Published online Nov 26, 2025. doi: 10.4330/wjc.v17.i11.110537
Revised: July 13, 2025
Accepted: October 13, 2025
Published online: November 26, 2025
Processing time: 165 Days and 20.9 Hours
Glycated hemoglobin (HbA1c) is a well-established biomarker for diagnosing and managing diabetes. However, its prognostic significance in patients without diag
To investigate the association between elevated HbA1c levels in the prediabetic range and adverse outcomes in patients without diagnosed diabetes undergoing PCI.
We systematically searched PubMed, EMBASE, and Cochrane Central through April 2025 for studies comparing clinical outcomes in coronary artery disease (CAD) patients without a prior diabetes diagnosis, stratified by HbA1c levels (≥ 5.7% vs < 5.7%). Risk ratios (RR) with 95% confidence intervals (CI) were pooled using a random-effects model. Statistical analysis was performed using R software (version 4.3.2). Primary outcomes were long-term all-cause mortality and major adverse cardiovascular events (MACE); secondary outcomes included short-term mortality and cardiac death.
Ten studies involving 32403 patients (mean age: 60 years; 29% female) were included. Elevated HbA1c levels in patients without diagnosed diabetes were significantly associated with increased risk of long-term all-cause mortality (RR: 1.30; 95%CI: 1.10-1.54; P < 0.01; I2 = 41%) and MACEs (RR: 1.31; 95%CI: 1.01-1.69; P = 0.04; I2 = 61%). Although the risks of short-term all-cause mortality (RR: 1.16; 95%CI: 0.88-1.53; P = 0.29; I2 = 1%) and cardiac mortality (RR: 1.76; 95%CI: 0.85-3.67; P = 0.13; I2 = 94%) were elevated, they did not reach statistical significance. Sensitivity analyses confirmed the robustness of the findings despite moderate to high heterogeneity in some outcomes.
Among CAD patients without diagnosed diabetes, elevated HbA1c levels in the prediabetic range (≥ 5.7%) are independently associated with worse long-term outcomes following PCI. HbA1c may serve as a valuable bio
Core Tip: Patients without a formal diabetes diagnosis may still exhibit elevated glycated hemoglobin (HbA1c) levels in the prediabetic range (5.7%-6.4%), which may contribute to worse cardiovascular outcomes. This systematic review and meta-analysis examined the prognostic impact of elevated HbA1c in non-diabetic patients undergoing percutaneous coronary intervention (PCI). We found that HbA1c ≥ 5.7% is independently associated with increased long-term all-cause mortality and major adverse cardiovascular events. These findings highlight the potential value of HbA1c as a simple, cost-effective marker for post-PCI risk stratification and emphasize the need for early intervention in this high-risk group.
- Citation: Shahid S, Sethi FA, Ahmed S, Kumar A, Shahid MH, Raja HAA, Usama M, Mughal HMF. Prognostic impact of prediabetic glycated hemoglobin levels in nondiabetic patients undergoing percutaneous coronary intervention: A systematic review and meta-analysis. World J Cardiol 2025; 17(11): 110537
- URL: https://www.wjgnet.com/1949-8462/full/v17/i11/110537.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i11.110537
Coronary artery disease (CAD) remains a leading cause of global morbidity and mortality, despite substantial adv
Importantly, individuals without a formal diagnosis of diabetes may still present with elevated HbA1c levels within the range defined as prediabetes by international guidelines (5.7%-6.4%)[4]. This subclinical dysglycemia is linked to insulin resistance, endothelial dysfunction, and chronic inflammation-all of which may contribute to adverse cardi
Previous meta-analyses, such as the one by Li et al[8], attempted to address this issue but were limited by smaller sample sizes, a narrower study population [e.g., acute myocardial infarction (AMI) patients], and lack of stratification by follow-up duration. Given these limitations and the growing body of new evidence, we conducted an updated and comprehensive meta-analysis to evaluate whether elevated HbA1c levels-specifically in the prediabetic range-are independently associated with adverse clinical outcomes in patients without diagnosed diabetes undergoing PCI. This study aimed to incorporate a broader, contemporary dataset, distinguish between short- and long-term outcomes, and provide more definitive insights into this clinically relevant question.
This meta-analysis adhered to the PRISMA[9] and was prospectively registered on the international prospective register of systematic reviews (PROSPERO) (CRD420251041323).
A systematic search of studies listed in the PubMed, EMBASE and Cochrane Central databases from inception to April 2025 was conducted using the following search terms: ("Percutaneous Coronary Intervention" OR "PCI" OR "Coronary Angioplasty" OR "Coronary Revascularization" OR "Coronary Stenting" OR "Stent Placement" OR "Balloon Angioplasty" OR "Angioplasty, Balloon, Coronary") AND ("Hemoglobin A1c" OR "HbA1c" OR "Glycated Hemoglobin" OR "Glycosylated Hemoglobin" OR "Glycohemoglobin" OR "Hemoglobin A1*" OR "HbA1*" OR "A1c"). All references in the retrieved articles were also scanned to identify other potentially available studies. The detailed search strategy is provided in Supplementary Table 1.
Studies were considered eligible to be included in our systematic review and meta-analysis if they met the following inclusion criteria: (1) Enrolled patients without a prior diagnosis of diabetes with CAD undergoing PCI; (2) Evaluated elevated HbA1c levels (defined as > 5.7%) as the intervention group; (3) Included a control group with normal HbA1c levels (< 5.7%); (4) Reported at least one of the outcomes of interest; and (5) Provided raw data to allow estimation of risk ratios (RR) with 95% confidence intervals (CI).
We defined elevated HbA1c as ≥ 5.7%, corresponding to the internationally recognized threshold for prediabetes, in accordance with American Diabetes Association guidelines. This allowed us to investigate outcomes in patients within the prediabetic spectrum, despite the absence of a formal diabetes diagnosis. Patients without diagnosed diabetes were defined as individuals with no prior diagnosis of diabetes at the time of admission and no history of receiving anti-diabetic therapy. Exclusion criteria were: (1) Experimental studies; (2) Conference articles, case reports, systematic reviews and meta-analyses; (3) Insufficient outcome data provided; (4) Duplicate reports of literature research; and (5) Animal studies.
The following data were reviewed and extracted from each study: (1) Study details such as the first author's last name, year of publication, country, and sample size; (2) Baseline characteristics of the patient population, including age, gender, body mass index, smoking habits, presence of hypertension, dyslipidemia, and family history of CAD; (3) The timing of HbA1c measurement; (4) Clearly stated inclusion and exclusion criteria; (5) Potential for selection bias; (6) Follow-up completeness; (7) Reported adverse clinical outcomes; and (8) Whether confounding variables were adjusted for in mul
The primary adverse clinical outcomes evaluated in our study were long-term all-cause mortality and major adverse cardiovascular events (MACEs), while the secondary outcomes were short-term all-cause and cardiac mortality. Long-term outcomes were defined as those with a follow-up period of at least one year, whereas short-term outcomes included events occurring during hospitalization or within 30 days post-procedure. MACEs encompassed all-cause mortality, non-fatal myocardial infarction, target lesion revascularization, target vessel revascularization, recurrent AMI, hospitalization for heart failure, and stent thrombosis. Cardiac mortality was defined as any death due to any cardiac cause.
Two independent reviewers (Shahid MH and Raja HAA) evaluated the risk of bias using the Newcastle-Ottawa Scale (NOS) for observational studies. Observational studies were assessed using the NOS, which evaluates methodological quality based on three broad perspectives: (1) Selection of study groups; (2) Comparability of groups; and (3) Asc
We used R software (version 4.3.2) to perform data synthesis for all reported outcomes. A P value of < 0.05 was con
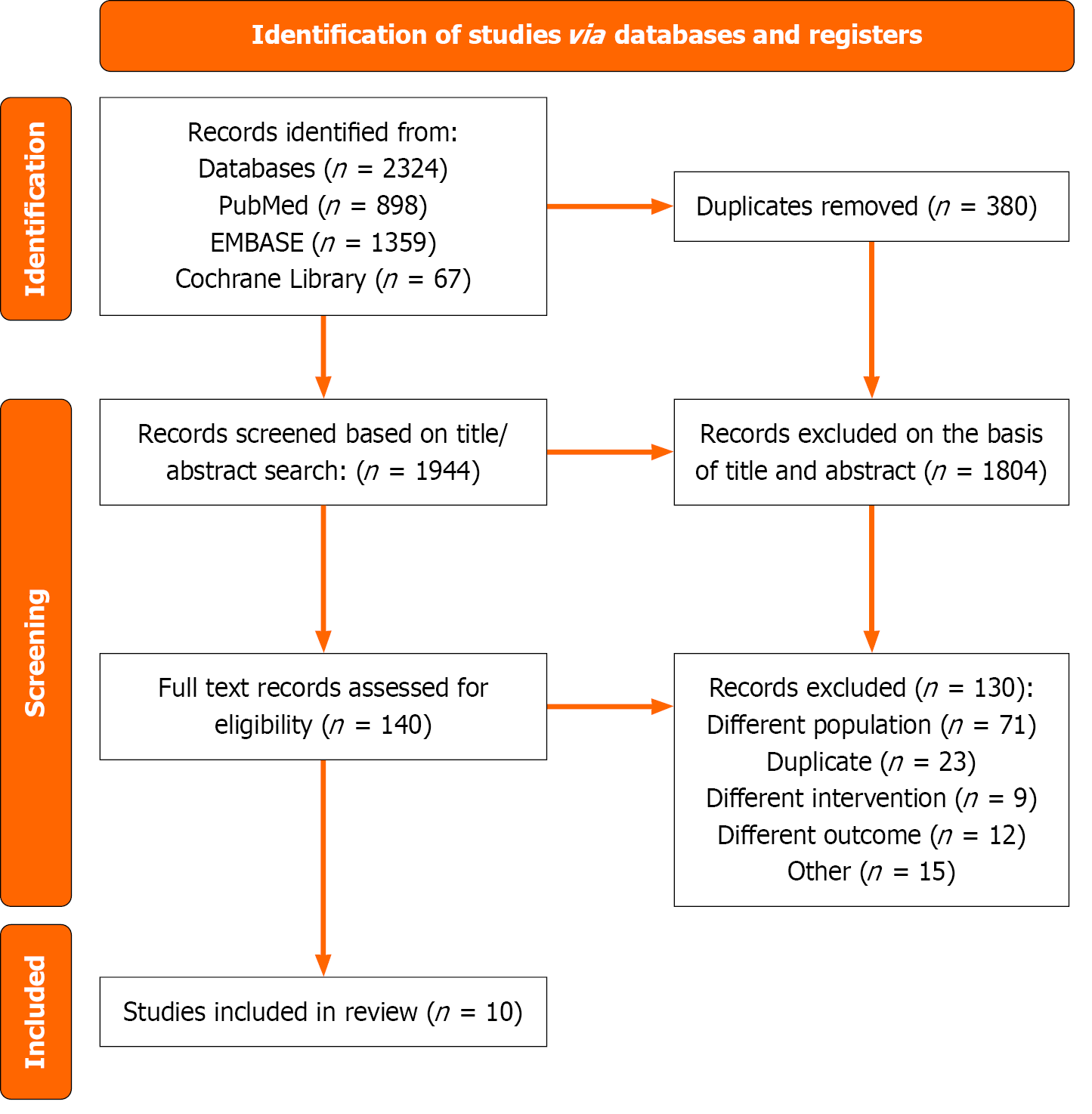
The preliminary search yielded 2324 articles. After removing duplicates, 1944 articles remained for title and abstract screening. Of these, 1798 were excluded based on irrelevance or failure to meet the inclusion criteria. The remaining 140 articles were subjected to full-text review to assess eligibility. Ultimately, ten studies comprising a total of 32403 patients met the inclusion criteria and were included in the final analysis[11-20]. The process of identifying and selecting relevant studies is illustrated in the flow chart (Figure 1).
Eight of the included ten studies reported long-term all-cause mortality[11-16,19,20], five studies had long-term MACE outcomes at follow-up[14-16,18,19], three of the ten studies investigated short-term all-cause mortality[11,15,16] and two studies assessed cardiac mortality[19,20]. Among the ten studies, five were from Asia (three from China, one from Japan, and one from South Korea), two from the Netherlands, one from Germany, and one from the United States. Baseline characteristics of the included studies are shown in Table 1.
| Ref. | Country | Sample size | Mean age in years (MD) | Males % | Normal HbA1c | Abnormal HbA1c | Follow up | Cardiac diagnosis | Study outcomes |
| Aggarwal et al[13] | United States | 1163 (652 vs 511) | NR | NR | < 5.7 | 5.7 < HbA1c < 6.5 | 3 years | STEMI | All-cause deaths |
| Chen et al[15] | China | 267 (64 vs 263) | NR | 83.5 | < 6 | 6.0 < HbA1c < 6.5 | 178 days | STEMI/NSTEMI | All-cause deaths, repeated PCI, recurrent AMI, HF requiring hospitalization |
| Dykun et al[17] | Germany | 1692 (1188 vs 510) | 66.1 (11.4) | 77.1 | 6.3 (1.2) | ≥ 7.8% | 2.8 years | STEMI/NSTEMI | association of HbA1c with ACM in unadjusted and multivariable adjusted modeling |
| Kok et al[16] | Netherland | 2103 (234 vs 1869) | NR | NR | < 6 | 6.0 < HbA1c < 6.5 | 1 year | Obstructive coronary disease | All-cause deaths, MI, revascularization, and stent thrombosis) |
| Naito et al[12] | Japan | 452 (231 vs 221) | NR | NR | < 5.7 | 5.7 < HbA1c < 6.5 | 4.7 years | ACS | Primary endpoint was a composite of all-cause death and non-fatal MI |
| Park et al[18] | South Korea | 1318 (120 vs 1198) | 64.8 (8.9) vs 60.6 (11.3) | 48.02 | < 5.7 | 6.5 ≤ HbA1c < 7.0 | 1.83 years | CAD | Survival free from MACE |
| Shin et al[14] | South Korea | 2470 (1475 vs 995) | NR | NR | < 5.7 | 5.7 < HbA1c < 6.5 | 1 year | STEMI | All-cause deaths, MACEs (all-cause mortality, non-fatal MI, TLR, TVR) |
| Timmer et al[11] | Netherland | 4176 (1024 vs 3152) | 62 (13) vs 63 (13) | 74 | < 5.8 | > 5.8 | 3.3 ± 1.5 years | STEMI | 30-day mortality, 1-year mortality, and infarct size |
| Wen et al[19] | China | 7033 (3530 vs 3503) | NR | 74.3 | < -0.506 | ≥ 0.179 | 2 years | ACS + SCAD | Mortality, ACM, CM, MACEs, MACCEs |
| Zhou et al[20] | China | 11729 (4976 vs 6753) | 51.78 (6.97) vs 49.07 (7.21) | 71.9 | < 5 | > 6 | 4.62 years | Premature CAD | Long-term all-cause mortality, cardiac mortality |
Risk of bias was generally low across studies based on the NOS, with most scoring 8 out of 9 stars. Two studies[12,19] scored 9, reflecting high methodological quality. One study[20] scored 7, indicating a slightly higher risk of bias in at least one domain (Supplementary Table 2). Publication bias was assessed using funnel plots and quantified via the LFK asymmetry index. For short-term all-cause mortality (LFK = -0.35) and MACE (LFK = 0.62), funnel plots showed minimal asymmetry, indicating low risk of publication bias. However, the analysis for long-term all-cause mortality (LFK = 3.62) revealed significant asymmetry, suggesting potential publication bias. This may reflect under-representation of small neutral/negative studies in the literature (Supplementary Figures 1-4).
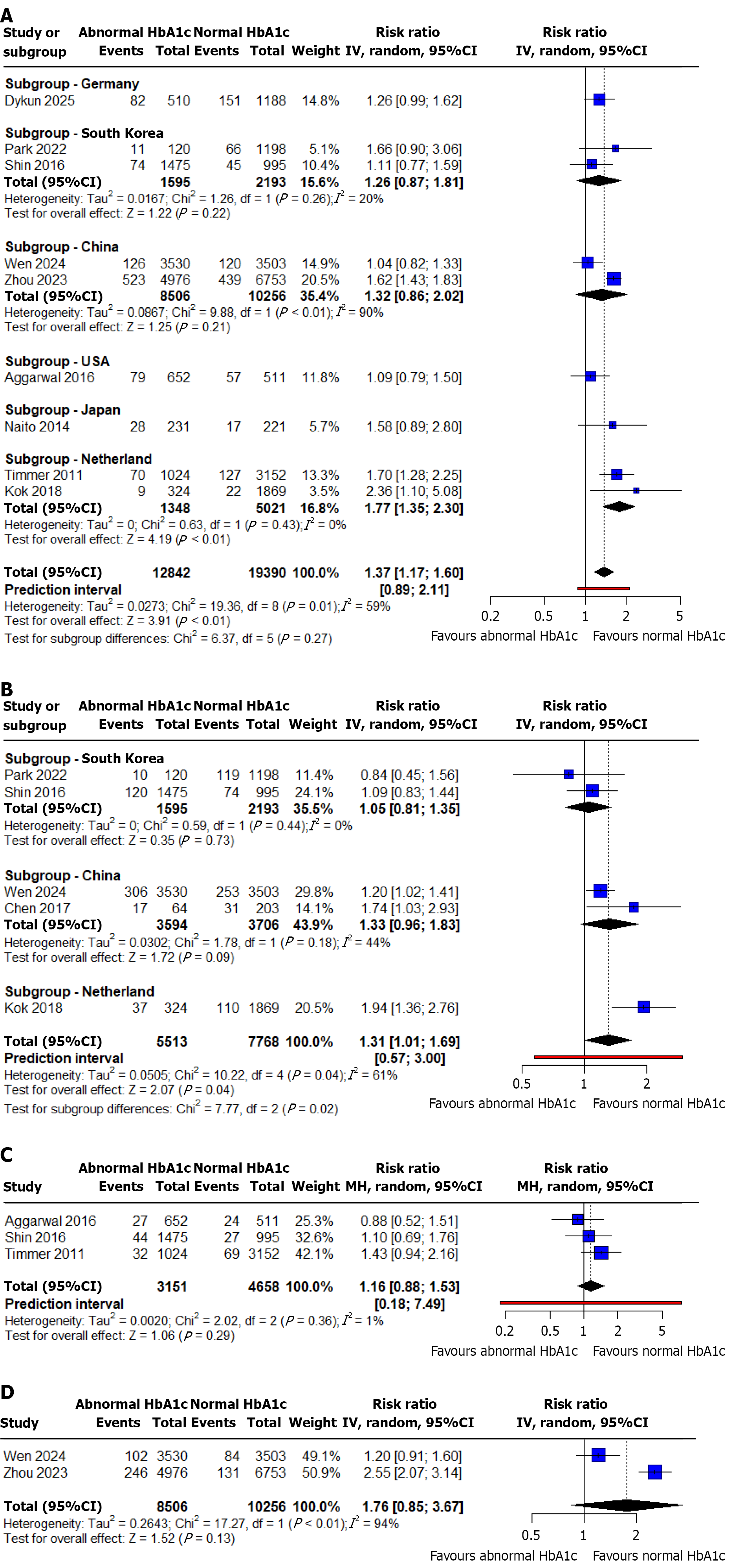
Long-term all-cause mortality: The outcome of long-term all-cause mortality was reported by 8 out of 10 selected studies with a total of 20503 patients[11-16,19,20]. The pooled analysis demonstrated that abnormal HbA1c was associated with increased risk of long-term all-cause deaths compared to those with normal HbA1c levels (RR: 1.30; 95%CI: 1.10-1.54; P value < 0.01; I2 = 41%) (Figure 2A). There was a moderate statistical heterogeneity among the studies. We performed a sensitivity analysis by excluding each study in turn, with no significant impact on heterogeneity (Supplementary Figure 5).
MACE: The outcome of MACE was reported by 5 out of 10 selected studies with a total of 13281 patients[14-16,18,19]. The pooled analysis demonstrated that abnormal HbA1c was associated with an increased risk of MACEs compared to those with normal HbA1c levels (RR: 1.31; 95%CI: 1.01-1.69; P value = 0.04; I2 = 61%) (Figure 2B). There was a marked heterogeneity in the data (I2 = 61%). We conducted a sensitivity analysis by excluding each study in turn for heterogeneity. We found that the marked heterogeneity originated from Kok et al's study[16], likely due to the HbA1c cut-off levels of 6.0% to 6.5% used in their analysis (Supplementary Figure 6).
Short-term all-cause mortality: The outcome of short-term all-cause mortality was reported by 3 out of 10 selected studies with a total of 7809 patients[11,15,16]. The pooled analysis demonstrated that abnormal HbA1c were associated with increased risk of short-term all-cause deaths compared to those with normal HbA1c levels. However, this association was not statistically significant (RR: 1.16; 95%CI: 0.88-1.53; P value = 0.29; I2 = 1%) (Figure 2C). There was no significant statistical heterogeneity observed among the included studies. Sensitivity analysis confirmed the robustness of the findings, as exclusion of individual studies did not substantially alter the overall effect estimates or heterogeneity levels (Supplementary Figure 7).
Cardiac mortality: Cardiac mortality was reported by 2 out of the 10 selected studies[19,20]. The pooled analysis showed that abnormal HbA1c was associated with a higher risk of cardiac mortality compared to normal HbA1c levels; however, this association did not reach statistical significance (RR: 1.76; 95%CI: 0.85-3.67; P value = 0.13; I2 = 94%) (Figure 2D). Considerable heterogeneity was observed among the included studies. Sensitivity analysis demonstrated that excluding individual studies did not significantly change the overall results or reduce heterogeneity (Supplementary Figure 8).
Subgroup analysis was performed based on country of origin. It demonstrated that, elevated admission HbA1c levels were significantly associated with an increased risk of long-term all-cause mortality (RR 1.77, 95%CI: 1.32-2.30, P < 0.01) and MACE (RR 1.94, 95%CI: 1.36-2.76, P < 0.01) in patients from the Netherlands. In contrast, no statistically significant associations were observed in other countries, including China, South Korea, Germany, the United States, and Japan, suggesting possible geographic variation in the prognostic impact of abnormal HbA1c levels (Figure 2A and B). Subgroup analysis was not performed for the outcomes of short term all-cause and cardiac mortality due to an insufficient number of studies, with fewer than two studies available per subgroup.
This systematic review and meta-analysis indicate that elevated HbA1c levels (≥ 5.7%) are substantially correlated with an increased risk of long-term all-cause mortality and MACE in non-diabetic patients receiving PCI. Short-term all-cause and cardiac mortality risks were raised, although not statistically significant. Small sample sizes (three studies for short-term mortality and two for cardiac death), short follow-up periods, and few events may explain this. These limitations diminish statistical power and may obscure a true association, necessitating validation in larger prospective cohorts. The findings suggest that even modest increases in HbA1c below the diabetic threshold may reflect underlying metabolic or vascular dysfunction that predisposes patients to adverse long-term cardiovascular outcomes.
Our results validate and expand upon the findings of Li et al[8], who first identified the prognostic relevance of increased HbA1c levels in non-diabetic individuals after PCI. Nonetheless, their analysis was limited by a reduced sample size (n = 8385), a lower number of included studies (n = 5), and a primary emphasis on AMI patients, without differentiating outcomes based on follow-up time. Conversely, our research included three supplementary trials and included 12118 more patients, culminating in a combined cohort of 20503 people for long-term mortality assessment and 13281 for MACE. We investigated outcomes across both short- and long-term durations and incorporated cardiac death as an additional endpoint, which was not addressed in the previous meta-analysis. Our dataset encompasses a diverse group of CAD individuals with differing manifestations, hence enhancing the generalizability of our findings.
Furthermore, the results align with individual studies conducted by Aggarwal et al[13] and Geng et al[21], both of which recognized HbA1c as an independent predictor of worse cardiovascular outcomes in non-diabetic patients following PCI. Xu et al[5] also documented an increase in in-hospital and long-term mortality linked to higher HbA1c levels in non-diabetic patients having PCI for ST-segment elevation myocardial infarction (STEMI). In contrast, Cicek et al[22] identified no independent correlation between HbA1c and significant poor outcomes; however, their results may be constrained by a smaller sample size, a predominance of stable CAD patients, a shorter follow-up period, and variations in the scheduling of HbA1c evaluation.
A significant aspect of our study is the inclusion of patients from various geographic locations, especially from China, which was lacking in the previous meta-analysis by Li et al[8]. Subgroup analysis indicated a statistically significant correlation between elevated HbA1c levels and adverse outcomes in Dutch patients-specifically regarding long-term mortality (RR: 1.77, 95%CI: 1.32-2.30, P < 0.01) and MACE (RR: 1.94, 95%CI: 1.36-2.76, P < 0.01)-but not in other nations such as China, South Korea, the United States, Germany, or Japan, implying possible regional disparities in the prog
Sensitivity analyses corroborated the robustness of our results. Sequential elimination of individual studies did not significantly modify impact estimates for both long-term mortality and MACE. The heterogeneity in the MACE analysis (I2 = 61%) was mostly affected by the study done by Kok et al[16], which utilised a broader HbA1c range (6.0%-6.5%) in contrast to the standardised criteria of ≥ 5.7% applied in other segments of our analysis. The elimination of this study led to a decrease in heterogeneity, hence confirming its designation as an outlier. Conversely, minimal heterogeneity was noted for short-term mortality (I2 = 1%) and moderate heterogeneity for long-term mortality (I2 = 41%), highlighting the uniformity of results across the majority of included studies.
Nonetheless, additional sources of heterogeneity should be considered. Variability in HbA1c cutoff values across studies may reflect differing definitions of prediabetes, which could influence the pooled effect sizes. Similarly, inconsistency in follow-up durations might affect the detection and timing of outcomes, thereby contributing to between-study variability. While we explored heterogeneity through sensitivity analyses, further subgroup analyses stratified by HbA1c thresholds or follow-up duration were limited by the availability and consistency of data across included studies. Future meta-analyses with access to individual participant data may help better delineate these sources and strengthen the validity of findings.
Several molecular pathways may link high HbA1c to unfavourable cardiovascular events in non-diabetics. Endothelial dysfunction due to reduced nitric oxide bioavailability, low-grade chronic inflammation, oxidative stress, and enhanced platelet reactivity are likely contributors. These mechanisms promote atherogenesis, thrombogenicity, and vascular injury, even in the absence of overt diabetes. Periodic HbA1c monitoring in non-diabetic PCI patients may help stratify risk. Integrating HbA1c into existing risk scoring systems, such as the SYNTAX score, could improve their predictive performance. A ≥ 5.7% threshold might serve as a target for early intervention in this population.
Several limitations of this meta-analysis must be acknowledged. First, the majority of included studies were observational in nature, which inherently increases the risk of residual confounding despite multivariable adjustments. Importantly, key confounders such as markers of inflammation (e.g., C-reactive protein, interleukin-6) and insulin resistance indices (e.g., homeostatic model assessment for insulin resistance) were not consistently adjusted for, pot
This meta-analysis highlights that HbA1c levels within the non-diabetic range (≥ 5.7%) may serve as a valuable biomarker for cardiovascular risk stratification following PCI. Given its affordability, widespread availability, and prognostic value, incorporating HbA1c into routine follow-up protocols could help identify high-risk individuals who may benefit from targeted interventions. These could include structured lifestyle programs, closer monitoring, and potentially early pharmacological therapy to improve metabolic health. The frequency of HbA1c monitoring and the optimal threshold for triggering interventions should be evaluated in future research.
Future research should also explore several directions: (1) Prospective studies measuring serial HbA1c changes post-PCI; (2) Randomized controlled trials targeting pre-diabetic HbA1c levels to assess benefit from early intervention; and (3) Mechanistic studies investigating HbA1c's role in endothelial dysfunction, plaque instability, and thrombosis risk.
In conclusion, increased HbA1c levels, defined as ≥ 5.7%, correlate with worse long-term results in non-diabetic CAD patients after PCI. Regular evaluation of HbA1c may provide a straightforward and efficient method for risk stratification, even in non-diabetic patients. Future prospective trials are warranted to ascertain if targeted therapies in individuals with increased HbA1c might enhance cardiovascular outcomes.
| 1. | Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7073] [Cited by in RCA: 6924] [Article Influence: 865.5] [Reference Citation Analysis (1)] |
| 2. | Han YL. De-escalation of anti-platelet therapy in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a narrative review. Chin Med J (Engl). 2019;132:197-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Cui KY, Lyu SZ, Zhang M, Song XT, Yuan F, Xu F. Drug-Eluting Balloon versus New-Generation Drug-Eluting Stent for the Treatment of In-Stent Restenosis: An Updated Systematic Review and Meta-Analysis. Chin Med J (Engl). 2018;131:600-607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Nathan DM, Turgeon H, Regan S. Relationship between glycated haemoglobin levels and mean glucose levels over time. Diabetologia. 2007;50:2239-2244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 337] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 5. | Xu X, Wang R, Wang Y, Cai S. Glycosylated hemoglobin levels and clinical outcomes in diabetic patients receiving percutaneous coronary interventions: A meta-analysis of cohort studies. Int J Cardiol. 2015;190:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Gustafsson I, Kistorp CN, James MK, Faber JO, Dickstein K, Hildebrandt PR; OPTIMAAL Study Group. Unrecognized glycometabolic disturbance as measured by hemoglobin A1c is associated with a poor outcome after acute myocardial infarction. Am Heart J. 2007;154:470-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 7. | Taly AB, Meenakshi-Sundaram S, Sinha S, Swamy HS, Arunodaya GR. Wilson disease: description of 282 patients evaluated over 3 decades. Medicine (Baltimore). 2007;86:112-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 181] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 8. | Li Y, Li XW, Zhang YH, Zhang LM, Wu QQ, Bai ZR, Si J, Zuo XB, Shi N, Li J, Chu X. Prognostic significance of the hemoglobin A1c level in non-diabetic patients undergoing percutaneous coronary intervention: a meta-analysis. Chin Med J (Engl). 2020;133:2229-2235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 51705] [Article Influence: 10341.0] [Reference Citation Analysis (2)] |
| 10. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 27059] [Article Influence: 1127.5] [Reference Citation Analysis (0)] |
| 11. | Timmer JR, Hoekstra M, Nijsten MW, van der Horst IC, Ottervanger JP, Slingerland RJ, Dambrink JH, Bilo HJ, Zijlstra F, van 't Hof AW. Prognostic value of admission glycosylated hemoglobin and glucose in nondiabetic patients with ST-segment-elevation myocardial infarction treated with percutaneous coronary intervention. Circulation. 2011;124:704-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 186] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 12. | Naito R, Miyauchi K, Ogita M, Kasai T, Kawaguchi Y, Tsuboi S, Konishi H, Okazaki S, Kurata T, Daida H. Impact of admission glycemia and glycosylated hemoglobin A1c on long-term clinical outcomes of non-diabetic patients with acute coronary syndrome. J Cardiol. 2014;63:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Aggarwal B, Shah GK, Randhawa M, Ellis SG, Lincoff AM, Menon V. Utility of Glycated Hemoglobin for Assessment of Glucose Metabolism in Patients With ST-Segment Elevation Myocardial Infarction. Am J Cardiol. 2016;117:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Shin D, Ahn J, Cha KS, Park JS, Oh JH, Lee HW, Hong JY, Kim BW, Hong TJ; Korea Working Group on Myocardial Infarction Investigators. Impact of initial glycosylated hemoglobin level on cardiovascular outcomes in prediabetic patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Coron Artery Dis. 2016;27:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Chen CL, Yen DH, Lin CS, Tsai SH, Chen SJ, Sheu WH, Hsu CW. Glycated hemoglobin level is an independent predictor of major adverse cardiac events after nonfatal acute myocardial infarction in nondiabetic patients: A retrospective observational study. Medicine (Baltimore). 2017;96:e6743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Kok MM, von Birgelen C, Sattar N, Zocca P, Löwik MM, Danse PW, Schotborgh CE, Scholte M, Hartmann M, Kant GD, Doelman C, Tjon Joe Gin M, Stoel MG, van Houwelingen G, Linssen GCM, IJzerman MJ, Doggen CJM, van der Heijden LC. Prediabetes and its impact on clinical outcome after coronary intervention in a broad patient population. EuroIntervention. 2018;14:e1049-e1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Dykun I, Kappo N, Kampf J, Babinets O, Jánosi RA, Totzeck M, Rassaf T, Mahabadi AA. Association of Hemoglobin A1c Levels With All-Cause Mortality in Patients With Coronary Artery Disease: The Essen Coronary Artery Disease Registry. JACC Adv. 2025;4:101624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Park HW, Her SH, Jung J, Chun H, Chung WS. Association of Glycosylated Hemoglobin with Long-Term Adverse Cardiac Events after Percutaneous Coronary Intervention in Non Diabetes and Controlled Diabetes Patients: An Observational Study from the Korean COACT Registry. Life (Basel). 2022;12:1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Wen ZY, Li FP, Wu TT, Hou XG, Pan Y, Deng CJ, Li YX, He XC, Gao WT, Chen HX, Zheng YY, Xie X. Association of hemoglobin glycation index with clinical outcomes in patients with coronary artery disease: a prospective cohort study. Diabetol Metab Syndr. 2024;16:241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 20. | Zhou Z, Qiao L, Ling Y, He Y, Chang T, Lu H, Yu S, Liu J, Guo W, Chen S, Liu Y, Chen J. Intermediate Hyperglycemia Increases the Risk of All-Cause Mortality in Premature Coronary Artery Disease Patients Undergoing Percutaneous Coronary Intervention. Rev Cardiovasc Med. 2023;24:352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Geng J, Zhang Y, Wang B, Xie J, Xu B, Li J. Glycosylated hemoglobin levels and clinical outcomes in nondiabetic patients with coronary artery disease: A meta-analysis. Medicine (Baltimore). 2017;96:e6784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Cicek G, Uyarel H, Ergelen M, Ayhan E, Abanonu GB, Eren M, Gibson CM. Hemoglobin A1c as a prognostic marker in patients undergoing primary angioplasty for acute myocardial infarction. Coron Artery Dis. 2011;22:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/