Published online Sep 26, 2025. doi: 10.4330/wjc.v17.i9.110220
Revised: July 3, 2025
Accepted: August 14, 2025
Published online: September 26, 2025
Processing time: 108 Days and 9.5 Hours
Peripheral endovascular intervention (PEVI) is performed using radiation. Radiation has deleterious health consequences for patients and operators.
To investigate the gender radiation disparities and procedural outcomes in PEVI.
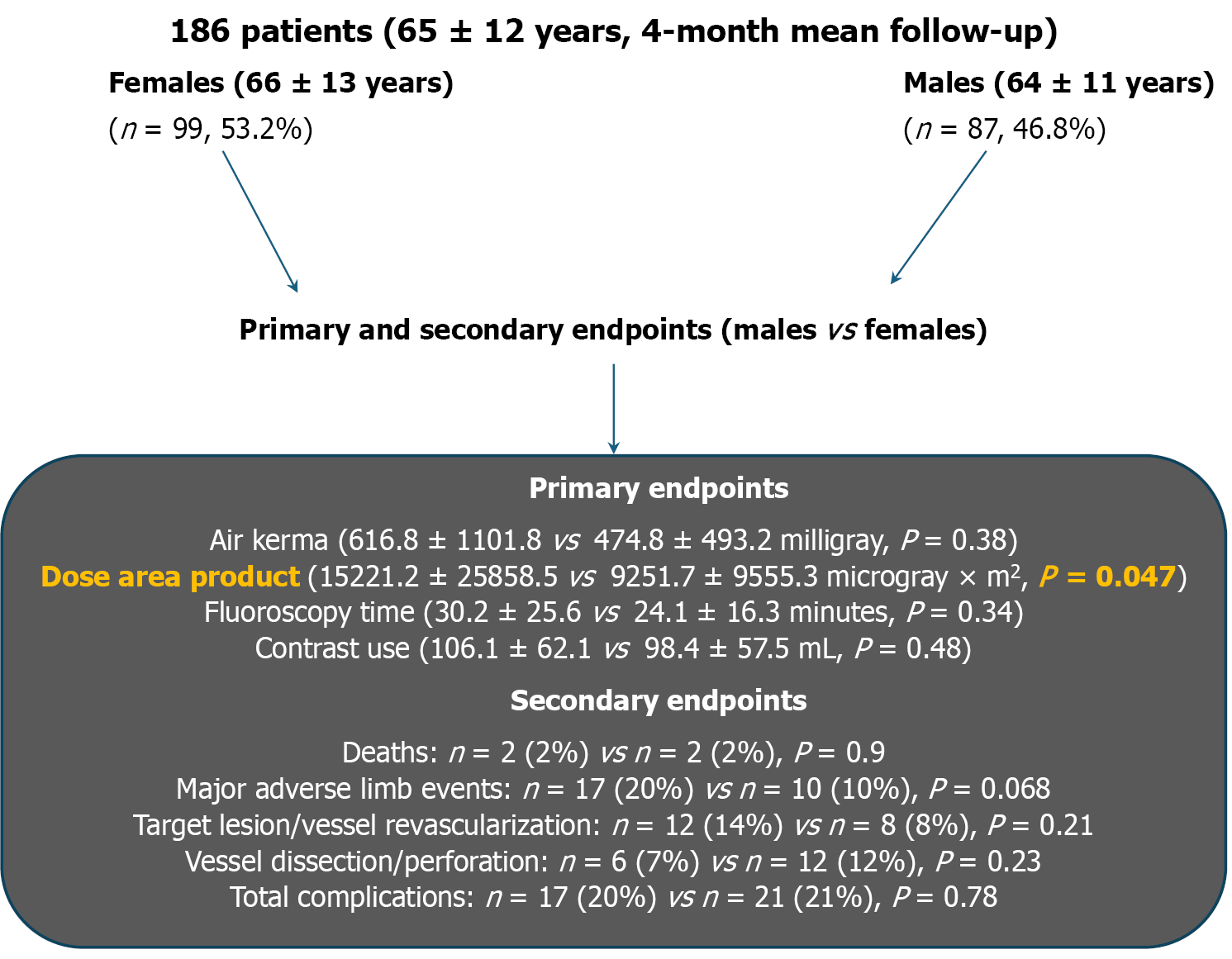
A prospective observational study was performed in 186 consecutive patients (65 ± 12 years) at an academic medical center from January 2019 to April 2020 (mean follow-up of 3.9 ± 3.6 months) comparing the gender radiation disparity and outcomes of PEVI (n = 147 underwent intervention, 79.0%). Groups were divided into women (n = 99, 53.2%) and men (n = 87, 48.4%). Primary endpoints included air kerma, dose area product (DAP), fluoroscopy time, and contrast use. Secondary endpoints included all-cause mortality, acute myocardial infarction, acute kidney injury, stroke, repeat revascularization, major adverse limb event, and the composite of complications.
Men showed increased DAP compared with women (15221.2 ± 25858.5 µGy × m2vs 9251.7 ± 9555.3 µGy × m2, P = 0.047), but no significant difference in air kerma or any other primary endpoints. In the secondary endpoints, no significant diffe
Men had increased DAP indicating more radiation absorption in the exposed area. Gender outcomes showed no difference in complications. Thus, PEVI can be safely performed in men or women.
Core Tip: Radiation is harmful to patients and operators. During peripheral angiogram and peripheral endovascular intervention (PEVI), radiation is produced. Our study showed that men had a greater dose area product compared with women, but no difference in procedural outcomes. These findings are important, so that we may consider more radiation prudent practices in men to minimize the patient’s and operator’s radiation exposure. Such a study has not been previously conducted in PEVI and provides important real-world information.
- Citation: Kar S, Espinoza C. Gender-based radiation exposure and clinical outcomes in peripheral endovascular intervention for limb ischemia: A prospective study. World J Cardiol 2025; 17(9): 110220
- URL: https://www.wjgnet.com/1949-8462/full/v17/i9/110220.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i9.110220
Peripheral arterial disease (PAD) occurs in a substantial portion of the world’s population. In 2010, 202 million people were afflicted with PAD[1]. In a 2023 scientific statement from the American Heart Association, it was reported that PAD affects 200 million people worldwide and over 12 million Americans[2]. Treatment for PAD involves peripheral endo
We conducted a prospective observational study of 186 consecutive patients (65 ± 12 years) between January 2019 to April 2020 at a university medical center and compared the radiation and gender outcomes of PEVI. PEVI was defined as any patient who underwent a PEVI with balloon angioplasty, drug-coated balloon angioplasty, and/or peripheral endovascular stent. The majority of patients (n = 160, 87.0%) underwent procedures using 7.5-10 frames/second (f/s) fluoroscopy and 7.5-10 f/s cine angiography. Radiation shielding, frame rate, and collimation adjustment was used at the discretion of the interventional cardiologist. All the interventional cardiologists were experienced and blinded to the study outcomes. Peripheral angiographic images were reviewed by the 4 interventional cardiologists performing the procedures. Figure 1 displays the study protocol. All procedures were performed using Siemens Artis CC X-ray equip
The primary endpoints included air kerma, DAP, fluoroscopy time, and contrast use. In ionizing radiation air kerma is a measure of the kinetic energy released by air per unit mass. DAP is a calculated variable derived from air kerma multiplied by the irradiated area. DAP is the calculated total energy received by the patient during PEVI[16]. The x-ray equipment automatically calculated the radiation parameters. In fluoroscopic procedures, air kerma denotes the kinetic energy that is transferred to the electrons in air and the absorbed dose of radiation in that volume of air. DAP is an important variable for risk of radiation related malignancy (stochastic), whereas air kerma is an important measurement for tissue injury risk (deterministic effect)[17]. Secondary endpoints included all-cause mortality, cardiac mortality, acute myocardial infarction, stroke, acute kidney injury, repeat target lesion/repeat vessel revascularization, vessel dissection/perforation, major adverse limb event (MALE), composite of complications, and access site complications such as major bleeding, arteriovenous fistula, pseudoaneurysm, retroperitoneal bleeding, compartment syndrome, and limb amputation. Patient follow-up was performed via clinic evaluation. The university institutional review board approved our study and informed consent was obtained from patients.
Quantitative variables were summarized using mean and standard deviation. Categorical variables were summarized using frequency and percentages. The student’s t-test and χ2 test were used in normal distributions and the Wilcoxon sum rank test was used for non-normal distributions. Variables not fitting the normal distribution were converted using a natural log transformation with P values representing the analysis of the log transformed variables. Comparison between groups were analyzed using χ2 and Fisher’s exact test. The main outcome variables (fluoroscopy time, DAP, air kerma, and contrast) were analyzed using a linear regression model and compared across the radiation groups while correcting for age, gender, body mass index (BMI), and diabetes. The model also compensated for clustering effect caused by patients who underwent staged or repeat intervention. The study was powered to detect difference in radiation parame
The baseline patient characteristics showed that women (n = 99, 53.2%, 65.5 ± 12.7 years) had lower body weight (73.2 ± 16.8 kg vs 80.1 ± 16.4 kg; P = 0.005) and height (158.8 ± 7.5 cm vs 169.4 ± 14.3 cm; P < 0.001) compared with men (n = 87, 46.8%, 64.4 ± 10.9 years). Women also had lower body surface area (1.8 ± 0.2 m2vs 1.9 ± 0.2 m2; P < 0.001). However, no significant difference was found between men or women in BMI (27.6 ± 5.4 kg/m2vs 29.0 ± 6.5 kg/m2; P = 0.11). Smoking was more common in men (n = 54, 62%) compared with women (n = 36, 36%, P < 0.001). The most common indications for PEVI were CLI (n = 89, 47.8%), ALI (n = 32, 17.2%), and intermittent claudication (n = 27, 14.5%) with no significant difference in the incidence between gender. Most of the patients underwent infrainguinal (n = 94, 50.5%) and infra
Men had more symptomatic lesions with greater number of patients having Rutherford Grade III (n = 30, 34% vs n = 21, 21%; P = 0.043) and Fontaine Class IV lesions (n = 31, 36% vs n = 19, 19%; P = 0.012) compared with women. However, women had more complex lesions with greater number having Transatlantic Intersociety Consensus C lesions (TASC) than men (n = 36, 36% vs n = 20, 23%; P = 0.047). The most common risk factors included the following: Hypertension (n = 140, 75.3%), diabetes (n = 126, 67.7%), hyperlipidemia (n = 98, 52.7%), current or past history of smoking (n = 90, 48.4%), and history of coronary artery disease (n = 59, 31.7%). No significant difference in these risk factors was found between men vs women, except smoking. Patient characteristics are listed in Table 1. The TASC, Rutherford, and Fontaine classifications for PAD are listed in Table 1.
| Male, n = 87 | Female, n = 99 | P value | |
| Age | 64.4 ± 10.9 | 65.5 ± 12.7 | 0.52 |
| Weight (kg) | 80.1 ± 16.4 | 73.2 ± 16.8 | 0.005 |
| Height (cm) | 169.4 ± 14.3 | 158.8 ± 7.5 | < 0.001 |
| Body mass index (kg/m2) | 27.6 ± 5.4 | 29.0 ± 6.5 | 0.11 |
| Body surface area (m2) | 1.9 ± 0.2 | 1.8 ± 0.2 | < 0.001 |
| Total fluoro time (minutes) | 30.2 ± 25.6 | 24.1 ± 16.3 | 0.34 |
| DAP (µGy × m2) | 15221.2 ± 25858.5 | 9251.7 ± 9555.3 | 0.047 |
| Air Kerma (mGy) | 616.8 ± 1101.8 | 474.8 ± 493.2 | 0.38 |
| Contrast (cc) | 106.1 ± 62.1 | 98.4 ± 57.5 | 0.48 |
| Follow-up (month) | 3.5 ± 3.3 | 4.3 ± 3.8 | 0.18 |
| Diabetes | 53 (61) | 73 (74) | 0.062 |
| Hypertension | 63 (72) | 77 (78) | 0.40 |
| Hyperlipidemia | 44 (51) | 54 (55) | 0.59 |
| Past medical history of CAD | 25 (29) | 34 (34) | 0.41 |
| History of MI/PCI | 23 (26) | 28 (28) | 0.78 |
| Atrial fibrillation/flutter | 4 (5) | 9 (9) | 0.23 |
| History of chronic kidney disease/end stage renal disease on hemodialysis | 1 (1) | 7 (7) | 0.047 |
| History of peripheral intervention | 26 (30) | 22 (22) | 0.23 |
| History of vascular surgery | 3 (3) | 2 (2) | 0.55 |
| History of acute limb ischemia/critical limb ischemia | 14 (16) | 17 (17) | 0.84 |
| Current/former smoker | 54 (62) | 36 (36) | < 0.001 |
| History of amputation | 13 (15) | 15 (15) | 0.97 |
| Intermittent claudication | 14 (16) | 13 (13) | 0.57 |
| Critical limb ischemia | 41 (47) | 48 (48) | 0.85 |
| Acute limb ischemia | 12 (14) | 20 (20) | 0.25 |
| Lower extremity edema/deep venous thrombosis/may Thurner/chronic total occlusion vein | 14 (16) | 11 (11) | 0.32 |
| Venous stenosis | 1 (1) | 1 (1) | 0.93 |
| Re-look angiography | 6 (7) | 7 (7) | 0.96 |
| Left heart cath with peripheral angio | 1 (1) | 5 (5) | 0.13 |
| Peripheral aneurysm | 4 (5) | 0 (0) | 0.031 |
| Suprainguinal | 20 (23) | 21 (21) | 0.77 |
| Infrainguinal | 42 (48) | 52 (53) | 0.56 |
| Infrapopliteal | 29 (33) | 37 (37) | 0.57 |
| Intervention | 71 (82) | 75 (76) | 0.33 |
| Chronic total occlusion intervention | 23 (26) | 31 (31) | 0.46 |
| Staged intervention | 10 (11) | 8 (8) | 0.43 |
| Femoral access | 70 (80) | 88 (89) | 0.11 |
| Pedal | 13 (15) | 5 (5) | 0.023 |
| Retrograde | 84 (97) | 95 (96) | 0.83 |
| Antegrade | 0 (0) | 1 (1) | 0.35 |
| Brachial/radial | 20 (23) | 8 (8) | 0.005 |
| Venous intervention | 12 (14) | 7 (7) | 0.13 |
| Tasc A | 7 (8) | 9 (9) | 0.80 |
| Tasc B | 33 (38) | 23 (23) | 0.029 |
| Tasc C | 20 (23) | 36 (36) | 0.047 |
| Tasc D | 6 (7) | 7 (7) | 0.96 |
| Ruth grade I | 12 (14) | 15 (15) | 0.79 |
| Ruth grade II | 22 (25) | 35 (35) | 0.14 |
| Ruth grade III | 30 (34) | 21 (21) | 0.043 |
| Ruth grade IV | 1 (1) | 0 (0) | 0.28 |
| Fontaine I | 0 | 0 | |
| Fontaine II | 13 (15) | 20 (20) | 0.35 |
| Fontaine III | 12 (14) | 25 (25) | 0.051 |
| Fontaine IV | 31 (36) | 19 (19) | 0.012 |
| Complications | 17 (20) | 21 (21) | 0.78 |
| Major adverse limb event | 17 (20) | 10 (10) | 0.068 |
| Myocardial infarction | 3 (3) | 1 (1) | 0.25 |
| Stroke | 0 (0) | 1 (1) | 0.35 |
| Hematoma | 4 (5) | 3 (3) | 0.58 |
| Dissection/perforation | 6 (7) | 12 (12) | 0.23 |
| Acute kidney injury | 9 (10) | 17 (17) | 0.18 |
| Repeat intervention/target lesion revascularization | 12 (14) | 8 (8) | 0.21 |
| Death | 2 (2) | 2 (2) | 0.90 |
In the entire study population (diagnostic and interventional procedures), men (mean follow-up 3.5 ± 3.3 months) compared with women (mean follow-up 4.3 ± 3.8 months) showed an increase in DAP (15221.2 ± 25858.5 µGy × m2vs 9251.7 ± 9555.3 µGy × m2, P = 0.047), but no significant increase in air kerma (616.8 ± 1101.8 mGy vs 474.8 ± 493.2 mGy; P = 0.38), fluoroscopy time (30.2 ± 25.6 minutes vs 24.1 ± 16.3 minutes; P = 0.34), or contrast use (106.1 ± 62.1 cc vs 98.4 ± 57.5 cc; P = 0.48).
During PEVI (n = 147, 79.0%), men (n = 71, 48.3%) compared with women (n = 76, 51.7%) also had higher DAP (16371.7 ± 28148.2 µGy × m2vs 8916.7 ± 9855.1 µGy × m2; P = 0.005). None of the other primary endpoints differed between gender. No significant difference was found in the number of men vs women undergoing PEVI (n = 71 vs n = 75; P = 0.33), location of intervention (suprainguinal, infrainguinal, or infrapopliteal), or treatment for such lesions. CTO intervention was also performed without any difference between gender. Retrograde femoral access was the most common approach (n = 179, 96.2%). Patient characteristics of those who underwent PEVI are listed in Table 2.
| Male, n = 71 | Female, n = 76 | P value | |
| Age | 64.4 ± 11.6 | 65.8 ± 13.4 | 0.49 |
| Weight (kg) | 80.3 ± 16.2 | 73.6 ± 16.8 | 0.016 |
| Height (cm) | 169.7 ± 14.8 | 158.7 ± 7.9 | < 0.001 |
| Body mass index (kg/m2) | 27.5 ±5.3 | 29.3 ± 6.5 | 0.069 |
| Body surface area (m2) | 1.9 ± 0.2 | 1.8 ± 0.2 | < 0.001 |
| Follow-up (month) | 4.0 ± 3.5 | 4.0 ± 3.6 | 0.84 |
| Diabetes | 42 (59) | 58 (76) | 0.026 |
| Hypertension | 50 (70) | 62 (82) | 0.11 |
| Hyperlipidemia | 32 (45) | 40 (53) | 0.36 |
| Past history of coronary artery disease | 18 (25) | 30 (39) | 0.068 |
| History of MI/PCI | 16 (23) | 25 (33) | 0.16 |
| Atrial fibrillation/flutter | 4 (6) | 8 (11) | 0.28 |
| History of chronic kidney disease/end stage renal disease on hemodialysis | 1 (1) | 5 (7) | 0.11 |
| History of peripheral intervention | 23 (32) | 19 (25) | 0.32 |
| History of vascular surgery | 2 (3) | 2 (3) | 0.94 |
| History of acute limb ischemia/critical limb ischemia | 11 (15) | 16 (21) | 0.38 |
| Current/former smoker | 45 (63) | 25 (33) | < 0.001 |
| History of amputation | 8 (11) | 13 (17) | 0.31 |
| Intermittent claudication | 7 (10) | 10 (13) | 0.53 |
| Critical limb ischemia | 37 (52) | 39 (51) | 0.92 |
| Acute limb ischemia | 11 (15) | 19 (25) | 0.15 |
| Lower extremity edema/deep venous thrombosis/May Thurner/chronic total occlusion vein | 14 (20) | 7 (9) | 0.069 |
| Venous stenosis | 0 (0) | 1 (1) | 0.33 |
| Re-look angiography | 5 (7) | 5 (7) | 0.91 |
| Peripheral aneurysm | 2 (3) | 0 (0) | 0.14 |
| Suprainguinal | 20 (28) | 21 (28) | 0.94 |
| Infrainguinal | 42 (59) | 52 (68) | 0.24 |
| Infrapopliteal | 29 (41) | 37 (49) | 0.34 |
| Intervention | 71 (100) | 75 (99) | 0.33 |
| Chronic total occlusion intervention | 23 (32) | 31 (41) | 0.29 |
| Dissection/perforation | 6 (8) | 12 (16) | 0.17 |
| Femoral access | 60 (85) | 69 (91) | 0.25 |
| Pedal | 11 (15) | 5 (7) | 0.083 |
| Retrograde | 68 (96) | 73 (96) | 0.93 |
| Antegrade | 0 (0) | 1 (1) | 0.33 |
| Brachial/radial | 12 (17) | 3 (4) | 0.010 |
| Venous intervention | 12 (17) | 7 (9) | 0.16 |
| Tasc A | 5 (7) | 8 (11) | 0.46 |
| Tasc B | 27 (38) | 21 (28) | 0.18 |
| Tasc C | 19 (27) | 33 (43) | 0.035 |
| Tasc D | 6 (8) | 6 (8) | 0.90 |
| Ruth grade I | 7 (10) | 12 (16) | 0.28 |
| Ruth grade II | 20 (28) | 32 (42) | 0.077 |
| Ruth grade III | 27 (38) | 16 (21) | 0.024 |
| Ruth grade IV | 1 (1) | 0 (0) | 0.30 |
| Fontaine I | 0 | 0 | |
| Fontaine II | 7 (10) | 17 (22) | 0.040 |
| Fontaine III | 10 (14) | 23 (30) | 0.019 |
| Fontaine IV | 28 (39) | 14 (18) | 0.005 |
| Staged intervention | 9 (13) | 8 (11) | 0.68 |
In the secondary endpoints, no significant difference was found between gender in any complication or the composite of complications. The incidence of MALE, acute kidney injury, or death was not significantly different. Table 3 Lists the primary and secondary endpoints between gender.
| Primary endpoints | Male | Female | P value |
| Total fluoro time (minute) | 33.3 ± 25.4 | 26.9 ± 15.7 | 0.071 |
| DAP (μGy × m2) | 16371.7 ± 28148.2 | 8916.7 ± 9855.1 | 0.005 |
| Air kerma (mGy) | 661.2 ± 1198.5 | 470.8 ± 511.6 | 0.11 |
| Contrast (cc) | 114.2 ± 63.3 | 105.4 ± 58.9 | 0.39 |
| Secondary endpoints complications | 16 (23) | 20 (26) | 0.59 |
| Major adverse limb event | 17 (24) | 10 (13) | 0.091 |
| MI | 2 (3) | 1 (1) | 0.52 |
| Stroke | 0 | 0 | |
| Hematoma | 3 (4) | 2 (3) | 0.59 |
| Dissection/perforation | 6 (8) | 12 (16) | 0.17 |
| Acute kidney injury | 9 (13) | 14 (18) | 0.34 |
| Repeat intervention/target lesion revascularization | 11 (15) | 8 (11) | 0.37 |
| Death | 2 (3) | 2 (2) | 0.52 |
PEVI is associated with elevated radiation which is detrimental to patients[3,6,7,9,13]. PEVI in aortoiliac lesions is associated with even higher radiation compared with the femoropopliteal or infrapopliteal regions[11,18,19]. Radiation is increased in PEVI so that tissue injury can develop in 1 out of 14 patients along with the inherent risk of malignancy[20]. The stochastic effects of radiation can induce genetic damage and cancer. Deterministic effects can induce patient skin injury and the development of cataracts in operators, specifically posterior subcapsular cataracts from ionizing radiation in the catheterization laboratory[21].
PEVI in women is associated with worse outcomes than men[14,15]. In the K-VIS registry[22], women had higher rates of death, myocardial infarction, major amputation, MALE, and procedural complications during PEVI than men. In the National Inpatient Sample database from 2002 to 2015, CLI outcomes between gender were evaluated for revascularization and in-hospital outcomes among 2400778 CLI hospitalizations (43.6% women). This retrospective study showed that women were older with a greater prevalence of hypertension, heart failure, obesity, and prior stroke. Also, women had lower incidence of any revascularization (34.7% vs 35.4%, P < 0.001). On multivariable analysis, women had a greater incidence of in-hospital mortality, bleeding, blood transfusion requirement, ischemic stroke, and postoperative infection[23].
In a post-hoc analysis of the EUCLID trial (Examining Use of Ticagrelor in PAD), sex specific differences in MACE and limb events were evaluated in 13885 patients (30-month follow-up) with PAD (28% women, n = 3888). Women had a lower risk of MACE [9.5% vs 11.2%; adjusted hazard ratio: 0.77; 95% confidence interval (95%CI): 0.68-0.88; P < 0.001] and all-cause-mortality (7.6% vs 9.7%; adjusted hazard ratio: 0.61; 95%CI: 0.53 to 0.71; P < 0.001). However, no differences occurred between women and men in MALE (2.6% vs 3.0%) and hospitalization for ALI (1.6% vs 1.7%)[24].
A retrospective analysis of the National Cardiovascular Data Registry Peripheral Vascular Intervention Registry from 2014-2017 (85 United States sites, n = 27119) found that 7.1% undergoing PEVI have the potential for tissue injury and are all exposed to the potential for subsequent malignancy. Multivariate logistic and linear regression models found that the most powerful predictors for DAP (DAP > 500 Gy × m2 and increased overall DAP) was lesion location, procedure duration, BMI, male sex, bifurcation lesion, diabetes, and hypertension[20].
In a German population-based registry (2004-2015) of procedures performed by vascular surgeons, interventional cardiologists, interventional radiologists, and angiologists, DAP was higher in men and lower when performed by vascular surgeons[16]. This was a prospective study of 24000 patients who underwent PEVI for PAD in Germany[16]. However, no other radiation parameters were evaluated in the study.
Furthermore, no study has evaluated the radiation differences between men and women undergoing PEVI comparing air kerma, DAP, and fluoroscopy time, nor the discrepancy in contrast use. These are important outcomes due to the pernicious effects of radiation and to discover if more radiation precautions should be implemented in a particular gender. The KAR RAD study[25] evaluated the effects of low dose radiation during cardiac catheterization and percu
Our study showed that DAP was increased in men during PEVI, which may be secondary to their increased weight and height compared with women (Tables 1 and 2). Also, men had a significantly greater body surface area, which may have elevated their DAP. Procedural complexity was actually significantly more in women (more TASC C), so this cannot account for the increase in DAP. Moreover, PEVI techniques were performed and adjusted based on lesion findings and complexity. However, the air kerma was not significantly different nor was fluoroscopy time or contrast utilization. Thus, men who undergo PEVI with either normal BMI or elevated BMI, consideration should be given to reduce radiation parameters such as lowering the frame rate during fluoroscopy and cineangiography since their DAP was increased. Hence, men had increased radiation absorption over the irradiated area. Furthermore, the scatter radiation to the operator will increase, which is an important factor during PEVI for the operator and the catheterization staff. Consequently, the operator can contemplate reducing radiation parameters to minimize potential harm to the patient and also scatter radiation to the operator and catheterization staff. Monitoring the operator’s radiation dose during PEVI in men and especially with elevated BMI can be considered. Therefore, in men and particularly, men with high BMI, meticulous care should be instituted to monitor and control radiation during PEVI. Even though men had greater weight than women, they did not experience more complications suggesting safety. No study in PEVI has specifically evaluated radiation reduction strategies. In cardiac catheterization and percutaneous coronary intervention, the KAR RAD study[25] showed the benefits of radiation reduction techniques which significantly reduced the radiation endpoints of air kerma and DAP.
Women underwent similar treatment for PAD including CTO PEVI as men. Women had no significant difference in the number of PEVI procedures performed compared with men. Even though women were significantly lower in weight and height, they did not experience more complications than men. Moreover, the increased severity of lesions in women did not contribute to enhanced complications. Women may have developed more severe disease since they were less symptomatic. Nevertheless, women did not have more risk factors than men. In actuality, men had an additional risk factor - more commonly smokers. Men also had more severe symptomatic disease even though they had lesions which were less complex.
Our prospective study reports an important finding of increased DAP in men. The combined radiation endpoints of air kerma, DAP, fluoroscopy time, and contrast utilization has not been previously studied in PEVI, so it answers a critical knowledge gap. Our results are relevant to interventional cardiologists, general cardiologists, vascular surgeons, vascular medicine physicians, and interventional radiologists who either perform PEVI or manage these patients. Awareness of the medical community of the potentially increased DAP in men during PEVI can suggest the performance of staged PEVI and more meticulous monitoring for the detrimental deterministic or stochastic effects of radiation. Furthermore, in men undergoing PEVI, practicing radiation reduction techniques should be considered to reduce potential harm. Since patients may undergo multiple procedures or tests which expose them to radiation in their lifetime, mitigating radiation during PEVI should be considered, especially in men. Collimation, fluoroscopy frame rate, filtration, and shielding are techniques which should be implemented for radiation reduction.
The prospective study was performed in a single academic center so it may potentially lack generalizability. Long-term follow-up on radiation effects were not studied. Unmeasured confounders may be procedure time which can vary by operator. Nonetheless, this is a meaningful study since all patients were included for PEVI (all-comers study). Moreover, no patients were excluded for gender analyses, so it provides practical data on radiation parameters, gender outcomes, and complications in PEVI.
To our knowledge, this is the first prospective study comparing radiation differences in air kerma and DAP between women and men undergoing PEVI in PVD including ALI and CLI. Men had increased DAP compared with women, but no significant difference in air kerma, contrast use, or fluoroscopy time. Women underwent PEVI with similar outcomes as men even though they had lower body weight and more complex lesions. Men had more symptomatic PAD. No gender difference in complications was found, but men had an extra risk factor (smoking). Consequently, PEVI may be safely performed in men and women regardless of gender and lesion severity. However, DAP was higher in men so they should be monitored closely during the procedure to minimize radiation to the patient and operator. Moreover, radiation reduction techniques to decrease DAP in men should be considered, especially in men with elevated BMI. In conclusion, our study showed that PEVI is safe and effective regardless of gender. However, meticulous radiation monitoring and practices are necessary, particularly in men. Further clinical research on radiation disparity and their long-term outcomes may be worthwhile in a larger cohort study.
We would like to thank Alok Dwivedi, PhD, and Luis A. Alvarado, MS, for their statistical analyses.
| 1. | Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2122] [Cited by in RCA: 2542] [Article Influence: 195.5] [Reference Citation Analysis (0)] |
| 2. | Allison MA, Armstrong DG, Goodney PP, Hamburg NM, Kirksey L, Lancaster KJ, Mena-Hurtado CI, Misra S, Treat-Jacobson DJ, White Solaru KT; American Heart Association Council on Peripheral Vascular Disease; Council on Hypertension; and Council on Lifestyle and Cardiometabolic Health. Health Disparities in Peripheral Artery Disease: A Scientific Statement From the American Heart Association. Circulation. 2023;148:286-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 102] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 3. | Andreassi MG, Piccaluga E, Guagliumi G, Del Greco M, Gaita F, Picano E. Occupational Health Risks in Cardiac Catheterization Laboratory Workers. Circ Cardiovasc Interv. 2016;9:e003273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 204] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 4. | Karatasakis A, Brilakis HS, Danek BA, Karacsonyi J, Martinez-Parachini JR, Nguyen-Trong PJ, Alame AJ, Roesle MK, Rangan BV, Rosenfield K, Mehran R, Mahmud E, Chambers CE, Banerjee S, Brilakis ES. Radiation-associated lens changes in the cardiac catheterization laboratory: Results from the IC-CATARACT (CATaracts Attributed to RAdiation in the CaTh lab) study. Catheter Cardiovasc Interv. 2018;91:647-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Matsubara K, Lertsuwunseri V, Srimahachota S, Krisanachinda A, Tulvatana W, Khambhiphant B, Sudchai W, Rehani M. Eye lens dosimetry and the study on radiation cataract in interventional cardiologists. Phys Med. 2017;44:232-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Elmaraezy A, Ebraheem Morra M, Tarek Mohammed A, Al-Habaa A, Elgebaly A, Abdelmotaleb Ghazy A, Khalil AM, Tien Huy N, Hirayama K. Risk of cataract among interventional cardiologists and catheterization lab staff: A systematic review and meta-analysis. Catheter Cardiovasc Interv. 2017;90:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 7. | Roguin A, Goldstein J, Bar O, Goldstein JA. Brain and neck tumors among physicians performing interventional procedures. Am J Cardiol. 2013;111:1368-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 415] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 8. | Klein LW, Tra Y, Garratt KN, Powell W, Lopez-Cruz G, Chambers C, Goldstein JA; Society for Cardiovascular Angiography and Interventions. Occupational health hazards of interventional cardiologists in the current decade: Results of the 2014 SCAI membership survey. Catheter Cardiovasc Interv. 2015;86:913-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 9. | Venneri L, Rossi F, Botto N, Andreassi MG, Salcone N, Emad A, Lazzeri M, Gori C, Vano E, Picano E. Cancer risk from professional exposure in staff working in cardiac catheterization laboratory: insights from the National Research Council's Biological Effects of Ionizing Radiation VII Report. Am Heart J. 2009;157:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 251] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 10. | Attigah N, Oikonomou K, Hinz U, Knoch T, Demirel S, Verhoeven E, Böckler D. Radiation exposure to eye lens and operator hands during endovascular procedures in hybrid operating rooms. J Vasc Surg. 2016;63:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Sigterman TA, Bolt LJ, Snoeijs MG, Krasznai AG, Heijboer R, Schurink GW, Bouwman LH. Radiation Exposure during Percutaneous Transluminal Angioplasty for Symptomatic Peripheral Arterial Disease. Ann Vasc Surg. 2016;33:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Balter S, Hopewell JW, Miller DL, Wagner LK, Zelefsky MJ. Fluoroscopically guided interventional procedures: a review of radiation effects on patients' skin and hair. Radiology. 2010;254:326-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 421] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 13. | Kirkwood ML, Arbique GM, Guild JB, Timaran C, Valentine RJ, Anderson JA. Radiation-induced skin injury after complex endovascular procedures. J Vasc Surg. 2014;60:742-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Jackson EA, Munir K, Schreiber T, Rubin JR, Cuff R, Gallagher KA, Henke PK, Gurm HS, Grossman PM. Impact of sex on morbidity and mortality rates after lower extremity interventions for peripheral arterial disease: observations from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium. J Am Coll Cardiol. 2014;63:2525-2530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Lo RC, Bensley RP, Dahlberg SE, Matyal R, Hamdan AD, Wyers M, Chaikof EL, Schermerhorn ML. Presentation, treatment, and outcome differences between men and women undergoing revascularization or amputation for lower extremity peripheral arterial disease. J Vasc Surg. 2014;59:409-418.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 16. | Behrendt CA, Rieß HC, Heidemann F, Diener H, Rohlffs F, Hohnhold R, Debus ES. Radiation Dosage for Percutaneous PAD Treatment is Different in Cardiovascular Disciplines: Results From an Eleven Year Population Based Registry in the Metropolitan Area of Hamburg. Eur J Vasc Endovasc Surg. 2017;53:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Nickoloff EL, Lu ZF, Dutta AK, So JC. Radiation dose descriptors: BERT, COD, DAP, and other strange creatures. Radiographics. 2008;28:1439-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Segal E, Weinberg I, Leichter I, Klimov A, Giri J, Bloom AI. Patient radiation exposure during percutaneous endovascular revascularization of the lower extremity. J Vasc Surg. 2013;58:1556-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Kaya IÇ, Kaya FA. Radiation doses in endovascular revascularisation of lower-extremity arterial diseases. Cardiovasc J Afr. 2023;34:212-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Goldsweig AM, Kennedy KF, Abbott JD, Jones WS, Velagapudi P, Rutar FJ, Curtis JC, Tsai TT, Aronow HD. Patient Radiation Dosage During Lower Extremity Endovascular Intervention. JACC Cardiovasc Interv. 2019;12:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Picano E, Vano E. The radiation issue in cardiology: the time for action is now. Cardiovasc Ultrasound. 2011;9:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 22. | Choi KH, Park TK, Kim J, Ko YG, Yu CW, Yoon CH, Lee JH, Min PK, Koh YS, Chae IH, Choi D, Choi SH; K‐VIS Investigators. Sex Differences in Outcomes Following Endovascular Treatment for Symptomatic Peripheral Artery Disease: An Analysis From the K- VIS ELLA Registry. J Am Heart Assoc. 2019;8:e010849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Elbadawi A, Barssoum K, Megaly M, Rai D, Elsherbeeny A, Mansoor H, Shishehbor MH, Abdel-Latif A, Gulati M, Elgendy IY. Sex Differences in Trends and In-Hospital Outcomes Among Patients With Critical Limb Ischemia: A Nationwide Analysis. J Am Heart Assoc. 2021;10:e022043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Haine A, Kavanagh S, Berger JS, Hess CN, Norgren L, Fowkes FGR, Katona BG, Mahaffey KW, Blomster JI, Patel MR, Jones WS, Rockhold FW, Hiatt WR, Baumgartner I; International Steering Committee and Investigators of the EUCLID Trial. Sex-Specific Risks of Major Cardiovascular and Limb Events in Patients With Symptomatic Peripheral Artery Disease. J Am Coll Cardiol. 2020;75:608-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 25. | Kar S, Teleb M, Albaghdadi A, Ibrahim A, Mukherjee D. Efficacy of Low-Dose Compared With Standard-Dose Radiation for Cardiac Catheterization and Intervention (KAR RAD Study). J Invasive Cardiol. 2019;31:187-194. [PubMed] [DOI] [Full Text] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/