Published online Dec 26, 2025. doi: 10.4330/wjc.v17.i12.112396
Revised: September 4, 2025
Accepted: November 13, 2025
Published online: December 26, 2025
Processing time: 152 Days and 1.6 Hours
Cardiac myxoma (CM) is the most common type of primary cardiac tumor and a major embolic source of cardioembolic stroke. Two potential causative mecha
Core Tip: Cardiac myxoma, although rare, is a significant cause of cardioembolic stroke in young adults, often due to embolism from tumor fragments or metastatic spread. The link between tumor mobility, morphology, and stroke risk remains unclear. This article explores the relationship between cardiac myxoma and ischemic stroke (CM-IS), highlighting two potential pathogenetic mechanisms and identifying tumor morphology and mobility as critical risk factors. CM-IS predominantly affects younger patients presenting with acute neurological symptoms, necessitating tailored diagnostic and therapeutic approaches. The analysis highlights the complexity of CM-IS and calls for further research to refine its management and support clinical decision making.
- Citation: Cai B, Qiao ML, Miao D, Liu GZ. Cardiac myxoma and its implications for cardioembolic stroke. World J Cardiol 2025; 17(12): 112396
- URL: https://www.wjgnet.com/1949-8462/full/v17/i12/112396.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i12.112396
Cardiac myxoma (CM) is the most common type of cardiac tumor, accounting for 50%-85% of all benign cardiac tumors[1,2]. The overall low incidence of cardiac tumors (only 0.02% of all tumors during autopsies[3]) renders CM a rare disease, with an incidence of approximately 0.5 to 1 per million individuals. A prospective study of 402 patients with cardioembolic cerebral infarction revealed that only 4 cases were associated with cardiac tumors[4]. Although the overall incidence of cardiac tumors and their related strokes is low in the general population, embolic events remain common and serious complications of CM, occurring in 20%-45% of cases[5], with cerebral embolism constituting 50% of all embolic events and representing a significant cause of cardioembolic stroke[6]. Considerable controversy persists regarding the causative mechanisms, risk factors, optimal timing of surgery, pharmacological treatment, and short- and long-term complications of CM-related ischemic stroke (CM-IS).
For this updated review, we systematically searched PubMed, Embase, and Web of Science databases for studies published between January 2000 and July 2025 using the keywords “cardiac myxoma”, “ischemic stroke”, “cardioembolic stroke”, and “neurological complications”. We included original research articles, systematic reviews, and large case series on CM-IS pathogenesis, risk factors, clinical characteristics, diagnostic approaches, or treatment. Single-case reports without an accompanying literature review and non-English publications were excluded. This article summarizes the pathogenesis, risk factors, clinical manifestations, and recent advances in the diagnosis and treatment of CM-IS.
To date, two potential causative mechanisms have been proposed for the pathogenesis of CM-IS: Embolism from detached tumor debris and metastatic infiltration. The first mechanism suggests that tissue fragments, thrombi, or a combination of both detach from the tumor mass owing to its gelatinous structure, migrate into the circulation, and occlude the cerebral vessels[7-9]. The second mechanism proposes that myxomatous cells shed from the tumor tissue, spread to the brain, infiltrate cerebral vessels, and cause focal alterations of the internal elastic lamina, leading to tumor seeding and growth, which can result in central nervous system metastases and recurrent brain infarctions[10,11]. Intriguingly, in our recent study, we identified multiple differentially expressed genes (miRNAs, lncRNAs, and mRNAs) in the plasma and tumor-derived exosomes of patients with CM-IS using RNA-seq datasets[12]. This finding seems significant because tumor-derived exosomes have been reported to play critical roles in tumor growth, metastasis, and immunoregulation[13,14]. Further research is needed to elucidate the pathogenesis and mechanistic actions of these exosomes in the context of CM-IS.
Previous studies have identified several risk factors for embolic complications in CM, including tumor type, size, location, and mobility; patient sex; platelet count; and lower B-type natriuretic peptide levels[15-18]. Among these factors, tumor fragments or tumors with high mobility have been associated with an increased incidence of cerebral embolism[18,19]. A general consensus exists across studies regarding the relationship between tumor mobility and morphology and CM-related stroke[19-22]. However, the influence of tumor size on the occurrence of stroke related to CM remains contentious[18,21,23,24], necessitating further research.
Although CM is relatively rare (< 0.5%), it remains a significant source of emboli, particularly in young adults presenting with stroke[25]. Several studies have reported that stroke caused by CM mainly affects young women in their third and sixth decades of life[17,26,27]; however, our recent multicenter study showed no female predominance, demonstrating a female-to-male ratio of 1:1[18]. Unlike cerebral aneurysms or metastatic brain tumors, CM-IS is typically associated with an acute onset, where initial symptoms mainly comprise hemiplegia, aphasia, altered consciousness, dysarthria, and ataxia, while some cases present with concomitant constitutional, cardiac, and/or neurological symptoms[18,26,28]. In the acute phase, more than half of patients presented with relatively mild or moderate clinical deficits[18,21,27], with a low incidence of early neurological deterioration[18]. Additionally, the involvement of a single cerebral vessel (predominantly the middle cerebral artery) and multiple territories were common among patients with CM-IS[18,21,26]. The most frequently affected areas are the basal ganglion and frontal, parietal, temporal, and cerebellar areas, all of which correspond well with neurologic deficits such as hemiplegia, hypoesthesia, and aphasia[18]. Consequently, brain imaging should be performed in all patients with CM who present with immediate neurological deficits. However, the clinical features of CM-IS related to metastatic infiltration remain largely unexplored owing to the rarity of such cases[10,11].
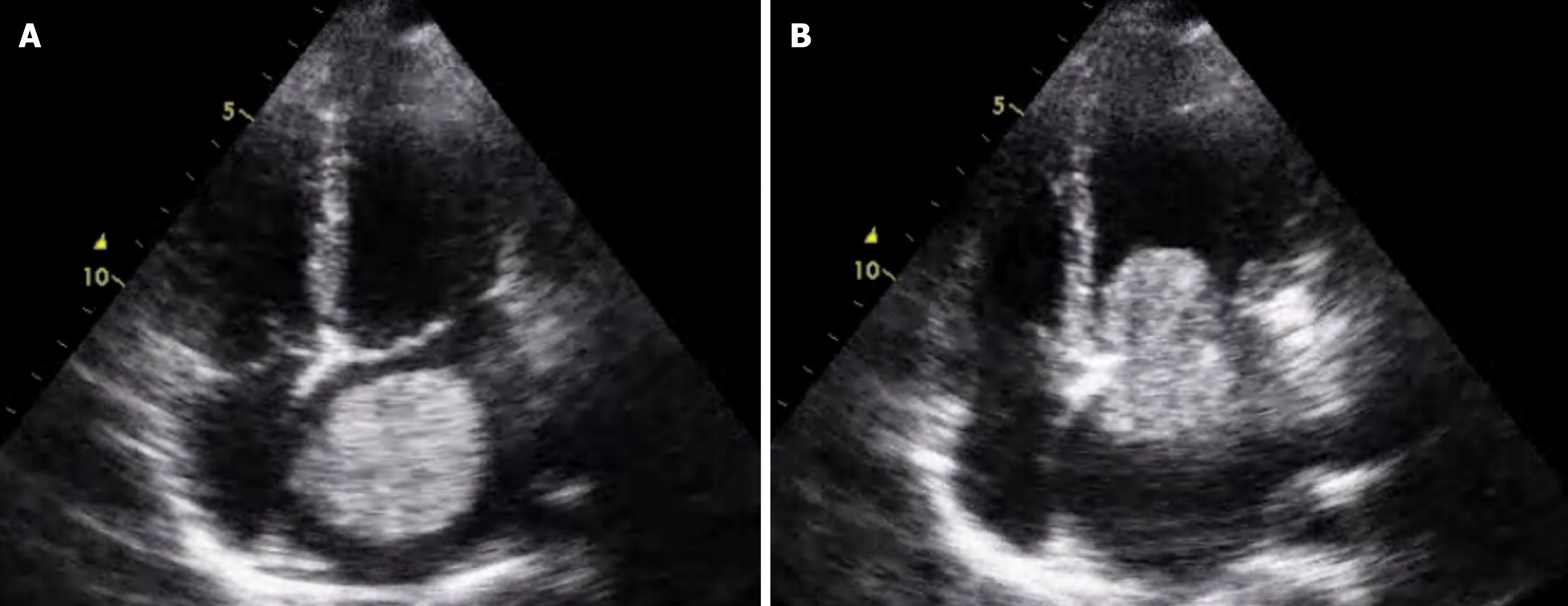
The diagnosis of CM-IS relies on clinical features (non-specific cardiac, embolic, and constitutional symptoms), imaging (cranial and cardiac), and laboratory investigations, with definitive diagnosis established through histopathological examination of the mass[29]. When CM-related stroke is suspected, cranial magnetic resonance imaging (MRI) should be performed early, especially during the hyperacute phase, to facilitate further management and gain valuable time. Comprehensive cranial MRI and vascular assessments should be completed prior to tumor resection because CM can metastasize intracranially, leading to intracranial aneurysms or myxomas. Echocardiography is the preferred diagnostic modality for CM and is capable of delineating the size, location, attachment, and mobility of the atrial mass as well as the degree of circulatory obstruction caused by the tumor, thereby aiding in the assessment of embolic event risk. Representative transthoracic echocardiographic images are shown (Figure 1). Among echocardiographic assessments, transesophageal echocardiography is superior to transthoracic echocardiography in characterizing atrial masses, despite being relatively simpler to perform[30]. Cardiac MRI can also assist in diagnosing CM, visualizing the tumor's anatomy, pericardial and pleural effusion, and providing insight into intratumoral vascularization via T1- and T2-weighted imaging[27,31]. For patients undergoing surgical resection of a CM, coronary angiography should be integrated into the routine preoperative evaluation[32]. Additionally, laboratory tests, such as those that measure serum interleukin-6 levels, can reflect the anatomical characteristics of the CM (e.g., tumor diameter, volume, and irregular tumor surface)[33,34]. However, to date, no universal diagnostic criteria for CM-IS have been established, although we first proposed cardioembolic stroke-based diagnostic criteria along with key points for the clinical diagnosis of CM-IS (Table 1)[35].
| Key points | Substance |
| Clinical features | More common in mid-aged and young women; typical symptoms of cardioembolic stroke |
| Brain imaging (CT/MRI) | Unilateral multi-territory infarcts (basal ganglia, cerebellum, parietal lobe, and temporal lobe), predominantly involving middle cerebral artery; cerebral hemorrhage or mucinous metastases are rare |
| Vascular imaging (CTA/MRA, DSA) | A single cerebral artery (especially the MCA) or multiple cerebral arteris stenosis, presenting as multi-segmental occlusion; accompanied by cerebral aneurysms or pseudoaneurysms |
| Cardiac findings | Confirmed cardiac myxoma |
| Laboratory findings | Increased serum level of erythrocyte sedimentation rate and C-reactive protein |
| Differential diagnosis | Exclusion of other diseases |
To date, no clear guidelines exist regarding the treatment options for CM-IS. The use of intravenous thrombolysis and mechanical thrombectomy (MT) in these patients was based solely on previously published case reports, with no reliable evidence supporting its clinical utilization. Thrombolysis has been administered on several occasions in patients with CM-IS, yielding variable degrees of success or failure[21,36-42]. We hypothesize that the composition and heterogeneity of the clot in CM-IS patients may influence the efficacy of both treatment modalities, as emboli can consist of tissue, thrombi, or a combination of both[43,44]. Theoretically, thrombotic embolisms could benefit more from thrombolysis. MT appears to be more effective for treating these patients and producing symptomatic alleviation[45-49]. More importantly, it can provide relevant information regarding the etiology of CM-IS through pathological examination of the retrieved clot. Currently, the evidence-based time window for thrombectomy and endovascular methods is not well defined. Upon review, MT was performed in patients with anterior circulation strokes within 4.5-6 hours[45,50] and posterior circulation strokes within 23 hours of symptom onset[47]. Moreover, MT with stent retrievers appears safe and effective for the rapid recanalization of large vessels secondary to CM, yielding favorable functional outcomes[47,50,51].
The definitive therapy for CM is complete surgical resection of the tumor to prevent further complications[9,52]. However, no consensus exists regarding the appropriate timing for cardiac surgery or surgical approaches following ischemic stroke in these cases. Some studies favor surgical intervention immediately or within 48 hours after ischemic stroke onset[53,54], albeit with uncertainty regarding the exact timing[55]. Conversely, other studies support delayed surgical intervention at 5 days or 7 days[36,45] or 3-4 weeks[21] following stroke onset. da Silva and de Freitas[56] suggested that for patients with CM-IS with severe symptoms and large infarcts, surgical resection should be postponed, while in patients with mild symptoms and small infarcts, early surgery may be warranted.
Advancements in surgical techniques have enabled myxoma excision using various methods, including classical surgery (median sternotomy), minimally invasive approaches (right anterolateral minithoracotomy), and robot-assisted interventions. Among these techniques, minimally invasive approaches and robotic surgery have demonstrated advantages over median sternotomy in relation to hospital stay, blood loss, and the risk of complications such as stroke, although data on these two methods remain limited due to the rarity of CM[52]. We propose that the timing and selection of surgical resection should be individualized, taking into account the tumor size, location, patient condition (e.g., disease severity and infarct size), and surgeon’s expertise. Nevertheless, further studies are needed to develop individualized, multidisciplinary, and comprehensive treatment protocols and to validate the long-term efficacy of surgical methods, particularly minimally invasive and robotic approaches.
The present case also has important clinical relevance. It highlights the diagnostic challenges of CM-IS, especially in patients presenting with acute ischemic stroke but lacking conventional vascular risk factors. This scenario underscores the necessity of early cardiac evaluation, including echocardiography, in cryptogenic stroke cases to promptly identify potential cardiac sources of embolism. Furthermore, it illustrates the importance of integrating neurological and cardiological assessments to determine the optimal timing for intervention. By placing this case in the context of the existing literature, it reinforces the need for heightened clinical awareness and multidisciplinary collaboration to improve patient outcomes in CM-IS. A summary of the major case series and cohort studies on CM-IS, highlighting their diagnostic and therapeutic characteristics, is provided in Table 2.
| Ref. | Country | Sample size | Mean age (years) | Sex (male/female) | Tumor location | Neurological manifestations | Diagnostic modalities |
| Ekinci EI and Donnan[57], 2004 | Australia | 72 | 48 | 28/44 | LA (90%), RA (10%) | Ischemic stroke, TIA, seizures | TTE, TEE, MRI |
| Zhang et al[21], 2021 | China | 37 | 46 | 16/21 | LA (95%), RA (5%) | Multiple territory infarcts, recurrent emboli | MRI, CTA, TEE |
| Wu et al[45], 2024 | China | 15 | 50 | 6/9 | LA (87%), RA (13%) | Large vessel occlusion | MRI, DSA, MT |
| El Sabbagh et al[29], 2017 | United States | 12 | 49 | 5/7 | LA (100%) | Stroke, systemic embolism | TTE, TEE, MRI |
| Takach et al[58], 1998 | United States | 19 | 45 | 8/11 | LA (84%), RA (16%) | Stroke, embolic events | Echo, MRI |
Despite significant research progress in the field of CM-IS over the past decades, substantial uncertainty or controversy remains regarding its pathogenesis, risk factors, diagnosis, and therapy, primarily owing to the rarity of the disease. Two potential causative mechanisms, embolism from detached tumor debris and metastatic infiltration, may underlie CM-IS pathogenesis. The risk factors for embolism caused by CM include tumor mobility and morphology, although the role of tumor size is subject to significant debate across studies. CM-IS often initially presents with central nervous system complications, and its diagnosis requires a comprehensive assessment of clinical manifestations, imaging findings, and laboratory tests, with histopathological examination of the CM required for confirmation. Although surgical resection of myxoma remains the most effective treatment for CM-IS, the optimal timing and surgical approach have been contested. Large-sample studies are required to clarify these issues. However, the present review has certain limitations. The literature is constrained by the rarity of CM-IS, predominance of retrospective case series, lack of large-scale prospective studies, and heterogeneity of diagnostic and therapeutic approaches. These factors restrict the generalizability of the current evidence and preclude definitive recommendations in some areas. Additionally, our work may have been influenced by selection bias despite the use of a systematic literature search strategy owing to the narrative review design.
Several avenues warrant further investigation. Large-scale, multicenter, prospective studies are essential to better define the incidence, risk factors, and natural history of CM-IS. Molecular and genetic profiling of CMs may help to elucidate the mechanisms underlying embolic events and potential metastatic behavior. The development of standardized diagnostic algorithms that integrate neurological and cardiological evaluations could facilitate timely and accurate diagnosis. Finally, comparative studies evaluating the timing and surgical techniques, including minimally invasive and robot-assisted approaches, are needed to optimize patient outcomes.
| 1. | Gošev I, Paić F, Durić Z, Gošev M, Ivčević S, Jakuš FB, Biočina B. Cardiac myxoma the great imitators: comprehensive histopathological and molecular approach. Int J Cardiol. 2013;164:7-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 2. | Islam AKMM. Cardiac myxomas: A narrative review. World J Cardiol. 2022;14:206-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (5)] |
| 3. | Reynen K. Frequency of primary tumors of the heart. Am J Cardiol. 1996;77:107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 552] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 4. | Pujadas Capmany R, Arboix A, Casañas-Muñoz R, Anguera-Ferrando N. Specific cardiac disorders in 402 consecutive patients with ischaemic cardioembolic stroke. Int J Cardiol. 2004;95:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Raicea VC, Suciu H, Raicea AD, Macarie GC, Mezei T, Maier MS. Giant left atrial myxoma - literature review and case presentation. Rom J Morphol Embryol. 2021;62:361-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Guo F, Jing ZC. [Modified classification, screening, prevention and treatment of cardioembolic stroke]. Zhonghua Yi Xue Za Zhi. 2022;102:3563-3568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Koyalakonda SP, Mediratta NK, Ball J, Royle M. A rare case of aortic valve myxoma: an unusual cause of embolic stroke. Cardiology. 2011;118:101-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Yuan L, Ge L, Zhu Y, Chen C, Zhou Z, Yang Q. Cardiac myxoma and ischemic stroke. QJM. 2020;113:674-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Butany J, Nair V, Naseemuddin A, Nair GM, Catton C, Yau T. Cardiac tumours: diagnosis and management. Lancet Oncol. 2005;6:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 548] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 10. | Rajeshwari M, Subramanian P, Suri V, Nambirajan A, Garg A, Vibha D, Phalak M, Sharma MC. Metastatic lesions of atrial myxoma. A pathologist can clinch them all. Neuropathology. 2020;40:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Solís-Gómez R, Dávalos Cabral N, Arrieta Limón G, Hurtado Presa BA, Salgado Alvear A, Reyes Martínez LM, Serrano Arias FE. Brain metastases in a patient with antecedent of cardiac myxoma: a case report and review of literature. Rev Fac Cien Med Univ Nac Cordoba. 2024;81:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Ma L, Liu YH, Liu C, Wang SQ, Ma J, Li XQ, Ren M, Yang TT, Liu GZ. lncRNA, miRNA, and mRNA of plasma and tumor-derived exosomes of cardiac myxoma-related ischaemic stroke. Sci Data. 2025;12:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 13. | Poggio M, Hu T, Pai CC, Chu B, Belair CD, Chang A, Montabana E, Lang UE, Fu Q, Fong L, Blelloch R. Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory. Cell. 2019;177:414-427.e13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 822] [Cited by in RCA: 1072] [Article Influence: 153.1] [Reference Citation Analysis (0)] |
| 14. | Huang M, Ji J, Xu X, Jin D, Wu T, Lin R, Huang Y, Qian J, Tan Z, Jiang F, Hu X, Xu W, Xiao M. Known and unknown: Exosome secretion in tumor microenvironment needs more exploration. Genes Dis. 2025;12:101175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 15. | Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine (Baltimore). 2001;80:159-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 593] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 16. | He DK, Zhang YF, Liang Y, Ye SX, Wang C, Kang B, Wang ZN. Risk factors for embolism in cardiac myxoma: a retrospective analysis. Med Sci Monit. 2015;21:1146-1154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Lee SJ, Kim JH, Na CY, Oh SS. Eleven years' experience with Korean cardiac myxoma patients: focus on embolic complications. Cerebrovasc Dis. 2012;33:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Qiao ML, Ma L, Wang CB, Fang LB, Fan ZX, Niu TT, Wang ZY, Lu JF, Yuan BY, Liu GZ. Clinical features, risk factors and survival in cardiac myxoma-related ischemic stroke: A multicenter case-control study. J Neurol Sci. 2023;444:120517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 19. | Wold LE, Lie JT. Cardiac myxomas: a clinicopathologic profile. Am J Pathol. 1980;101:219-240. [PubMed] |
| 20. | Wen XY, Chen YM, Yu LL, Wang SR, Zheng HB, Chen ZB, Ma L, Liao XP, Li QF. Neurological manifestations of atrial myxoma: A retrospective analysis. Oncol Lett. 2018;16:4635-4639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Zhang Y, Ye Z, Fu Y, Zhang Z, Ye Q, Chen F, Cheng J. Characterizations of Ischemic Stroke Complications in Cardiac Myxoma Patients at a Single Institution in Eastern China. Neuropsychiatr Dis Treat. 2021;17:33-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Liao WH, Ramkalawan D, Liu JL, Shi W, Zee CS, Yang XS, Li GL, Li J, Wang XY. The imaging features of neurologic complications of left atrial myxomas. Eur J Radiol. 2015;84:933-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Yin L, Wang J, Li W, Ling X, Xue Q, Zhang Y, Wang Z. Usefulness of CHA(2)DS(2)-VASc Scoring Systems for Predicting Risk of Perioperative Embolism in Patients of Cardiac Myxomas Underwent Surgical Treatment. Sci Rep. 2016;6:39323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Wang Z, Chen S, Zhu M, Zhang W, Zhang H, Li H, Yuan G, Zou C. Risk prediction for emboli and recurrence of primary cardiac myxomas after resection. J Cardiothorac Surg. 2016;11:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Saaf S, Miqdadi A, Merzouk FZ, El Aidaoui K, Hazim A. Cardiac Myxoma as a Rare Cause of an Ischemic Stroke of the Vertebrobasilar Territory in a Young Adult: A Case Report. Cureus. 2022;14:e24792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 26. | Yuan SM, Humuruola G. Stroke of a cardiac myxoma origin. Rev Bras Cir Cardiovasc. 2015;30:225-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Stefanou MI, Rath D, Stadler V, Richter H, Hennersdorf F, Lausberg HF, Lescan M, Greulich S, Poli S, Gawaz MP, Ziemann U, Mengel AM. Cardiac Myxoma and Cerebrovascular Events: A Retrospective Cohort Study. Front Neurol. 2018;9:823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Singh PK, Sureka RK, Sharma AK, Bhuyan S, Gupta V. Recurrent stroke in a case of left atrial myxoma masquerading vasculitis. J Assoc Physicians India. 2013;61:912, 917-920. [PubMed] |
| 29. | El Sabbagh A, Al-Hijji MA, Thaden JJ, Pislaru SV, Pislaru C, Pellikka PA, Arruda-Olson AM, Grogan M, Greason KL, Maleszewski JJ, Klarich KW, Nkomo VT. Cardiac Myxoma: The Great Mimicker. JACC Cardiovasc Imaging. 2017;10:203-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Swartz MF, Lutz CJ, Chandan VS, Landas S, Fink GW. Atrial myxomas: pathologic types, tumor location, and presenting symptoms. J Card Surg. 2006;21:435-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Hoffmeier A, Sindermann JR, Scheld HH, Martens S. Cardiac tumors--diagnosis and surgical treatment. Dtsch Arztebl Int. 2014;111:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 32. | Nguyen T, Vaidya Y. Atrial Myxoma. 2023 Jul 3. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 33. | Gavrielatos G, Letsas KP, Pappas LK, Dedeilias P, Sioras E, Kardaras F. Large left atrial myxoma presented as fever of unknown origin: a challenging diagnosis and a review of the literature. Cardiovasc Pathol. 2007;16:365-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 34. | Yuan SM, Lin HZ. Predictors of Normalization of Circulating Interleukin-6 after Cardiac Myxoma Resection. Braz J Cardiovasc Surg. 2019;34:22-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Liu GZ, Hu R, Peng DT; Geriatric Neurology Group, Geriatric Branch of Chinese Medical Association; Writing Group of Chinese expert consensus on diagnosis of cardiogenic stroke. Chinese expert consensus on the diagnosis of cardiogenic stroke (2019). Chin Med J (Engl). 2021;134:505-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Shrestha GS, Rimal A, Shrestha SK, Shrestha PS, Acharya SP. Recurrent Ischemic Stroke in a Patient with Atrial Myxoma: A Case Report. JNMA J Nepal Med Assoc. 2022;60:969-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 37. | Nagy CD, Levy M, Mulhearn TJ 4th, Shapland M, Sun H, Yuh DD, Cheung D, Chandra-Strobos N. Safe and effective intravenous thrombolysis for acute ischemic stroke caused by left atrial myxoma. J Stroke Cerebrovasc Dis. 2009;18:398-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Ibrahim M, Iliescu C, Safi HJ, Buja ML, McPherson DD, Fuentes F. Biatrial myxoma and cerebral ischemia successfully treated with intravenous thrombolytic therapy and surgical resection. Tex Heart Inst J. 2008;35:193-195. [PubMed] |
| 39. | Vidale S, Comolli F, Tancredi L, Campana C, Arnaboldi M. Intravenous thrombolysis in a patient with left atrial myxoma. Neurol Sci. 2017;38:1345-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Dong M, Ge Y, Li J, Fu K, Zhang L, Teng W, Tian L. Intravenous thrombolysis for pure pontine infarcts caused by cardiac myxoma: a case report and literature review. Int J Neurosci. 2020;130:635-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Esmaeili S, Shojaei SF, Bahadori M, Mojtahed M, Mehrpour M. Intravenous Thrombolysis for Acute Ischemic Stroke Due to Cardiac Myxoma. Basic Clin Neurosci. 2020;11:855-859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Zapata-Arriaza E, Pardo-Galiana B, González-García A, Montaner Villalonga J. Intravenous thrombolysis and thrombectomy in young patients with ischaemic stroke due to undetected atrial myxoma: Do recent clinical trials provide sufficient evidence to support reperfusion in these cases? Neurologia. 2017;32:404-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Alkuwaiti FA, Elghoneimy Y, Ghazal S. Aortic Valve Myxoma Presenting with a Stroke: A case report and review of the literature. Sultan Qaboos Univ Med J. 2018;18:e537-e540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Chang WS, Li N, Liu H, Yin JJ, Zhang HQ. Thrombolysis and embolectomy in treatment of acute stroke as a bridge to open-heart resection of giant cardiac myxoma: A case report. World J Clin Cases. 2021;9:7572-7578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Wu X, Chen T, Han Y, Wang K, Zhou J. Left atrial myxoma as a rare cause of stroke. Heliyon. 2024;10:e23897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 46. | Martin O, Devejian N, Paul A, Randall J. Early Surgical Resection of Left Atrial Myxoma After Thromboembolic Stroke. World J Pediatr Congenit Heart Surg. 2024;15:245-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 47. | Bhatia V, Jain C, Ray S, Gupta O, Chatterjee D, Kumar A. Mechanical Thrombectomy in Embolic Cardiac Myxoma: Case Report and Literature Review. Neurol India. 2021;69:707-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Zhou B, Huang S, Liu S, Ren L, Huang C, Lian Z. Thrombectomy for Stroke Caused by Cardiac Myxoma. J Stroke Cerebrovasc Dis. 2020;29:105407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 49. | Bandlamuri S, Custozzo A, Silva J, Bandlamuri SK, Qian J, Paul AR. Systematic Review and Case of Thrombectomy for Pediatric Stroke Due to Myxoma Embolism. World Neurosurg. 2024;183:e761-e771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Vega RA, Chan JL, Anene-Maidoh TI, Grimes MM, Reavey-Cantwell JF. Mechanical thrombectomy for pediatric stroke arising from an atrial myxoma: case report. J Neurosurg Pediatr. 2015;15:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 51. | Zander T, Maynar J, López-Zárraga F, Herrera R, Timiraos-Fernández JJ, Saraceni A, Maynar M. Mechanical thrombectomy in patients with tumour-related ischaemic stroke. Interv Neuroradiol. 2016;22:705-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Mendyka D, Płonek T, Jędrasek T, Korman A, Złotowska A, Jędrasek A, Skalik R, Kustrzycki W. The Therapeutic Potential of Different Surgical Approaches in the Management of Cardiac Myxoma: A Systematic Review. J Clin Med. 2024;14:121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 53. | Chiarello G, Garzya M, Donateo M, Marazia S, Soldato N, Cucurachi MR, Guaricci AI, Colonna G. Giant left atrial myxoma causing acute ischemic stroke. Future Cardiol. 2023;19:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 54. | Salam H, Reddy MK, Ganaraja VH, V S, Kodapala S. Cardioembolic Stroke in Young: A Case of Atrial Myxoma Origin. Cureus. 2022;14:e27890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 55. | Sharma P, Kumble YA, Shrestha AB, Jaiswal V. A case report of left atrial myxoma presenting as embolic stroke. Clin Case Rep. 2023;11:e8022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 56. | da Silva IR, de Freitas GR. Is it safe to proceed with thrombolytic therapy for acute ischemic stroke in a patient with cardiac myxoma? Case report and review of the literature. Eur Neurol. 2012;68:185-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 57. | Ekinci EI, Donnan GA. Neurological manifestations of cardiac myxoma: a review of the literature and report of cases. Intern Med J. 2004;34: 243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 58. | Takach TJ, Reul GJ Jr, Cooley DA, Livesay JJ, Duncan JM, Ott DA, Hallman GL. Concomitant occlusive disease of the coronary arteries and great vessels. Ann Thorac Surg. 1998;65: 79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/