Published online Nov 26, 2025. doi: 10.4330/wjc.v17.i11.110178
Revised: June 17, 2025
Accepted: October 21, 2025
Published online: November 26, 2025
Processing time: 174 Days and 23 Hours
Pulmonary embolism (PE) is a leading cause of cardiovascular mortality. Altho
To assess whether prehospital aspirin use is associated with improved outcomes in patients hospitalized with acute PE.
We conducted a retrospective case-control study of 323 adult patients admitted with computed tomography-confirmed acute PE from January 2020 to December 2023. Patients were stratified according to documented daily aspirin use for ≥ 7 days prior to hospital admission. Primary outcomes included right ventricular strain, intensive care admission, shock, mechanical ventilation, and in-hospital mortality. Univariate logistic regression was used. A P value < 0.05 was consi
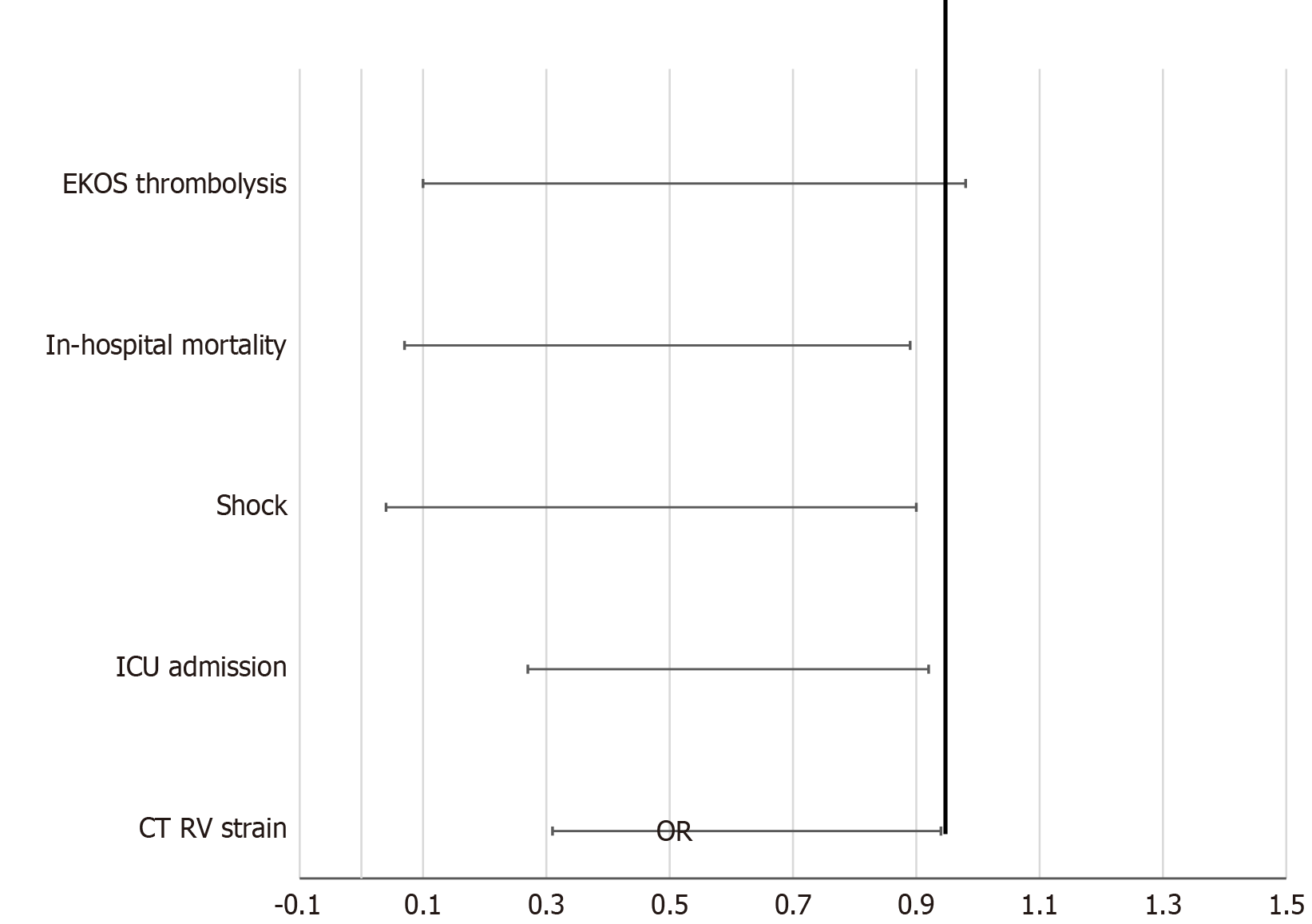
Total of 323 patients, 90 (27.9%) used aspirin prehospital. Aspirin users were older (74.2 ± 14.3 years vs 66.9 ± 16.7 years, P < 0.001) and had more coronary artery disease. Aspirin use was associated with significantly lower rates of right ventricular strain on computed tomography [22.2% vs 34.8%, odds ratio (OR) = 0.536, 95% confidence interval (CI): 0.305-0.944, P = 0.029], Intensive care admission (16.7% vs 28.8%, OR = 0.496, 95%CI: 0.266-0.924, P = 0.025), shock (2.2% vs 9.9%, OR = 0.208, 95%CI: 0.048-0.899, P = 0.021), and in-hospital mortality (3.3% vs 11.6%, OR = 0.260, 95%CI: 0.080-0.889, P = 0.022).
Prehospital aspirin use is associated with reduced severity and mortality in acute PE. These findings support a potential protective role for aspirin and warrant validation in prospective, multicenter trials.
Core Tip: In this retrospective case-control study, we demonstrate that patients with pulmonary embolism who were on aspirin prior to hospitalization had significantly lower incidence of right ventricular strain, intensive care unit admission, shock, and mortality. These results suggest a protective role of aspirin in pulmonary embolism pathophysiology and highlight the need for further prospective validation.
- Citation: Suresh MG, Mohamed S, Shanmugavel Geetha H, Sekar A, Prabhu S, Sargent J, Abraham GM, Hatwal J, Batta A, Mohan B. Prehospital aspirin use is associated with improved clinical outcomes in pulmonary embolism: A retrospective case-control study. World J Cardiol 2025; 17(11): 110178
- URL: https://www.wjgnet.com/1949-8462/full/v17/i11/110178.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i11.110178
Pulmonary embolism (PE), a major manifestation of venous thromboembolism (VTE), is a leading cause of cardiovascular morbidity and mortality, accounting for approximately 300000 deaths annually in the United States alone. It ranks third among causes of cardiovascular death after myocardial infarction and stroke. The annual incidence of PE ranges from 60 cases to 112 cases per 100000 population, with significantly higher rates observed in older adults. In-hospital mortality is estimated at 8%-12% for the general population but can exceed 25% in patients with high-risk features such as right ventricular (RV) dysfunction or hemodynamic instability[1-3].
Guidelines from the European Society of Cardiology and American College of Chest Physicians recommend risk stratification using clinical presentation, cardiac biomarkers, and imaging, particularly echocardiographic or computed tomography (CT) evidence of RV strain. Intermediate- and high-risk PE patients demonstrate increased rates of adverse outcomes, including shock, arrhythmias, need for intensive care, and death. Although anticoagulation is the standard of care, its prolonged use is limited by bleeding risks, particularly in elderly and comorbid populations[4-8].
Aspirin, a widely used antiplatelet agent, has demonstrated pleiotropic effects that may influence venous throm
Clinical trials such as WARFASA and ASPIRE demonstrated that low-dose aspirin reduced recurrent VTE risk by 26%-32% in patients completing anticoagulation, with a low incidence of major bleeding. A pooled analysis confirmed aspirin’s efficacy in extended secondary prevention for unprovoked VTE. Additionally, retrospective studies in acute coronary syndrome and ischemic stroke suggest that prehospital aspirin use is associated with smaller infarct size, reduced intensive care unit (ICU) admission, and improved survival[10-14].
Despite these findings, the role of aspirin in acute PE - particularly in non-surgical patients - remains poorly defined. Existing data are sparse and often focus on postoperative or mixed arterial/venous thrombotic settings. Whether aspirin can mitigate early complications such as RV dysfunction, shock, and death in acute PE has not been robustly examined[15,16].
This study aims to evaluate the association between prehospital aspirin use and clinical outcomes in non-surgical patients hospitalized with acute PE. We hypothesized that aspirin may reduce the severity of disease manifestations - specifically RV strain, ICU admission, shock, and mortality - through its antiplatelet and anti-inflammatory effects.
We conducted a retrospective, single-center, case-control study at Saint Vincent Hospital, a tertiary care academic center in Worcester, Massachusetts. The study included adult patients admitted with radiologically confirmed PE from January 1, 2020, to December 31, 2023. The study adhered to the STROBE checklist for observational studies.
Adults (≥ 18 years) with a confirmed diagnosis of acute PE on CT pulmonary angiography were eligible. PE diagnosis was defined by the presence of intraluminal filling defects in the pulmonary vasculature. We excluded patients with recent surgery (< 4 weeks), therapeutic anticoagulation for ≥ 7 consecutive days before admission, pregnancy or postpartum state (< 6 weeks), and those with post-surgical PE.
Patients were categorized according to documented prehospital aspirin use, defined as daily use of any aspirin dose for at least 7 days prior to admission, verified by outpatient records, medication reconciliation, or clinical documentation.
Baseline variables included age, sex, body mass index, and comorbidities (e.g., hypertension, coronary artery disease, chronic kidney disease, malignancy) as documented in Table 1. Additional variables included recent risk factors (e.g., trauma, travel), medication use, and presenting vital signs.
| Variable | Aspirin (n = 90) | No aspirin (n = 233) | P value |
| Age (year), mean ± SD | 74.2 ± 14.3 | 66.9 ± 16.7 | < 0.001 |
| Male sex | 49 (54.4) | 113 (48.5) | 0.338 |
| BMI (kg/m²), mean ± SD | 29.7 ± 7.4 | 29.7 ± 7.2 | 0.986 |
| Hypertension | 58 (64.4) | 125 (53.6) | 0.079 |
| Coronary artery disease | 44 (48.9) | 21 (9.0) | < 0.001 |
| Chronic kidney disease | 15 (16.7) | 23 (9.9) | 0.089 |
| Active malignancy | 18 (20.0) | 59 (25.3) | 0.314 |
| Smoking history | 47 (52.2) | 109 (46.8) | 0.380 |
| Heart rate on admission (bpm) | 89.0 ± 19.3 | 94.6 ± 21.4 | 0.032 |
The primary outcomes were: (1) Radiographic RV strain (RV/Left ventricle ratio > 1.0 on CT pulmonary angiography); (2) ICU admission during hospitalization; (3) Shock (systolic blood pressure < 90 mmHg or requiring vasopressors); (4) Mechanical ventilation; and (5) In-hospital mortality.
Secondary endpoints included echocardiographic RV strain, use of catheter-directed thrombolysis (Ekosonic endo
Categorical variables were analyzed using Fisher’s exact test or χ2 test. Continuous variables were compared using independent samples t-test. Logistic regression was used to estimate odds ratios (OR) with 95% confidence intervals (CI) for associations between aspirin use and outcomes. A two-tailed P value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS Version 28.0 (IBM Corp., Armonk, NY, United States).
A formal a priori sample size calculation was not performed due to the retrospective design. However, with 323 patients and outcome event rates exceeding 10% for several primary endpoints, the study was sufficiently powered to detect moderate effect sizes (OR = 0.4-0.6) with statistical confidence.
A total of 323 patients were included: 90 (27.9%) with documented prehospital aspirin use and 233 (72.1%) without (Table 2). Aspirin users were significantly older (74.2 ± 14.3 years vs 66.9 ± 16.7 years; P < 0.001) and had a higher prevalence of coronary artery disease (48.9% vs 9.0%; P < 0.001; OR = 9.66, 95%CI: 5.25-17.77). Other baseline characteristics, including sex distribution, body mass index, hypertension, chronic kidney disease, malignancy, and smoking status, were similar between groups.
| Outcome | Aspirin (n = 90) | No aspirin (n = 233) | P value | OR (95%CI) |
| ICU admission | 16.7% | 28.8% | 0.025 | 0.496 (0.266-0.924) |
| Shock | 2.2% | 9.9% | 0.021 | 0.208 (0.048-0.899) |
| RV strain (CT) | 22.2% | 34.8% | 0.029 | 0.536 (0.305-0.944) |
| In-hospital mortality | 3.3% | 11.6% | 0.022 | 0.260 (0.080-0.889) |
| Mechanical ventilation | 6.7% | 4.7% | 0.483 | 1.442 (0.517-4.021) |
| EKOS therapy | 3.3% | 11.2% | 0.047 | 0.275 (0.081-0.931) |
| Cardiac arrest | 6.7% | 6.0% | 0.826 | 1.117 (0.416-3.004) |
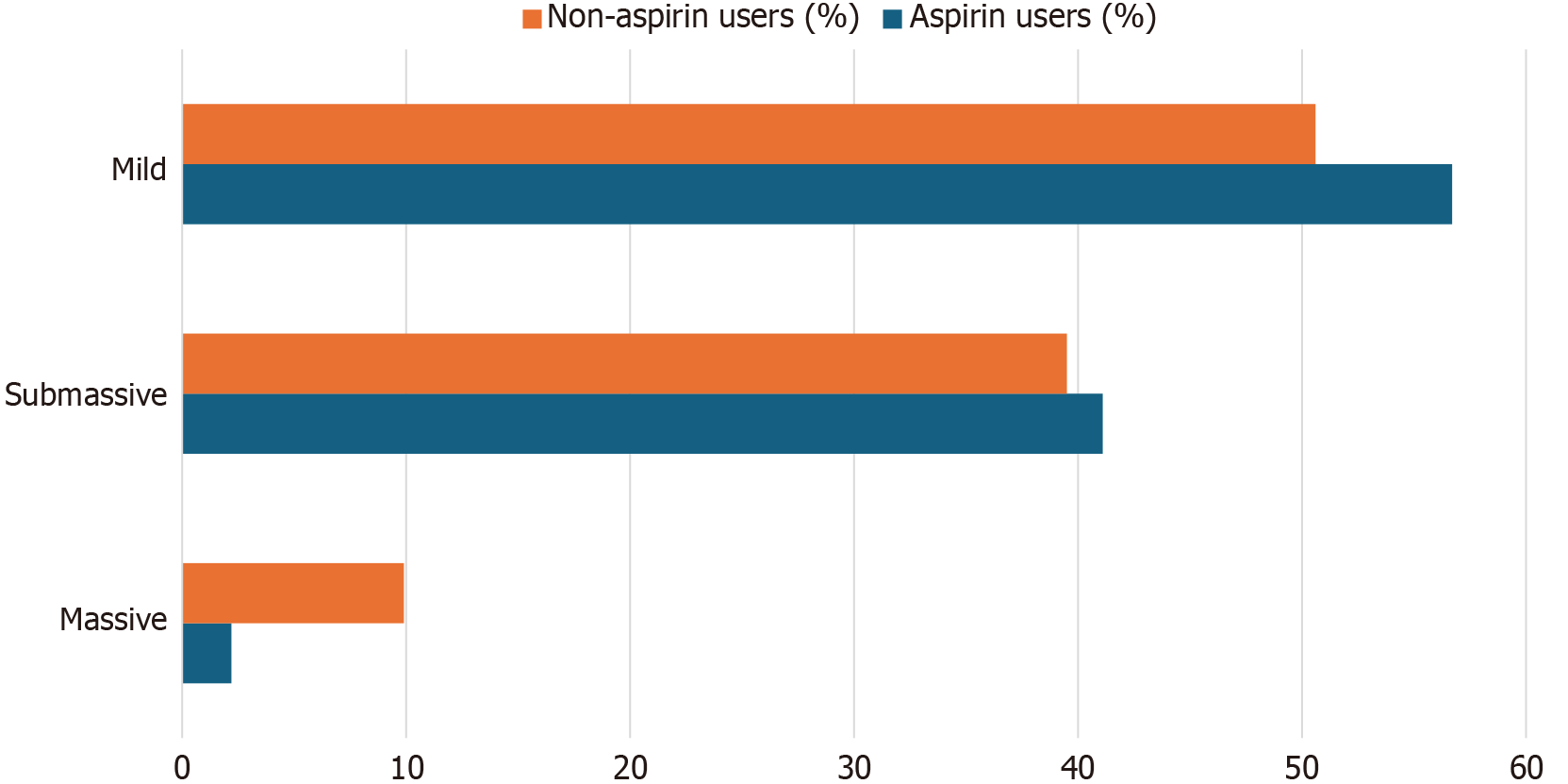
Prehospital aspirin use was associated with improved clinical outcomes (Table 3, Figure 1): (1) ICU admission: 16.7% vs 28.8% (P = 0.025; OR = 0.496, 95%CI: 0.266-0.924); (2) Shock: 2.2% vs 9.9% (P = 0.021; OR = 0.208, 95%CI: 0.048-0.899); (3) In-hospital mortality: 3.3% vs 11.6% (P = 0.022; OR = 0.260, 95%CI: 0.080-0.889); and (4) PE severity (Figure 2). There was a trend toward less severe PE among aspirin users, with a lower rate of massive PE (2.2% vs 9.9%; P = 0.067). Submassive (41.1% vs 39.5%) and mild PE (56.7% vs 50.6%) rates were comparable.
| Outcome | Aspirin (n = 90) | No aspirin (n = 233) | P value | OR (95%CI) |
| RV strain on CT | 20 (22.2) | 81 (34.8) | 0.029 | 0.54 (0.31-0.94) |
| RV strain on echocardiogram | 12 (13.3) | 36 (18.9) | 0.246 | 0.66 (0.32-1.34) |
| ICU admission | 15 (16.7) | 67 (28.8) | 0.025 | 0.50 (0.27-0.92) |
| Pressor requirement | 2 (2.2) | 23 (9.9) | 0.021 | 0.21 (0.04-0.90) |
| In-hospital mortality | 3 (3.3) | 27 (11.6) | 0.022 | 0.260 (0.080-0.889) |
| EKOS therapy | 3 (3.3) | 26 (11.2) | 0.047 | 0.28 (0.08-0.93) |
| Mechanical ventilation | 6 (6.7) | 11 (4.7) | 0.483 | 1.44 (0.52-4.02) |
| Cardiac arrest | 6 (6.7) | 14 (6.0) | 0.826 | 1.12 (0.42-3.00) |
Radiographic RV strain on CT was significantly lower in the aspirin group (22.2% vs 34.8%; P = 0.029; OR = 0.536, 95%CI: 0.305-0.944) (Table 3). However, echocardiographic RV dysfunction, including strain, dilation, and hypokinesis, did not significantly differ between groups (P = 0.587). There were no significant differences in: (1) Mechanical ventilation: 6.7% vs 4.7% (P = 0.483; OR = 1.456, 95%CI: 0.517-4.021); (2) Cardiac arrest: 6.7% vs 6.0% (P = 0.826; OR = 1.117, 95%CI: 0.416-3.004); and (3) Pressor use: 2.2% vs 6.0% (P = 0.160; OR = 0.356, 95%CI: 0.079-1.597). Use of catheter-directed thrombolysis (Ekosonic endovascular system) was also significantly lower in aspirin users (3.3% vs 11.2%; P = 0.047; OR = 0.275, 95%CI: 0.081-0.931).
Aspirin users presented with lower mean heart rate (89.0 ± 19.3 bpm vs 94.6 ± 21.4 bpm; P = 0.032). Peak lactate values were also significantly lower among aspirin users (3.34 ± 2.57 mmol/L vs 6.25 ± 10.79 mmol/L; P = 0.029). No significant differences were noted in admission or peak pro-brain natriuretic peptide levels. Total hospital and ICU lengths of stay were comparable.
This retrospective case-control study is one of the first to evaluate the association between prehospital aspirin use and clinical outcomes in patients hospitalized with acute PE outside of surgical contexts. While aspirin has been well-established as a secondary preventive agent in arterial thrombotic diseases, including myocardial infarction and stroke, its role in VTE has remained largely confined to recurrence prevention following cessation of anticoagulation. Our findings offer a new perspective by suggesting that aspirin exposure prior to hospitalization may influence the initial severity and in-hospital trajectory of acute PE[17,18].
Aspirin users in our study exhibited a significantly lower incidence of RV strain on imaging, reduced need for intensive care support, lower rates of shock, and improved in-hospital survival. While computed tomography demonstrated significant differences in RV strain, echocardiographic parameters did not show corresponding changes. This may be explained by differences in test timing, operator variability, and the more standardized, quantitative nature of CT-based assessments compared to the qualitative interpretation of echocardiographic strain. These associations were observed despite the aspirin group comprising older individuals with a higher prevalence of cardiovascular comorbidities, particularly coronary artery disease. In typical clinical scenarios, these factors would be expected to confer worse outcomes. That the aspirin cohort demonstrated better clinical endpoints despite higher baseline risk supports the hypothesis that aspirin may exert a disease-modifying effect in the pathogenesis of PE[19]. The observation that older, more comorbid patients experienced better outcomes paradoxically strengthens the case against confounding by indication and supports a potential protective effect rather than aspirin merely serving as a surrogate marker of baseline health.
The biological plausibility for this observation is supported by a growing body of mechanistic literature implicating platelets in venous thrombosis and thromboinflammatory cascades. Platelets contribute to clot propagation not only through aggregation but also through the promotion of NETs, cytokine release, and endothelial injury - all of which can amplify pulmonary vascular resistance and strain on the right ventricle. Aspirin’s inhibition of platelet activation and downstream thromboxane-mediated signaling may thus blunt these processes, leading to reduced clot burden or inflammatory injury at the time of presentation[20,21]. Recent evidence also implicates platelet-leukocyte interactions and NETs in the propagation of thromboinflammation, providing further biologic plausibility for aspirin’s protective effects in venous thromboembolic disease.
Unlike prior studies that examined aspirin exclusively as a tool for secondary prevention after cessation of anticoagulant therapy, our study explores its role during the acute phase of disease - a setting with significant clinical implications. In contrast to the WARFASA and ASPIRE trials, which demonstrated reduced recurrence of VTE over months following initial treatment, our findings suggest a possible benefit at the time of hospital presentation, when the thrombotic and inflammatory burdens are highest and risk stratification is most critical. Moreover, while earlier observational studies in myocardial infarction and stroke populations have demonstrated improved perfusion and outcomes with prehospital aspirin use, no prior study, to our knowledge, has evaluated these relationships in the context of acute PE in a non-surgical population[10,15,16,18].
The strengths of our study are several. First, it addresses an underexplored clinical question with meaningful implications for risk mitigation in a common and potentially fatal cardiovascular condition. Second, the inclusion criteria ensured a clearly defined population, excluding surgical patients and those on prior therapeutic anticoagulation, thus minimizing confounding from perioperative thrombotic risk or baseline antithrombotic therapy. Third, outcome definitions were objective and based on imaging, hemodynamics, and clinical events rather than subjective measures, increasing the reliability of our findings. Additionally, the associations observed were not isolated to a single endpoint but extended across a spectrum of markers reflecting disease severity - supporting internal consistency.
However, our study also has important limitations. As a retrospective, single-center analysis, it is inherently subject to residual confounding and selection bias. We were unable to perform multivariable regression or propensity score matching due to sample size limitations, which restricts our ability to fully adjust for baseline differences. Nonetheless, the consistency of the observed protective trends across multiple endpoints lends internal validity to our findings. Medication reconciliation, though carefully documented, may have missed undocumented over-the-counter aspirin use or nonadherence. Aspirin dosing was not standardized across patients and was not stratified for analysis, precluding assessment of a dose-response relationship. We were also unable to systematically evaluate bleeding-related complications due to lack of consistent documentation, which is critical when considering the risk-benefit profile of any antithrombotic therapy. Furthermore, although we excluded patients on long-term anticoagulation, some patients may have initiated aspirin for cardiovascular indications that are independently associated with both thrombotic and protective outcomes, raising the possibility of confounding by indication. Finally, the relatively small sample size and single-center design may limit generalizability to other populations with different baseline characteristics or treatment protocols. As such, our results should be interpreted as hypothesis-generating and warrant validation in larger, prospective, multicenter studies.
Nonetheless, these limitations must be weighed against the strength of the observed associations and the biological plausibility of the effect. Unlike many retrospective studies that explore associations without mechanistic grounding, our findings are supported by a robust scientific rationale, aligning with contemporary understanding of thromboinflammation in venous disease. In fact, our data may help catalyze a shift in how aspirin is conceptualized - not merely as a secondary prevention tool, but as a potential modifier of early disease trajectory in patients with acute PE.
These results have direct implications for clinical practice and future research. If corroborated in prospective studies, prehospital aspirin use could represent a simple, inexpensive, and globally scalable adjunct to reduce initial disease severity in PE. This is particularly relevant in resource-limited settings where access to emergent anticoagulation, thrombolysis, or intensive care may be delayed or constrained. It also raises the question of whether aspirin should be considered as part of a broader prophylactic strategy in high-risk ambulatory populations, such as those with prior cardiovascular disease, chronic immobility, or systemic inflammation. Prospective validation could ultimately redefine aspirin’s role in the acute management of PE, extending its utility beyond secondary prevention.
Prehospital aspirin use is independently associated with significantly lower risks of right ventricular strain, intensive care unit admission, shock, and in-hospital mortality among patients presenting with acute PE. These findings support the hypothesis that aspirin may have a protective, disease-modifying effect in the early stages of VTE. Given aspirin's favorable safety profile, global accessibility, and low cost, these results warrant further validation through large-scale, prospective, and mechanistically informed studies. If confirmed, aspirin could represent a pragmatic adjunctive strategy to mitigate morbidity and mortality in acute PE.
| 1. | Expert Panel on Cardiac Imaging, Kirsch J, Wu CC, Bolen MA, Henry TS, Rajiah PS, Brown RKJ, Galizia MS, Lee E, Rajesh F, Raptis CA, Rybicki FJ, Sams CM, Verde F, Villines TC, Wolf SJ, Yu J, Donnelly EF, Abbara S. ACR Appropriateness Criteria® Suspected Pulmonary Embolism: 2022 Update. J Am Coll Radiol. 2022;19:S488-S501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Freund Y, Cohen-Aubart F, Bloom B. Acute Pulmonary Embolism: A Review. JAMA. 2022;328:1336-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 154] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 3. | Goldberg JB, Giri J, Kobayashi T, Ruel M, Mittnacht AJC, Rivera-Lebron B, DeAnda A Jr, Moriarty JM, MacGillivray TE. Surgical Management and Mechanical Circulatory Support in High-Risk Pulmonary Embolisms: Historical Context, Current Status, and Future Directions: A Scientific Statement From the American Heart Association. Circulation. 2023;147:e628-e647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 4. | Becattini C, Agnelli G, Lankeit M, Masotti L, Pruszczyk P, Casazza F, Vanni S, Nitti C, Kamphuisen P, Vedovati MC, De Natale MG, Konstantinides S. Acute pulmonary embolism: mortality prediction by the 2014 European Society of Cardiology risk stratification model. Eur Respir J. 2016;48:780-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 204] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 5. | Kahn SR, de Wit K. Pulmonary Embolism. N Engl J Med. 2022;387:45-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 6. | Piazza G. Advanced Management of Intermediate- and High-Risk Pulmonary Embolism: JACC Focus Seminar. J Am Coll Cardiol. 2020;76:2117-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 7. | Andreotti F, Geisler T, Collet JP, Gigante B, Gorog DA, Halvorsen S, Lip GYH, Morais J, Navarese EP, Patrono C, Rocca B, Rubboli A, Sibbing D, Storey RF, Verheugt FWA, Vilahur G. Acute, periprocedural and longterm antithrombotic therapy in older adults: 2022 Update by the ESC Working Group on Thrombosis. Eur Heart J. 2023;44:262-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 8. | Frei AN, Stalder O, Limacher A, Méan M, Baumgartner C, Rodondi N, Aujesky D. Comparison of Bleeding Risk Scores in Elderly Patients Receiving Extended Anticoagulation with Vitamin K Antagonists for Venous Thromboembolism. Thromb Haemost. 2021;121:1512-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Tarantino E, Amadio P, Squellerio I, Porro B, Sandrini L, Turnu L, Cavalca V, Tremoli E, Barbieri SS. Role of thromboxane-dependent platelet activation in venous thrombosis: Aspirin effects in mouse model. Pharmacol Res. 2016;107:415-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Simes J, Becattini C, Agnelli G, Eikelboom JW, Kirby AC, Mister R, Prandoni P, Brighton TA; INSPIRE Study Investigators (International Collaboration of Aspirin Trials for Recurrent Venous Thromboembolism). Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration. Circulation. 2014;130:1062-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 210] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 11. | Brighton TA, Eikelboom JW, Mann K, Mister R, Gallus A, Ockelford P, Gibbs H, Hague W, Xavier D, Diaz R, Kirby A, Simes J; ASPIRE Investigators. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med. 2012;367:1979-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 377] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 12. | Becattini C, Agnelli G, Schenone A, Eichinger S, Bucherini E, Silingardi M, Bianchi M, Moia M, Ageno W, Vandelli MR, Grandone E, Prandoni P; WARFASA Investigators. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366:1959-1967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 451] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 13. | Ryu WS, Schellingerhout D, Hong KS, Jeong SW, Kim BJ, Kim JT, Lee KB, Park TH, Park SS, Park JM, Kang K, Cho YJ, Park HK, Lee BC, Yu KH, Oh MS, Lee SJ, Kim JG, Cha JK, Kim DH, Lee J, Han MK, Park MS, Choi KH, Nahrendorf M, Lee J, Bae HJ, Kim DE. Relation of Pre-Stroke Aspirin Use With Cerebral Infarct Volume and Functional Outcomes. Ann Neurol. 2021;90:763-776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Lansberg MG, O'Donnell MJ, Khatri P, Lang ES, Nguyen-Huynh MN, Schwartz NE, Sonnenberg FA, Schulman S, Vandvik PO, Spencer FA, Alonso-Coello P, Guyatt GH, Akl EA. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e601S-e636S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 323] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 15. | Mekaj YH, Daci FT, Mekaj AY. New insights into the mechanisms of action of aspirin and its use in the prevention and treatment of arterial and venous thromboembolism. Ther Clin Risk Manag. 2015;11:1449-1456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Van Galen J, Pava L, Wright C, Elbadawi A, Hamer A, Chaturvedi A, Cameron SJ. Effect of platelet inhibitors on thrombus burden in patients with acute pulmonary embolism. Platelets. 2021;32:138-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, Stevens SM, Vintch JRE, Wells P, Woller SC, Moores L. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3526] [Article Influence: 352.6] [Reference Citation Analysis (2)] |
| 18. | Stevens SM, Woller SC, Baumann Kreuziger L, Bounameaux H, Doerschug K, Geersing GJ, Huisman MV, Kearon C, King CS, Knighton AJ, Lake E, Murin S, Vintch JRE, Wells PS, Moores LK. Executive Summary: Antithrombotic Therapy for VTE Disease: Second Update of the CHEST Guideline and Expert Panel Report. Chest. 2021;160:2247-2259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 298] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 19. | Wang L, Wu J, Zhang W, Zhi Y, Wu Y, Jiang R, Yang R. Effects of aspirin on the ERK and PI3K/Akt signaling pathways in rats with acute pulmonary embolism. Mol Med Rep. 2013;8:1465-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Undas A, Brummel-Ziedins K, Mann KG. Why does aspirin decrease the risk of venous thromboembolism? On old and novel antithrombotic effects of acetyl salicylic acid. J Thromb Haemost. 2014;12:1776-1787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Crescente M, Armstrong PC, Kirkby NS, Edin ML, Chan MV, Lih FB, Jiao J, Maffucci T, Allan HE, Mein CA, Gaston-Massuet C, Cottrell GS, Mitchell JA, Zeldin DC, Herschman HR, Warner TD. Profiling the eicosanoid networks that underlie the anti- and pro-thrombotic effects of aspirin. FASEB J. 2020;34:10027-10040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/