Published online Feb 26, 2026. doi: 10.4330/wjc.v18.i2.111861
Revised: August 1, 2025
Accepted: January 6, 2026
Published online: February 26, 2026
Processing time: 206 Days and 16.9 Hours
Premature ventricular contractions (PVCs) originating from the left ventricular summit (LVS) could be challenging to ablate due to anatomical reasons.
To study the role of new irrigation technologies, like the TactiFlex catheter, could facilitate deeper radiofrequency (RF) penetration and thus increase success in the ablative treatment of this PVC subset.
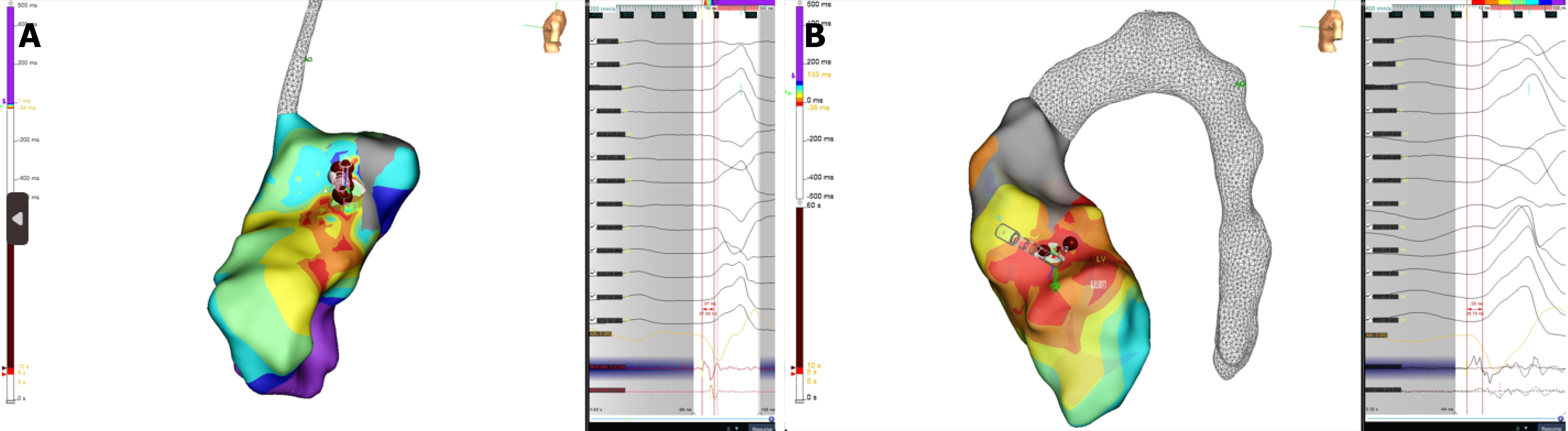
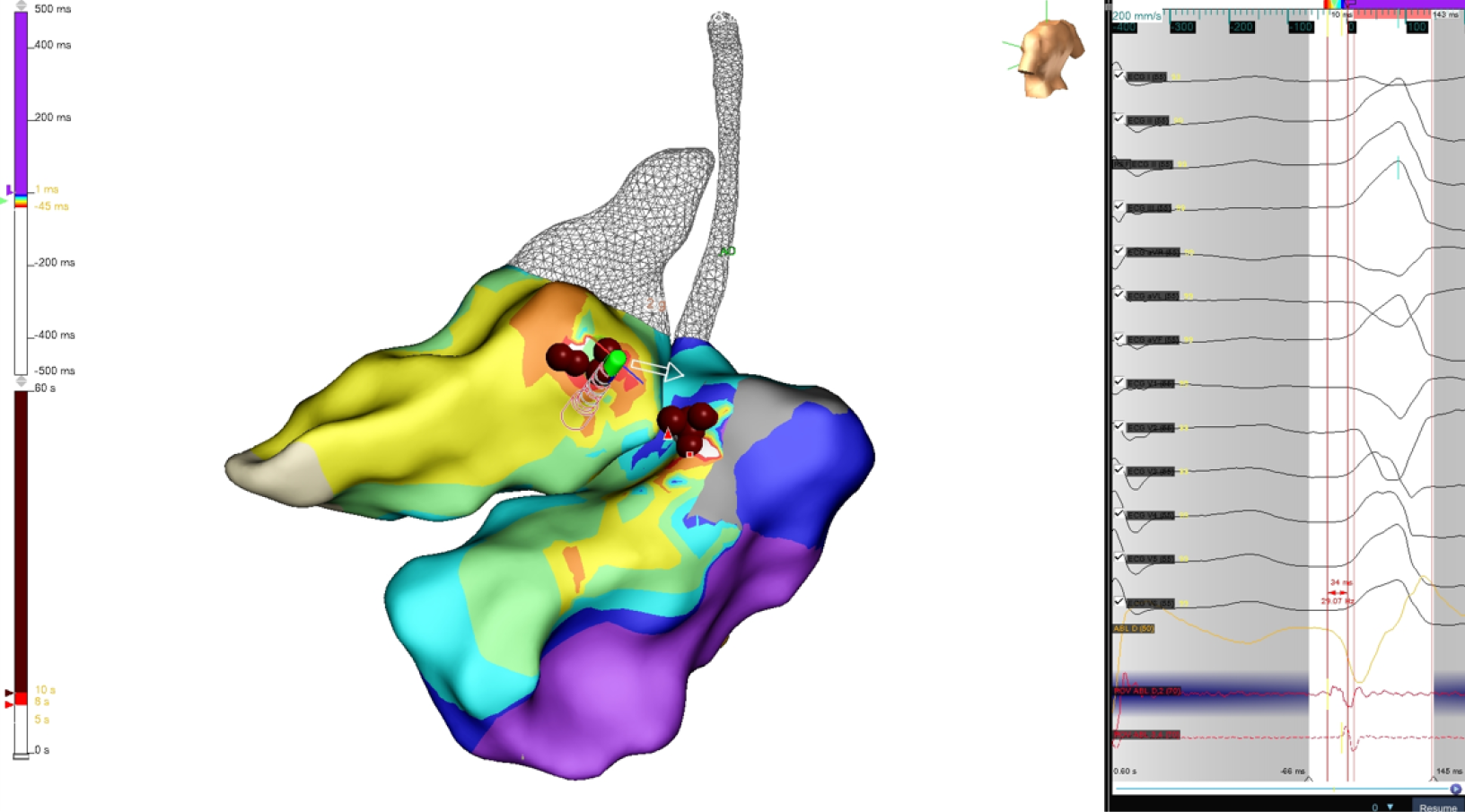
Three PVCs focus (left bundle branch block morphology, inferior axis on the frontal plane, early R/S transition in V1-V2) were accurately mapped with Ensite X omnipolar technology.
RF was delivered at mitroaortic continuity by TactiFlex catheter (4-9 lesions, max 35 W, 43 °C, 13 mL/minute, max 60 seconds, mean impedance drop 11.9 ± 1.7 ohms) with acute PVCs suppression but early recurrence in all cases. In one case, an anatomical approach in the posteroseptal right ventricular outflow tract was performed without acute success. After 6-10 hours, no PVCs/ventricular arrhy
Late PVCs elimination could be due to the porous flexible distal tip design of the TactiFlex catheter that allows deeper RF penetration in the myocardium due to a greater adhesion of the saline irrigation system to tissue. It is reasonable to assume that this new technology makes lesions more transmural, determining a delayed lesion maturation, thus not limited to the duration of energy delivery.
Core Tip: Premature ventricular contractions from left ventricular summit could be challenging to ablate due to anatomical reasons. New irrigation technologies, like the TactiFlex catheter, could facilitate deeper radiofrequency penetration and thus increase success in the ablative treatment. Late premature ventricular contractions elimination could be due to the porous flexible distal tip design of the TactiFlex catheter that allows deeper radiofrequency penetration in the myocardium due to a greater adhesion of the saline irrigation system to tissue. It is reasonable to assume that this new technology makes lesions more transmu-ral, determining a delayed lesion maturation, thus not limited to the duration of energy delivery.
- Citation: Palamà Z, Tricarico G, Scarà A, Robles AG, De Masi De Luca G, Nesti M, Romano S, Sciarra L. Late left ventricular summit premature ventricular contractions elimination with new TactiFlex irrigation technology. World J Cardiol 2026; 18(2): 111861
- URL: https://www.wjgnet.com/1949-8462/full/v18/i2/111861.htm
- DOI: https://dx.doi.org/10.4330/wjc.v18.i2.111861
Symptomatic premature ventricular contractions (PVCs) originating from the left ventricular outflow tract (LVOT), including the left ventricular summit (LVS), in an otherwise normal heart, can be treated by catheter ablation if antiarrhythmic medications are ineffective, not tolerated, or not the patient’s preference. The most common sites of idiopathic endocardial LVOT-PVCs are the aortic root, aortomitral continuity, and the mitral annulus, whereas the most common epicardial structure involved in ventricular arrhythmias (VAs) genesis is the LVS.
LVS is a triangular epicardial region of the left ventricular surface bounded by the bifurcation between the left anterior descending and the left circumflex coronary arteries, bisected by the great cardiac vein into superior and inferior regions. Given the proximity to major coronary vessels and the presence of a thick layer of epicardial fat, catheter ablation by the epicardial approach of the basal region of LVS is particularly challenging, given safety and efficacy reasons[1]. Radiofrequency (RF) ablation is avoided within 5 mm of a coronary artery, and therefore, coronary angiography needs to be performed before epicardial ablation to confirm a safe distance between the catheter tip and coronary vessels. Ablation over epicardial fat results in decreased lesion depth because of inadequate RF penetration of conductive heating[2]. These factors reduce the safety and efficacy of LVS VAs ablation. Therefore, both epicardial ablation through the coronary sinus (CS) and endocardial ablation from anatomically opposite sites (anatomic approach) may be a good option to overcome these limitations, despite the limits of high impedance inside cardiac veins and, sometimes, inaccessibility to the site of the earliest activation with the ablation catheter[3]. In case of persistence or recurrence of LVS-PVCs after a first ablation procedure, the patient should be followed up rather than treated by repeated ablation approaches that could only increase the risk of complications[4]. In 1992, Klein et al[5] reported a case in which idiopathic VAs could not be induced 6 weeks after an unsuccessful ablation. Subsequently, several studies showed the possibility of delayed disappearance of VAs after a single ablation procedure. The main mechanism of the RF delayed effect concerns lesion maturation. At first, the RF lesion is an area of central coagulative necrosis surrounded by inflammatory cells and diffused microhemorrhages. The inflammatory response and microcirculatory damage may lead to fibrosis of this peripheral zone, therefore resulting in lesion size increase. Besides, ultrastructural disorders have been reported up to 6 mm beyond the macroscopic RF lesion because of plasma membrane and microvasculature alterations[6].
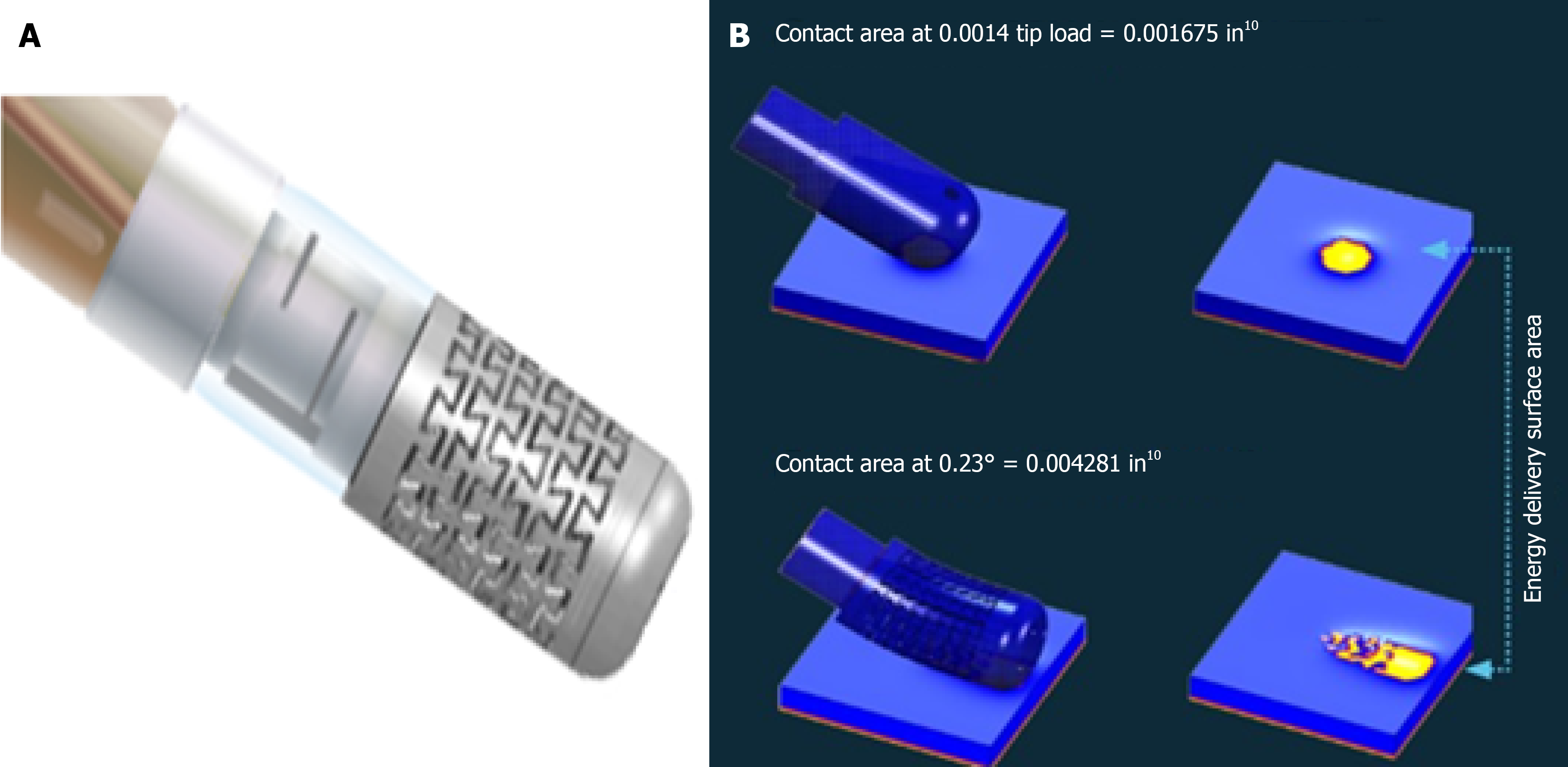
TactiFlex™ Sensor Enabled™ catheter is made with a flexible porous tip electrode to facilitate saline irrigation during the ablation procedure. The flexible tip helps reduce procedural risk by directing and ensuring uniform flow orientation, potentially favoring a deeper lesion with an irrigated circuit. The flexible tip of the TactiFlex catheter, compared to the fixed tip, is able to atraumatically rest on the cardiac tissue and increase the surface area of the scaling catheter in contact with the tissue (Figure 1). The catheter has been studied in the treatment of atrial fibrillation with a high-power short-duration approach, demonstrating shortening of procedural times, greater stability, and greater impedance drop[7].
We present 3 consecutive cases of LVS PVCs ablations treated with TactiFlex catheter ablation. All 3 cases underwent ablation for drug-refractory and highly symptomatic PVCs presenting left bundle branch block morphology, inferior axis on the frontal plane, and early R/S transition in V1-V2. The pre-procedural mean PVC burden was 25000/24 hours with repetitive forms (couples, non-sustained ventricular tachycardia). High-density mapping with Ensite X omnipolar te
To further investigate the potential predictive role of impedance drop in lesion efficacy, we created a comparison group of 10 patients with similar clinical and electrophysiological characteristics [PVCs from LVS, same electrocardiogram (ECG) morphology, high PVC burden, mapping and ablation strategy], but in which the ablation resulted in immediate and lasting suppression of PVCs, treated with a Tactiflex catheter. The V2S/V3R amplitude ratio of the PVC on the surface ECG described in detail by Yoshida was used for lateralization of the source of origin[8]. All cases were treated with normal saline irrigation solution (0.9% NaCl). The clinical and instrumental characteristics of the two groups are summarized in Table 1.
| Case study (n = 3) | Control group (n = 10) | |
| Age (years) | 46.3 | 52.3 |
| LVEF (%) | 52.3 | 53.5 |
| V2S/V3R ratio | 1.61 | 1.68 |
| Beta blockers | 3/3 | 8/10 |
| Flecainide | 3/3 | 9/10 |
| Amiodarone | 0/3 | 1/10 |
| Total RF delivery time (minutes) | 5.0 ± 1 | 4.3 ± 1.8 |
| Single lesion impedance drop (ohms) | 11.9 ± 1.7 | 13.5 ± 2.1 |
| PVCs recurrence (6 months follow-up) | 0/3 | 1/10 |
In all three cases (Figures 2 and 3), a set of ablation lesions (4-9 lesions, max 35 W, 43 °C, 13 mL/minutes, max 60 seconds) has been performed in mitroaortic continuity if these hallmarks were achieved: (1) The local ventricular activation time recorded at the ablation site on bipolar signal preceded the onset of the Q, R, and S peaks complex with a precocity between -25 milliseconds and -34 milliseconds; (2) QS morphology at the unipolar mapping; and (3) Optimal pa
Several clinical and electrophysiological predictors of the success of LVS ablation have been described, among them: The absence of structural heart disease, gender, and the earliest activation > 26 milliseconds before Q, R, and S peaks onset on bipolar electrogram[9,10]. However, the mechanism of the late elimination of LVS arrhythmias after ablation has not been completely understood and can be ascribed to a delayed RF effect related to lesion maturation[6]. RF catheters with new systems of irrigation flow to the tip-tissue interface have been developed, aiming to complete lesion transmurality and thus elimination of deep substrates. The porous distal end of the TactiFlex catheter also has a combination of a flexible tip with a fiber optic-based contact force sensing, which allows for greater adhesion between the saline irrigation system and myocardial tissue. As demonstrated by Ptaszek et al[11], TactiFlex has 2x greater stability during intracardiac movement of the catheter and during the ablation itself, allowing better contact to be achieved more easily, performing more ef
This case-control study clearly presents the major limitation of a small sample size. Further controlled randomized trials are certainly needed to assess the efficacy of irrigated catheter ablation for PVCs and to determine whether differences exist according to the technology used. Moreover, demonstrating a consistent response to RF ablation in a larger series of patients with LVS PVCs could potentially lead to a change in clinical practice, reserving more invasive (i.e., epicardial) approaches only for cases of recurrence. Another limitation of the study - although it also paves the way for future comparative research - is the use of a single catheter type to evaluate differences between the two groups. Future studies should include various RF technologies, including devices from different manufacturers, to comprehensively evaluate their efficacy and safety.
LVS ablation is a challenging procedure with life-threatening risks. New catheter technologies, with new irrigation systems (with or without an anatomical approach in the RVOT region), could be a treatment option, avoiding more aggressive treatments like the epicardial/CS approach.
| 1. | Santangeli P, Lin D, Marchlinski FE. Catheter Ablation of Ventricular Arrhythmias Arising from the Left Ventricular Summit. Card Electrophysiol Clin. 2016;8:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Shen B, Hu WM, Shao JM, Shen Y, Yan Y, James SM, D'Angelo L, Xu GJ, Zheng C, Lin JF. Ventricular arrhythmias originating from different portions of the communicating vein of the left ventricular summit: electrocardiographic characteristics and catheter ablation. BMC Cardiovasc Disord. 2024;24:421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Chen YH, Lin JF. Catheter Ablation of Idiopathic Epicardial Ventricular Arrhythmias Originating from the Vicinity of the Coronary Sinus System. J Cardiovasc Electrophysiol. 2015;26:1160-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Ding L, Hou B, Wu L, Qiao Y, Sun W, Guo J, Zheng L, Chen G, Zhang L, Zhang S, Yao Y. Delayed efficacy of radiofrequency catheter ablation on ventricular arrhythmias originating from the left ventricular anterobasal wall. Heart Rhythm. 2017;14:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Klein LS, Shih HT, Hackett FK, Zipes DP, Miles WM. Radiofrequency catheter ablation of ventricular tachycardia in patients without structural heart disease. Circulation. 1992;85:1666-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 307] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Fenelon G, Brugada P. Delayed effects of radiofrequency energy: mechanisms and clinical implications. Pacing Clin Electrophysiol. 1996;19:484-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Dello Russo A, D'Angelo L, Compagnucci P, Cipolletta L, Parisi Q, Valeri Y, Campanelli F, Volpato G, Carboni L, Ciliberti G, Stronati GE, Barbarossa A, La Piscopia V, Bondavalli B, Guerra F, Natale A, Casella M. High-power short-duration catheter ablation of atrial fibrillation: is it really a new era? Comparison between new and old radiofrequency contact force-sensing catheters. J Interv Card Electrophysiol. 2024;67:549-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Yoshida N, Yamada T, McElderry HT, Inden Y, Shimano M, Murohara T, Kumar V, Doppalapudi H, Plumb VJ, Kay GN. A novel electrocardiographic criterion for differentiating a left from right ventricular outflow tract tachycardia origin: the V2S/V3R index. J Cardiovasc Electrophysiol. 2014;25:747-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 9. | Enriquez A, Malavassi F, Saenz LC, Supple G, Santangeli P, Marchlinski FE, Garcia FC. How to map and ablate left ventricular summit arrhythmias. Heart Rhythm. 2017;14:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 10. | Yamada T. Predictors of Successful Endocardial Ablation of Epicardial Left Ventricular Summit Arrhythmias. Card Electrophysiol Clin. 2023;15:15-24. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Ptaszek LM, Koruth J, Santangeli P, Piccini JP, Ranjan R, Mahapatra S, Pipenhagen C, Fish JM, Moon LB, Ambrosius NM, Boudlali H, Jensen JA. Safe and effective delivery of high-power, short-duration radiofrequency ablation lesions with a flexible-tip ablation catheter. Heart Rhythm O2. 2023;4:42-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 12. | Ábrahám P, Ambrus M, Herczeg S, Szegedi N, Nagy KV, Salló Z, Osztheimer I, Széplaki G, Tahin T, Merkely B, Gellér L. Similar outcomes with manual contact force ablation catheters and traditional catheters in the treatment of outflow tract premature ventricular complexes. Europace. 2021;23:596-602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Chinitz JS, Michaud GF, Stephenson K. Impedance-guided Radiofrequency Ablation: Using Impedance to Improve Ablation Outcomes. J Innov Card Rhythm Manag. 2017;8:2868-2873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |