Published online Sep 26, 2025. doi: 10.4330/wjc.v17.i9.110061
Revised: June 18, 2025
Accepted: August 27, 2025
Published online: September 26, 2025
Processing time: 111 Days and 23.8 Hours
Stable angina pectoris, a clinical manifestation of coronary artery disease (CAD), is commonly evaluated using non-invasive diagnostic tools. Traditionally, stress testing modalities such as exercise electrocardiography (ECG), myocardial per
To compare the diagnostic and prognostic performance of CCTA with various forms of stress testing in adult patients presenting with suspected or confirmed stable angina.
A comprehensive literature search was performed across PubMed, EMBASE, Scopus, and the Cochrane Central Register of Controlled Trials in accordance with the PRISMA guidelines. Only randomized controlled trials (RCT) published in English within the last 15 years were included. Studies involving adult patients (≥ 18 years) with stable angina or low-risk chest pain were selected. The intervention was CCTA, and the comparators included ECG, MPI, and stress echocardiography. Data were extracted using a standardized process, and study quality was assessed using the Cochrane Risk of Bias 2.0 tool. Due to heterogeneity in outcome measures and modalities, narrative synthesis was employed.
Five high-quality RCTs encompassing a total of 5551 patients were included. CCTA demonstrated superior diagnostic accuracy and prognostic capability across multiple studies. It was more effective in predicting major adverse cardiac events, including myocardial infarction and cardiac death, and was associated with fewer un
CCTA offers a diagnostically superior and clinically impactful strategy for the initial evaluation of patients with stable angina, especially those with intermediate pretest probability of CAD. Compared to conventional stress testing, it enhances risk stratification, reduces unnecessary procedures, and may improve long-term outcomes. These findings support its broader integration into diagnostic pathways for stable angina.
Core Tip: This systematic review compares coronary computed tomography angiography (CCTA) and stress testing in patients with stable angina. Analyzing five randomized controlled trials, the study highlights the superior diagnostic accuracy and prognostic value of CCTA. CCTA was associated with fewer unnecessary invasive procedures, better event-free survival, and more appropriate revascularization decisions compared to stress testing modalities. These findings support the integration of CCTA into first-line diagnostic pathways for intermediate-risk patients, promoting earlier diagnosis and optimized patient management.
- Citation: Gundareddy V, Singla S, Mounika J, Owona O, Singla B, Singh T, Anwar S, Ramachandran V, Ullah H, Mazari S. Coronary computed tomography angiography vs stress testing for stable angina evaluation: Diagnostic and prognostic superiority. World J Cardiol 2025; 17(9): 110061
- URL: https://www.wjgnet.com/1949-8462/full/v17/i9/110061.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i9.110061
Stable angina pectoris is a common clinical manifestation of coronary artery disease (CAD), characterized by predictable chest pain resulting from myocardial ischemia during exertion or stress[1]. Accurate and timely diagnosis is essential to guide risk stratification, therapeutic decision-making, and to prevent adverse cardiovascular events. Currently, first-line evaluation strategies for stable angina rely on non-invasive functional testing such as exercise electrocardiography (ECG), which detects ST-segment changes during physical activity; myocardial perfusion imaging (MPI), which uses nuclear tracers to assess blood flow mismatch; and stress echocardiography, which evaluates regional wall motion abnormalities induced by stress[2]. These functional tests assess inducible ischemia and have long served as gatekeepers for invasive coronary angiography[3].
However, the diagnostic landscape has shifted with the advent of coronary computed tomography angiography (CCTA), a noninvasive anatomic imaging modality that directly visualizes coronary artery stenoses and plaque burden[4]. Multiple randomized controlled trials (RCT) have suggested that CCTA not only improves diagnostic certainty but may also enhance clinical outcomes through early implementation of preventive therapies. Furthermore, the incorpora
Despite its growing use, the comparative effectiveness of CCTA vs stress testing in patients with stable angina remains a subject of ongoing debate. While some studies support the superior diagnostic yield and prognostic value of CCTA, others highlight concerns about radiation exposure, cost, and overdiagnosis[6]. The interpretation of "intermediate-risk" or "low-risk" chest pain varies across guidelines, with some using the Diamond-Forrester model, while others incorporate the CAD Consortium Clinical Score or guideline-based pre-test probability estimates. This uncertainty necessitates a comprehensive evaluation of current evidence to guide clinicians in selecting the optimal initial testing strategy for stable angina[7].
Previous reviews have compared CCTA and stress testing for stable angina; however, many included heterogeneous study types and lacked focus on high-quality RCTs published in the past decade. Our review builds upon the existing literature by exclusively analyzing recent RCTs with robust methodologies, focusing on downstream clinical outcomes, and proposing future integration of advanced CCTA metrics for better risk stratification.
The objective of this systematic review is to evaluate and compare the diagnostic performance, prognostic value, and clinical outcomes associated with CCTA vs stress testing in patients with stable angina. This analysis is guided by the PICO framework[8], which defines the population as adults with suspected or confirmed stable angina; the intervention as CCTA; and the comparator as stress testing modalities, including ECG, MPI, or stress echocardiography. The outcomes of interest include diagnostic accuracy, downstream testing requirements, major adverse cardiovascular events such as MI and death, as well as patient-centered measures like quality of life and cost-effectiveness.
A comprehensive literature search was conducted in accordance with the PRISMA guidelines[9] to ensure methodological rigor and transparency. Databases including PubMed, EMBASE, Scopus, and the Cochrane Central Register of Controlled Trials (CENTRAL) were systematically searched for RCTs comparing CCTA with various forms of stress testing-including ECG, MPI, and stress echocardiography-in adult patients with suspected or confirmed stable angina. The search strategy was developed using a combination of Medical Subject Headings (MeSH) and relevant keywords, with filters applied to include studies published in English within the past 15 years. Reference lists of relevant articles were also manually screened to capture any additional eligible studies. This approach yielded a final selection of five high-quality RCTs for evidence synthesis, each of which underwent detailed quality assessment using the Cochrane Risk of Bias 2.0 tool[10].
Studies were selected based on predefined eligibility criteria guided by the PICO framework. The population included adult patients (≥ 18 years) with suspected or confirmed stable angina or equivalent low-risk chest pain. The intervention was diagnostic assessment using CCTA, either as a standalone modality or part of a tiered protocol. The comparator was any form of stress testing, including ECG, MPI, or stress echocardiography. The primary outcomes of interest were diagnostic accuracy, prognostic performance (e.g., prediction of MI or death), downstream testing, rates of revascularization, and other clinical endpoints such as event-free survival. Only RCTs published in English and reporting original outcome data were included. Studies focused on acute coronary syndromes, case reports, editorials, observational studies, or non-randomized trials were excluded. Risk stratification varied slightly among the included studies; however, most defined "intermediate-risk" populations based on pretest probability estimates using clinical scoring systems such as the Diamond-Forrester model or modified Duke criteria, while "low-risk" patients typically presented with atypical chest pain and minimal risk factors. These classifications were either explicitly reported or inferred based on study inclusion protocols and baseline characteristics.
A structured data extraction process was employed to ensure consistency and accuracy across included studies. Two independent reviewers screened titles, abstracts, and full texts to determine eligibility. For each study, key variables were extracted into a standardized table, including study design, sample size, population characteristics, type of intervention and comparator, outcomes measured, key findings, and duration of follow-up. Where necessary, discrepancies in extracted data were resolved through discussion or consultation with a third reviewer. In addition, each study underwent independent quality assessment using the Cochrane Risk of Bias 2.0 tool, evaluating randomization, outcome measure
Given the heterogeneity in comparator modalities, outcome measures, and follow-up durations, a narrative synthesis approach was adopted rather than a meta-analysis. Study findings were qualitatively compared based on clinical out
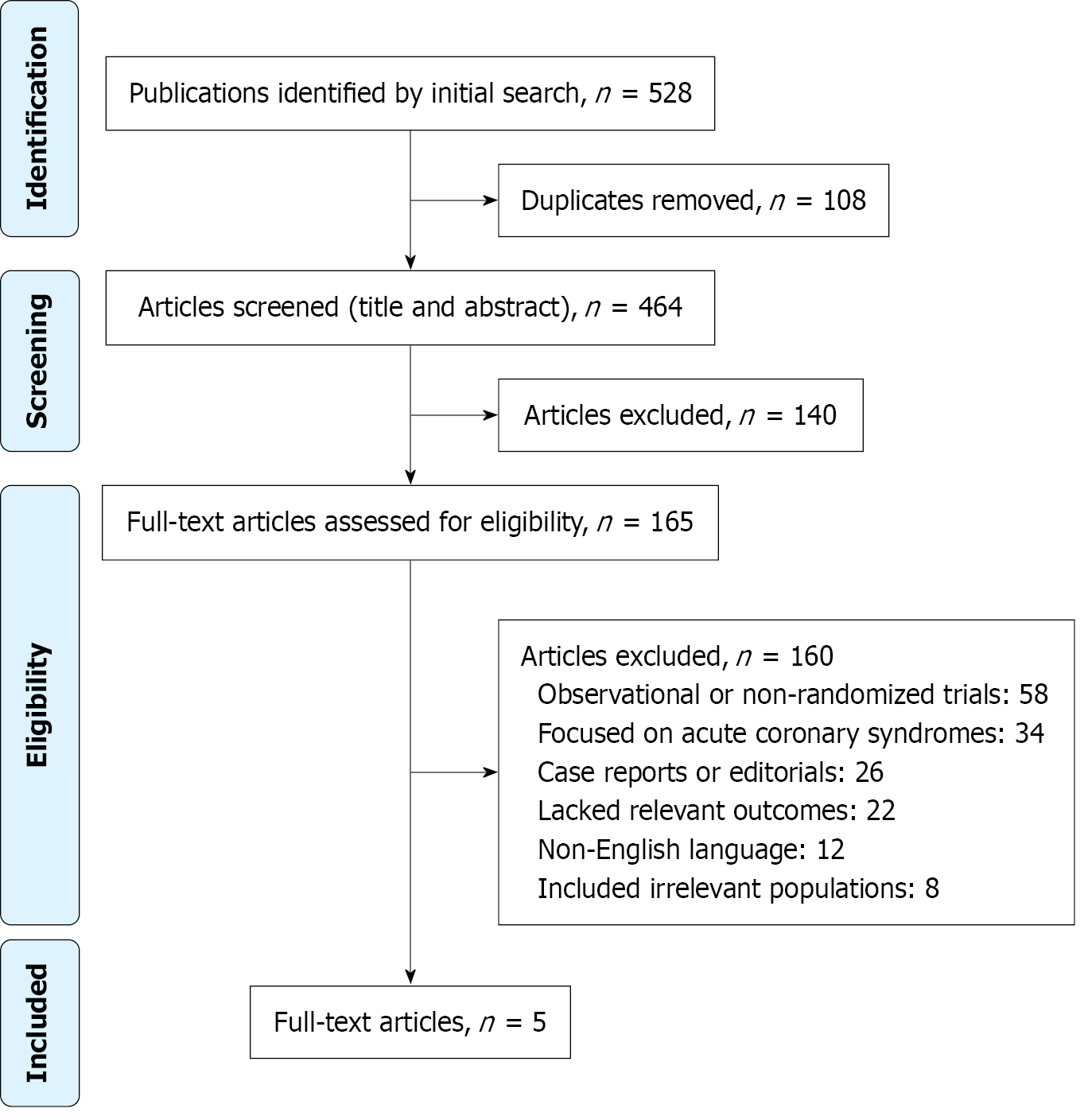
The study selection process followed the PRISMA 2009 guidelines and is illustrated in Figure 1. A total of 528 records were identified through systematic searches of four major databases: PubMed (n = 186), EMBASE (n = 152), Scopus (n = 118), and CENTRAL (n = 72). After the removal of 64 duplicate records, 464 unique records were screened based on titles and abstracts. Of these, 140 were excluded for not meeting basic relevance criteria. The remaining 324 reports were sought for full-text retrieval, but 159 could not be accessed due to availability or access issues. A total of 165 full-text articles were assessed for eligibility. Based on predefined inclusion and exclusion criteria, 160 reports were excluded for reasons such as being observational or non-randomized trials (n = 58), focusing on acute coronary syndromes (n = 34), being case reports or editorials (n = 26), lacking relevant outcomes (n = 22), non-English or duplicate publications (n = 12), or including an irrelevant population (n = 8). Ultimately, five high-quality RCTs were included in the final evidence synthesis.
The characteristics of the five RCTs included in this review are summarized in Table 1. All studies evaluated adult patients with suspected or confirmed stable angina or low-risk chest pain presentations. Sample sizes ranged from 268 to 3283 participants, with populations enrolled across outpatient cardiology clinics and multi-institutional trial networks. The intervention in each study involved the use of CCTA, either as a standalone modality or integrated into a tiered diagnostic protocol, while comparators included various forms of functional stress testing such as ECG, MPI, and stress echocardiography. Across studies, outcomes assessed included diagnostic accuracy, major adverse cardiac events (MACE), event-free survival, rates of invasive angiography, revascularization indications, and healthcare utilization. Follow-up durations ranged from 6 months to 5 years, enabling both short- and long-term outcome evaluation. Overall, these trials provided robust comparative data on the diagnostic and clinical impact of CCTA vs functional testing in the stable angina population.
| Ref. | Study design | Population characteristics | Sample size | Intervention (CCTA) | Comparator (stress testing) | Outcomes measured | Key findings | Follow-up duration |
| Singh et al[11] | Post hoc of RCT | Adults aged 18-75 with suspected stable angina; Scotland clinics | 3283 | CCTA as add-on to standard care | Exercise ECG | CHD death, nonfatal MI, diagnostic accuracy | CCTA more predictive of CHD death/MI (HR 10.63); ECG: 39% sensitivity, 91% specificity | 5 years |
| Stillman et al[12] | Multicenter RCT | Stable angina, intermediate risk; 44 sites | 1050 | CCTA to guide therapy/revascularization | SPECT MPI | MACE (MI, cardiac death), revascularization | Similar outcomes (HR 1.03); CCTA better predicted MACE; fewer events in CCTA-negative patients | Mean 16.2 months |
| Lubbers et al[13] | Multicenter RCT | Stable angina; Dutch outpatient clinics | 350 | Tiered: CAC → CCTA | Functional (ECG, MPI, echo) | Event-free survival, symptoms, downstream testing, cost | Higher survival (96.7% vs 89.8%, P = 0.011); less downstream testing; lower cost | 1.2 years |
| Lubbers et al[14] | Multicenter RCT | Stable angina; mean pretest CAD probability 54% | 268 | Tiered: CAC → CCTA → CT perfusion (if needed) | Functional (mostly exercise ECG) | Angiograms w/ and w/o revascularization, further testing, efficiency | Fewer unnecessary angiograms (1.5% vs 7.2%, P = 0.035); more revascularizations (88% vs 50%, P = 0.017) | 6 months |
| Linde et al[15] | RCT | Acute chest pain with normal ECG/troponin | 600 | CCTA-guided; functional added if needed | Standard: Bicycle ECG or MPI | Composite: Death, MI, UAP, revascularization, readmission | Follow-up |
To enhance clarity and facilitate direct comparison, the primary outcomes and key metrics reported in the five included RCTs are summarized in Table 2. This table presents a side-by-side evaluation of CCTA and various stress testing modalities, highlighting sample sizes, diagnostic strategies, follow-up durations, and statistical outcomes such as HRs and event rates. Across all studies, CCTA consistently demonstrated either superior or comparable performance to functional stress testing in predicting MACE, reducing unnecessary invasive procedures, and improving event-free survival. These findings reinforce the diagnostic and prognostic advantages of CCTA, particularly in intermediate-risk populations.
| Ref. | Sample Size | CCTA modality | Stress test comparator | Primary outcome(s) | Hazard ratio/statistics | Follow-up duration | Key findings |
| Singh et al[11] | 3283 | CCTA as add-on to standard care | Exercise ECG | CHD death or nonfatal MI; diagnostic accuracy | HR for CHD death/MI: 10.63; ECG Sensitivity: 39%, Specificity: 91% | 5 years | CCTA strongly predicted CHD events; superior diagnostic accuracy over ECG |
| Stillman et al[12] | 1050 (CCTA: 518; SPECT: 532) | CCTA to guide therapy and revascularization | SPECT MPI | MACE (MI or cardiac death); revascularization | HR 1.03 (NS); Event rate: 1.2% (CCTA-negative) vs 3.2% (SPECT-negative) | Mean 16.2 months | CCTA better identified patients at low risk and those needing revascularization |
| Lubbers et al[13] | 350 | Tiered approach: CAC → CCTA | ECG, MPI, or Echo | Event-free survival; anginal symptoms; cost | Event-free survival: 96.7% (CCTA) vs 89.8% (Functional), P = 0.011 | 1.2 years | Higher event-free survival and cost-effectiveness in the CCTA group |
| Lubbers et al[14] | 268 (CT: 130; Functional: 138) | CAC → CCTA → CT perfusion (if needed) | Mostly Exercise ECG | Angiograms (with/without revascularization); further testing | Unnecessary angiograms: 1.5% vs 7.2%, P = 0.035; Revascularization: 88% vs 50%, P = 0.017 | 6 months | CCTA reduced unnecessary procedures; improved selection for revascularization |
| Linde et al[15] | 600 (CCTA: 299; Control: 301) | CCTA-guided strategy | Bicycle ECG or MPI | Composite: MI, cardiac death, UAP, revascularization, readmission | Composite events: 11% (CCTA) vs 16% (Control), HR 0.62, P = 0.04; MACE HR: 0.36 | Median 18.7 months | Control |
Quality assessment of the included studies was conducted using the Cochrane Risk of Bias 2.0 (RoB 2) tool, and the results are summarized in Table 3. All five RCTs demonstrated low risk of bias across most domains, including randomization, adherence to interventions, outcome measurement, and data completeness. Four studies were rated as having an overall low risk of bias, reflecting strong methodological quality and internal validity. One study showed some concerns in the domain of selective reporting, primarily due to a limited number of outcome events, which may affect the robustness of statistical conclusions. Nevertheless, the overall quality of evidence was high, supporting the reliability of the synthesized findings in this reviews.
| Ref. | Randomization process | Deviations from intended interventions | Missing outcome data | Measurement of outcome | Selection of the reported result | Overall risk of bias |
| Singh et al[11] | Low | Low | Low | Low | Low | Low |
| Stillman et al[12] | Low | Low | Low | Low | Some concerns (limited outcome events may impact reporting) | Some concerns |
| Lubbers et al[13] | Low | Low | Low | Low | Low | Low |
| Lubbers et al[14] | Low | Low | Low | Low | Low | Low |
| Linde et al[15] | Low | Low | Low | Low | Low | Low |
This systematic review synthesized evidence from five high-quality RCTs to compare the clinical effectiveness of CCTA and stress testing in the evaluation of patients with suspected or confirmed stable angina. Across all studies, CCTA consistently demonstrated superior prognostic value and diagnostic efficiency. In the SCOT-HEART analysis[11], CCTA showed a markedly stronger association with the risk of coronary heart disease death or nonfatal MI, with a HR of 10.63, while exercise ECG had limited sensitivity (39%) despite high specificity (91%). Similarly, in the RESCUE trial[12], although MACE rates were statistically comparable between CCTA and SPECT arms (HR 1.03), CCTA was a better predictor of future events and associated with fewer adverse outcomes in patients with negative test results (1.2% vs 3.2%, P = 0.03).
Further supporting the diagnostic and clinical utility of CCTA, both the CRESCENT[13] and CRESCENT-II trials[14] highlighted the benefits of a tiered CCTA approach involving calcium scoring and perfusion imaging when needed. These studies reported significantly fewer unnecessary invasive angiograms (1.5% vs 7.2%, P = 0.035), faster time to diagnosis, and reduced downstream testing compared to functional modalities. In the CATCH trial[15], which included patients with acute chest pain and stable features, CCTA-guided strategy led to a significant reduction in composite clinical events (11% vs 16%, HR 0.62, P = 0.04) and MACE (HR 0.36). Collectively, these findings emphasize that CCTA is not only diagnostically robust but also clinically impactful, supporting its integration into diagnostic pathways for stable angina, in line with recent updates in clinical guidelines.
Our findings are consistent with prior large-scale studies and meta-analyses that have demonstrated the diagnostic superiority and prognostic value of CCTA in patients with suspected CAD. Landmark trials such as PROMISE[16] and SCOT-HEART have previously shown that anatomical imaging with CCTA leads to better diagnostic certainty and risk stratification compared to functional testing. However, some earlier reviews suggested that stress testing remains sufficient for lower-risk populations due to lower cost and radiation exposure. The trials included in our review bridge this gap by highlighting that in intermediate-risk and diagnostically uncertain cases, CCTA not only improves accuracy but also informs more appropriate downstream management. Variations in outcomes between studies may be attributed to differences in study populations, imaging protocols, and clinical settings.
The results of this review support a shift toward favoring CCTA as a first-line diagnostic tool in patients with suspected stable angina, particularly in those with intermediate pretest probability of CAD[17-19]. CCTA not only im
Nonetheless, certain limitations of CCTA should be acknowledged. These include exposure to ionizing radiation, risk of contrast-induced nephropathy-particularly in patients with reduced glomerular filtration rate-and decreased diagno
Limited access to CCTA, particularly in rural or resource-constrained healthcare settings, also poses a barrier to widespread adoption. Disparities in equipment availability, specialized training, and reimbursement models can hinder its routine clinical use in non-tertiary centers. From a cost perspective, CCTA may initially appear more expensive due to the need for advanced imaging infrastructure. However, several trials, including CRESCENT and SCOT-HEART, demonstrated its ability to reduce overall healthcare expenditures by minimizing unnecessary invasive procedures and repeat testing. This aligns with principles of value-based care and supports its long-term cost-effectiveness.
Emerging technologies such as fractional flow reserve derived from computed tomography (CT) and myocardial blood flow quantification offer the ability to bridge anatomical and functional assessments noninvasively. These metrics have been shown to enhance diagnostic accuracy and improve patient selection for revascularization. As integration of these tools becomes more widespread, the diagnostic value of CCTA may be further amplified[22].
In several of the included studies, diagnostic findings directly influenced management pathways. For instance, in SCOT-HEART and RESCUE, CCTA-guided strategies led to earlier initiation of preventive therapies including high-intensity statins and aspirin, while CRESCENT-II demonstrated more efficient identification of patients requiring percutaneous coronary intervention. These results suggest that improved visualization of coronary anatomy can drive more targeted and timely interventions. Stress testing may still have utility in certain populations, such as those with contraindications to contrast or where perfusion data is specifically needed. Overall, the integration of CCTA into diagnostic algorithms can enhance clinical decision-making, patient outcomes, and health system efficiency.
This review was conducted with methodological rigor, adhering closely to PRISMA guidelines and focusing exclusively on high-quality RCTs to ensure the reliability and applicability of findings. The comprehensive literature search spanned multiple databases and was designed to capture the most recent and relevant evidence, minimizing selection bias. Data extraction and quality assessment were systematically performed using validated tools such as RoB 2.0[21], which reinforced the internal validity of the included studies. The consistency of findings across diverse geographic and clinical settings further strengthens the generalizability of our conclusions.
Despite the strengths of this review, several limitations must be acknowledged. The included studies varied in terms of follow-up duration, comparator modalities (e.g., ECG, SPECT, MPI), and diagnostic thresholds, introducing some degree of heterogeneity. Some trials, such as RESCUE[12], had relatively low event rates, which may limit the statistical power to detect differences in major outcomes. Furthermore, publication bias cannot be entirely excluded, and our focus on English-language RCTs may have led to the exclusion of relevant studies. Additionally, while most studies included intermediate-risk patients, generalizability to low-risk or high-risk populations may be limited.
Future research should aim to evaluate the long-term clinical outcomes of CCTA-guided strategies beyond 5 years and in diverse patient subgroups such as diabetics, women, and patients with prior inconclusive testing. Head-to-head trials comparing CCTA with newer functional imaging modalities, including positron emission tomography and magnetic resonance imaging-based techniques, would also help clarify optimal diagnostic pathways[22]. Additionally, the role of hybrid protocols that integrate anatomical and perfusion imaging warrants further investigation. Artificial intelligence-driven platforms for CCTA interpretation and cost-effectiveness analyses across various healthcare systems may also help refine the use of CCTA in routine cardiovascular care[23].
This systematic review demonstrates that CCTA offers superior diagnostic accuracy and prognostic value compared to stress testing in patients with stable angina. It enables earlier diagnosis, better risk stratification, and reduced unnecessary testing, supporting its role as a preferred first-line tool, especially in intermediate-risk populations. However, stress testing retains value in select scenarios-such as patients with advanced CKD, contrast allergy, or limited access to CCTA-highlighting the need for individualized diagnostic strategies. Future studies should evaluate the clinical utility of CT-derived fractional flow reserve and hybrid protocols (e.g., CCTA plus perfusion) in large, diverse cohorts to further optimize care.
| 1. | Gillen C, Goyal A. Stable Angina. 2022 Dec 19. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 2. | Senior R, Monaghan M, Becher H, Mayet J, Nihoyannopoulos P; British Society of Echocardiography. Stress echocardiography for the diagnosis and risk stratification of patients with suspected or known coronary artery disease: a critical appraisal. Supported by the British Society of Echocardiography. Heart. 2005;91:427-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Ananthasubramaniam G, Ananthasubramaniam K. Stress electrocardiography testing in coronary artery disease: Is it time for its swan song or to redefine its role in the modern era? Indian Heart J. 2022;74:81-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Parikh R, Patel A, Lu B, Senapati A, Mahmarian J, Chang SM. Cardiac Computed Tomography for Comprehensive Coronary Assessment: Beyond Diagnosis of Anatomic Stenosis. Methodist Debakey Cardiovasc J. 2020;16:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Budoff MJ, Lakshmanan S, Toth PP, Hecht HS, Shaw LJ, Maron DJ, Michos ED, Williams KA, Nasir K, Choi AD, Chinnaiyan K, Min J, Blaha M. Cardiac CT angiography in current practice: An American society for preventive cardiology clinical practice statement(✰). Am J Prev Cardiol. 2022;9:100318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Rahsepar AA, Arbab-Zadeh A. Cardiac CT vs. Stress Testing in Patients with Suspected Coronary Artery Disease: Review and Expert Recommendations. Curr Cardiovasc Imaging Rep. 2015;8:29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Barbosa MF, Canan A, Xi Y, Litt H, Diercks DB, Abbara S, Kay FU. Comparative Effectiveness of Coronary CT Angiography and Standard of Care for Evaluating Acute Chest Pain: A Living Systematic Review and Meta-Analysis. Radiol Cardiothorac Imaging. 2023;5:e230022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 8. | Brown D. A Review of the PubMed PICO Tool: Using Evidence-Based Practice in Health Education. Health Promot Pract. 2020;21:496-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 199] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 9. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 48652] [Article Influence: 2861.9] [Reference Citation Analysis (3)] |
| 10. | Cochrane Bias. RoB 2: A revised Cochrane risk-of-bias tool for randomized trials. [cited 2 April 2025]. Available from: https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials. |
| 11. | Singh T, Bing R, Dweck MR, van Beek EJR, Mills NL, Williams MC, Villines TC, Newby DE, Adamson PD. Exercise Electrocardiography and Computed Tomography Coronary Angiography for Patients With Suspected Stable Angina Pectoris: A Post Hoc Analysis of the Randomized SCOT-HEART Trial. JAMA Cardiol. 2020;5:920-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Stillman AE, Gatsonis C, Lima JAC, Liu T, Snyder BS, Cormack J, Malholtra V, Schnall MD, Udelson JE, Hoffmann U, Woodard PK; RESCUE investigators *. Coronary Computed Tomography Angiography Compared With Single Photon Emission Computed Tomography Myocardial Perfusion Imaging as a Guide to Optimal Medical Therapy in Patients Presenting With Stable Angina: The RESCUE Trial. J Am Heart Assoc. 2020;9:e017993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Lubbers M, Dedic A, Coenen A, Galema T, Akkerhuis J, Bruning T, Krenning B, Musters P, Ouhlous M, Liem A, Niezen A, Hunink M, de Feijter P, Nieman K. Calcium imaging and selective computed tomography angiography in comparison to functional testing for suspected coronary artery disease: the multicentre, randomized CRESCENT trial. Eur Heart J. 2016;37:1232-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 14. | Lubbers M, Coenen A, Kofflard M, Bruning T, Kietselaer B, Galema T, Kock M, Niezen A, Das M, van Gent M, van den Bos EJ, van Woerkens L, Musters P, Kooij S, Nous F, Budde R, Hunink M, Nieman K. Comprehensive Cardiac CT With Myocardial Perfusion Imaging Versus Functional Testing in Suspected Coronary Artery Disease: The Multicenter, Randomized CRESCENT-II Trial. JACC Cardiovasc Imaging. 2018;11:1625-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 15. | Linde JJ, Hove JD, Sørgaard M, Kelbæk H, Jensen GB, Kühl JT, Hindsø L, Køber L, Nielsen WB, Kofoed KF. Long-Term Clinical Impact of Coronary CT Angiography in Patients With Recent Acute-Onset Chest Pain: The Randomized Controlled CATCH Trial. JACC Cardiovasc Imaging. 2015;8:1404-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Mark DB, Anstrom KJ, Sheng S, Baloch KN, Daniels MR, Hoffmann U, Patel MR, Cooper LS, Lee KL, Douglas PS; PROMISE Investigators. Quality-of-Life Outcomes With Anatomic Versus Functional Diagnostic Testing Strategies in Symptomatic Patients With Suspected Coronary Artery Disease: Results From the PROMISE Randomized Trial. Circulation. 2016;133:1995-2007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Woods E, Bennett J, Chandrasekhar S, Newman N, Rizwan A, Siddiqui R, Khan R, Khawaja M, Krittanawong C. Efficacy of Diagnostic Testing of Suspected Coronary Artery Disease: A Contemporary Review. Cardiology. 2025;150:111-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Trost J, Ferraro RA, Sharma G, Hays AG, Boden WE, Blumenthal RS, Arbab-Zadeh A. CCTA Should Be the New Diagnostic Gateway for Evaluating Intermediate-Risk Stable Angina Patients. JACC Adv. 2022;1:100116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Marwick TH, Cho I, Ó Hartaigh B, Min JK. Finding the Gatekeeper to the Cardiac Catheterization Laboratory: Coronary CT Angiography or Stress Testing? J Am Coll Cardiol. 2015;65:2747-2756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | van Rosendael SE, Shiyovich A, Cardoso RN, Souza Freire CV, van Rosendael AR, Lin FY, Larocca G, Bienstock SW, Blankstein R, Shaw LJ. The Role of Cardiac Computed Tomography Angiography in Risk Stratification for Coronary Artery Disease. J Soc Cardiovasc Angiogr Interv. 2024;3:102230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 21. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 18833] [Article Influence: 2690.4] [Reference Citation Analysis (0)] |
| 22. | Danad I, Raijmakers PG, Driessen RS, Leipsic J, Raju R, Naoum C, Knuuti J, Mäki M, Underwood RS, Min JK, Elmore K, Stuijfzand WJ, van Royen N, Tulevski II, Somsen AG, Huisman MC, van Lingen AA, Heymans MW, van de Ven PM, van Kuijk C, Lammertsma AA, van Rossum AC, Knaapen P. Comparison of Coronary CT Angiography, SPECT, PET, and Hybrid Imaging for Diagnosis of Ischemic Heart Disease Determined by Fractional Flow Reserve. JAMA Cardiol. 2017;2:1100-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 382] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 23. | Thribhuvan Reddy D, Grewal I, García Pinzon LF, Latchireddy B, Goraya S, Ali Alansari B, Gadwal A. The Role of Artificial Intelligence in Healthcare: Enhancing Coronary Computed Tomography Angiography for Coronary Artery Disease Management. Cureus. 2024;16:e61523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/