvic descent | 54 | 94.7 |
| Pelvic floor widening | 45 | 78.9 |
| Cystocele | 37 | 65.9 |
| Anorectal junction descent | 54 | 94.7 |
| Vaginal and uterine prolapse | 22 | 59.4 |
| Rectocele | 45 | 78.9 |
| Grade 1 | 15 | 26.3 |
| Grade 2 | 23 | 40.4 |
| Grade 3 | 7 | 12.3 |
| Enterocele | 15 | 26.3 |
| Grade 1 | 12 | 21.1 |
| Grade 2 | 3 | 5.3 |
| Grade 3 | 0 | 0.0 |
| Paradoxical contraction | 4 | 7.0 |
| Sigmoidocele | 0 | 0.0 |
| Peritoneocele | 0 | 0.0 |
| Intussusception | 23 | 40.4 |
| Intra-rectal | 10 | 17.6 |
| Intra-anal | 11 | 19.3 |
| Rectal prolapse | 2 | 3.5 |
| Urethal mobility | ||
| Normal | 42 | 73.7 |
| Hypermobility | 15 | 26.3 |
| Levator muscle symmetry | ||
| Symmetrical | 44 | 77.2 |
| Asymmetrical | 13 | 22.8 |
| Atrophy | 2 | 3.5 |
| Focal defects | ||
| Scarring | 2 | 3.5 |
| Ballooning | 2 | 3.5 |
| Focal eventration | 1 | 1.8 |
| Perineal body tear | 2 | 3.5 |
| Endopelvic facia | ||
| Not deformed | 45 | 78.9 |
| Deformed | ||
| Level I | 4 | 7.0 |
| Level II | 3 | 5.3 |
| Level III | 5 | 8.8 |
Among patients with rectocele, grade 2 was most prevalent (23, 40.4%), followed by grade 1 in 15 (26.3%) cases and grade 3 in 7 (12.3%) cases. Enterocele was present in 12 (21.1%) patients with grade 1 and 3 (5.3%) patients with grade 2. No peritoneocele or sigmoidocele was identified (Table 4).
Rectal intussusception was found in 23 (40.4%) cases, comprising 10 (17.6%) cases intra-rectal, 11 (19.3%) cases intra-anal, and 2 (3.5%) cases rectal prolapse cases. Urethral hypermobility was seen in 15 (26.3%) patients; the remaining patients showed normal urethral mobility (Table 4).
The levator muscle appeared symmetrical in 44 (77.2%) cases, whereas asymmetry was observed in 13 (22.8%) cases. Atrophy and perineal tear were evident in 2 (3.5%) cases each. Focal atrophy was noted in 5 patients (scarring in 2 patients, ballooning in 2 patients, and focal eventration in 1 patient). Deformity of the endopelvic fascia was found in 11 (20.1%) cases, involving level I (4 cases, 7%), level II (3 cases, 5.3%), and level III (5 cases, 8.8%) (Table 4).
Table 5 shows the findings from ARA measurements. At rest, the mean ARA was 97.09° ± 24.80° (range: 45°–145°), decreasing to 80.18° ± 23.36° during Kegel exercises, and increasing to 116.82° ± 31.23° during defecation (P < 0.001). ARA remained within the normal range in 46 (80.7%) cases. Expected narrowing during Kegel exercises was seen in 32 (56.1%) cases, and expected widening during defecation was observed in 37 (64.9%) patients. Abnormal ARA during the resting phase was present in 11 patients (9 patients with narrowing, 2 patients with widening). This number increased to 23 (43.9%) during Kegel exercises and 20 (35.1%) during defecation. During defecation, 15 (26.3%) patients showed diminished widening, 1 (1.8%) patient had no change, and 4 (7.0%) patients demonstrated paradoxical narrowing of the ARA (Table 6).
| Functional parameters | mean ± SD | Range | P value |
| Anorectal angle | |||
| Resting | 97.09 ± 24.80 | 45-145 | |
| Squeeze (Kegel) | 80.18 ± 23.36 | 35-138 | < 0.001 |
| Defecation | 116.82 ± 31.23 | 53-185 |
| Resting | Kegel | Defecation | ||||||
| ARA condition | n | % | ARA condition | n | % | ARA condition | n | % |
| Normal | 46 | 80.7 | Expected narrowing | 32 | 56.1 | Expected widening | 37 | 64.9 |
| Narrowed | 9 | 15.8 | Diminished narrowing | 25 | 43.9 | Diminished widening | 15 | 26.3 |
| Widened | 2 | 3.5 | No change | 1 | 1.8 | |||
| Paradoxical narrowing | 4 | 7.0 | ||||||
Table 7 illustrates pelvic floor movement parameters. The mean H-line length at rest was 5.28 cm ± 1.43 cm (range: 3.20-7.90 cm), increasing to 7.56 cm ± 1.69 cm (range: 4.4–12.2 cm) during defecation, with a statistically significant difference
| The H-line, M-line, organ prolapse parameters | Resting | Defecation | P value | ||||
| n | % | mean ± SD | n | % | mean ± SD | ||
| H-line in cm | |||||||
| Grade 0 | 40 | 70.2 | 5.28 ± 1.43 (3.20-7.90) | 12 | 21.1 | 7.56 ± 1.69 (4.40-12.20) | 0.001 |
| Grade 1 | 17 | 29.8 | 19 | 33.3 | |||
| Grade 2 | 0 | 0.0 | 25 | 43.9 | |||
| Grade 3 | 0 | 0.0 | 1 | 1.8 | |||
| M-line | |||||||
| Grade 0 | 33 | 57.9 | 1.93 ± 1.07 (0.20-3.90) | 3 | 5.3 | 5.17 ± 2.28 (1.0-9.60) | 0.001 |
| Grade 1 | 24 | 42.1 | 16 | 28.1 | |||
| Grade 2 | 0 | 0.0 | 20 | 35.1 | |||
| Grade 3 | 0 | - | 18 | 31.6 | |||
| Cystocele | |||||||
| Grade 0 | 57 | 100 | 20 | 35.1 | 0.001 | ||
| Grade 1 | - | - | 28 | 49.1 | |||
| Grade 2 | - | - | 6 | 10.5 | |||
| Grade 3 | - | - | 3 | 5.3 | |||
| Uterine/vaginal prolapse | |||||||
| Grade 0 | 37 | 100 | 15 | 40.5 | 0.001 | ||
| Grade 1 | - | - | 17 | 45.9 | |||
| Grade 2 | - | - | 5 | 13.5 | |||
| Grade 3 | - | - | - | - | |||
At rest, 17 (29.8%) cases exhibited grade 1 pelvic floor widening (H-line), while the remainder were normal. During defecation, widening increased to grade 1 in 19 (33.3%) patients, grade 2 in 25 (43.9%) patients, and grade 3 in 1 (1.8%) patient (P < 0.001).
Regarding M-line measurements, grade 1 pelvic floor descent was observed in 24 (42.1%) cases at rest. During defecation, grade 1 descent was seen in 16 cases (28.1%), grade 2 in 20 (35.1%) cases, and grade 3 in 18 (31.6%) cases (P < 0.001).
Cystocele was not detected at rest but became evident in 37 (64.9%) patients during defecation. Among these, 28 (49.1%) patients had grade 1, 6 (10.5%) patients had grade 2, and 3 (5.3%) patients had grade 3 cystocele. This difference was highly significant (P < 0.001). Among the 37 female patients, no uterine/vaginal vault prolapse was observed at rest. However, during defecation, 17 (45.9%) patients had grade 1 and 5 (13.5%) patients had grade 2 prolapse, a highly significant finding compared to the resting phase (P < 0.001) (Table 7).
This study revealed that ODS is more common in younger individuals, with a lower incidence among older patients. However, age-related changes in the pelvic floor may contribute to ODS in older adults. This age distribution underscores the importance of early diagnosis, particularly in younger patients (Table 3). Compared to previous research, the mean age of participants in this study was slightly lower. For instance, Ahmed and Mfom[12] reported a mean age of 40.2 years, whereas Elshazly et al[13] observed a mean age of 48.15 years ± 14.3 years. These differences may reflect variations in populations or regional characteristics of the cohorts. The younger mean age in this study suggests that ODS may affect individuals earlier than traditionally expected, emphasizing the need to identify and address the condition in younger patients who might not typically be considered at risk.
The female predominance observed in our study is consistent with previous research. Several study findings[16,17] highlight the higher prevalence of ODS in women, indicating a potential sex-related susceptibility to the condition. In Bangladesh a cross sectional survey in the Sylhet District reported functional constipation prevalence 4.9% with higher rate for the elderly (at 10.1%)[18]. This sex difference may be attributed to several factors, including dietary habits like low consumption of vegetables and spices, as well as hormonal influences such as the effects of estrogen and progesterone, which can affect gastrointestinal motility and pelvic floor function. Additionally, anatomical variances in the pelvic region and a higher prevalence of conditions such as pelvic organ prolapse and irritable bowel syndrome in women may increase their susceptibility to ODS[5,6,16,17].
A national study found that 35.3% of Bangladeshi women aged 30 years to 59 years experienced at least one symptomatic pelvic floor disorder, influenced by aspects such as higher parity, early marriage, and low socioeconomic status. Further research is needed to determine whether the predominance of ODS in younger women is associated with pelvic floor disorders[19].
T2WI proved highly effective in visualizing the levator ani muscle, a key component of pelvic floor function. Muscle asymmetry and focal defects may contribute to pelvic floor dysfunction by weakening the levator ani, affecting organ support and anorectal function[1,5,9,20,21]. These findings also focus on the value of T2WI in detecting levator ani muscle abnormalities. Similar results were reported by Bamboriya et al[20] and Loubeyre et al[21], who observed levator muscle symmetry on MRI in 71.9% of patients, with asymmetry noted in 28.1%. In 2011, Loubeyre et al[21] concluded that 56% of patients had at least one structural variant (thinning, aplasia) of muscles of levator ani complex. Focal defects in the levator ani also were identified in 13.4% of patients, with scarring, ballooning, and focal eventration[20].
Table 4 highlights the importance of endopelvic fascia integrity in patients with ODS, as fascial abnormalities may contribute to the condition and should be incorporated into treatment planning. El Sayed et al[22] found level I and II fascial defects in 21% of patients (7 of 34), and Bamboriya et al[20] observed defects in 37% of patients (34 of 82), with level I and III defects in 13.4% and level II defects in 9.7%. These findings align with the current study, emphasizing the role of fascial defects in pelvic floor dysfunction, with level I defects linked to organ descent and level II and III defects affecting vaginal and perineal support.
In the current study, posterior compartment descent, including conditions like rectocele, enterocele, peritoneocele, sigmoidocele, and rectal intussusception, was evaluated using the ARA (Tables 4, 5 and 6). The incidence of anismus (paradoxical narrowing) in this study was comparable to that reported by Colaiacomo et al[3], Li et al[23], Maglinte et al[24], and Ramage et al[25]. These findings suggest that ARA reduction and anismus are common features of pelvic floor dysfunction, particularly in patients with ODS. The variation in ARA reduction observed across studies may reflect differences in patient populations or diagnostic techniques, but the overall trend indicates the importance of evaluating the ARA and its role in ODS. In this study, rectocele was the most frequent anatomical abnormality observed in patients with pelvic floor disorders. Failure to diagnose enteroceles can lead to missed opportunities for appropriate intervention, which may help alleviate symptoms associated with pelvic floor dysfunction and ODS. Internal forms of intussusception are often linked to ODS, as the protruding rectal tissue obstructs stool passage during bowel movements. Studies evaluating anorectal dynamics in patients with ODS have reported varying findings. Bamboriya et al[20] observed that 78.1% of patients maintained a normal ARA at rest, Rentsch et al[4] and Paetzel et al[26] reported ARA reductions in 33.3% and 60% of cases, respectively. Regarding pelvic organ prolapse, Bamboriya et al[20] identified enterocele in 28.1% of cases and peritoneocele in 84.5% of cases, with no instances of sigmoidocele. By contrast, Elshazly et al[13] found enteroceles in 15% of patients, whereas Rentsch et al[4] and Paetzel et al[26] reported rates of 13.3% and 20%, respectively. Additionally, Bamboriya et al[20] documented intra-rectal intussusception in 48.3% of cases, intra-anal intussusception in 44.8%, and rectal prolapse in 6.9%. Ahmed and Mfom[12] reported similar findings, supporting the association between rectal intussusception and ODS. The high prevalence of rectocele in this study, particularly grade 2, further supports its significant role in the pathophysiology of ODS. These results align with those of Hetzer et al[27], who observed similar patterns of pelvic floor descent during defecation. Similarly, Thapar et al[28] reported pelvic floor descent in 37.1% of patients at grade 1, 30.9% at grade 2, and 33.9% at grade 3. In this study, pelvic floor descent was detected more than twice as often during defecation (94.8%) compared to the resting state (42.1%), with statistical significance (P < 0.001).
A marked difference in MRD functional parameters was detected between the resting state and during defecation or maximal strain, evaluated using the health maintenance organization system for pelvic floor relaxation and descent. Significant differences (P < 0.05) were found in all parameters, including the H-line, M-line, and the descent of the cervical/vaginal and anorectal junction in both states (Table 7). The marked increase during defecation (P < 0.001) emphasizes the influence of dynamic factors such as intra-abdominal pressure and muscle relaxation on pelvic floor dysfunction in ODS, helping to identify patients at higher risk. Similar findings were reported by Piloni et al[29] who noted an increased hiatus in 67.6% of patients. Additionally, Bamboriya et al[20] observed a significant increase in abnormal pelvic floor relaxation during defecation (81%) compared to the resting state (34%) (P < 0.001).
Cystoceles can contribute to outlet obstruction by impairing bladder and urethral function, resulting in incomplete evacuation and prolonged straining. Elshazly et al[13] reported anterior compartment defect in 30% of patients radiologically. The incidence of cystoceles and urethral hypermobility associated with ODS in these studies was notably lower than in ours. Conversely, Bamboriya et al[20] found cystoceles in 60.9% of Indian patients, consistent with our findings. This suggests a higher incidence of cystoceles associated with ODS in South Asian populations, possibly due to genetic, cultural, or healthcare-related factors unique to this region. These findings underscore the importance of developing tailored diagnostic and therapeutic approaches for managing ODS in South Asian populations.
Pelvic floor dysfunction affects approximately 50% of women over the age of 50 years. Among those presenting for medical evaluation, nearly 11% have undergone prior surgical intervention, with approximately 30% requiring repeat surgical procedures[10,11,13]. Surgical options for ODS include stapled transanal rectal resection, laparoscopic ventral rectopexy, transperineal repair, the Delorme or Altemeier procedure, sacral nerve stimulation, and hemorrhoidopexy[10,13,16]. Indications of surgery include persistent symptoms such as chronic constipation and straining, which significantly impact quality of life despite non-surgical treatment, as well as structural (e.g., large rectocele, rectal prolapse) or functional pelvic floor issues, often resulting from damage to the endopelvic fascia and levator ani muscle[10,13,16,17,20]. Patient selection is critical, guided by imaging and functional assessment, with long-term follow-up to monitor outcomes. A previous study showed that MRD findings changed the treatment decision in 20% of patients[13]. A number of patients experience recurrence due to the involvement of multiple compartments. One study mentioned a recurrence rate of 50% after 5.5 years post surgery[30]. Therefore, a multi-compartment diagnostic evaluation before surgery is essential for preoperative planning, which can be facilitated effectively by MRD[3-7]. Integrating a validated symptom scoring system, such as the Wexner constipation score, in future studies will enhance the ability to correlate radiological findings with patient-reported outcomes[31]. This approach will provide a more inclusive understanding of the clinical significance of imaging results and improve patient-centered assessments.
This study had limitations that must be considered in the interpretation of our findings. The supine position used for MRD is not physiological; thus, it is best to perform MRD while sitting or squatting. However, the institute's MR scanners were made to be used in a supine position. It is important to note, though, that even if a seated setup is permitted for open MRI, the posture does not entirely align with natural processes. Additionally, open MRI systems typically have a lower resolution, leading to images with reduced detail. Moreover, it is expensive, not widely available, and takes longer to scan. A further limitation is that an intelligence quotient test was not conducted before the examination, which might have revealed information on cognitive variables influencing patient compliance and comprehension of the process[10,25].
This study primarily found pelvic organ prolapse and anorectal descent in patients with ODS. ODS was more prevalent in females than males, particularly in the younger age group (under 30 years). Based on the findings of this study, it is recommended that special attention be paid to young female patients when evaluating ODS. Policymakers should con
We are grateful to the authority of Bangabandhu Sheikh Mujib Medical University for their technical support and active cooperation in continuation of this research. We are also thankful to Mr. MD Shafiqul Islam for his technical support. We further express our gratitude to the patients, doctors, and paramedics in the colorectal clinic for their invaluable support.
| 1. | Zbar AP. Posterior pelvic floor disorders and obstructed defecation syndrome: clinical and therapeutic approach. Abdom Imaging. 2013;38:894-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Ganeshan A, Anderson EM, Upponi S, Planner AC, Slater A, Moore N, D'Costa H, Bungay H. Imaging of obstructed defecation. Clin Radiol. 2008;63:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Colaiacomo MC, Masselli G, Polettini E, Lanciotti S, Casciani E, Bertini L, Gualdi G. Dynamic MR imaging of the pelvic floor: a pictorial review. Radiographics. 2009;29:e35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Rentsch M, Paetzel C, Lenhart M, Feuerbach S, Jauch KW, Fürst A. Dynamic magnetic resonance imaging defecography: a diagnostic alternative in the assessment of pelvic floor disorders in proctology. Dis Colon Rectum. 2001;44:999-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Arshad Rashid SK. Obstructed Defecation Syndrome: A Treatise on Its Functional Variant. Intern Med. 2014;s1. [DOI] [Full Text] |
| 6. | Bharucha AE, Wald A, Enck P, Rao S. Functional anorectal disorders. Gastroenterology. 2006;130:1510-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 345] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 7. | Snooks SJ, Setchell M, Swash M, Henry MM. Injury to innervation of pelvic floor sphincter musculature in childbirth. Lancet. 1984;2:546-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 515] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 8. | Peschers UM, Schaer GN, DeLancey JO, Schuessler B. Levator ani function before and after childbirth. Br J Obstet Gynaecol. 1997;104:1004-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 101] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Gill EJ, Hurt WG. Pathophysiology of pelvic organ prolapse. Obstet Gynecol Clin North Am. 1998;25:757-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Megha K, Sushil G K, Dilip L L. Applications and Limitations of Magnetic Resonance Defecography in Evaluation of Pelvic Floor Dysfunction Disorders. Int J Radiol Imaging Technol. 2019;5. [DOI] [Full Text] |
| 11. | Bitti GT, Argiolas GM, Ballicu N, Caddeo E, Cecconi M, Demurtas G, Matta G, Peltz MT, Secci S, Siotto P. Pelvic floor failure: MR imaging evaluation of anatomic and functional abnormalities. Radiographics. 2014;34:429-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Ahmed F, Mfom M. Role of MR Defecography in the Assessment of Obstructed Defecation Syndrome. MJCU. 2018;86:927-931. [DOI] [Full Text] |
| 13. | Elshazly WG, El Nekady Ael A, Hassan H. Role of dynamic magnetic resonance imaging in management of obstructed defecation case series. Int J Surg. 2010;8:274-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Korula DR, Chandramohan A, John R, Eapen A. Barium Defecating Proctography and Dynamic Magnetic Resonance Proctography: Their Role and Patient's Perception. J Clin Imaging Sci. 2021;11:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Shetty A, Walizai T, Murphy A. MR defaecating proctography. Radiopaedia. 2016. [DOI] [Full Text] |
| 16. | Rosen A. Obstructed defecation syndrome: diagnosis and therapeutic options, with special focus on the STARR procedure. Isr Med Assoc J. 2010;12:104-106. [PubMed] |
| 17. | Ferreira-Santos R, Gonçalves RB, Marques I, Pereira CC, Martins S, Pereira JC. Lessons from Magnetic Resonance Defecography in Obstructed Defecation Syndrome: A Cornerstone for Adequate Surgical Planning. World J Colorectal Surg. 2024;13:117-123. [DOI] [Full Text] |
| 18. | Perveen I, Rahman MM, Saha M, Parvin R, Chowdhury M. Functional constipation - prevalence and life style factors in a district of Bangladesh. Mymensingh Med J. 2015;24:295-304. [PubMed] |
| 19. | Islam RM, Bell RJ, Billah B, Hossain MB, Davis SR. The prevalence of symptomatic pelvic floor disorders in women in Bangladesh. Climacteric. 2016;19:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Bamboriya R, Jaipal U, Jakhar S. A Descriptive Study of Mr Defecography for Evaluation of Obstructed Defecation Syndrome. IJMBS. 2020;4. [DOI] [Full Text] |
| 21. | Loubeyre P, Copercini M, Petignat P, Dubuisson JB. Levator ani muscle complex: anatomic findings in nulliparous patients at thin-section MR imaging with double opacification. Radiology. 2012;262:538-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | El Sayed RF, El Mashed S, Farag A, Morsy MM, Abdel Azim MS. Pelvic floor dysfunction: assessment with combined analysis of static and dynamic MR imaging findings. Radiology. 2008;248:518-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Li M, Jiang T, Peng P, Yang XQ, Wang WC. Association of compartment defects in anorectal and pelvic floor dysfunction with female outlet obstruction constipation (OOC) by dynamic MR defecography. Eur Rev Med Pharmacol Sci. 2015;19:1407-1415. [PubMed] |
| 24. | Maglinte DD, Hale DS, Sandrasegaran K. Comparison between dynamic cystocolpoproctography and dynamic pelvic floor MRI: pros and cons: which is the "functional" examination for anorectal and pelvic floor dysfunction? Abdom Imaging. 2013;38:952-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Ramage L, Georgiou P, Qiu S, McLean P, Khan N, Kontnvounisios C, Tekkis P, Tan E. Can we correlate pelvic floor dysfunction severity on MR defecography with patient-reported symptom severity? Updates Surg. 2018;70:467-476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Paetzel C, Strotzer M, Fürst A, Rentsch M, Lenhart M, Feuerbach S. [Dynamic MR defecography for diagnosis of combined functional disorders of the pelvic floor in proctology]. Rofo. 2001;173:410-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Hetzer FH, Andreisek G, Tsagari C, Sahrbacher U, Weishaupt D. MR defecography in patients with fecal incontinence: imaging findings and their effect on surgical management. Radiology. 2006;240:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Thapar RB, Patankar RV, Kamat RD, Thapar RR, Chemburkar V. MR defecography for obstructed defecation syndrome. Indian J Radiol Imaging. 2015;25:25-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Piloni V, Tosi P, Vernelli M. MR-defecography in obstructed defecation syndrome (ODS): technique, diagnostic criteria and grading. Tech Coloproctol. 2013;17:501-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 30. | Gagliardi G, Pescatori M, Altomare DF, Binda GA, Bottini C, Dodi G, Filingeri V, Milito G, Rinaldi M, Romano G, Spazzafumo L, Trompetto M; Italian Society of Colo-Rectal Surgery (SICCR). Results, outcome predictors, and complications after stapled transanal rectal resection for obstructed defecation. Dis Colon Rectum. 2008;51:186-95; discussion 195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 31. | Agachan F, Chen T, Pfeifer J, Reissman P, Wexner SD. A constipation scoring system to simplify evaluation and management of constipated patients. Dis Colon Rectum. 1996;39:681-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 875] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/