Published online May 28, 2025. doi: 10.4329/wjr.v17.i5.106084
Revised: March 12, 2025
Accepted: April 11, 2025
Published online: May 28, 2025
Processing time: 100 Days and 12.3 Hours
This narrative review examines the use of imaging biomarkers for diagnosing and monitoring hydrocephalus from birth through childhood. Early detection and longitudinal follow-up are essential for guiding timely interventions and asse
Core Tip: This narrative review highlights the role of imaging biomarkers in diagnosing and monitoring hydrocephalus from birth through childhood. It explores the utility of cranial ultrasound and magnetic resonance imaging, with emphasis on key ventricular indices, volumetric analysis, and diffusion-based techniques. The integration of artificial intelligence enhances measurement precision and reproducibility, addressing interobserver variability. The review also discusses the need for standardized imaging protocols and reliable normative values to improve diagnostic consistency. By synthesizing recent advancements, it underscores the importance of longitudinal imaging in assessing neurodevelopmental outcomes and optimizing patient management.
- Citation: Navarro-Ballester A, Álvaro-Ballester R, Lara-Martínez MÁ. Imaging biomarkers for detection and longitudinal monitoring of ventricular abnormalities from birth to childhood. World J Radiol 2025; 17(5): 106084
- URL: https://www.wjgnet.com/1949-8470/full/v17/i5/106084.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i5.106084
Hydrocephalus is a complex neurological condition characterized by an abnormal accumulation of cerebrospinal fluid (CSF) within the ventricular system, leading to increased intracranial pressure and progressive ventricular dilation, which can cause significant structural and functional brain changes. While it can occur at any stage of life, its effects are particularly pronounced in early development, especially during the neonatal period, when the brain is still in critical stages of maturation. Without timely diagnosis and effective management, hydrocephalus can lead to severe and potentially irreversible neurodevelopmental impairments.
The identification of robust imaging parameters to diagnose and monitor hydrocephalus from birth through childhood is critical for guiding timely interventions and evaluating treatment outcomes. These parameters are invaluable in tracking ventricular size, assessing CSF dynamics, and identifying signs of progressive ventricular enlargement. Early and ongoing assessment of these biomarkers helps clinicians make informed decisions about surgical or medical interventions, such as ventriculoperitoneal shunts or endoscopic third ventriculostomy, and their long-term effectiveness.
Imaging modalities play a central role in the management of hydrocephalus across the pediatric age spectrum. Cranial ultrasound (CUS), widely used in neonates due to its safety, portability, and ability to provide bedside imaging, is indispensable for initial evaluations and routine follow-ups in the neonatal population[1-3]. For older children and in more complex cases, magnetic resonance imaging (MRI) offers superior resolution, allowing detailed assessment of ventricular anatomy, white matter changes, and CSF flow[4]. Advances in imaging technologies, such as phase-contrast MRI[5] and automated volumetric analyses[6], have further refined the ability to diagnose and monitor hydrocephalus, providing critical information on disease progression and treatment outcomes.
This narrative review focuses on the imaging biomarkers and parameters that are essential for diagnosing and monitoring hydrocephalus from birth to adolescence. It provides an overview of their clinical applications, recent technological advancements, and the ongoing challenges in achieving standardized imaging protocols and reliable normative data. By exploring these aspects, the review aims to contribute to a better understanding of hydrocephalus management across the pediatric age range.
CUS remains the cornerstone imaging modality for the evaluation of ventricular abnormalities in neonates, particularly preterm infants. Its widespread use is driven by its accessibility, non-invasiveness, and the ability to perform bedside imaging in real time. This makes CUS an ideal tool for routine screening and early diagnosis in neonatal intensive care units.
One of the key strengths of CUS is its ability to provide real-time measurements of ventricular size and morphology, which are essential for assessing and monitoring changes in hydrocephalus. These metrics are essential for assessing ventricular size and monitoring the progression of hydrocephalus or other conditions. Recent advances, such as the use of very high-frequency transducers, have further enhanced the spatial resolution of CUS, enabling a more detailed evaluation of small structures, including the periventricular white matter and subtle ventricular abnormalities[1].
Contrast-enhanced transfontanellar ultrasound (CEUS) has been explored as an imaging technique in neonates and infants, offering both qualitative and quantitative assessments of cerebral perfusion. This method shows promise in detecting abnormalities such as ischemia, hemorrhage, and intracranial shunts, due to its ability to visualize micro
Additionally, ultrafast Doppler imaging plays a key role in evaluating cerebral vascularization and hemodynamics, enabling detailed measurements of resistivity indices across vessels of various sizes. Unlike conventional Doppler methods, ultrafast Doppler offers higher sensitivity and spatial resolution, facilitating the discrimination between arteries and veins with greater precision. These capabilities are particularly valuable for identifying and monitoring conditions such as white matter injuries in neonates, where early detection of cerebrovascular abnormalities is crucial[8]. Fur
Three-dimensional CUS (3D CUS) has emerged as a promising tool for volumetric assessment of the ventricular system, addressing some of the limitations of traditional two-dimensional (2D) measurements. Studies have demon
Emerging techniques such as transfontanellar elastography offer a novel approach to evaluating the mechanical properties of brain tissue, with potential applications in assessing intracranial pressure and tissue elasticity in hydro
MRI offers unparalleled spatial resolution and structural detail, making it an essential tool for the evaluation of ven
Advanced MRI techniques, such as diffusion tensor imaging (DTI) and phase-contrast MRI, are important tools for both structural and functional assessment in pediatric hydrocephalus. DTI is particularly useful for evaluating white matter microstructural integrity, helping to identify disruptions linked to neurodevelopmental impairments in affected children. Metrics such as fractional anisotropy and diffusivity allow for the detection of brain tissue alterations caused by the disease, providing a better understanding of its impact on neural connectivity[13,14]. In addition, phase-contrast MRI serves as a non-invasive method for quantifying CSF flow dynamics, particularly in children with ventriculomegaly. Studies have demonstrated that hyper-oscillating CSF flow patterns in the Sylvian aqueduct correlate with reduced brain compliance and clinical symptoms such as increased intracranial pressure[5].
While CUS remains indispensable for routine evaluations in neonates, its limitations in detecting subtle lesions, such as mild white matter abnormalities, are well documented. Nevertheless, its high predictive value for severe injuries like grade III and IV hemorrhages and hydrocephalus underscores its relevance in neonatal care. MRI, in contrast, proves essential in scenarios requiring detailed anatomical evaluation or prognostic insights, particularly in cases of suspected white matter injury or complex ventricular abnormalities. Despite its logistical challenges, including cost and the need for sedation in young children, MRI serves as a critical complement to CUS in tailoring management strategies for pediatric hydrocephalus. Together, these modalities ensure a robust diagnostic approach, adapting to the needs of patients as they progress through different stages of development[15,16]. Key imaging biomarkers are shown in Table 1.
| Biomarker | Benefits | Applicability |
| Levene’s index | Tailored for neonates (≤ 40 weeks of gestational age); correlates with gestational age. Useful for defining early intervention thresholds (e.g., > 4 mm above the 97th percentile) | Used in preterm infants via transfontanellar ultrasound. Requires population-specific nomograms |
| Thalamo-occipital distance | Assesses posterior ventricular dilation. Pathological values vary by population and age (e.g., > 24 mm is considered pathological in some studies) | Measured via parasagittal ultrasound or MRI. Complements anteriorly focused indices |
| Evans’ index | Simple and quick measurement; considers cranial size proportionality. Widely used in clinical practice | Applicable in MRI, CT, and transfontanellar ultrasound. Lacks standardized pediatric cut-off values |
| Cella media index | Relates ventricular size to brain tissue volume. Normal values > 4; correlates with third ventricle size | Applied in MRI/CT. Useful for assessing global ventricular dilation |
| Fronto-occipital horn ratio | Evaluates global ventricular dilation (includes frontal and occipital horns). The mean value in healthy individuals is approximately 0.37; a value above 0.44 indicates pathology | Validated in MRI, CT, and ultrasound. Age-independent reference ranges |
| Bicaudate index | Focuses on frontal horn size; useful for assessing ventricular symmetry | Limited to MRI/CT (technical challenges in ultrasound). Less reliable in asymmetry or congenital malformations |
| Anteroposterior lateral ventricle index | Measures the anteroposterior ventricular diameter relative to intracranial size. Has potential for detecting subtle pathological changes | Used in MRI/CT (axial plane reconstruction based on the AC-PC line). Not yet validated in pediatric populations, but it shows promise |
| Volumetric analysis | Quantifies total CSF volume; detects progressive changes that may be missed by linear indices. Shows better correlation with functional impairment | Requires high-resolution MRI and specialized software. Limited by cost and clinical workflow constraints |
Direct measurements of ventricular structures, such as the thickness of the anterior horns or the diameter of the third ventricle, are valuable tools for diagnosing and monitoring hydrocephalus due to their simplicity and ease of acquisition. However, compared to indices like the Evans’ index or bicaudate ratio, direct measurements are less comprehensive, as indices account for proportional relationships, making them less dependent on patient size and age. This is particularly important given the variability in cranial and body size not only between age groups but also among children of the same age. Incorporating parameters such as age, weight, and cranial perimeter can help contextualize direct measurements, improving their reproducibility and clinical reliability[17].
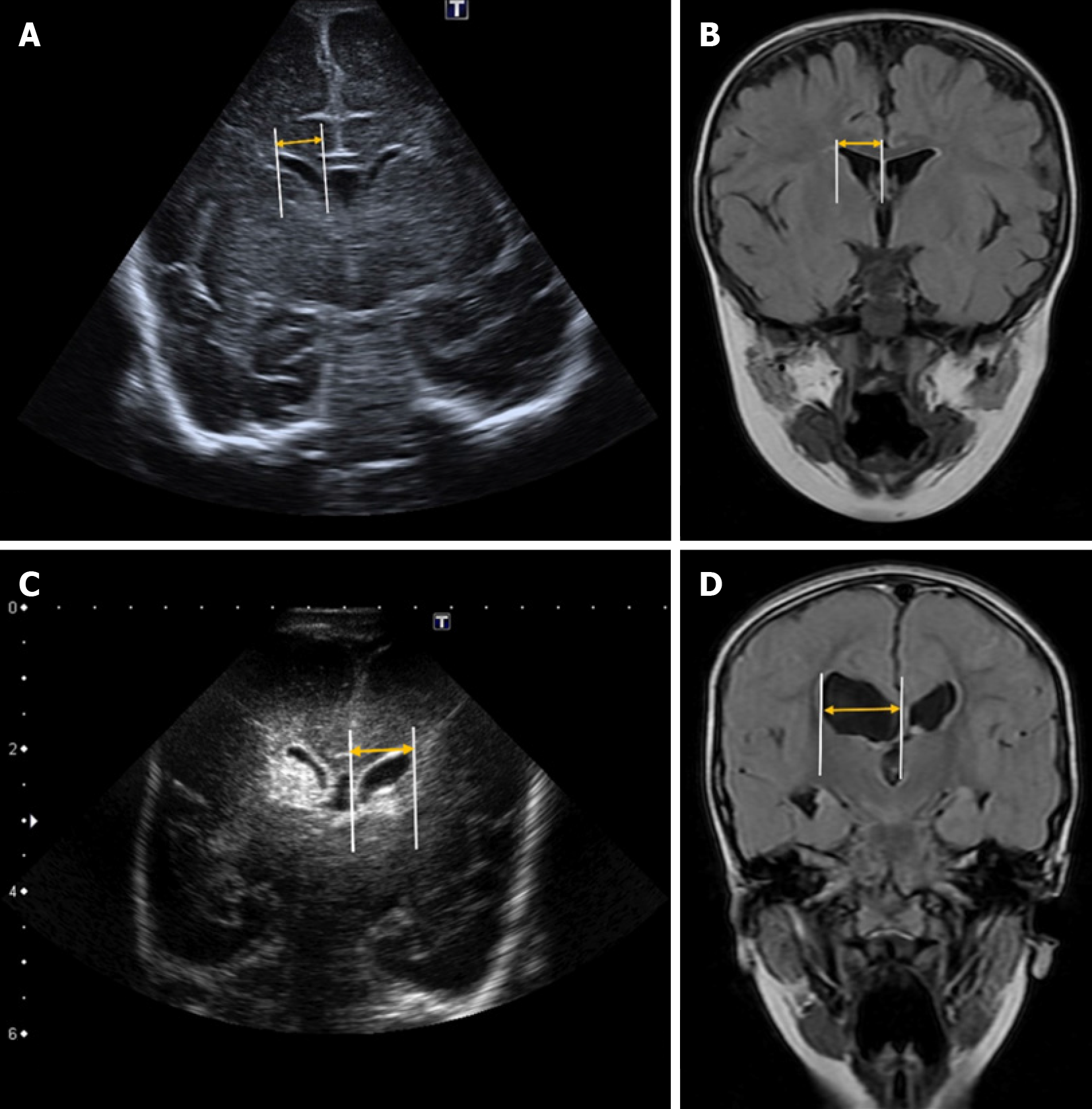
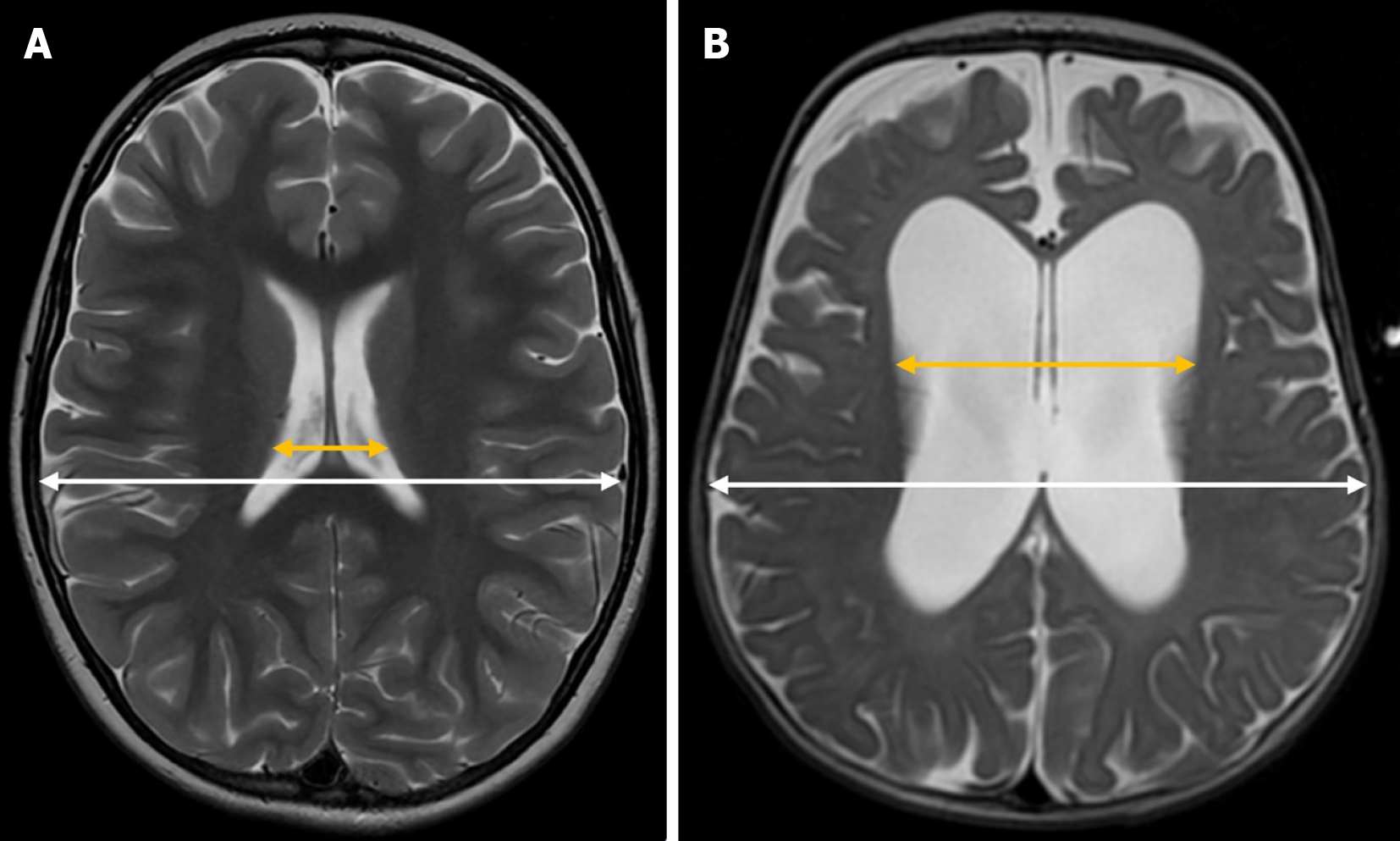
Levene’s index is a quantitative measure used to assess the size of the lateral ventricles in neonates, particularly during the first 40 weeks of gestational age[18]. It is calculated on a coronal plane at the level of the foramen of Monro by measuring the distance from the midline (falx cerebri) to the lateral wall of the anterior horn of the lateral ventricles on both sides (Figure 1)[3].
Reference values vary depending on gestational age. Some authors suggest that hydrocephalus treatment should be considered when this distance exceeds 4 mm above the 97th percentile[19,20]. However, it is essential to develop updated reference nomograms tailored to neonatal populations with shared phylogenetic characteristics to ensure standardized values for each group[3].
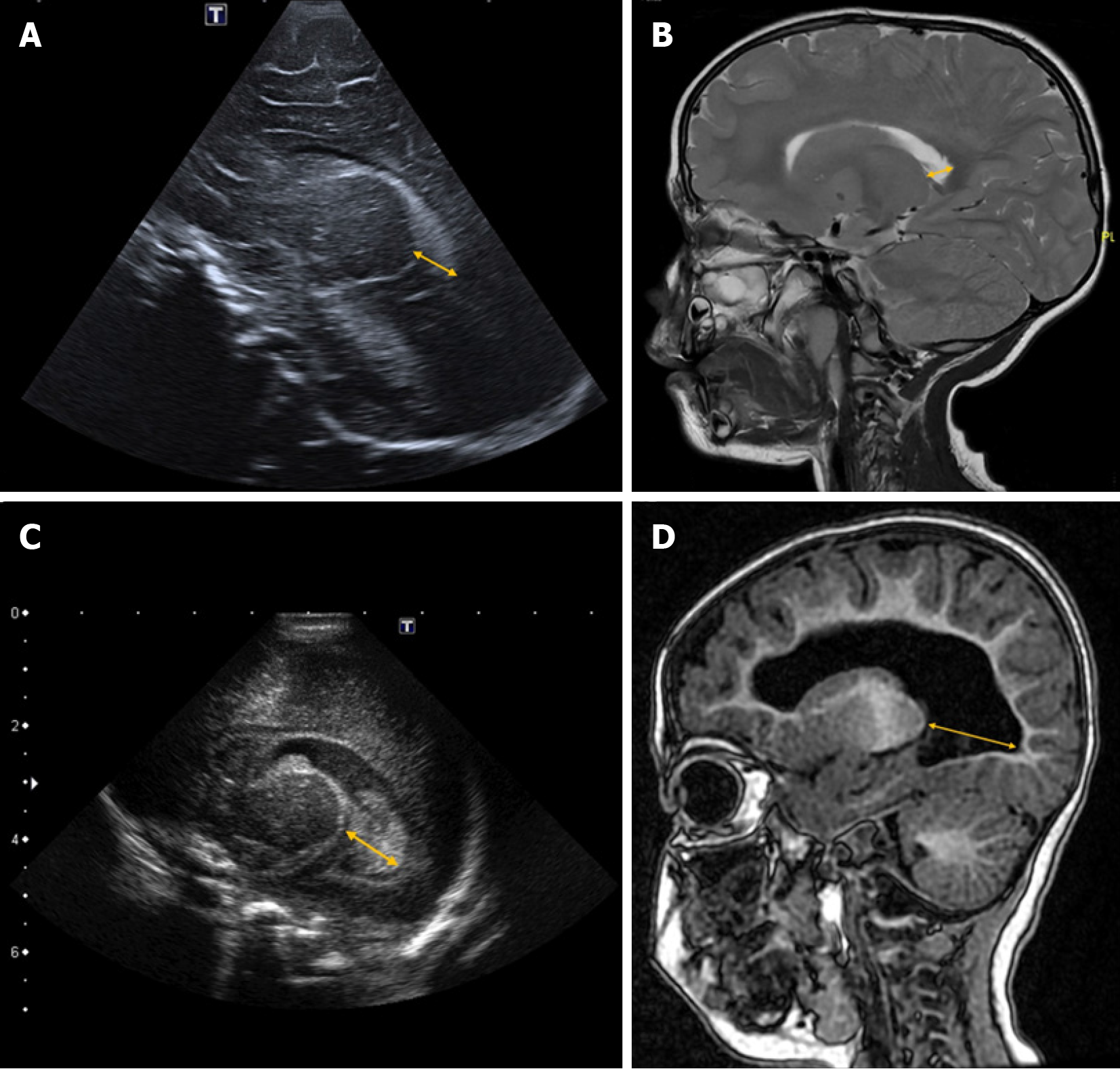
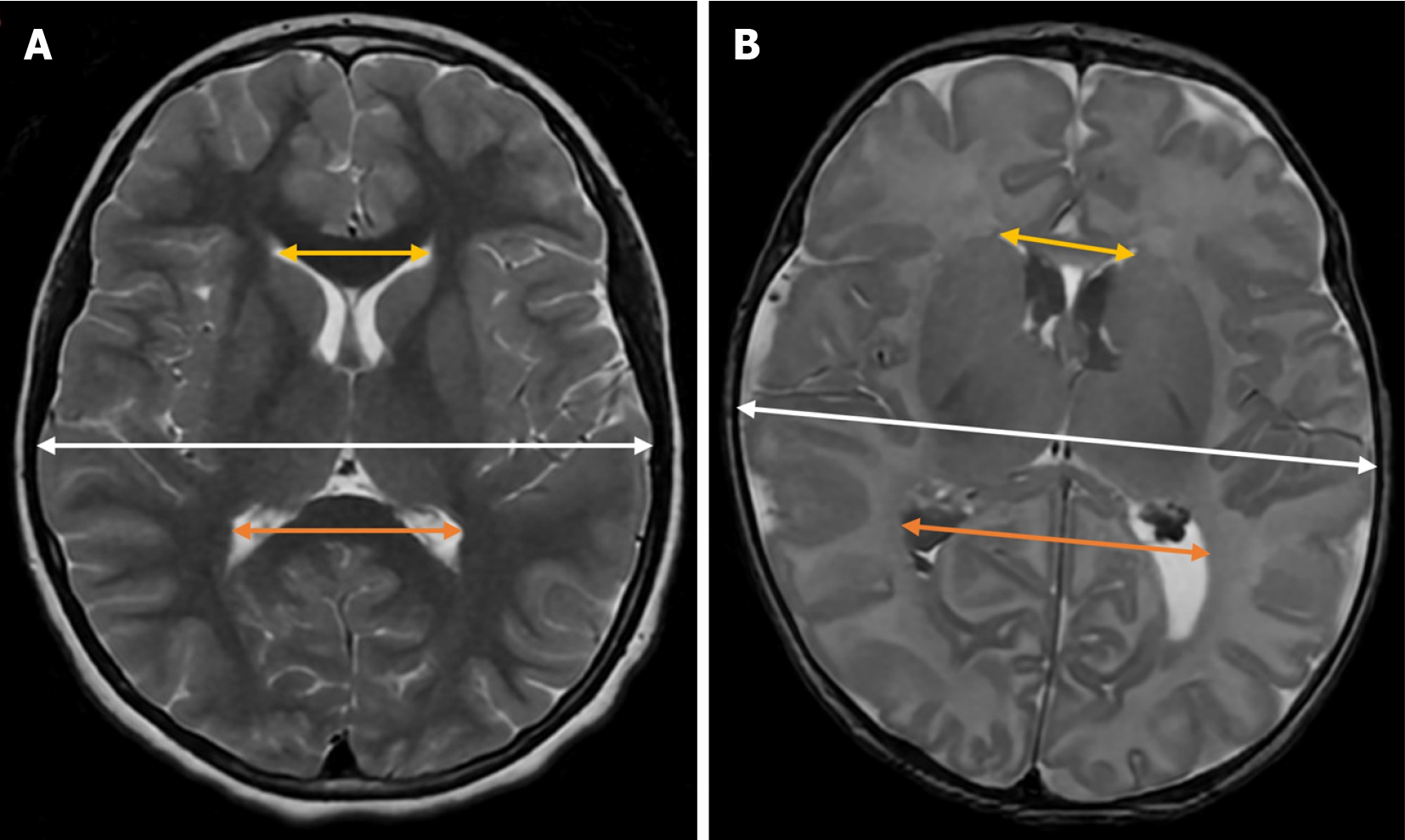
This index measures the distance between the posterior edge of the thalamus at its junction with the choroid plexus and the outermost point of the occipital horn of the lateral ventricle in the parasagittal plane[20] (Figure 2).
Although some authors consider a thalamo-occipital distance greater than 24 mm to be pathological[19,21], reference values can vary depending on the specific population and gestational age[22].
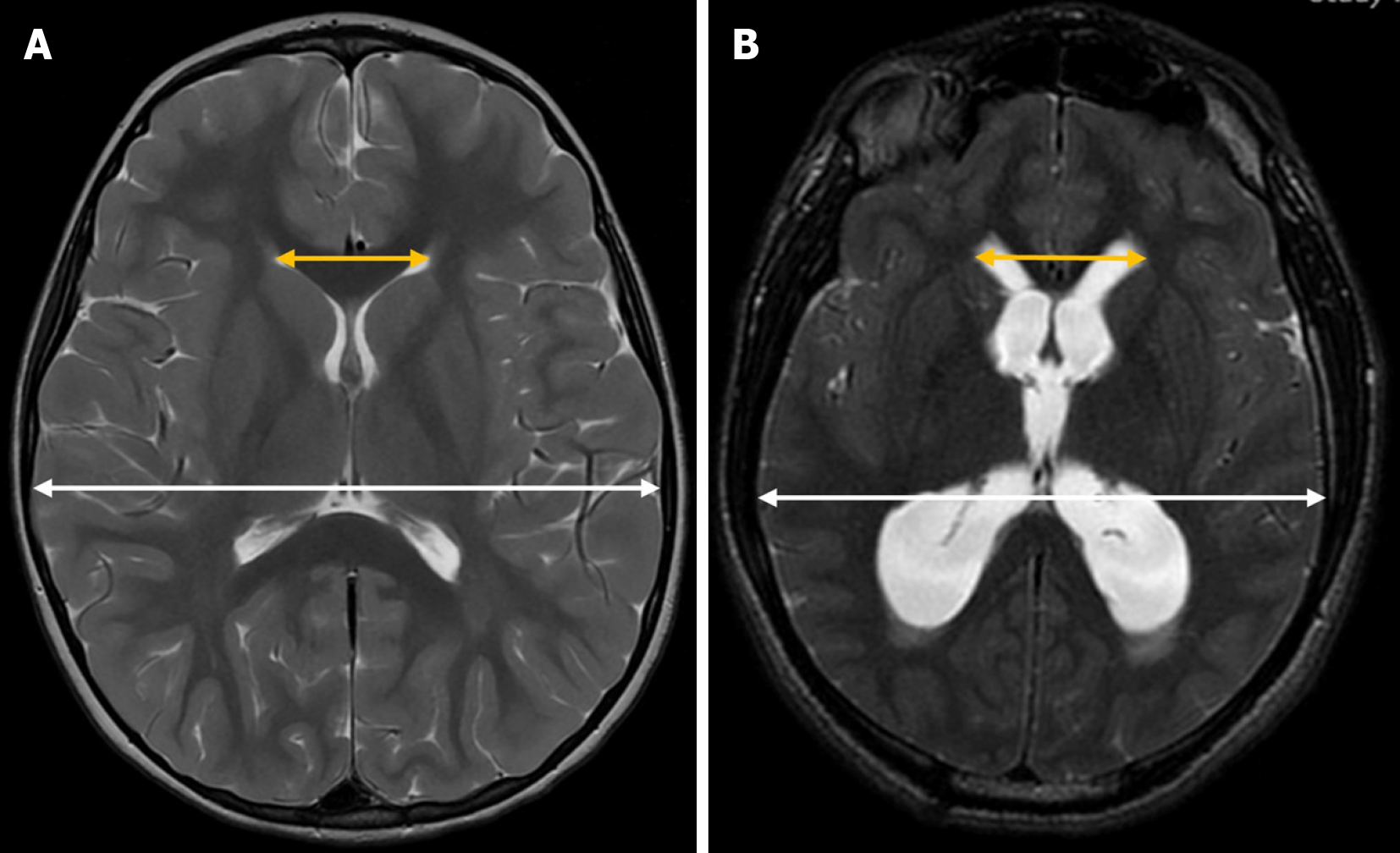
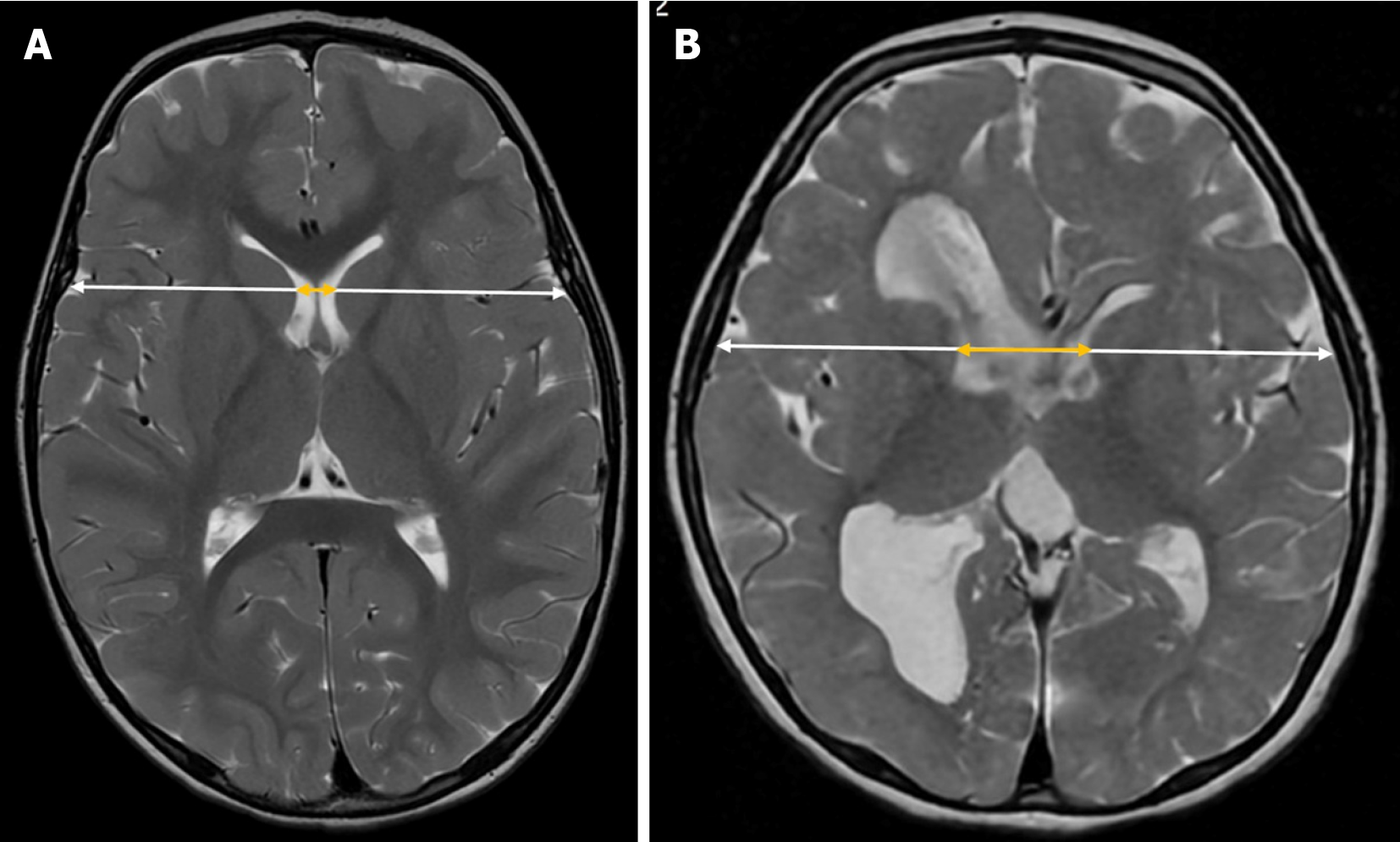
Evans’ index is a simple neuroimaging measurement used to assess the relative size of the lateral ventricles in proportion to the cranial size. It is calculated by dividing the maximum width of the frontal horns of the lateral ventricles by the maximum inner diameter of the skull in the same axial plane on brain MRI or CT scans[23] (Figure 3). In pediatric patients, it is also applied using transfontanellar ultrasound[3,24], where it is obtained from a strict coronal plane.
In adults, ventricular dilation is generally assumed when this index exceeds 0.3. However, in the pediatric population, standardized cutoff values are not well established, as they tend to vary depending on the specific population studied[25].
The anterior-posterior lateral ventricle diameter index is another quantitative measure used to assess lateral ventricle size. To calculate this index, an axial image must be reconstructed based on the anterior commissure-posterior commissure line (Figure 4). Once this plane is obtained, the most caudal axial slice that includes the entire body of the lateral ventricle, without including the thalami, is selected. In this slice, the maximum anteroposterior diameter of the lateral ventricle is measured and divided by the maximum intracranial anteroposterior diameter, measured along the falx cerebri.
This index has been used in adult populations, where a value greater than 0.5 is considered pathological[26,27]. Although its use has not yet been validated in pediatric patients, it holds potential as a useful biomarker for future applications.
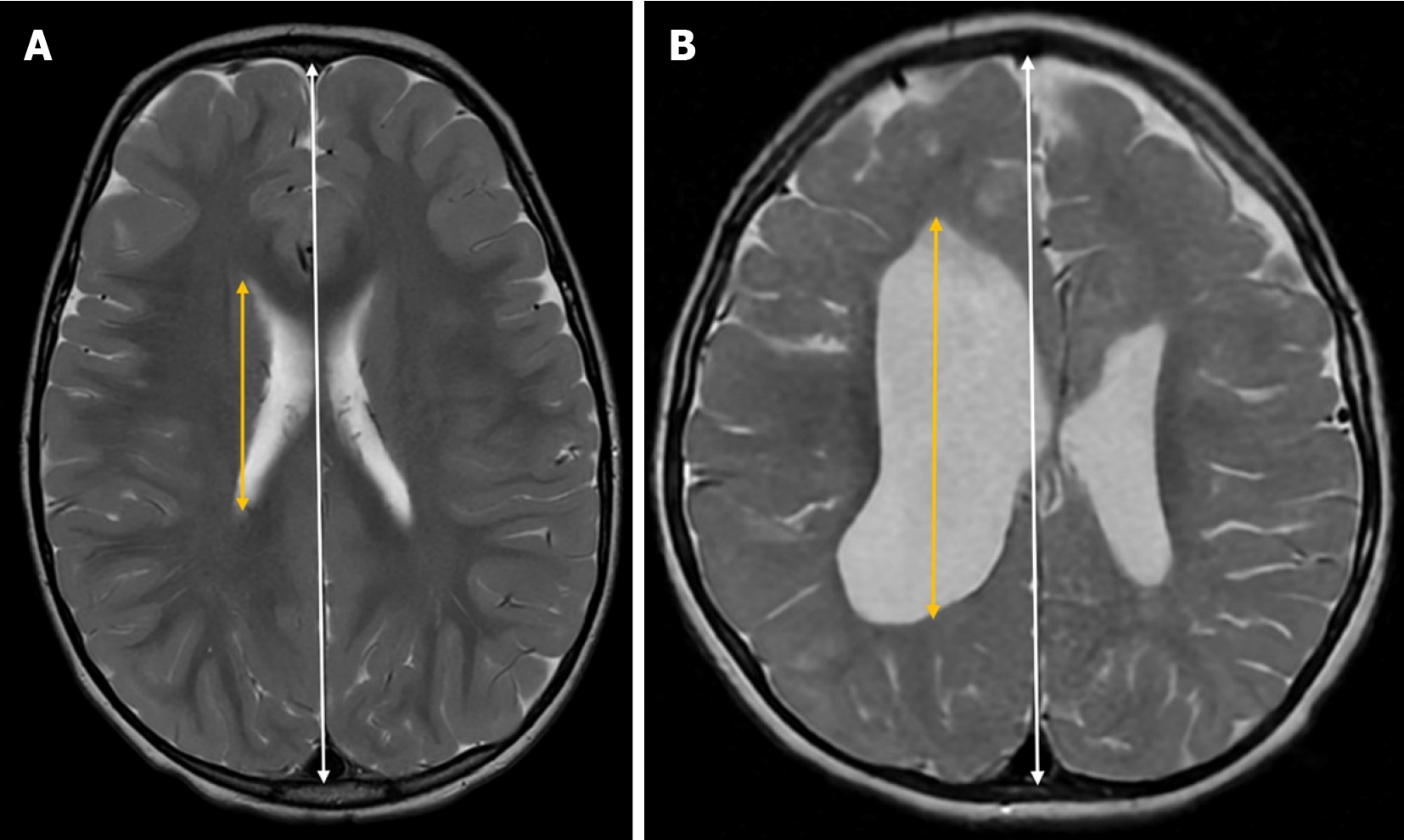
The Cella media index is a neuroimaging tool used in neuroradiology to assess the size of the lateral ventricles relative to brain tissue. It is calculated by dividing the biparietal diameter of the skull by the maximum external diameter of the central portions of the lateral ventricles (Cella media) (Figure 5).
A Cella media index greater than 4 is generally considered normal, while lower values may suggest ventricular dilation. Although reference values for the Cella media index are generally considered stable[28,29], studies have reported variations across different age groups[30]. Additionally, its correlation with third ventricle size supports its reliability as a biomarker for ventricular dilation[29].
Unlike other indices, such as Evans’ index, which focuses solely on the size of the frontal horns of the lateral ventricles, the Fronto-occipital horn ratio (FOHR) takes into account both the frontal and occipital horns, providing a more comprehensive assessment of global ventricular dilation.
To calculate this index, the maximum diameter of both the frontal and occipital horns of the lateral ventricles is measured in the same axial plane at the level of the foramen of Monro. The sum of these measurements is then divided by twice the maximum intracranial biparietal diameter[31] (Figure 6).
In healthy pediatric patients, the mean FOHR is approximately 0.37, with a 95% confidence interval ranging from 0.36 to 0.38, regardless of age[31]. A value exceeding 0.44 is considered indicative of ventricular enlargement[32].
The Bicaudate index is a neuroimaging tool used to assess the size of the frontal horns of the lateral ventricles in relation to the transverse diameter of the brain. To calculate this index, the distance between the most lateral portions of the frontal horns of the lateral ventricles at the level of the caudate nuclei is measured and then divided by the transverse brain diameter at the same level (along the same reference line used for ventricular measurements) in the axial plane on CT or MRI[33] (Figure 7).
There are no standardized reference values for this index. As with other ventricular indices, its application in transfontanellar ultrasound is technically challenging, limiting its use primarily to CT and MRI. Moreover, since it assumes ventricular symmetry, its diagnostic reliability decreases in cases of asymmetry or congenital ventricular malformations.
Volumetric analysis provides a more comprehensive evaluation of ventricular size by quantifying the total volume of CSF within the ventricular system, rather than relying on isolated linear dimensions. Unlike traditional indices, which measure specific distances or ratios from a single imaging slice, volumetric techniques assess the entire ventricular space, offering a more accurate representation of global ventricular enlargement.
One of the key advantages of volumetric measurements is their ability to detect subtle changes in ventricular expansion that may not be apparent using linear indices. This is particularly relevant in conditions such as chronic ventriculomegaly, where progressive ventricular dilation may occur without abrupt changes in shape that would be captured by traditional measurements. Additionally, volumetric assessments better correlate with functional impairment, providing a stronger link between radiological findings and clinical symptoms[34].
Despite these benefits, volumetric analysis has limitations that have slowed its widespread adoption. The technique requires high-resolution imaging, specialized software, and longer processing times, making it less practical in routine clinical workflows. Variability in segmentation protocols and differences in reference standards between institutions can also affect reproducibility.
Longitudinal imaging plays a crucial role in the assessment and management of hydrocephalus, providing valuable information about ventricular changes over time. Serial imaging allows clinicians to monitor disease progression, assess treatment response, and detect early signs of complications, such as shunt malfunction or progressive ventricular dilation. The ability to track ventricular size and fluid distribution over extended periods helps refine clinical decision-making, ensuring timely intervention when necessary. In addition to structural assessments, emerging evidence suggests that incorporating physiological parameters, such as cerebral oxygenation, cerebral blood flow, and cerebral metabolic rate of oxygen consumption, may improve the ability to predict long-term outcomes[35].
A key advantage of serial imaging is its capacity to reveal gradual ventricular changes that may not be apparent in a single imaging study. In some cases, ventricular dilation may remain stable for long periods, while in others, subtle enlargement can signal the need for closer monitoring or therapeutic adjustments. Tracking these variations is particularly important in infants and young children, whose brains are still developing and adapting to shifts in intracranial fluid homeostasis. However, current studies on longitudinal monitoring in pediatric hydrocephalus are limited in scope, often focusing on small cohorts or specific age groups. Expanding research across a broader pediatric population is essential to establish standardized imaging norms that can guide clinical management in different developmental stages.
Some studies have found that traditional ventricular indices show limited direct correlation with intracranial pressure[36,37], while optic nerve sheath diameter has demonstrated a moderate association with pressure dynamics[37]. These findings suggest that intracranial pressure monitoring in hydrocephalus may benefit from incorporating multiple imaging biomarkers rather than relying solely on ventricular size.
Furthermore, post-treatment brain growth, as assessed by longitudinal imaging, has been associated with better neurodevelopmental outcomes, suggesting that brain volume recovery could serve as a useful biomarker for treatment success. Studies indicate that infants with greater brain growth following intervention exhibit improved cognitive and motor development[38,39]. In addition, progressive ventricular enlargement, particularly when accompanied by white matter abnormalities, has been linked to an increased risk of neurodevelopmental impairment. Neonatal MRI findings have shown that white matter injury, including periventricular volume loss, signal abnormalities, and corpus callosal thinning, strongly predict cognitive and motor deficits later in childhood[40].
The application of artificial intelligence (AI) and machine learning in the diagnosis and management of hydrocephalus has gained significant attention due to its potential to improve accuracy, reproducibility, and efficiency in imaging-based assessments[41]. These technologies offer automated solutions for ventricular segmentation, volumetric analysis, and prediction of disease progression, reducing reliance on manual measurements, which are often time-consuming and prone to interobserver variability[42].
One of the most promising applications of AI in hydrocephalus management is longitudinal monitoring[41]. Tracking ventricular changes over time is essential for assessing disease progression and treatment response, yet traditional methods rely on manual measurements that may lack sensitivity to subtle variations. AI-driven tools can automate this process by integrating large-scale imaging datasets and applying deep learning models to identify early markers of deterioration. In particular, AI-powered predictive modeling has the potential to forecast ventricular dilation trends, allowing for earlier intervention and better individualized patient management[6,43].
However, the effectiveness of AI in longitudinal analysis depends on access to multicenter datasets spanning different pediatric age groups. Current studies often focus on specific cohorts, limiting the ability to establish standardized imaging norms across developmental stages. Expanding the use of AI through collaborative research networks and diverse datasets is critical to improving its applicability in routine clinical practice[44].
Machine learning models have been extensively developed for the segmentation of ventricular structures, enabling precise measurement of ventricular volume from MRI and CT scans[45]. Convolutional neural networks, such as U-Net, have demonstrated high accuracy in identifying and segmenting the lateral ventricles, even in complex cases where manual segmentation is challenging[41]. These models allow for objective quantification of ventricular size, which is critical for tracking hydrocephalus progression and treatment response.
Compared to traditional manual or semi-automated segmentation techniques, AI-driven algorithms offer several advan
AI-based imaging analysis is already being applied in clinical and research settings to improve hydrocephalus diagnosis and monitoring. Recent studies have demonstrated that deep learning models can accurately differentiate between normal ventricular anatomy and pathological enlargement, aiding in early intervention planning[45,46]. Moreover, AI-driven natural language processing systems can extract relevant clinical information from radiology reports to stan
The diagnosis and monitoring of hydrocephalus still face several challenges that must be addressed to improve clinical outcomes. One of the most pressing issues is the need for standardized imaging protocols, as variations in MRI angu
Imaging biomarkers are fundamental in the diagnosis and longitudinal monitoring of hydrocephalus, providing essential information about ventricular morphology, CSF dynamics, and neurodevelopmental outcomes. While traditional imaging techniques remain indispensable, the integration of AI and automated analysis has the potential to enhance measurement reproducibility and diagnostic efficiency. Overcoming current limitations, such as the lack of standardized imaging protocols and the need for pediatric-specific normative values, will be key to advancing the field and ensuring optimal clinical care for children with hydrocephalus.
| 1. | Miselli F, Guidotti I, Di Martino M, Bedetti L, Minotti C, Spaggiari E, Malmusi G, Lugli L, Corso L, Berardi A. Cranial ultrasound in preterm infants ≤ 32 weeks gestation-novel insights from the use of very high-frequency (18-5 MHz) transducers: a case series. Eur J Pediatr. 2024;183:3589-3598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 2. | Vinci F, Tiseo M, Colosimo D, Calandrino A, Ramenghi LA, Biasucci DG. Point-of-care brain ultrasound and transcranial doppler or color-coded doppler in critically ill neonates and children. Eur J Pediatr. 2024;183:1059-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Navarro-Ballester A, Rovira-Ferrando RE, Ródenas-Hernández JM, Bandura A, Fernández-García P, Marco Doménech SF. New reference nomograms for the study of ventricular size in preterm infants. Radiologia (Engl Ed). 2024;66:219-227. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Yang Q, Huang K, Zhang G, Li X, Gao Y, Zhao C. Relationship between the volume of ventricles, brain parenchyma and neurocognition in children after hydrocephalus treatment. Childs Nerv Syst. 2024;41:48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Nowosławska E, Gwizdała D, Barańska D, Grzelak P, Podgórski M, Zakrzewski K, Polis B, Stasiołek M, Polis L. The oscillatory flow of the cerebrospinal fluid in the Sylvian aqueduct and the prepontine cistern measured with phase contrast MRI in children with hydrocephalus-a preliminary report. Childs Nerv Syst. 2018;34:845-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Quon JL, Han M, Kim LH, Koran ME, Chen LC, Lee EH, Wright J, Ramaswamy V, Lober RM, Taylor MD, Grant GA, Cheshier SH, Kestle JRW, Edwards MSB, Yeom KW. Artificial intelligence for automatic cerebral ventricle segmentation and volume calculation: a clinical tool for the evaluation of pediatric hydrocephalus. J Neurosurg Pediatr. 2021;27:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Hwang M. Introduction to contrast-enhanced ultrasound of the brain in neonates and infants: current understanding and future potential. Pediatr Radiol. 2019;49:254-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Faure F, Baranger J, Alison M, Boutillier B, Frérot A, Lim C, Planchette G, Prigent M, Tanter M, Baud O, Biran V, Demené C. Quantification of brain-wide vascular resistivity via ultrafast Doppler in human neonates helps early detection of white matter injury. J Cereb Blood Flow Metab. 2024;44:1577-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Fakhari N, Aguet J, Nguyen MB, Zhang N, Mertens L, Jain A, Sled JG, Villemain O, Baranger J. Automated classification of cerebral arteries and veins in the neonate using ultrafast doppler spectrogram. Phys Med Biol. 2024;69. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Kishimoto J, de Ribaupierre S, Salehi F, Romano W, Lee DS, Fenster A. Preterm neonatal lateral ventricle volume from three-dimensional ultrasound is not strongly correlated to two-dimensional ultrasound measurements. J Med Imaging (Bellingham). 2016;3:046003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Faure F, Alison M, Francavilla M, Boizeau P, Guilmin Crepon S, Lim C, Planchette G, Prigent M, Frérot A, Tanter M, Demené C, Baud O, Biran V. Transfontanellar shear wave elastography of the neonatal brain for quantitative evaluation of white matter damage. Sci Rep. 2024;14:11827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | El-Ali AM, Subramanian S, Krofchik LM, Kephart MC, Squires JH. Feasibility and reproducibility of shear wave elastography in pediatric cranial ultrasound. Pediatr Radiol. 2020;50:990-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Knight MJ, Smith-Collins A, Newell S, Denbow M, Kauppinen RA. Cerebral White Matter Maturation Patterns in Preterm Infants: An MRI T2 Relaxation Anisotropy and Diffusion Tensor Imaging Study. J Neuroimaging. 2018;28:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Zhou L, Liu X, He G, Chen M, Zeng S, Sun C. Alteration of fractional anisotropy in preterm-born individuals: a systematic review and meta-analysis. J Obstet Gynaecol. 2024;44:2371956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 15. | Sartori JT, Ambros LE, Callegaro GIS. Alterations on magnetic resonance imaging of the neonatal brain: correlations with prenatal risk factors and transfontanellar ultrasound findings. Radiol Bras. 2022;55:280-285. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Burkitt K, Kang O, Jyoti R, Mohamed AL, Chaudhari T. Comparison of cranial ultrasound and MRI for detecting BRAIN injury in extremely preterm infants and correlation with neurological outcomes at 1 and 3 years. Eur J Pediatr. 2019;178:1053-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Agegnehu A, Tenaw B, Gebrewold Y, Jemberie M. Morphometric Study of Frontal Horn of Lateral Ventricle of Brain and Its Correlation with Age, Gender and Side among Adults in the University of Gondar Comprehensive Specialized Hospital, Gondar, Northwest Ethiopia, 2019. Austin J Anat. 2021;8:1096. [DOI] [Full Text] |
| 18. | Montero Yéboles R, Mayordomo Colunga J, Muñoyerro Sesmero M, Gómez Luque JM, Rodríguez Campoy P, González Cortés R. Ecografía transfontanelar. Hemorragia, isquemia cerebral e hidrocefalia. Protoc Diagn Ter Pediatr. 2021;1:447-462. |
| 19. | Llorens-Salvador R, Moreno-Flores A. The ABCs of transfontanellar ultrasound and more. Radiologia. 2016;58 Suppl 2:129-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Liu G, Nie C. Ultrasonic Diagnosis and Management of Posthemorrhagic Ventricular Dilatation in Premature Infants: A Narrative Review. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Valverde E, Ybarra M, Benito AV, Bravo MC, Pellicer A. Correction: Posthemorrhagic ventricular dilatation late intervention threshold and associated brain injury. PLoS One. 2024;19:e0315515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Khare A, Gaur AK, Mittal M, Bansal SC. Nomograms and Reference Ranges for Intra-Cranial Ventricular Dimensions in Indian Neonates. Indian J Pediatr. 2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Santhumayor BA, Mashiach E, Meng Y, Rotman L, Golub D, Bernstein K, Vasconcellos FN, Silverman JS, Harter DH, Golfinos JG, Kondziolka D. Predictors of Hydrocephalus Risk After Stereotactic Radiosurgery for Vestibular Schwannomas: Utility of the Evans Index. Neurosurgery. 2025;96:650-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Leijser LM, Scott JN, Roychoudhury S, Zein H, Murthy P, Thomas SP, Mohammad K; Calgary Neonatal Neuro-Critical Care Program. Post-hemorrhagic ventricular dilatation: inter-observer reliability of ventricular size measurements in extremely preterm infants. Pediatr Res. 2021;90:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Sarı E, Sarı S, Akgün V, Özcan E, Ìnce S, Babacan O, Saldır M, Açıkel C, Başbozkurt G, Yeşilkaya Ş, Kılıc C, Kara K, Vurucu S, Kocaoğlu M, Yeşilkaya E. Measures of ventricles and evans' index: from neonate to adolescent. Pediatr Neurosurg. 2015;50:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | He W, Fang X, Wang X, Gao P, Gao X, Zhou X, Mao R, Hu J, Hua Y, Xia J. A new index for assessing cerebral ventricular volume in idiopathic normal-pressure hydrocephalus: a comparison with Evans' index. Neuroradiology. 2020;62:661-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Zhou X, Xia J. Application of Evans Index in Normal Pressure Hydrocephalus Patients: A Mini Review. Front Aging Neurosci. 2021;13:783092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 28. | Lieb JM, Stippich C, Ahlhelm FJ. [Normal pressure hydrocephalus]. Radiologe. 2015;55:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Kerscher SR, Schweizer LL, Nägele T, Weichselbaum A, Haas-Lude K, Schuhmann MU. Changes of third ventricle diameter (TVD) mirror changes of the entire ventricular system after initial therapy and during follow-up in pediatric hydrocephalus. Eur J Paediatr Neurol. 2019;23:571-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Palla R. Evaluation of Cella Media Index by Computed Tomography in Hydrocephalic Children in Tertiary Hospital of TelanganaA Retrospective Study. J Clin Diagn Res. 2023;. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 31. | Radhakrishnan R, Brown BP, Kralik SF, Bain D, Persohn S, Territo PR, Jea A, Karmazyn B. Frontal Occipital and Frontal Temporal Horn Ratios: Comparison and Validation of Head Ultrasound-Derived Indexes With MRI and Ventricular Volumes in Infantile Ventriculomegaly. AJR Am J Roentgenol. 2019;213:925-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Retrouvey M, Vossough A, Zandifar A, Bellah RD, Heuer GG, Flibotte J, Tierradentro-Garcia LO, Saade-Lemus S, Kim JDU, Back SJ, Kaplan SL. Validation of Sonographic Fronto-Occipital Ratio Based on Anatomical Landmarks Compared to MR/CT-Derived Indexes in Children with Chiari II and Ventriculomegaly. Pediatr Neurosurg. 2022;57:71-77. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 33. | Ozdikici M. Morphometric Study of the Intracranial Fluid Spaces in Schizophrenia. Neurol India. 2024;72:817-823. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Crook JE, Gunter JL, Ball CT, Jones DT, Graff-Radford J, Knopman DS, Boeve BF, Petersen RC, Jack CR, Graff-Radford NR. Linear vs volume measures of ventricle size: Relation to present and future gait and cognition. Neurology. 2020;94:e549-e556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Vadset TA, Rajaram A, Hsiao CH, Kemigisha Katungi M, Magombe J, Seruwu M, Kaaya Nsubuga B, Vyas R, Tatz J, Playter K, Nalule E, Natukwatsa D, Wabukoma M, Neri Perez LE, Mulondo R, Queally JT, Fenster A, Kulkarni AV, Schiff SJ, Grant PE, Mbabazi Kabachelor E, Warf BC, Sutin JDB, Lin PY. Improving Infant Hydrocephalus Outcomes in Uganda: A Longitudinal Prospective Study Protocol for Predicting Developmental Outcomes and Identifying Patients at Risk for Early Treatment Failure after ETV/CPC. Metabolites. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Kim E, Lim YJ, Park HS, Kim SK, Jeon YT, Hwang JW, Lee YS, Park HP. The lack of relationship between intracranial pressure and cerebral ventricle indices based on brain computed tomography in patients undergoing ventriculoperitoneal shunt. Acta Neurochir (Wien). 2015;157:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Trakulpanitkit A, Tunthanathip T. Comparison of intracranial pressure prediction in hydrocephalus patients among linear, non-linear, and machine learning regression models in Thailand. Acute Crit Care. 2023;38:362-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 38. | Parikh NA. Advanced neuroimaging and its role in predicting neurodevelopmental outcomes in very preterm infants. Semin Perinatol. 2016;40:530-541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 39. | Lind A, Parkkola R, Lehtonen L, Munck P, Maunu J, Lapinleimu H, Haataja L; PIPARI Study Group. Associations between regional brain volumes at term-equivalent age and development at 2 years of age in preterm children. Pediatr Radiol. 2011;41:953-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 40. | Woodward LJ, Clark CA, Bora S, Inder TE. Neonatal white matter abnormalities an important predictor of neurocognitive outcome for very preterm children. PLoS One. 2012;7:e51879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 209] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 41. | Pahwa B, Bali O, Goyal S, Kedia S. Applications of Machine Learning in Pediatric Hydrocephalus: A Systematic Review. Neurol India. 2021;69:S380-S389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (1)] |
| 42. | Yepes-Calderon F, McComb JG. Eliminating the need for manual segmentation to determine size and volume from MRI. A proof of concept on segmenting the lateral ventricles. PLoS One. 2023;18:e0285414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Shahrestani S, Shlobin N, Gendreau JL, Brown NJ, Himstead A, Patel NA, Pierzchajlo N, Chakravarti S, Lee DJ, Chiarelli PA, Bullis CL, Chu J. Developing Predictive Models to Anticipate Shunt Complications in 33,248 Pediatric Patients with Shunted Hydrocephalus Utilizing Machine Learning. Pediatr Neurosurg. 2023;58:206-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 44. | Lee EH, Han M, Wright J, Kuwabara M, Mevorach J, Fu G, Choudhury O, Ratan U, Zhang M, Wagner MW, Goetti R, Toescu S, Perreault S, Dogan H, Altinmakas E, Mohammadzadeh M, Szymanski KA, Campen CJ, Lai H, Eghbal A, Radmanesh A, Mankad K, Aquilina K, Said M, Vossough A, Oztekin O, Ertl-Wagner B, Poussaint T, Thompson EM, Ho CY, Jaju A, Curran J, Ramaswamy V, Cheshier SH, Grant GA, Wong SS, Moseley ME, Lober RM, Wilms M, Forkert ND, Vitanza NA, Miller JH, Prolo LM, Yeom KW. An international study presenting a federated learning AI platform for pediatric brain tumors. Nat Commun. 2024;15:7615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 45. | Maragkos GA, Filippidis AS, Chilamkurthy S, Salem MM, Tanamala S, Gomez-Paz S, Rao P, Moore JM, Papavassiliou E, Hackney D, Thomas AJ. Automated Lateral Ventricular and Cranial Vault Volume Measurements in 13,851 Patients Using Deep Learning Algorithms. World Neurosurg. 2021;148:e363-e373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Kline JE, Sita Priyanka Illapani V, He L, Parikh NA. Automated brain morphometric biomarkers from MRI at term predict motor development in very preterm infants. Neuroimage Clin. 2020;28:102475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Ryska P, Slezak O, Eklund A, Salzer J, Malm J, Zizka J. Variability of Normal Pressure Hydrocephalus Imaging Biomarkers with Respect to Section Plane Angulation: How Wrong a Radiologist Can Be? AJNR Am J Neuroradiol. 2021;42:1201-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/