Published online Nov 26, 2025. doi: 10.4330/wjc.v17.i11.110339
Revised: July 19, 2025
Accepted: September 23, 2025
Published online: November 26, 2025
Processing time: 169 Days and 23.6 Hours
SRY-related high-mobility group box 9 (SOX9) is an indispensable transcription factor that regulates multiple developmental pathways related to stem cell differentiation and progenitor cell development. Several studies have investigated the role of SOX9 in chondrogenesis and oncogenesis. Significant research exists describing the role of SOX9 in embryological development of the cardiovascular system. However, there is limited research exploring the roles of SOX9 in deve
Core Tip: This article synthesizes evidence revealing the dual roles of SRY-related high-mobility group box 9 (SOX9) in cardiovascular biology. Crucially, SOX9 is indis
- Citation: Chadha AN, Cheng H, Yang J. Role of SOX9 in cardiovascular diseases: Evidence today. World J Cardiol 2025; 17(11): 110339
- URL: https://www.wjgnet.com/1949-8462/full/v17/i11/110339.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i11.110339
Cardiovascular diseases (CVD) are a barrier to sustainable human development, being among the notable causes of health loss globally, with an estimated 420 million cases and 18 million deaths. In China, CVD are attributed to be the leading cause of mortality[1-3]. Tremendous research has been poured into uncovering the specific causes of these diseases and providing targeted therapies to minimize the impact of these cardiac insults. Most myocardial insults result in cardiac fibrosis, which is a complicated task carried out by myocardial fibroblasts and although the degree of fibrosis can predict unfavorable outcomes, fibrosis itself is not the paramount cause of cardiac dysfunction[4,5].
Cardiac development is an extremely complex process driven by intricate transcriptional networks. Numerous transcription factors (TFs) have been identified that significantly affect cardiovascular development. To name a few, GATA binding protein, heart and neural crest derivatives expressed (Hand), NK2 homeobox, myocyte enhancer factor 2, serum response factor and T-box are considered to be core cardiac TFs[6]. Some other notable cardiac TFs include nuclear transcription factor Y subunit alpha, a TF that regulates metabolic state of the embryonic heart[7]; the Iroquois homeobox TF family plays prominent roles in regulating and fine-tuning the heart development as well as cardiac electrical patterning[8]; ETS variant transcription factor 1 is a critical regulator of the fast conduction phenotype and demonstrate the biological importance of this gene in cardiac conduction disease[9]; early B-cell factor 1 is involved in a previously unreported non-cell-autonomous pathway controlling cardiac growth and differentiation[10].
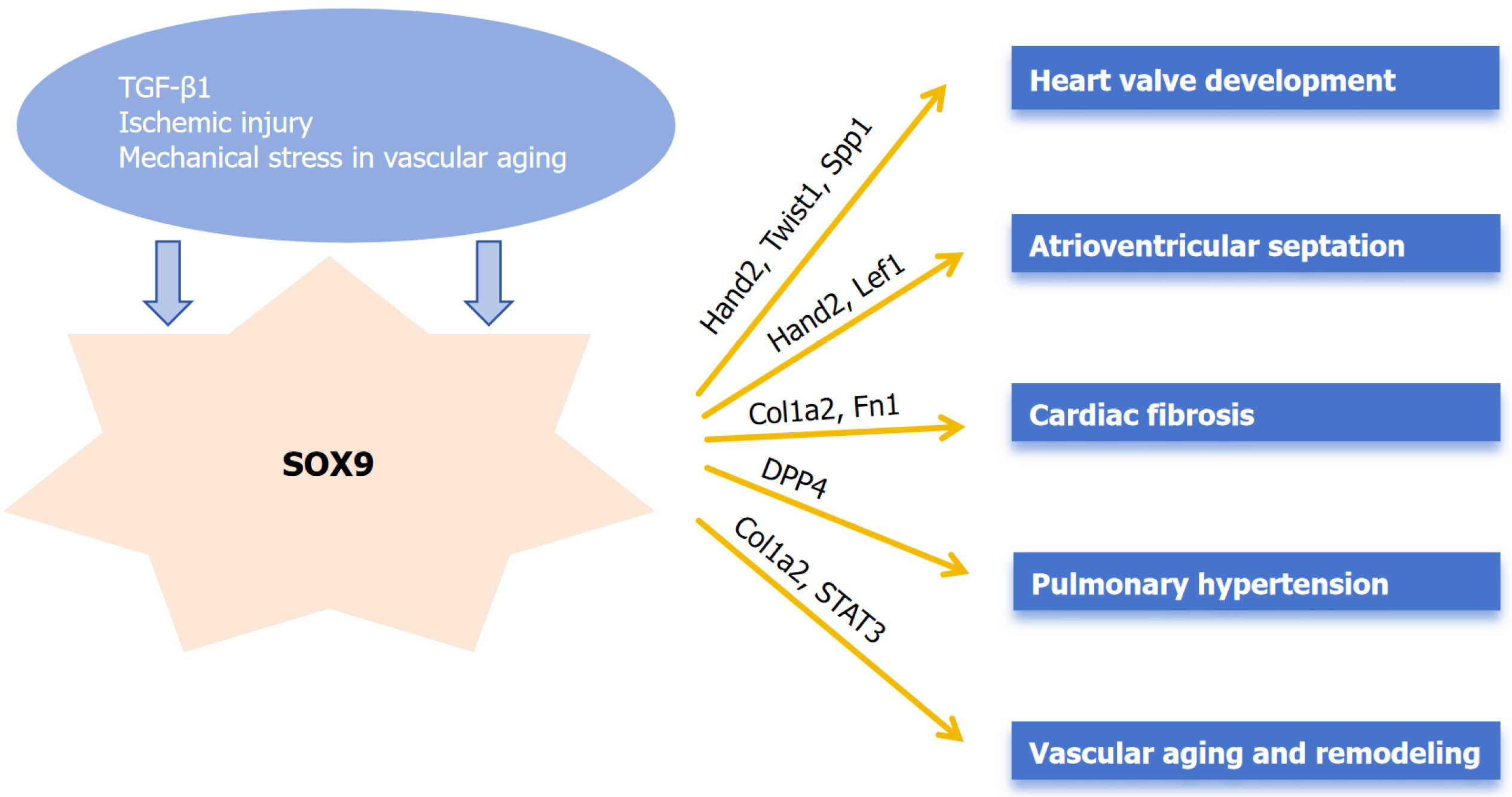
SRY-related high-mobility group box 9 (SOX9) is a TF with a high-mobility-group DNA-binding domain exhibiting a high degree of homology to the mammalian testis-determining factor, SRY. It plays a crucial role in sex determination, chondrogenesis, cardiogenesis, gliogenesis, and maintaining the pool of progenitor cells in the pancreas, among many other functions[11-13]. In cardiac development, SOX9 plays a role in regulation of extracellular matrix (ECM) components, progenitor cell proliferation and heart valve TF regulation[14]. SOX9 mediates proliferative activity in valvular interstitial cells (VICs) precursor cells during endocardial cushion formation, a critical process in initial valve ontogeny. It also plays an anti-calcific role in VICs[15].
The SOX9/Sox9 gene lies on human chromosome 17q and mouse chromosome 11q and is located in a gene desert containing long-range spatiotemporal specific enhancers. Protein sequence comparisons showed that the SOX genes fall into eight groups, A to H. SOX9 belongs to the group E of SOX proteins[13]. The human SOX9 protein comprises 509 amino acids and consists of a high-mobility-group box, a dimerization domain, two transactivation domains located in the middle and the C-terminus of the protein, and a proline/glutamine/alanine-rich domain[14]. SOX9 plays a major role in chondrogenesis, sex determination (development of the testis) and cardiac development.
The specific roles and targets of SOX9 during the process of cardiogenesis are listed below: (1) Regulation of ECM components: SOX9 targets aggrecan, elastin, hyaluronan and proteoglycan link protein 1, periostin, collagen type II alpha 1 chain (Col2a1) to carry out regulation of ECM[16]; (2) Proliferation of progenitor cells: SOX9 is involved in progenitor cell proliferation by acting on protein kinase B serine/threonine kinase 2, COP9 signalosome subunit 5, JunB proto-oncogene, Fos proto-oncogene (Fos), FosB proto-oncogene, Fos like 2, SRSF protein kinase 2, embryonic ectoderm development, histone deacetylase 1, histone deacetylase 2[16]; and (3) Regulation of cardiac valve TFs: SOX9 regulates these TFs by acting on Hand2, Twist family BHLH transcription factor 1, SOX4, MDS1 and EVI1 complex locus, lymphoid enhancer binding factor 1, paired like homeodomain 2[16].
The development of mitral valve pathologies, such as mitral valve prolapse and mitral valve regurgitation, is strongly influenced by genetic predisposition. Key genes implicated in these processes include fibrillin-1, filamin A, matrix metalloproteinase 2, and SOX9. Notably, filamin A and SOX9 are involved in promoting valve aging and modulating the hyalinization process[17].
Gallina et al[18] performed experiments to examine the dynamic expression pattern of SOX9 during valve formation with a focus on postnatal growth and maturation stages in mice. SOX9 had a restricted expression pattern in embryonic valve structures in the mesenchyme/VIC cell population, that is maintained after birth following their maturation into valve interstitial cells. Their study provided insights into the temporal and spatial distribution of SOX9 during differential stages of valve development, growth and maturation.
Epithelial-mesenchymal transformation (EMT) is a critical developmental process repeated in multiple organs throughout embryogenesis. The formation of endocardial cushions which act as primordial tissue for cardiac valves and septa, is a classic example of epithelial-mesenchymal transformation during cardiogenesis. To demonstrate the role of SOX9 in EMT in the heart, Akiyama et al[19] conditionally inactivated the SOX9 gene in male and female germ lines by using the Cre-loxP recombination system and generated embryos that were devoid of SOX9. Their study revealed that SOX9-deficient mutants had hypoplastic endocardial cushions.
To further examine the role of SOX9 during EMT in the heart, Lincoln et al[20] used conditional inactivation of SOX9 in mouse models driven by targeting endothelial-derived cells and targeting a subset of differentiating valve cells (Col2a1-cre) during early and late stages of valvulogenesis, respectively. Loss of SOX9 function in endothelial-derived cells showed that it was required for expansion and diversification of the valve precursor cell pool following EMT. Later inactivation of SOX9 with Col2a1-cre resulted in decreased expression of cartilage matrix-associated markers and abnormal ECM patterning in remodeling valve leaflets. In adult mice with Col2a1-cre mediated heterozygous loss of SOX9, histological analyses (including Von Kossa staining for calcium and Movat’s Pentachrome for ECM) revealed heart valve calcification and increased ECM production. These findings, supported by statistical analyses such as Student's t-test, indicate that SOX9 plays an early role in endothelial cell proliferation and later roles in differentiation, patterning and homeostasis of mature valve structures.
Peacock et al[21] reported that SOX9 played a crucial role in preventing calcification of heart valve leaflets. They conducted a study utilizing heterozygous SOX9fl/+; Col2a1-cre mice (targeting type II collagen-derived valve cells) in which these mice developed calcific lesions within heart valve leaflets from 3 months of age. Histological assessment using Von Kossa staining revealed calcium deposits, and quantitative analysis showed statistically significant increases in bone-related genes as measured by quantitative real-time polymerase chain reaction using TaqMan Low Density Array cards, alongside ECM remodeling and inflammatory processes. This osteogenic phenotype is recapitulated in vitro following direct SOX9 knockdown via adenoviral Cre infection in neonatal mouse heart valve explants.
Another study conducted by Peacock et al[22] suggested that SOX9 suppresses ECM mineralization in developing heart valves and chondrocytes by transcriptionally repressing secreted phosphoprotein 1 (Spp1). Knockdown experiments established Spp1 as essential for SOX9-deficiency-induced mineralization. Luciferase assays further confirmed SOX9 binds an SRY response element to significantly inhibit Spp1 promoter activity.
New evidence confirms SOX9’s essential role in valvular homeostasis through epicardial regulation. Harvey et al[23] demonstrated that epicardial-specific SOX9 deletion impairs cell invasion into developing atrioventricular valves, ultimately causing postnatal myxomatous mitral valve degeneration reminiscent of human disease. This work further identified CD109 as a novel SOX9-regulated gene associated with valve pathogenesis (Figure 1).
Deepe et al[24] found that SOX9 was critically important during the proper cardiac atrioventricular septation and played an extensive role in atrioventricular valvulo-septal morphogenesis. Their experimental design involved detailed histological and immunofluorescent analysis of embryonic hearts from embryonic day 9.5 to embryonic day 18.5, combined with quantitative 3D reconstruction using AMIRA software and cell counting with Cell Profiler. Strong evidence showed that second heart field-specific deletion of SOX9 Led to a high incidence (94%, 16/17 analyzed embryos) of septal defects, including complete atrioventricular septal defects and ventricular septal defects. Statistical analysis confirmed that the hypoplasia of the mesenchymal cap (a key structure in septation) was significant (P < 0.05) in knockout embryos compared to heterozygous controls, both in terms of cell number and tissue volume. Their study in mice provided strong evidence regarding the involvement of SOX9 in development of atrioventricular septation and its deletion leading to atrioventricular septal defect formation. Complementarily, Drummond et al[25] revealed SOX9’s compartmentalized roles in septation through lineage-specific studies, showing that second heart field-specific SOX9 deletion disrupts outflow tract mesenchymal development, causing ventricular septal defects via failed fusion of the outlet septum with the atrioventricular complex.
Cardiac fibrosis is the prevailing pathophysiologic companion of myocardial diseases to a great degree[26,27]. Notable TFs that prominently affect cardiac remodeling and fibrosis are small mothers against decapentaplegic[28], myocardin-related TF[29], nuclear factor of activated T-cells[30] and serum response factor[31].
Lacraz et al[32] identified SOX9 as a key transcriptional regulator of ECM-related genes. Their in vivo studies demonstrated that loss of SOX9 following myocardial infarction significantly attenuated pathological cardiac fibrosis in response to ischemic injury. Mechanistic studies revealed that SOX9 directly binds to enhancer regions of fibrosis-related genes (e.g., Col1a2, Fn1) and amplifies profibrotic signals in cardiac fibroblasts. Their data unveiled the unknown relevance of SOX9 as a key regulator of cardiac fibrosis and underscores that tomo-seq can be used to increase our mechanistic insights into cardiac remodeling to help guide the identification of novel therapeutic candidates.
Scharf et al[33] described that fibroblast-specific downregulation of SOX9 Led to reduction in ECM deposition in a mouse model of myocardial infarction in vivo and it averts the activation toward a proliferative and migrating fibroblast phenotype in cell culture in vitro. Additionally, they demonstrated that reduced SOX9 expression in fibroblasts ameliorates persistent inflammation within the infarct scar, prevents cardiac dilatation, and improves cardiac function after myocardial infarction.
To determine the role of SOX9 and transforming growth factor 1 (TGF-β1) mediated atrial fibrosis in human right atrial appendage and rat atrial fibroblasts, Wang et al[34] studied the effect of SOX9 on atrial fibrosis by revealing that SOX9 expression was increased by adenovirus or depleted by small interfering RNA, and fibroblasts were then treated with TGF-β1 for 24 hours. Western blot analysis quantified protein expression of SOX9, α-smooth muscle actin (α-SMA), connexin 43, and collagen I in each group. Notably, TGF-β1-treated fibroblasts exhibited significantly elevated levels of SOX9, α-SMA, and collagen I compared to controls. Crucially, their findings positioned SOX9 downstream of TGF-β1 signaling in atrial fibroblasts. Mechanistically, SOX9 overexpression drove fibroblasts toward an α-SMA-positive myofibroblast phenotype, enhanced deposition of collagen I, the principal ECM component, and accelerated cell migration, while lowering connexin 43 expression, a shift characteristic of the fibroblast-to-myofibroblast transition. This strongly suggests that SOX9 acts as a critical mediator within the TGF-β1 pathway to drive atrial fibrosis by regulating fibroblast activation, ECM production, and motility. Although their study had limitations, they provided significant evidence that SOX9, which acts as a regulator for the differentiation, collagen deposition, and cell migration of atrial fibroblasts, can become a potential target for treating atrial fibrosis and AF.
Another study provided evidence that Bellidifolin (BEL), a xanthone compound traditionally used to treat heart diseases in the Inner Mongolia region, inhibited SOX9 to block TGF-β1 signalling activation, ameliorating myocardial fibrosis. Yao et al[35] found that BEL alleviated myocardial fibrosis by inhibiting SOX9, which downregulates the TGF-β1 expression and impedes small mothers against decapentaplegic 3 phosphorylation resulting in the downregulation of α-SMA, collagen I, and collagen III. They concluded that BEL may provide a new therapeutic strategy by targeting SOX9 against myocardial fibrosis.
Guo et al[36] suggested that SOX9 could act as a potential marker for monitoring pulmonary hypertension (PH) prognosis and can also be a therapeutic target for the same. The study revealed that SOX9 was highly expressed in hypoxia-exposed pulmonary artery smooth muscle cells and pulmonary arteries of rats, promoting pulmonary artery smooth muscle cells proliferation and migration by stabilizing dipeptidyl peptidase 4, which led to pulmonary vascular remodeling and consequently exacerbated PH in rats.
The study conducted by Faleeva et al[37] described a novel role for SOX9 in human vascular aging. Their data indicate that SOX9 expression positively correlates with vascular smooth muscle cell (VSMC) senescence and exhibits mechanosensitive properties in aged VSMCs. Crucially, SOX9 mediates ECM stiffening, and the resultant SOX9-induced ECM phenocopies senescent ECM signatures. This modified ECM functions as a paracrine inhibitor of VSMC proliferation while concurrently inducing DNA damage and inflammatory responses to accelerate VSMC aging. Clinically relevant mechanisms are emerging regarding SOX9’s vascular impact. Jiang et al[38] identified SOX9 as a key mediator of in-stent restenosis, where it drives VSMC transformation via adenosine monophosphate-activated protein kinase-mediated nuclear translocation and signal transducer and activator of transcription 3 promoter binding. SOX9 knockdown significantly attenuated neointimal hyperplasia in carotid injury models[38].
Yu et al[39] used a well-established aortic interposition allograft model, and specifically showed potent restriction of SOX9 knockdown to VSMCs phenotypic modulation and proliferation, which is associated with impaired neointimal lesion formation and lumen stenosis in aortic allografts. Their studies describe a potential mechanistic basis for the development of transplant arteriosclerosis and shed light on a promising therapeutic strategy for chronic allograft failure via targeting SOX9.
Recent breakthrough research reveals SOX9 functions as a pioneer TF that fundamentally reprograms endothelial cells by reshaping the chromatin landscape. During endothelial-to-mesenchymal transition, a process pivotal for both embryonic development and vascular pathology, SOX9 unlocks silent chromatin loci, deposits active histone modifications and drives persistent cell fate changes[40]. Single-cell analyses now confirm that these SOX9 driven chromatin changes occur within human atherosclerotic lesions, providing direct clinical relevance[40].
Collectively, the evidence demonstrates SOX9’s dual roles in cardiac development and pathological remodeling. SOX9 mutations were first linked to campomelic dysplasia, a syndrome which often leads to death of patients during the neonatal period. Campomelic dysplasia typically presents with defects of the musculoskeletal system, craniofacial abnormalities, male-to-female sex reversal, but also congenital heart malformations including atrioventricular septal defects[41].
In this article, we briefly summarized the involvement of SOX9 in development of the heart valves and the at
Future work should employ timed gene editing and lineage tracing in mice to clarify how SOX9 switches roles between promoting heart fibrosis and exerting a protective effect against valvular calcification. Importantly, given the current over reliance on rodent models, subsequent validation in human patient-derived samples is imperative to confirm the clinical relevance of these findings. An incredible amount of detail is available on the involvement of SOX9 in chondrocyte differentiation. Limited studies limit the understanding of SOX9’s involvement in CVD, but there is unrealized potential for recognizing its implications in cardiovascular system development and its contribution in the pathogenesis of certain CVD. For that reason, it is reasonably crucial to keep unwrapping the actions of SOX9 and the processes it regulates.
| 1. | Clark H. NCDs: a challenge to sustainable human development. Lancet. 2013;381:510-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 2. | Liu S, Li Y, Zeng X, Wang H, Yin P, Wang L, Liu Y, Liu J, Qi J, Ran S, Yang S, Zhou M. Burden of Cardiovascular Diseases in China, 1990-2016: Findings From the 2016 Global Burden of Disease Study. JAMA Cardiol. 2019;4:342-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 522] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 3. | Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, Alla F, Alvis-Guzman N, Amrock S, Ansari H, Ärnlöv J, Asayesh H, Atey TM, Avila-Burgos L, Awasthi A, Banerjee A, Barac A, Bärnighausen T, Barregard L, Bedi N, Belay Ketema E, Bennett D, Berhe G, Bhutta Z, Bitew S, Carapetis J, Carrero JJ, Malta DC, Castañeda-Orjuela CA, Castillo-Rivas J, Catalá-López F, Choi JY, Christensen H, Cirillo M, Cooper L Jr, Criqui M, Cundiff D, Damasceno A, Dandona L, Dandona R, Davletov K, Dharmaratne S, Dorairaj P, Dubey M, Ehrenkranz R, El Sayed Zaki M, Faraon EJA, Esteghamati A, Farid T, Farvid M, Feigin V, Ding EL, Fowkes G, Gebrehiwot T, Gillum R, Gold A, Gona P, Gupta R, Habtewold TD, Hafezi-Nejad N, Hailu T, Hailu GB, Hankey G, Hassen HY, Abate KH, Havmoeller R, Hay SI, Horino M, Hotez PJ, Jacobsen K, James S, Javanbakht M, Jeemon P, John D, Jonas J, Kalkonde Y, Karimkhani C, Kasaeian A, Khader Y, Khan A, Khang YH, Khera S, Khoja AT, Khubchandani J, Kim D, Kolte D, Kosen S, Krohn KJ, Kumar GA, Kwan GF, Lal DK, Larsson A, Linn S, Lopez A, Lotufo PA, El Razek HMA, Malekzadeh R, Mazidi M, Meier T, Meles KG, Mensah G, Meretoja A, Mezgebe H, Miller T, Mirrakhimov E, Mohammed S, Moran AE, Musa KI, Narula J, Neal B, Ngalesoni F, Nguyen G, Obermeyer CM, Owolabi M, Patton G, Pedro J, Qato D, Qorbani M, Rahimi K, Rai RK, Rawaf S, Ribeiro A, Safiri S, Salomon JA, Santos I, Santric Milicevic M, Sartorius B, Schutte A, Sepanlou S, Shaikh MA, Shin MJ, Shishehbor M, Shore H, Silva DAS, Sobngwi E, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadele Atnafu N, Tesfay F, Thakur JS, Thrift A, Topor-Madry R, Truelsen T, Tyrovolas S, Ukwaja KN, Uthman O, Vasankari T, Vlassov V, Vollset SE, Wakayo T, Watkins D, Weintraub R, Werdecker A, Westerman R, Wiysonge CS, Wolfe C, Workicho A, Xu G, Yano Y, Yip P, Yonemoto N, Younis M, Yu C, Vos T, Naghavi M, Murray C. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2907] [Cited by in RCA: 2760] [Article Influence: 306.7] [Reference Citation Analysis (0)] |
| 4. | Frangogiannis NG. Cardiac fibrosis. Cardiovasc Res. 2021;117:1450-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 864] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 5. | Humeres C, Frangogiannis NG. Fibroblasts in the Infarcted, Remodeling, and Failing Heart. JACC Basic Transl Sci. 2019;4:449-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 282] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 6. | Grunert M, Dorn C, Rickert-Sperling S. Cardiac Transcription Factors and Regulatory Networks. Adv Exp Med Biol. 2024;1441:295-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Cui M, Bezprozvannaya S, Hao T, Elnwasany A, Szweda LI, Liu N, Bassel-Duby R, Olson EN. Transcription factor NFYa controls cardiomyocyte metabolism and proliferation during mouse fetal heart development. Dev Cell. 2023;58:2867-2880.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Kim KH, Rosen A, Bruneau BG, Hui CC, Backx PH. Iroquois homeodomain transcription factors in heart development and function. Circ Res. 2012;110:1513-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Shekhar A, Lin X, Liu FY, Zhang J, Mo H, Bastarache L, Denny JC, Cox NJ, Delmar M, Roden DM, Fishman GI, Park DS. Transcription factor ETV1 is essential for rapid conduction in the heart. J Clin Invest. 2016;126:4444-4459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Kim EE, Shekhar A, Ramachandran J, Khodadadi-Jamayran A, Liu FY, Zhang J, Fishman GI. The transcription factor EBF1 non-cell-autonomously regulates cardiac growth and differentiation. Development. 2023;150:dev202054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 11. | Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813-2828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1291] [Cited by in RCA: 1375] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 12. | Huang B, Wang S, Ning Y, Lamb AN, Bartley J. Autosomal XX sex reversal caused by duplication of SOX9. Am J Med Genet. 1999;87:349-353. [PubMed] [DOI] [Full Text] |
| 13. | Lefebvre V, Dumitriu B, Penzo-Méndez A, Han Y, Pallavi B. Control of cell fate and differentiation by Sry-related high-mobility-group box (Sox) transcription factors. Int J Biochem Cell Biol. 2007;39:2195-2214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 368] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 14. | Ming Z, Vining B, Bagheri-Fam S, Harley V. SOX9 in organogenesis: shared and unique transcriptional functions. Cell Mol Life Sci. 2022;79:522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 58] [Reference Citation Analysis (0)] |
| 15. | Kodigepalli KM, Thatcher K, West T, Howsmon DP, Schoen FJ, Sacks MS, Breuer CK, Lincoln J. Biology and Biomechanics of the Heart Valve Extracellular Matrix. J Cardiovasc Dev Dis. 2020;7:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Garside VC, Cullum R, Alder O, Lu DY, Vander Werff R, Bilenky M, Zhao Y, Jones SJ, Marra MA, Underhill TM, Hoodless PA. SOX9 modulates the expression of key transcription factors required for heart valve development. Development. 2015;142:4340-4350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Opris CE, Suciu H, Jung I, Flamand S, Harpa MM, Opris CI, Popa C, Kovacs Z, Gurzu S. Significance of Fibrillin-1, Filamin A, MMP2 and SOX9 in Mitral Valve Pathology. Int J Mol Sci. 2024;25:9410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | Gallina D, Lincoln J. Dynamic Expression Profiles of Sox9 in Embryonic, Post Natal, and Adult Heart Valve Cell Populations. Anat Rec (Hoboken). 2019;302:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Akiyama H, Chaboissier MC, Behringer RR, Rowitch DH, Schedl A, Epstein JA, de Crombrugghe B. Essential role of Sox9 in the pathway that controls formation of cardiac valves and septa. Proc Natl Acad Sci U S A. 2004;101:6502-6507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 219] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Lincoln J, Kist R, Scherer G, Yutzey KE. Sox9 is required for precursor cell expansion and extracellular matrix organization during mouse heart valve development. Dev Biol. 2007;305:120-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 21. | Peacock JD, Levay AK, Gillaspie DB, Tao G, Lincoln J. Reduced sox9 function promotes heart valve calcification phenotypes in vivo. Circ Res. 2010;106:712-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Peacock JD, Huk DJ, Ediriweera HN, Lincoln J. Sox9 transcriptionally represses Spp1 to prevent matrix mineralization in maturing heart valves and chondrocytes. PLoS One. 2011;6:e26769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Harvey AB, Wolters RA, Deepe RN, Tarolli HG, Drummond JR, Trouten A, Zandi A, Barth JL, Mukherjee R, Romeo MJ, Vaena SG, Tao G, Muise-Helmericks R, Ramos PS, Norris RA, Wessels A. Epicardial deletion of Sox9 leads to myxomatous valve degeneration and identifies Cd109 as a novel gene associated with valve development. J Mol Cell Cardiol. 2024;186:16-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 24. | Deepe RN, Drummond JR, Wolters RA, Fitzgerald EA, Tarolli HG, Harvey AB, Wessels A. Sox9 Expression in the Second Heart Field; A Morphological Assessment of the Importance to Cardiac Development with Emphasis on Atrioventricular Septation. J Cardiovasc Dev Dis. 2022;9:376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Drummond JR, Deepe RN, Tarolli HG, Wolters RA, Devji I, Harvey AB, Wessels A. Sox9 in the second heart field and the development of the outflow tract; implications for cardiac septation and valve formation. Dev Dyn. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117:568-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 715] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 27. | Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71:549-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1120] [Cited by in RCA: 1289] [Article Influence: 107.4] [Reference Citation Analysis (8)] |
| 28. | Lighthouse JK, Small EM. Transcriptional control of cardiac fibroblast plasticity. J Mol Cell Cardiol. 2016;91:52-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 29. | Small EM, Thatcher JE, Sutherland LB, Kinoshita H, Gerard RD, Richardson JA, Dimaio JM, Sadek H, Kuwahara K, Olson EN. Myocardin-related transcription factor-a controls myofibroblast activation and fibrosis in response to myocardial infarction. Circ Res. 2010;107:294-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 316] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 30. | Herum KM, Lunde IG, Skrbic B, Florholmen G, Behmen D, Sjaastad I, Carlson CR, Gomez MF, Christensen G. Syndecan-4 signaling via NFAT regulates extracellular matrix production and cardiac myofibroblast differentiation in response to mechanical stress. J Mol Cell Cardiol. 2013;54:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 31. | Bretherton R, Bugg D, Olszewski E, Davis J. Regulators of cardiac fibroblast cell state. Matrix Biol. 2020;91-92:117-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 32. | Lacraz GPA, Junker JP, Gladka MM, Molenaar B, Scholman KT, Vigil-Garcia M, Versteeg D, de Ruiter H, Vermunt MW, Creyghton MP, Huibers MMH, de Jonge N, van Oudenaarden A, van Rooij E. Tomo-Seq Identifies SOX9 as a Key Regulator of Cardiac Fibrosis During Ischemic Injury. Circulation. 2017;136:1396-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 33. | Scharf GM, Kilian K, Cordero J, Wang Y, Grund A, Hofmann M, Froese N, Wang X, Kispert A, Kist R, Conway SJ, Geffers R, Wollert KC, Dobreva G, Bauersachs J, Heineke J. Inactivation of Sox9 in fibroblasts reduces cardiac fibrosis and inflammation. JCI Insight. 2019;5:e126721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 34. | Wang H, Chen Y, Zhao S, Wang X, Lu K, Xiao H. Effect of Sox9 on TGF-β1-mediated atrial fibrosis. Acta Biochim Biophys Sin (Shanghai). 2021;53:1450-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Yao TT, Yang HX, Sun JH, Zhang Y, Zhang Y, Song QH, Liu WZ, Zhang JJ, Li AY. Bellidifolin Inhibits SRY-Related High Mobility Group-Box Gene 9 to Block TGF-β Signalling Activation to Ameliorate Myocardial Fibrosis. Evid Based Complement Alternat Med. 2022;2022:6841276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 36. | Guo YZ, Cui HY, Cai MY, Wang D, Deng WP, Hu CP. SOX9 promotes hypoxic pulmonary hypertension through stabilization of DPP4 in pulmonary artery smooth muscle cells. Exp Cell Res. 2024;442:114254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 37. | Faleeva M, Ahmad S, Theofilatos K, Lynham S, Watson G, Whitehead M, Marhuenda E, Iskratsch T, Cox S, Shanahan CM. Sox9 Accelerates Vascular Aging by Regulating Extracellular Matrix Composition and Stiffness. Circ Res. 2024;134:307-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 60] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 38. | Jiang C, Ye J, Huang J, Gao Y, Chen H, Guo F, Guo L, Yuan X. SOX9 mediates the phenotypic transformation of vascular smooth muscle cells in restenosis after carotid artery injury. Front Cell Dev Biol. 2025;13:1592594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 39. | Yu Q, Liu JX, Zheng X, Yan X, Zhao P, Yin C, Li W, Song Z. Sox9 mediates autophagy-dependent vascular smooth muscle cell phenotypic modulation and transplant arteriosclerosis. iScience. 2022;25:105161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 40. | Fuglerud BM, Drissler S, Lotto J, Stephan TL, Thakur A, Cullum R, Hoodless PA. SOX9 reprograms endothelial cells by altering the chromatin landscape. Nucleic Acids Res. 2022;50:8547-8565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 41. | Houston CS, Opitz JM, Spranger JW, Macpherson RI, Reed MH, Gilbert EF, Herrmann J, Schinzel A. The campomelic syndrome: review, report of 17 cases, and follow-up on the currently 17-year-old boy first reported by Maroteaux et al in 1971. Am J Med Genet. 1983;15:3-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 195] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 42. | Wang J, Wan X, Le Q. Cross-regulation between SOX9 and the canonical Wnt signalling pathway in stem cells. Front Mol Biosci. 2023;10:1250530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/