©The Author(s) 2025.
World J Radiol. Sep 28, 2025; 17(9): 110214
Published online Sep 28, 2025. doi: 10.4329/wjr.v17.i9.110214
Published online Sep 28, 2025. doi: 10.4329/wjr.v17.i9.110214
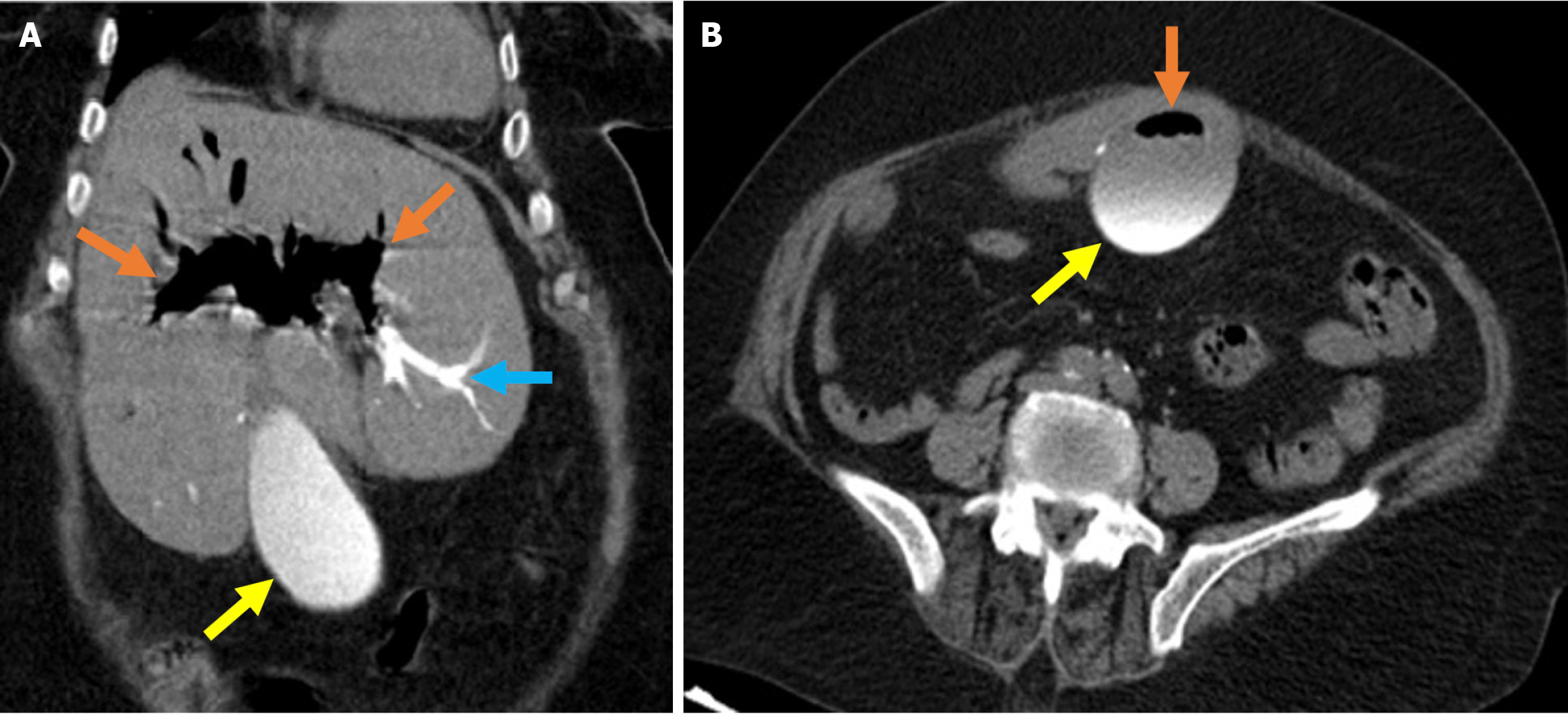
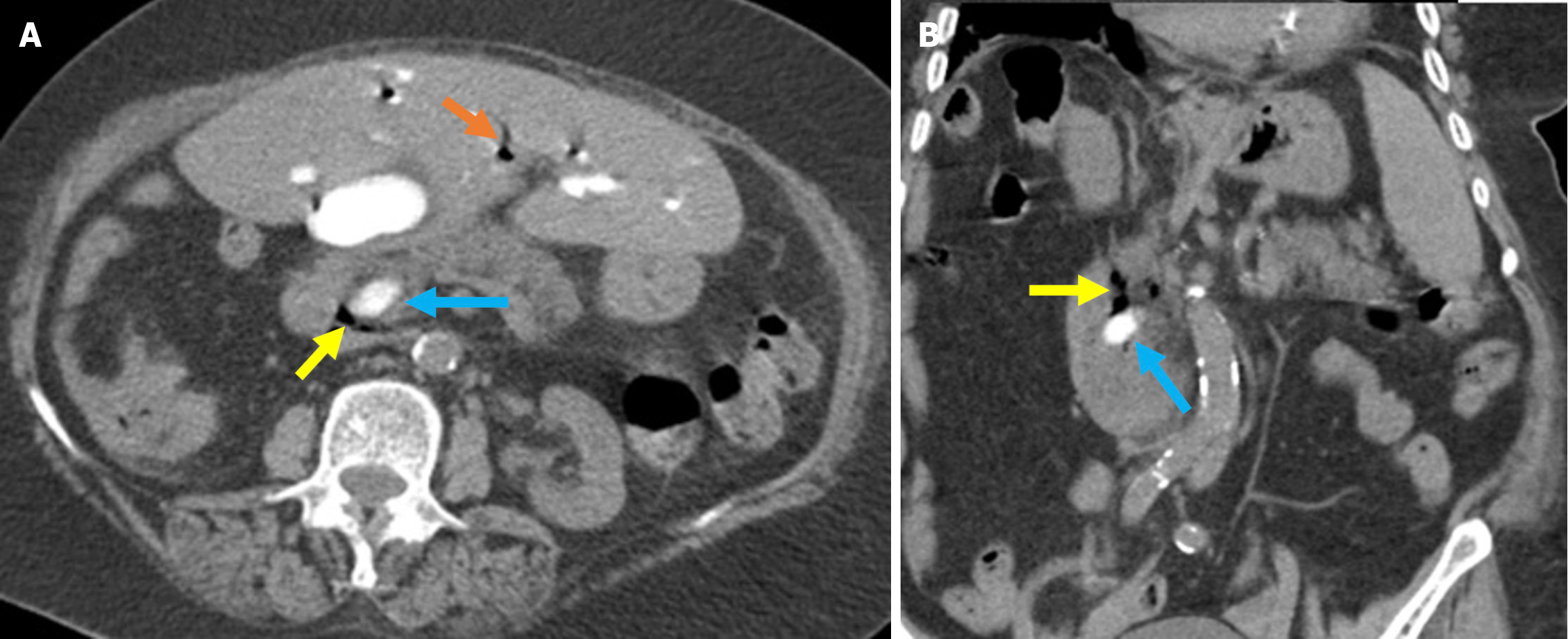
Figure 1 Typical imaging findings of air and contrast material in the biliary system after endoscopic retrograde cholangiopancreatogra
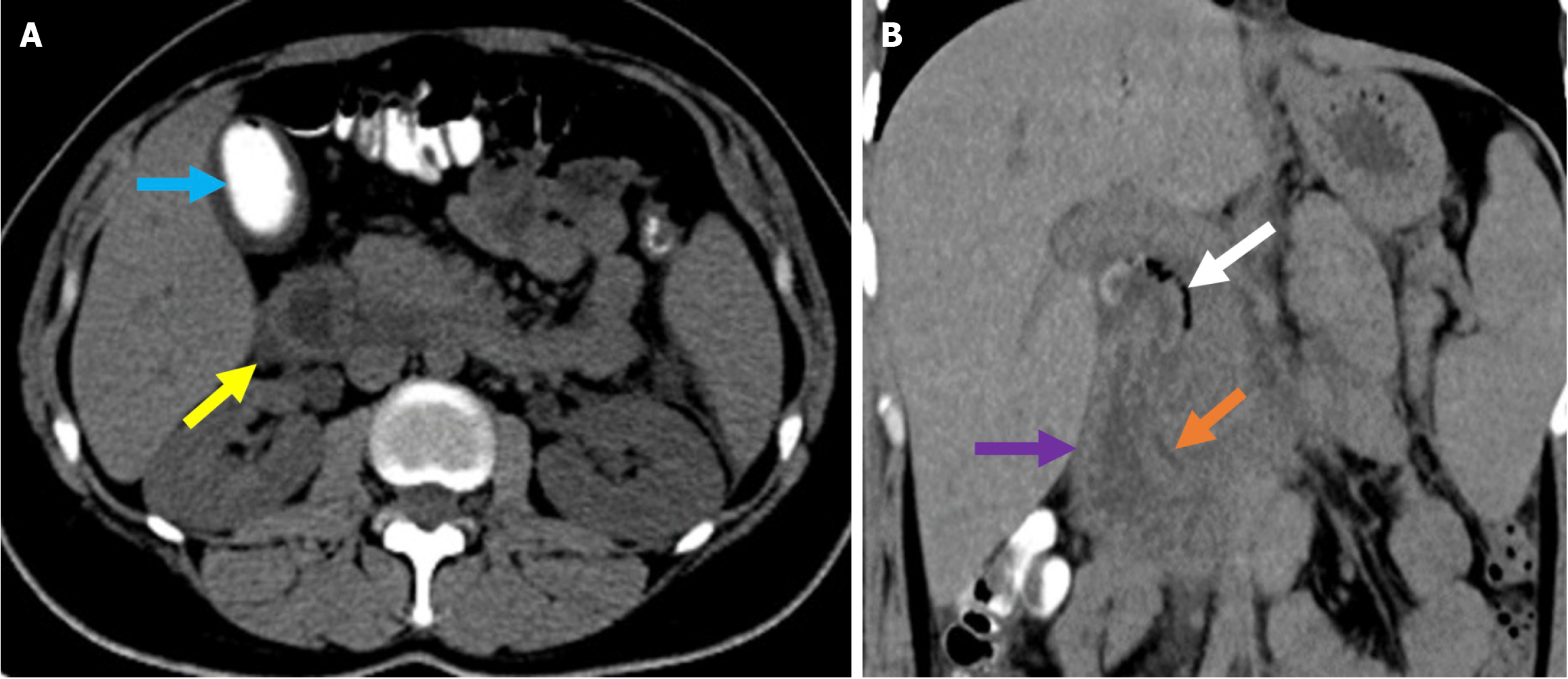
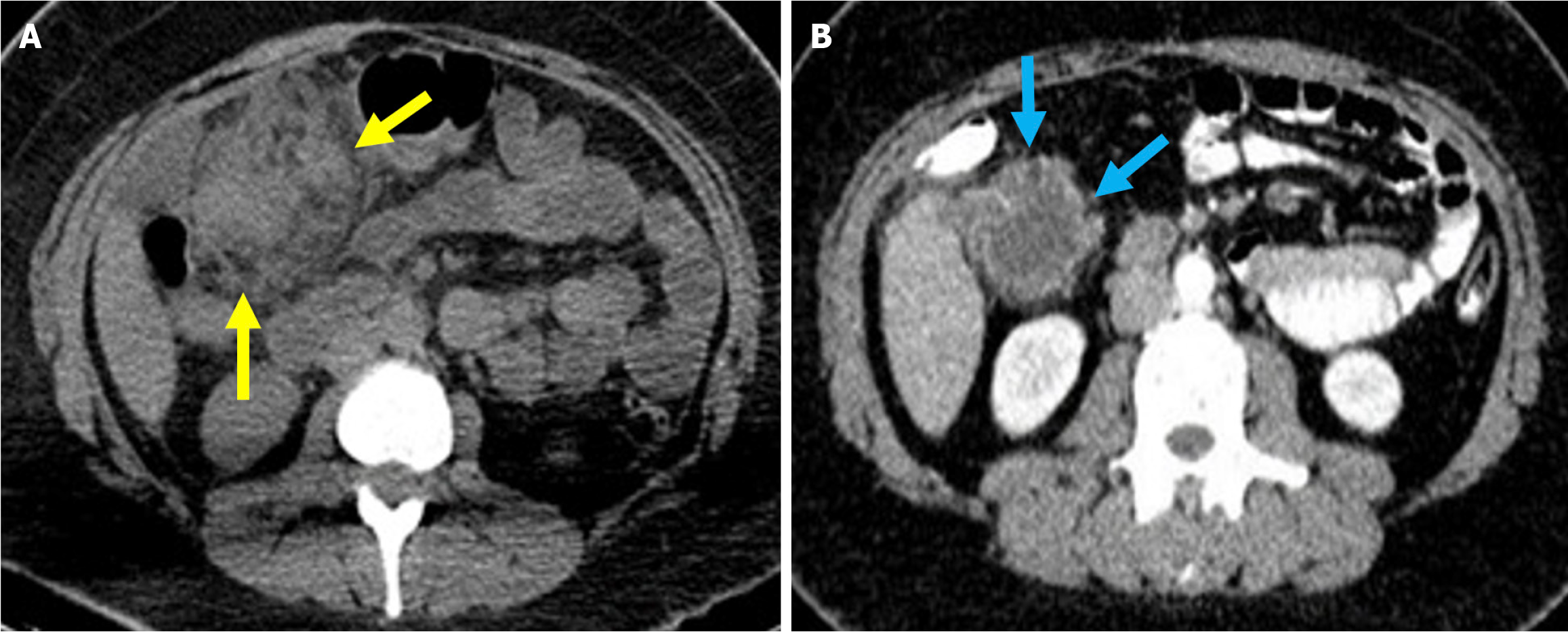
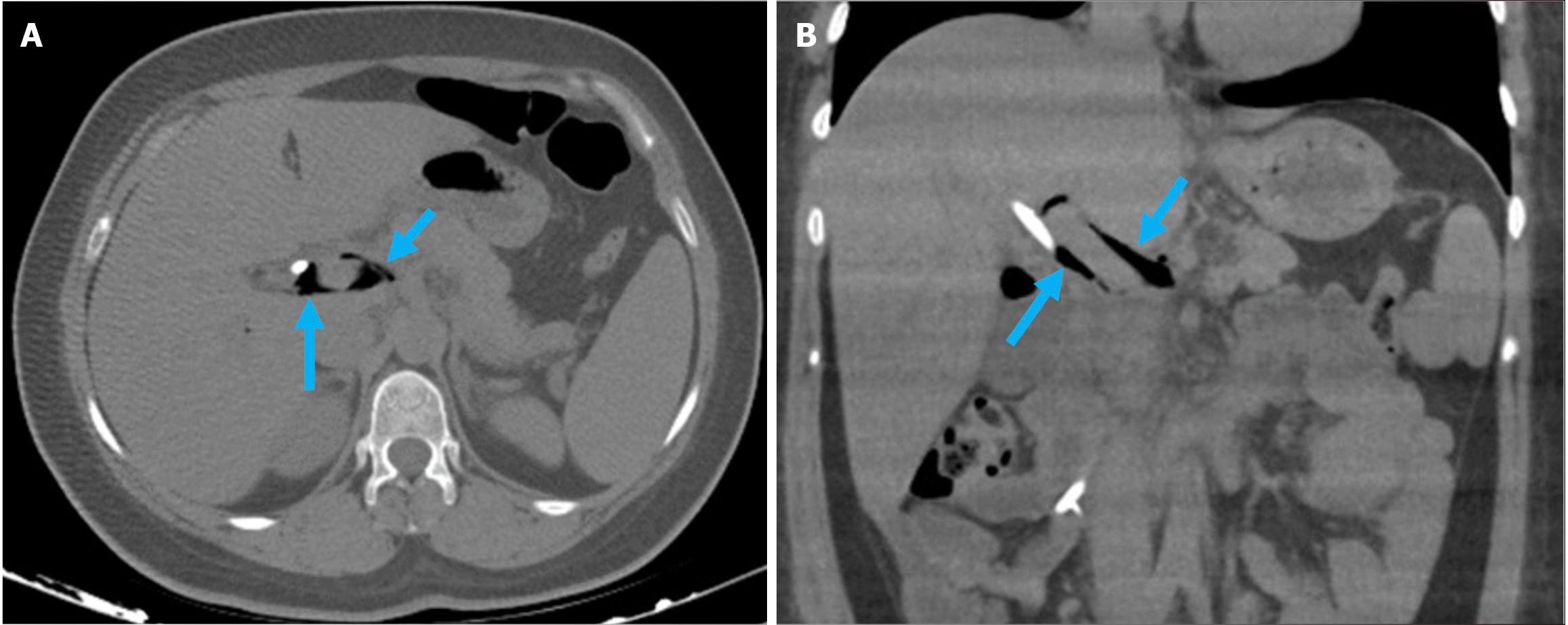
Figure 2 Typical imaging finding of acute duodenitis after endoscopic retrograde cholangiopancreatography.
A and B: Axial (A) and coronal (B) noncontrast computed tomography images revealed post-endoscopic retrograde cholangiopancreatography acute reversible duodenitis in a 26-year-old female patient. Following sphincterotomy, epigastric pain and dyspeptic symptoms developed the next day. Due to the persistence of symptoms during follow-up, imaging was performed. Periduodenal fat stranding (yellow arrow) and increased thickness with submucosal edema of the duodenal wall (purple arrow) were observed. Minimal fluid was noted in the pancreaticoduodenal groove (orange arrow). Expected post-procedural findings include intraductal air within the biliary tree (white arrow) and contrast material within the gallbladder (blue arrow).
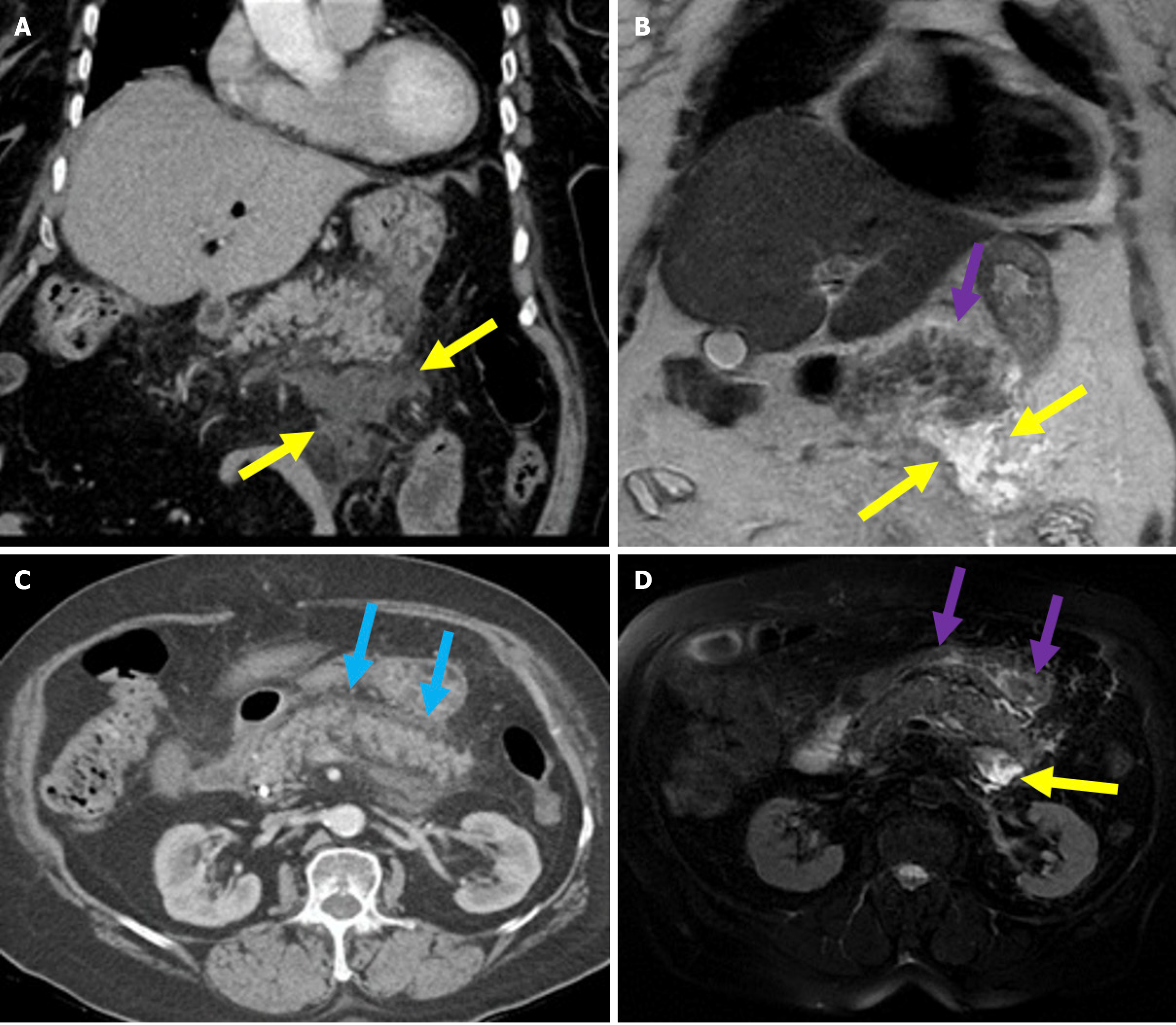
Figure 3 Necrotizing pancreatitis, acute necrotic collections, and walled-off necrosis.
A and B: Contrast-enhanced axial computed tomography (CT); C: T2-weighted magnetic resonance imaging (MRI); D: Post-contrast fat-suppressed T1-weighted MRI. A 76-year-old male patient underwent endoscopic retrograde cholangiopancreatography due to choledocholithiasis. On the evening of the procedure, the patient developed acute abdominal pain that was unresponsive to analgesics. Laboratory tests revealed elevated levels of aspartate aminotransferase, alanine aminotransferase, and lipase. Within the following 24 hours, the patient’s condition deteriorated and developed into shock. Subsequently, the patient required vasopressor support and intubation. Contrast-enhanced CT (A and B) was performed due to the severe abdominal pain. The images showed a markedly enlarged pancreas with areas of non-enhancement consistent with necrotizing pancreatitis that was accompanied by peripancreatic edema (yellow arrows). Heterogeneous acute necrotic collections were also observed in the peripancreatic region (blue arrows). Two months later, an MRI was performed for follow-up. On T2-weighted images (C) a collection with a heterogeneous internal structure and defined wall that was consistent with walled-off necrosis was visualized (orange double-sided arrow). There was a significant loss of pancreatic volume due to necrosis. In the post-contrast T1-weighted fat-suppressed image (D), contrast enhancement of the wall of the collection was noted. A small remnant of pancreatic tissue with normal contrast enhancement was observed (purple arrow).
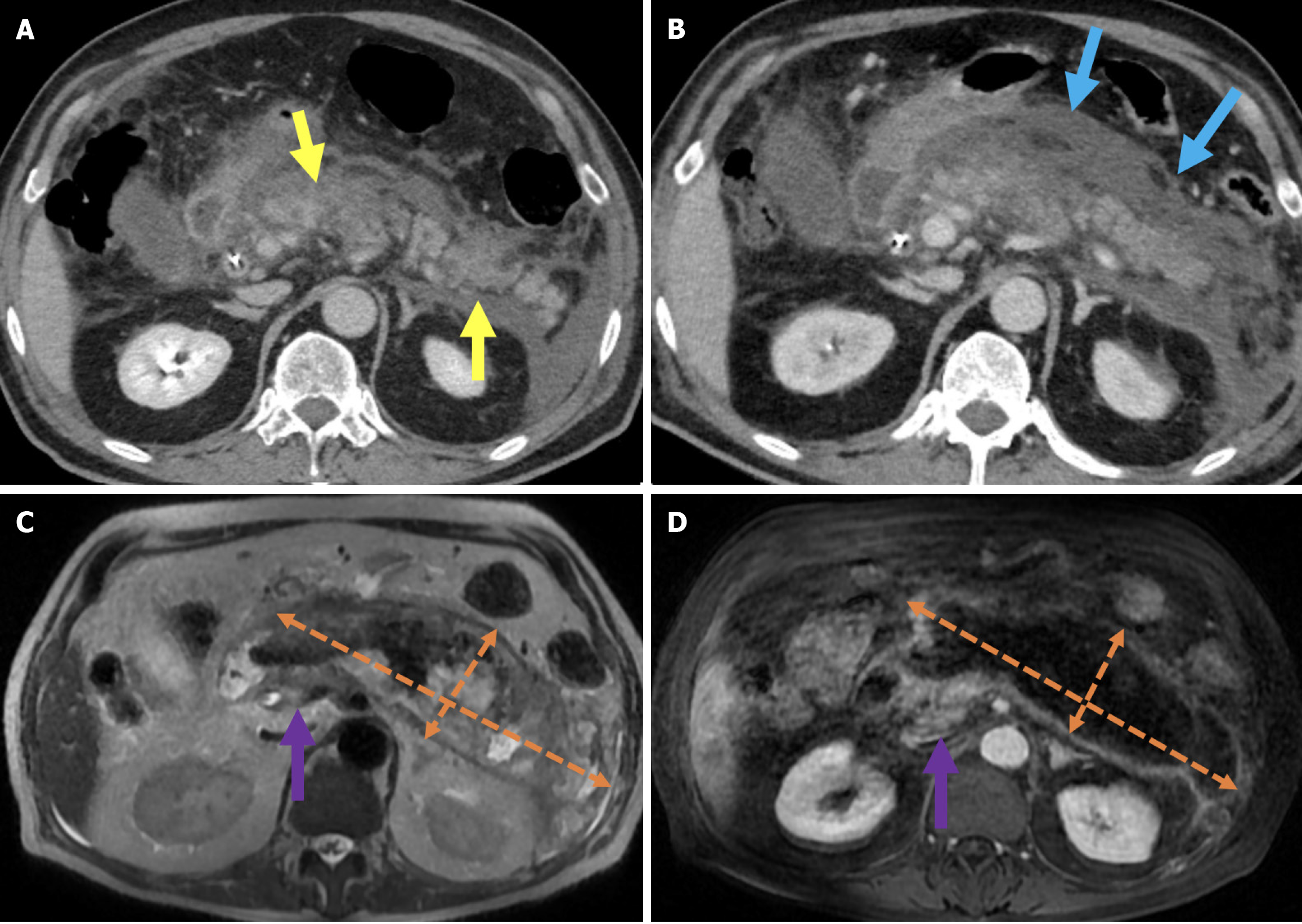
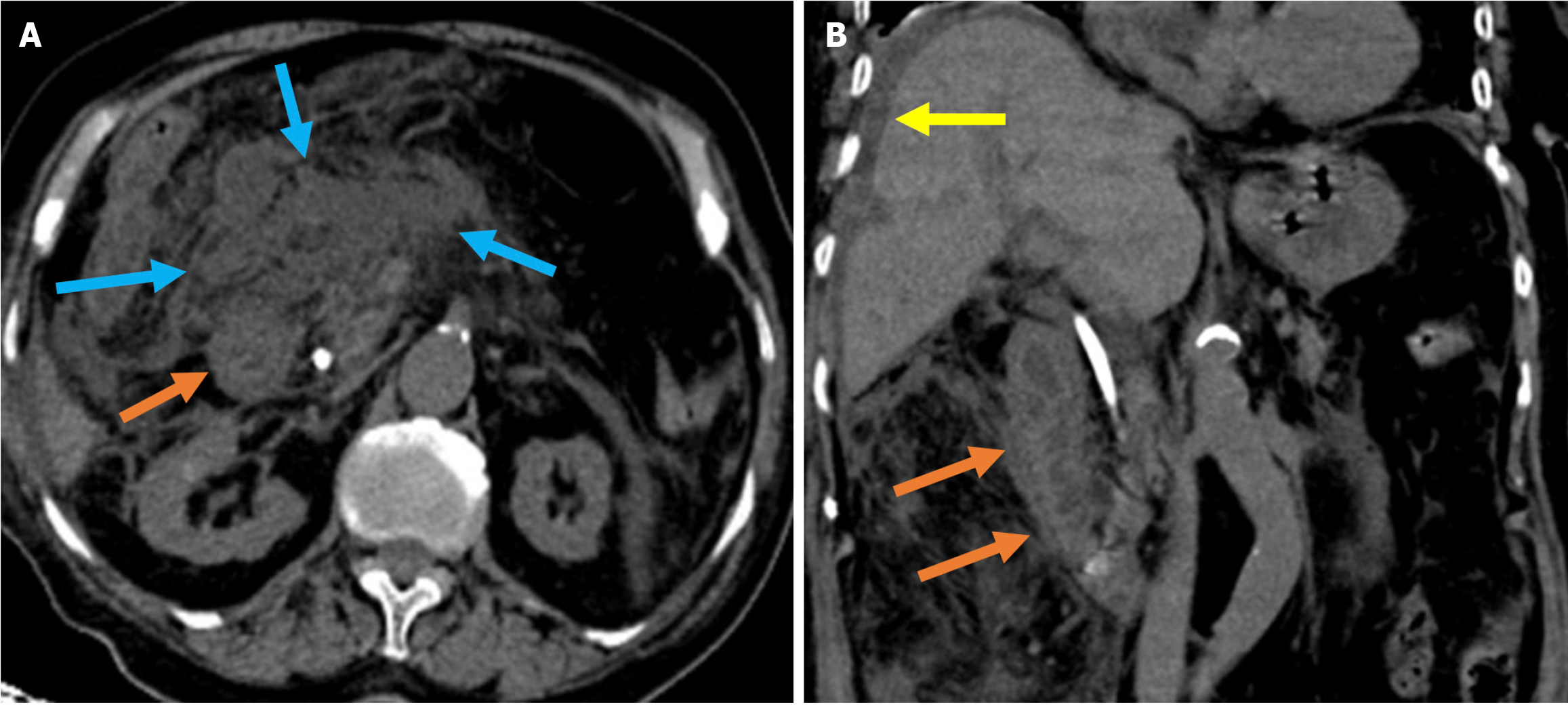
Figure 4 Interstitial edematous pancreatitis and acute peripancreatic fluid collections.
A-C: Contrast-enhanced coronal and axial computed tomography (CT) images (A and C), coronal T2-weighted magnetic resonance imaging (B); D: Fat-suppressed axial T2-weighted magnetic resonance imaging. The 81-year-old female patient diagnosed with post-endoscopic retrograde cholangiopancreatography pancreatitis had presented with epigastric pain and elevated amylase and C-reactive protein levels. CT images showed preserved pancreatic enhancement that was consistent with the absence of necrosis and peripancreatic fat stranding (blue arrow). The pancreatic enlargement and increased T2 signal intensity were suggestive of edema (purple arrow). The pancreas also exhibited mildly diffuse, lace-like T2 hyperintensity within the parenchyma that was associated with a small acute peripancreatic fluid collection (yellow arrow).
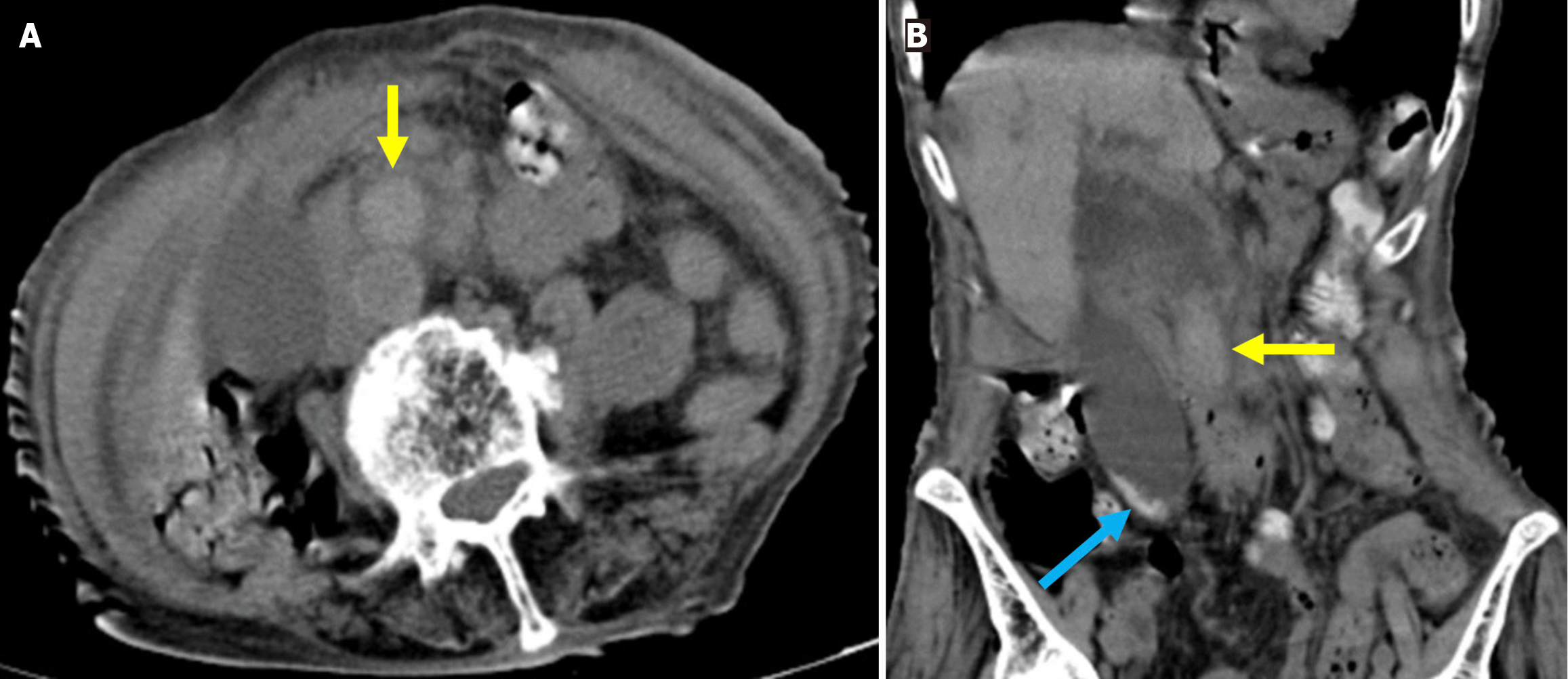
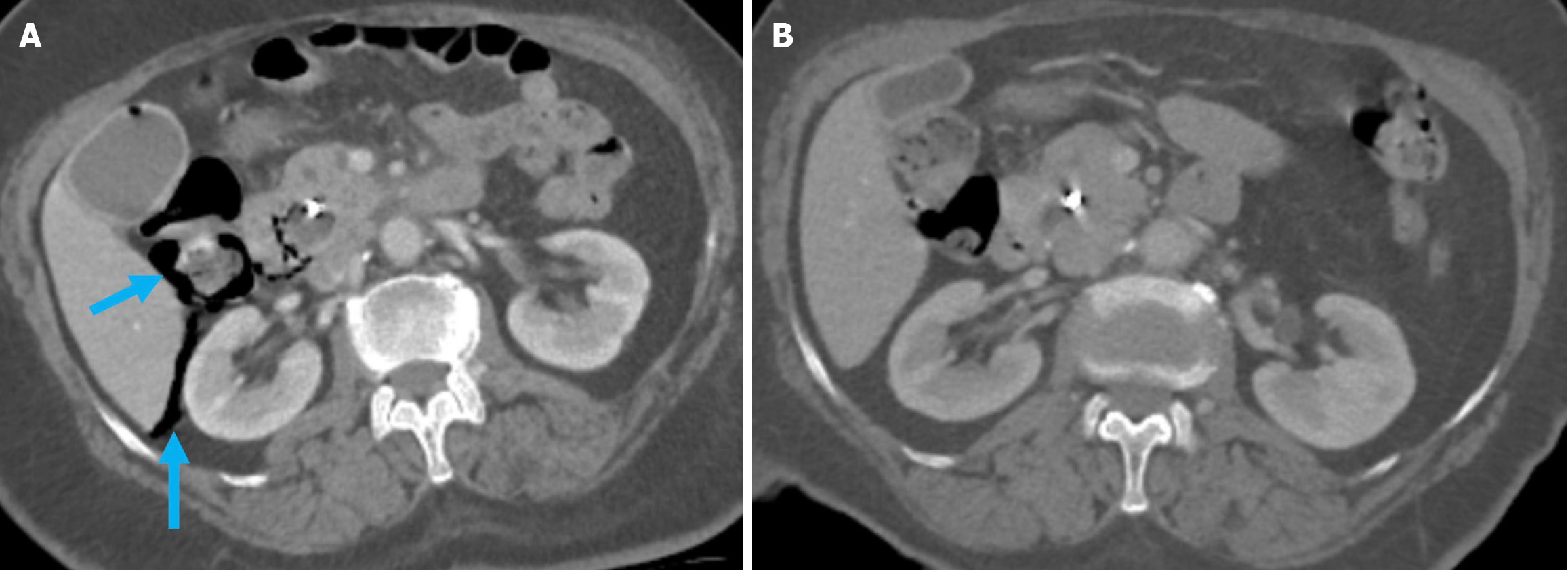
Figure 5 Acute peripancreatic fluid collections and pseudocyst.
A: Noncontrast axial computed tomography (CT); B: Contrast-enhanced axial CT after 15 months. A 28-year-old female patient underwent endoscopic retrograde cholangiopancreatography for choledocholithiasis. Ten hours after the procedure, the patient developed severe upper quadrant abdominal pain accompanied by fever. No signs of peritonitis were observed, and there was no evidence of hemodynamic instability. Laboratory tests revealed elevated levels of pancreatic enzymes and inflammatory markers. Due to suspected pancreatitis a noncontrast CT performed on post-procedure day 1 (A) demonstrated fluid loculation in the infrahepatic and paraduodenal region that was consistent with acute peripancreatic fluid collections (yellow arrow). The patient was diagnosed with post-endoscopic retrograde cholangiopancreatography edematous pancreatitis and was managed conservatively. A follow-up contrast-enhanced CT performed 1.5 months later (B) showed fluid collection in the exact location with pseudocapsule formation that was consistent with a pseudocyst (blue arrow).
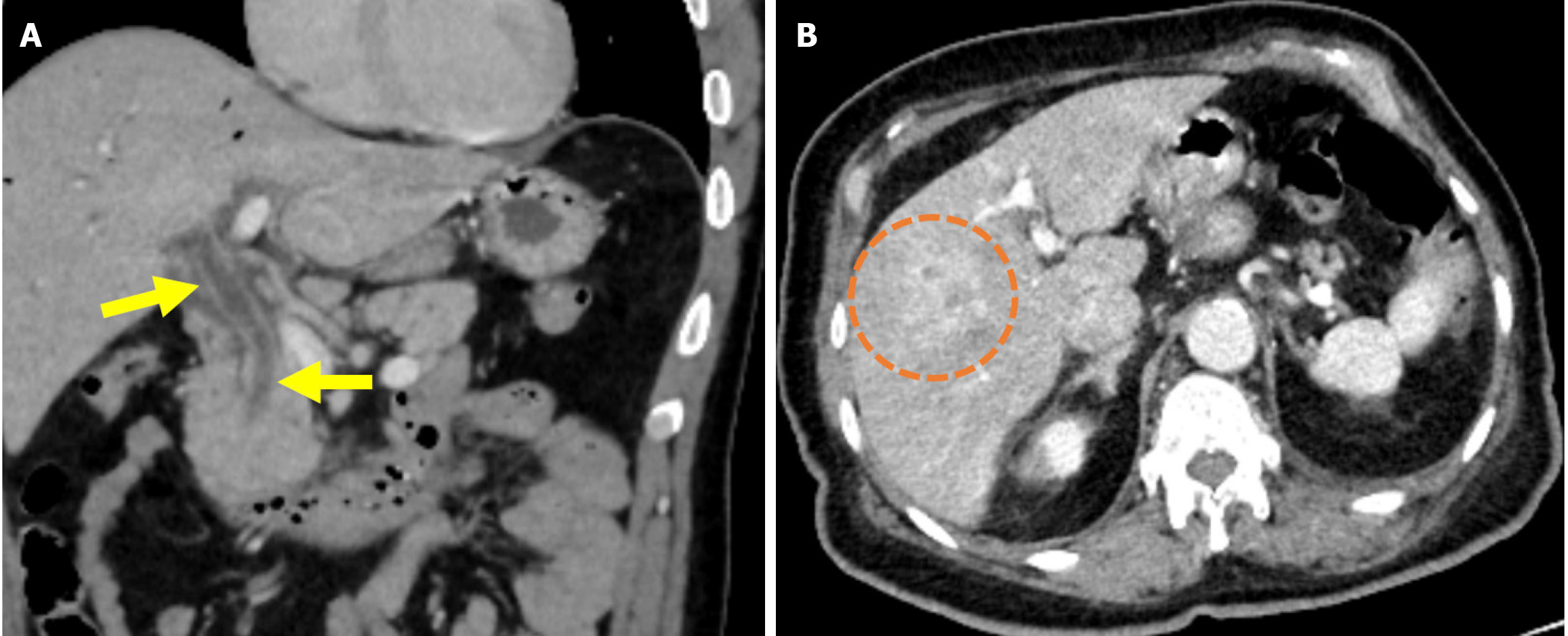
Figure 6 Cholangitis.
A and B: Coronal (A) and axial (B) contrast-enhanced computed tomography images showed findings in a 55-year-old male patient who presented with abdominal pain, chills, and jaundice 1 week after endoscopic retrograde cholangiopancreatography. The patient’s laboratory results revealed elevated levels of gamma-glutamyl transferase, alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, bilirubin, and acute phase reactants. The patient was subsequently diagnosed with cholangitis. The common bile duct appeared dilated with thickened and enhanced walls (yellow arrows). There was also dilatation of the intrahepatic bile ducts and periductal areas of increased perfusion, likely secondary to inflammation (orange circle).
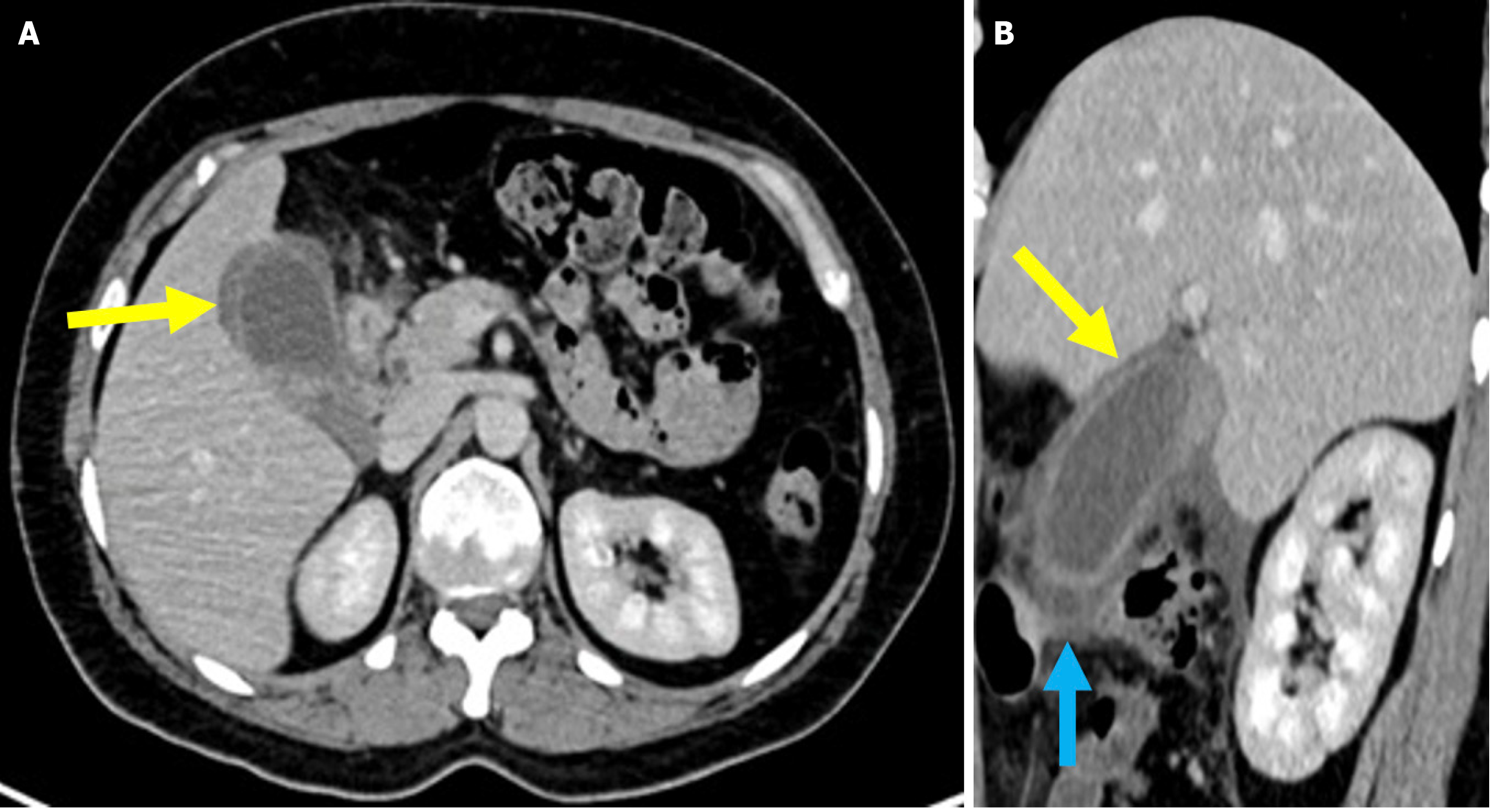
Figure 7 Cholecystitis.
A and B: Axial (A) and sagittal (B) contrast-enhanced computed tomography images showed findings consistent with post-endoscopic retrograde cholangiopancreatography cholecystitis in a 33-year-old female patient who presented 10 days after endoscopic retrograde cholangiopancreatography with abdominal pain, nausea, and a positive Murphy’s sign. The patient’s laboratory results revealed elevated levels of aspartate aminotransferase, alanine ami
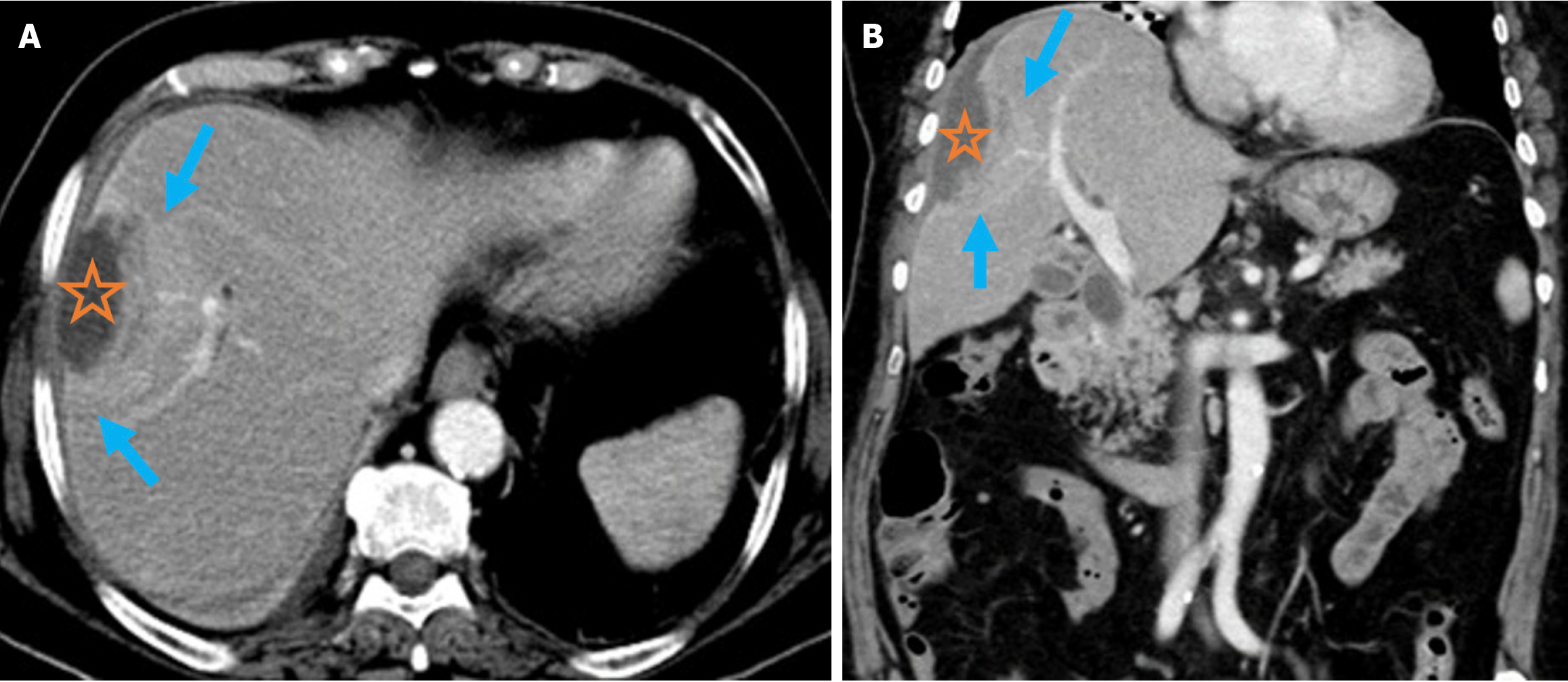
Figure 8 Pyogenic liver abscess.
A and B: Axial (A) and coronal (B) contrast-enhanced computed tomography images demonstrated pyogenic liver abscess secondary to endoscopic retrograde cholangiopancreatography in a 76-year-old female patient. She presented 2 months after endoscopic retrograde cho
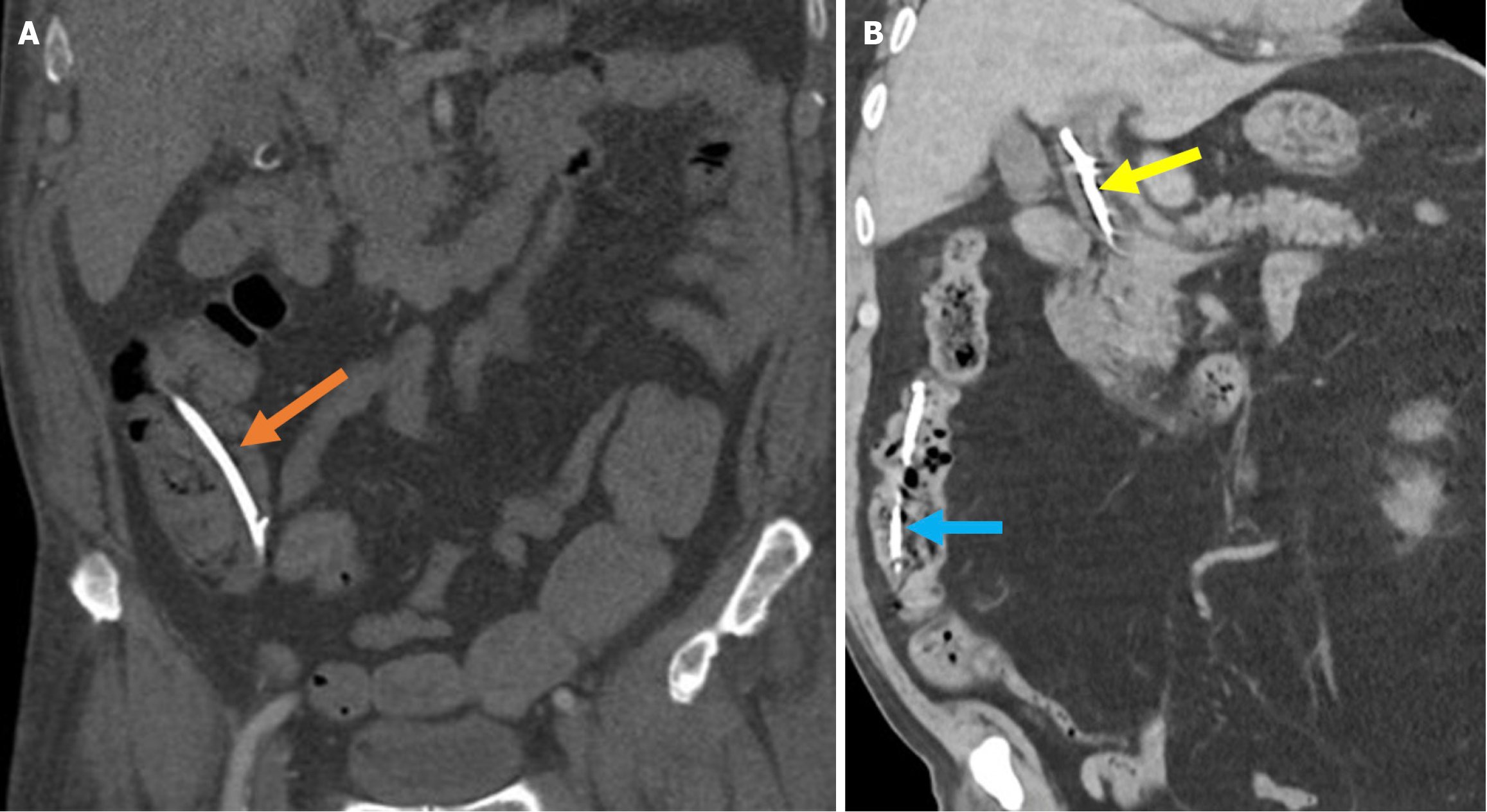
Figure 9 Acute duodenal wall and mesenteric hemorrhages.
A and B: Axial (A) and coronal (B) noncontrast computed tomography images revealed findings consistent with hemorrhage in an 81-year-old female patient who presented with abdominal pain and a drop in hemoglobin levels following endoscopic retrograde cholangiopancreatography. A high-density mesenteric collection and mesenteric fat stranding were observed and consistent with mesenteric hematoma (blue arrows). Hyperattenuating duodenal wall thickening, indicative of an intramural hematoma (orange arrows), was also present. Free fluid was noted in the perihepatic space (yellow arrow).
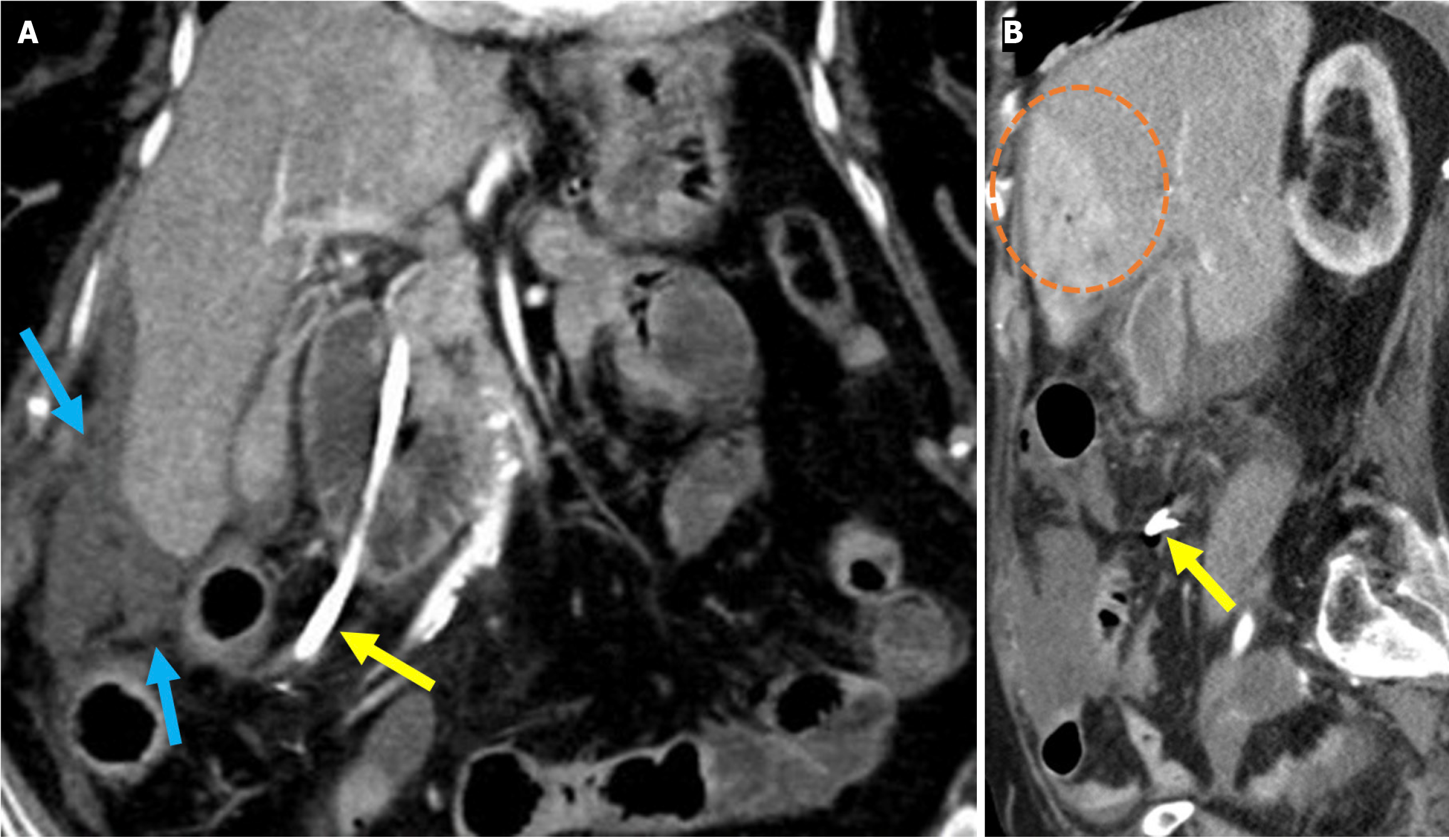
Figure 10 Hemobilia.
A and B: Axial (A) and coronal (B) noncontrast computed tomography images depicted findings of hemobilia in an 84-year-old female patient with coagulopathy who presented with abdominal pain and a drop in hemoglobin following endoscopic retrograde cholangiopancreatography. Dilatation of the common bile duct with hyperattenuating intraluminal material consistent with hemorrhage was observed (yellow arrows). Hyperdense gallstones were also visible within the gallbladder (blue arrow).
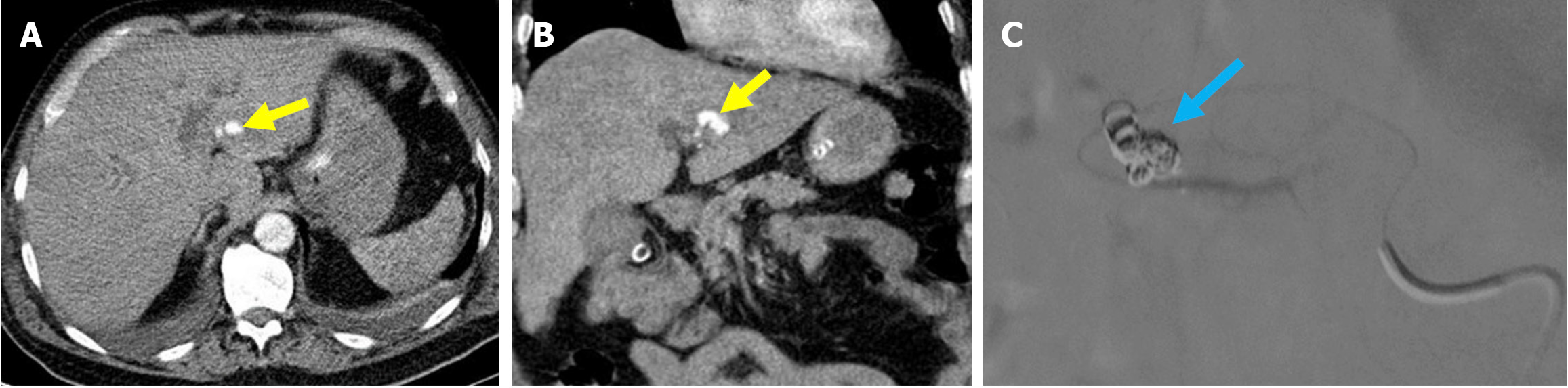
Figure 11 Hepatic pseudoaneurysm.
A and B: Axial (A) and coronal (B) computed tomography (CT) angiography in the arterial phase. C: Digital subtraction angiography during therapeutic intervention. A 63-year-old female patient presented with a rapid drop in hemoglobin and melena following the removal of a previous plastic stent. The patient developed progressively worsening periumbilical abdominal pain and exhibited signs of hypovolemic shock. Arterial phase CT angiography revealed a pseudoaneurysm originating from the left hepatic artery (yellow arrows). The digital subtraction angiography image showed successful embolization of the pseudoaneurysm with coil placement (blue arrow).
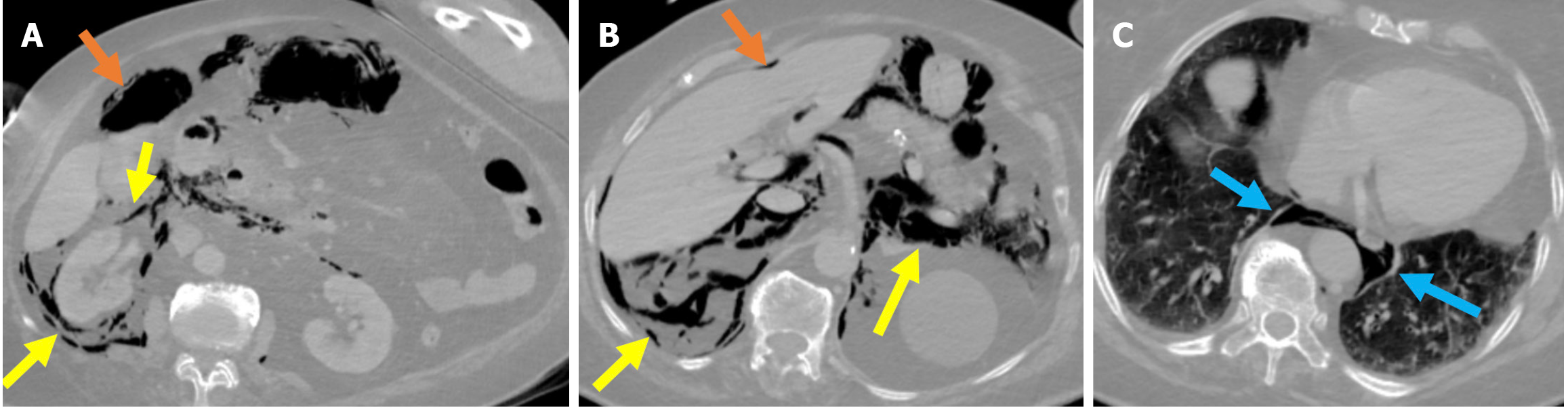
Figure 12 Stapfer type I perforation.
A-C: Contrast-enhanced computed tomography (CT) images in lung window settings at the duodenum (A), celiac artery (B), and basal thorax (C) revealed a lateral duodenal wall perforation in an 84-year-old female patient who had undergone sphincterotomy. During the procedure, suspicion of perforation arose due to suboptimal luminal insufflation during the endoscopic manipulation. A prompt CT scan was performed before the patient was returned to the ward. Findings included pneumoperitoneum (orange arrows) in the perihepatic and right paracolic regions and extensive retroperitoneal emphysema (yellow arrows) involving the right perirenal space, posterior and anterior pararenal spaces, and posterior pneumomediastinum (blue arrows). Due to worsening clinical signs of peritonitis and the results from imaging, an emergency laparotomy was required.
Figure 13 Stapfer type II perforation.
A and B: Axial (A) and coronal (B) noncontrast computed tomography (CT) images in a 70-year-old patient who de
Figure 14 Stapfer type III perforation.
A and B: Axial (A) and coronal (B) contrast-enhanced computed tomography images showed a choledochal micro
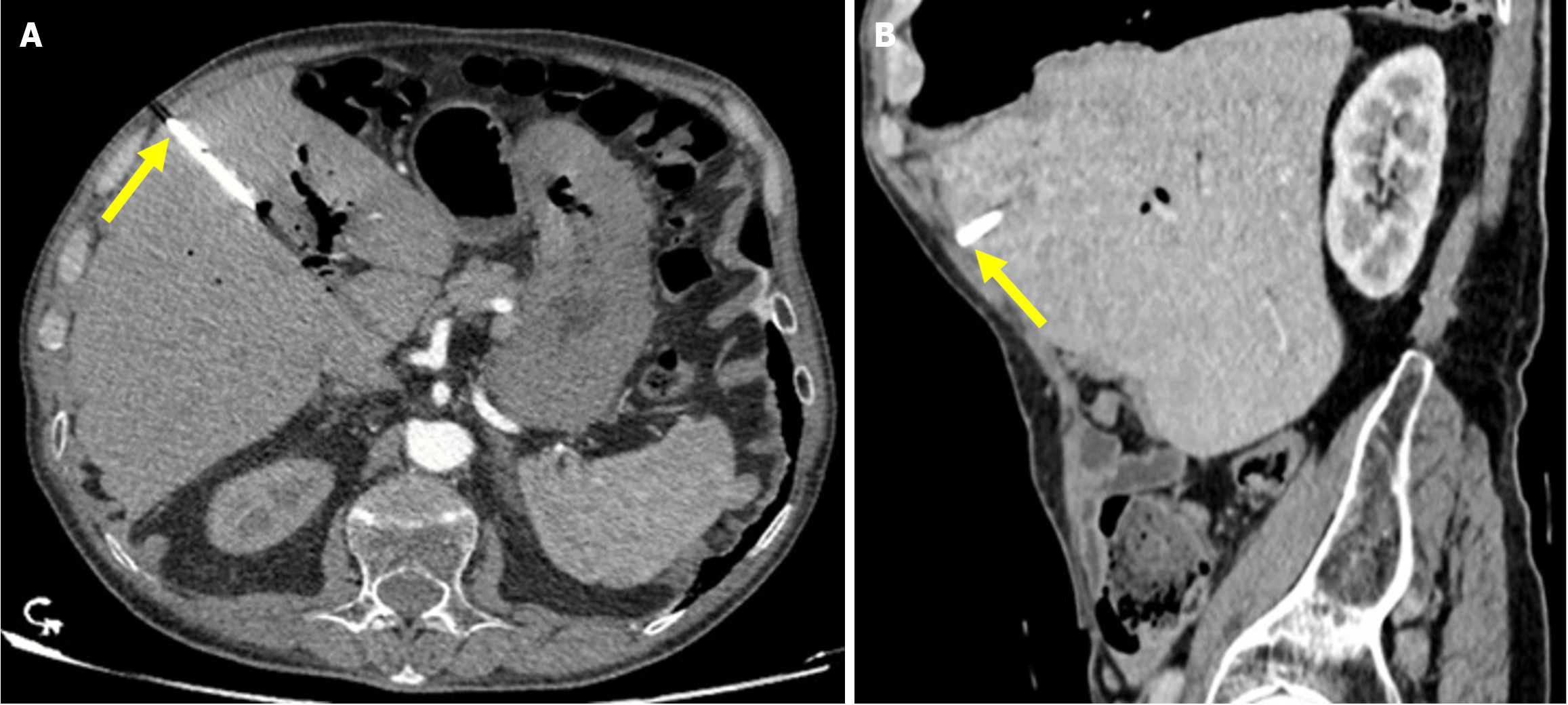
Figure 15 Stapfer type IV perforation.
A: Axial early post-endoscopic retrograde cholangiopancreatography contrast-enhanced computed tomography (CT); B: Axial contrast-enhanced CT on day 7 after endoscopic retrograde cholangiopancreatography. The 76-year-old patient who underwent sphincterotomy and placement of a plastic stent presented with mild abdominal pain and a slight elevation in serum amylase following the procedure. Despite the absence of intra-procedural suspicion and no clinical findings suggestive of perforation, free air was observed on CT in the right anterior pararenal and paraduodenal spaces (blue arrows). The patient was managed conservatively. A follow-up CT scan on day 7 demonstrated resolution of the free air. The patient’s clinical and laboratory findings were consistent with this benign course.
Figure 16 Proximal stent migration.
A and B: Axial (A) and sagittal (B) contrast-enhanced computed tomography of a 73-year-old male patient with a history of biliary stenting. He presented with persistent abdominal pain following revision of the common bile duct stent 1 week prior. The patient developed progressive jaundice, pruritus, and recurrent fever. Laboratory tests revealed elevated levels of bilirubin, aspartate aminotransferase, and alanine aminotransferase. The stent, which should be located within the common bile duct, migrated proximally into an intrahepatic bile duct and extended toward the subcapsular region of hepatic segment IV (yellow arrows).
Figure 17 Distal stent migration.
A: A 56-year-old male patient with a history of endoscopic retrograde cholangiopancreatography (ERCP) and biliary stenting presented with right lower quadrant pain lasting for 3 days. The coronal noncontrast computed tomography (CT) images demonstrated distal migration of the stent into the cecum (orange arrow); B: An 85-year-old patient with a history of ERCP and biliary stenting presented with jaundice. The previous stent was not visualized during ERCP for stent revision, and re-stenting was performed (yellow arrow). Post-procedural coronal contrast-enhanced CT revealed distal migration of the previous stent into the ascending colon (blue arrow).
Figure 18 Distal stent migration and duodenal perforation.
A and B: Coronal (A) and sagittal (B) contrast-enhanced computed tomography images in a 77-year-old female patient with a history of endoscopic retrograde cholangiopancreatography and biliary stenting for cholangitis, who presented with severe abdominal pain. Tachypnea and agitation were observed and were accompanied by nausea, fatigue, and impaired oral intake. Laboratory tests revealed elevated leukocyte counts, C-reactive protein levels, and lactate levels. The stent was found to have migrated distally into the duodenum and perforated the distal portion of the second part of the duodenum (yellow arrow). High attenuation-free fluid, suggestive of hemorrhage, was observed in the perihepatic region and mesentery (blue arrows). The liver exhibited heterogeneous contrast enhancement, consistent with impaired perfusion that was likely related to ischemia (orange circle). Emergent surgical intervention was required.
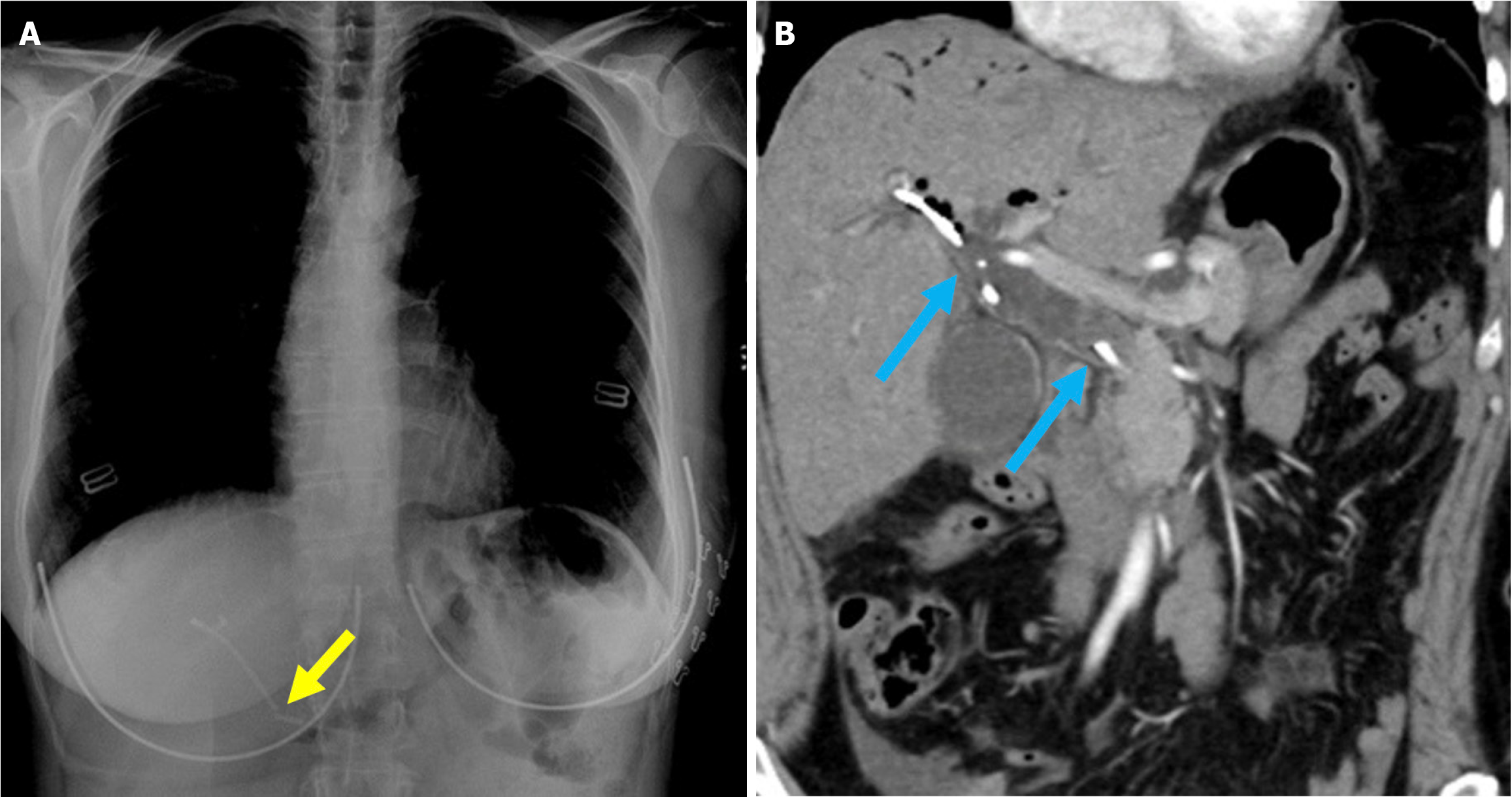
Figure 19 Stent fracture.
A: Posteroanterior chest radiograph; B: Contrast-enhanced coronal computed tomography scan. A 52-year-old female patient presented to the hospital 3 days after biliary stent placement via endoscopic retrograde cholangiopancreatography with complaints of abdominal pain and elevated levels of aspartate aminotransferase, alanine aminotransferase, and bilirubin. The imaging revealed a fracture of the previously placed plastic stent (yellow arrow). A repeat endoscopic retrograde cholangiopancreatography was performed to remove the fractured stent, but residual stent fragments remained within the common bile duct. The duct appeared dilated due to malfunction of the broken stent (blue arrow).
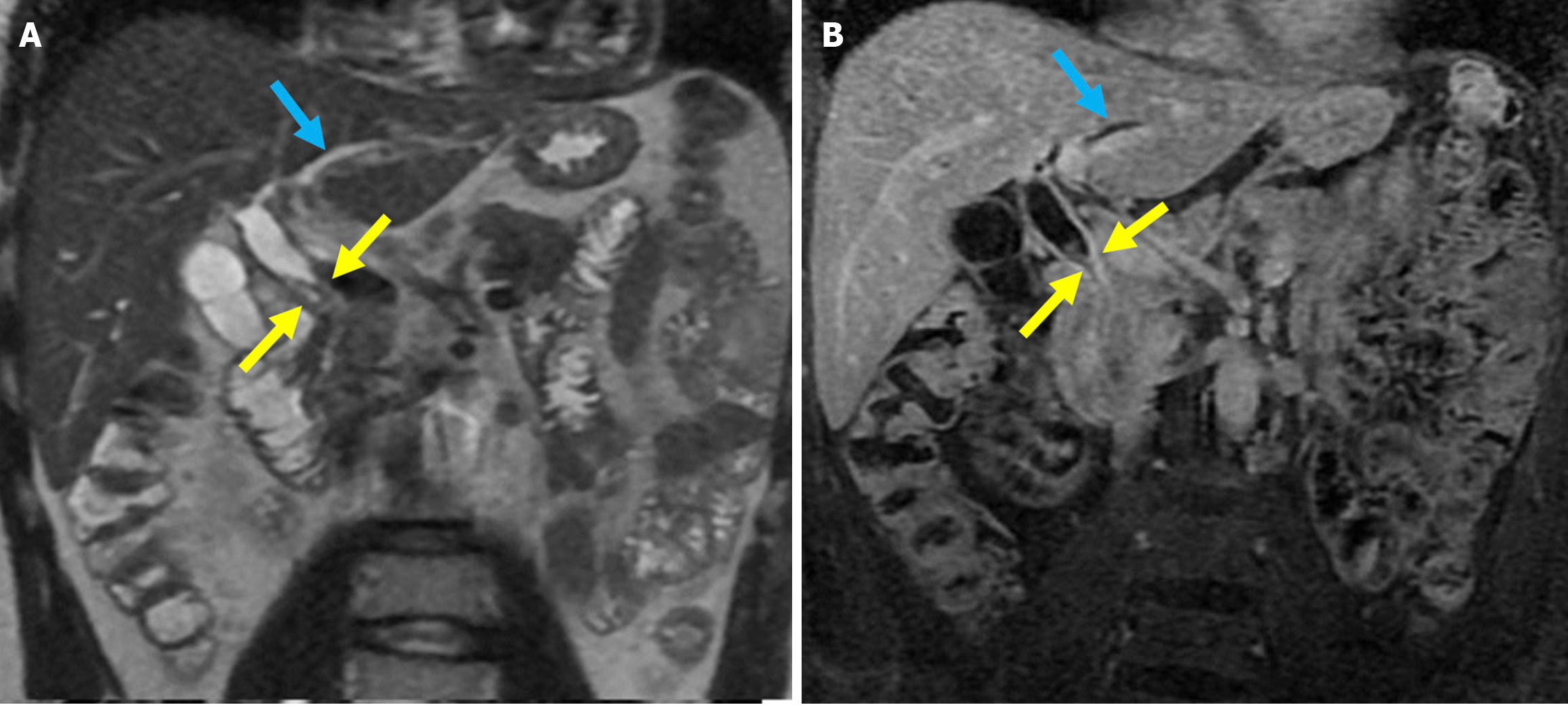
Figure 20 Post-sphincterotomy biliary stricture.
A: Coronal T2-weighted magnetic resonance imaging; B: Fat-suppressed post-contrast T1-weighted magnetic resonance imaging showed a biliary stricture in a 40-year-old male patient with a history of endoscopic retrograde cholangiopancreatography and sphincterotomy. Focal stenosis was observed in the mid portion of the common bile duct without any associated mass lesion (yellow arrows). There was associated mural thickening of the mid-bile duct and dilatation of the intrahepatic bile ducts (blue arrows). The findings were consistent with a benign biliary stricture secondary to prior endoscopic retrograde cholangiopancreatography.
- Citation: Simsar M, Yuruk YY, Sahin O, Sahin H. Complications and expected imaging findings after endoscopic retrograde cholangiopancreatography. World J Radiol 2025; 17(9): 110214
- URL: https://www.wjgnet.com/1949-8470/full/v17/i9/110214.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i9.110214