Published online Nov 26, 2025. doi: 10.4330/wjc.v17.i11.110563
Revised: July 7, 2025
Accepted: October 11, 2025
Published online: November 26, 2025
Processing time: 165 Days and 8.4 Hours
Colchicine is one of the most widely used drugs in the world. While it is most commonly used in the treatment and prevention of gout, it is also widely used to treat other chronic inflammatory diseases, such as familial Mediterranean fever and Behçet’s disease. Regarding cardiovascular disease, an established use of colchicine concerns pericarditis, both acute and chronic, and its effectiveness in this context is supported by multiple studies and robust evidence. Regarding coronary artery disease (CAD), colchicine use has been endorsed in both acute and chronic coronary syndromes (CCS), primarily because of two randomized controlled trials: The COLCOT trial for patients with acute coronary syndromes (ACS) and the LoDoCo2 trial for patients with CCS. Considering this robust evidence, CCS 2024 European Society of Cardiology (ESC) Guidelines re
Core Tip: This review aims to provide a practical, up-to-date resource on the utility of colchicine in atherosclerotic disease and is intended for cardiologists who confront the daily challenge of mitigating systemic inflammation in cardiovascular patients. Current guidelines support its use to reduce recurrent cardiovascular events, yet recent findings have questioned its benefit in acute settings. This highlights the need for clinicians to balance established recommendations with emerging evidence when considering colchicine as part of long-term cardiovascular risk management.
- Citation: Animati FM, Cappannoli L, Proietti S, Fracassi F, Montone RA, Ierardi C, Aurigemma C, Romagnoli E, Paraggio L, Lunardi M, Bianchini F, Leone AM, Trani C, Liuzzo G, Burzotta F. Colchicine in coronary artery and cerebrovascular disease: “Old skin for the new ceremony”. World J Cardiol 2025; 17(11): 110563
- URL: https://www.wjgnet.com/1949-8462/full/v17/i11/110563.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i11.110563
In the last decades, cardiology has achieved several outstanding goals in reducing the incidence and ameliorating prognosis of cardiovascular disease[1]. Among these, coronary artery disease (CAD) remains the most common and dangerous cardiac impairment, as it is the foremost single cause of mortality and loss of Disability Adjusted Life Years worldwide[2]. Moreover, despite good control of traditional risk factors, such as hypertension, diabetes mellitus, cigarette smoking and dislipidemia, some forms of atherosclerotic disease still progress. The reason behind this “residual risk” is often in large part due to inflammation[3]. Various studies have shown that CAD, in both its acute and chronic forms, corresponds to higher levels of inflammatory markers[4], and they stand as predictors of future vascular events[5]. Among these, C-reactive protein (CRP), serum amyloid A, interleukins (IL), myeloperoxidase, matrix metalloproteinases, selectins (E-selectin, P-selectin), vascular adhesion molecule 1, tumor necrosis factor (TNF)-α, systemic inflammation response index, are a few well-studied atherosclerosis-related inflammatory markers[6-11].
In recent years, colchicine, a low-cost yet effective drug long used across multiple medical indications, has emerged as a promising and potentially unique therapy for addressing the persistent and challenging problem of inflammation in CAD.
In line with this, the European Society of Cardiology (ESC) 2024 guidelines on chronic coronary syndromes (CCS) formalized the role of daily low dose colchicine (0.5 mg/day) to reduce myocardial infarction, stroke and need for revascularization in chronic CAD patients (Class of recommendation IIA, level of evidence A)[7]. Although several studies have affirmed the potential of colchicine in the treatment of cardiovascular disease and paved the way for its introduction in the latest ESC guidelines on CCS[5,8-11], some recent trials have reported contrasting results[12-14]. Among these studies, the recent CLEAR trial[15] showed that in patients with post-acute myocardial infarction (AMI), initiating colchicine therapy shortly after the event and continuing it for a median duration of 3 years did not reduce the incidence of the composite endpoint of cardiovascular death, recurrent myocardial infarction, stroke, or unplanned ischemia-driven revascularization.
In this review, we aim to summarize colchicine use, its mechanism of action and adverse reactions, and then to provide a deep examination on the most recent scientific evidence about colchicine application in cardiological clinical practice, providing a quick and effective guide for physicians who try to face the longstanding battle against inflammatory residual risk in ischemic heart disease patients.
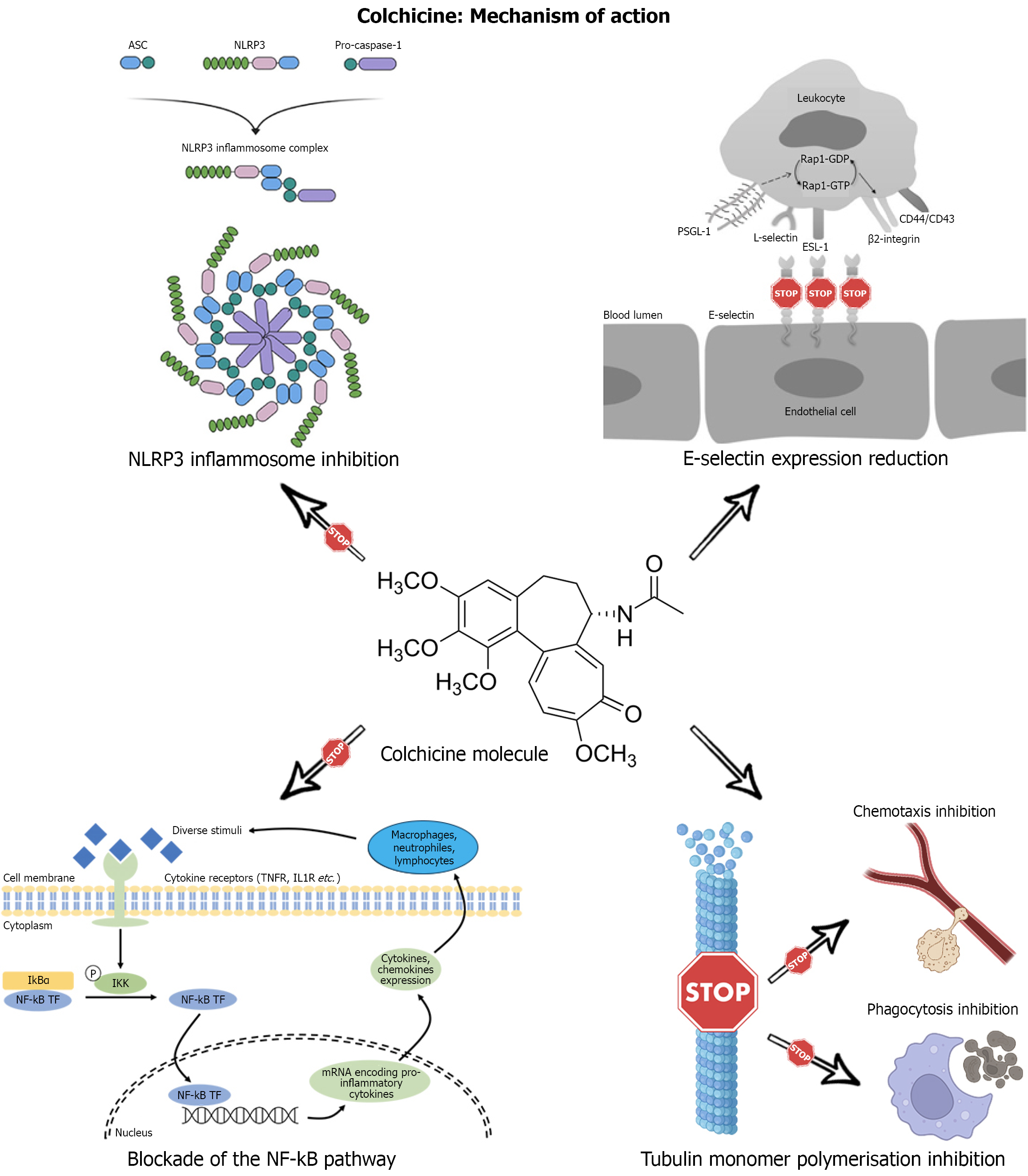
Colchicine is a plant alkaloid derived from the flower Colchicum Autumnale[16]. Its molecular structure is composed of three rings (A, B, C)[16]: The A and C rings are responsible for binding to tubulin, while the B ring keeps the other two in rigid conformation[16]. The mechanisms underlying colchicine's anti-inflammatory action are not yet fully understood. Among these, the most probable are the inhibition of tubulin monomer polymerization, the inhibition of NF-kB, the inhibition of NLRP3, and the modulation of E-selectin expression[16].
Microtubules are tubular structures based on tubulin heterodimers composed of α-tubulin and β-tubulin[17]. Microtubule polymerization and depolymerization processes underlie smooth muscle cell chemotaxis and migration[18]. Colchicine’s primary mechanism of action is the binding of the C-ring to β-tubulin so that the polymerization of tubulin monomers is prevented[19]; consequently, leukocyte phagocytosis and chemotaxis are inhibited[20] (Figure 1).
The NF-kB family is comprised of inducible transcription factors that play a role in atherosclerosis, heart failure and diabetes[21]. The NF-kB pathway is a crucial regulator of inflammatory processes, immune response and cell differentiation[22]. Upon activation, the NF-kB pathway induces pro-inflammatory cytokine secretion, such as IL-6, TNF-α and IL-1[23]. Several studies have documented how colchicine can reduce NF-kB activity through mechanisms related to the inhibition of microtubule polymerization[24,25] (Figure 1). In contrast, other studies have shown that high-dose colchicine (> 2 mg/day) can reduce CD14+/TLR2+ cells in patients with familial Mediterranean fever through direct NF-kB inhibition[26].
The NLRP3 inflammasome is a cellular multimeric protein complex capable of activating caspase-1 and stimulating the release of IL-1β/IL-18 cytokines[27]. It consists of NLRP3, the apoptosis-associated-speck-like protein containing a Caspase Activation and Recruitment Domain (CARD) (ASC) and procaspase-1[19]. Certain immunogenic molecules associated with pathogens (PAMPs) or cell damage (DAMPs) can activate NLRP3 and induce a conformation change such that ASC is recruited (CHEN link). Consequently, the CARD domain of ASC binds the CARD domain in procaspase-1, forming the NLRP3 inflammasome[28]. The NLRP3 inflammasome produces capsase-1, an active version of procaspase-1, which cleaves pro-IL-1β and pro-IL-18, producing the active version of these cytokines[29]. Several studies have shown that colchicine can inhibit the NLRP3 inflammasome[30,31] (Figure 1). More specifically, colchicine can reduce the expression of the pyrin gene (MEFV) in immune cells[32-34] and mature IL-1β and active caspase-1[35]. A sub-analysis conducted on the serum of participants in the LoDoCo2 trial showed that a lower level of NLRP3 in extracellular vesicles was observed in the colchicine-treated group[36].
E-selectin is a calcium-dependent transmembrane glycoprotein exclusively expressed on the surface of endothelial cells[37]. Its main function is to facilitate adhesion processes between the endothelium and neutrophils, monocytes, eosinophils and lymphocytes[38,39]. E-selectin is upregulated in response to several pro-inflammatory cytokines, such as TNF-α and IL-1β[40]. Colchicine can reduce E-selectin expression in endothelial cells[41] (Figure 1) through microtubule-dependent mechanisms[32]. Contextually, colchicine can inhibit macrophage release of TNF-α and down-regulate TNFR expression in macrophages and endothelial cells[42,43], indirectly inhibiting endothelial E-selectin expression.
Colchicine has been used since antiquity as an efficacious treatment against swelling and pain. During the VIth century, it was discovered as a potent gout treatment, and it remains a fundamental treatment against gouty flares and for gout prophylaxis today[44]. Those two conditions, along with familial Mediterranean fever, are the non-cardiovascular Food and Drug Administration (FDA)-approved uses of colchicine[44].
On January 20, 2023, colchicine received an FDA approval as the first anti-inflammatory drug for cardiovascular disease, meant for reducing cardiovascular events in patients who already have atherosclerotic cardiovascular disease (ASCVD) or those at risk of developing it. Its commercial name is “Lodoco”-which recalls the name of the pivotal clinical trials (LoDoCo and LoDoCo2) which, along with the COLCOT trial, brought the strongest evidence in favor of colchicine in ASCVD. The recommended dose is 0.5 mg/day[45]. Although not specifically approved by the FDA, there are several off-label uses for colchicine, in which it has shown efficacy and safety. These include acute and recurrent pericarditis, primary biliary cirrhosis and hepatic cirrhosis, Paget's disease of bone, chronic immune thrombocytopenia and idiopathic thrombocytopenic purpura, dermatitis herpetiformis, idiopathic pulmonary fibrosis and pseudogout[46].
Inflammation is a leading cause of CAD[47,48]. Different drugs have been previously tested to identify an effective agent able to reduce systemic inflammation in CAD without generating serious adverse effects. The CANTOS trial is one of the most important studies in this regard. It randomized 10061 patients with high-sensitivity CRP (hs-CRP) values ≥ 2 mg/L to take Canakinumab-a fully human monoclonal antibody that selectively binds and neutralizes IL-1β or placebo and showed a significant benefit in terms of nonfatal myocardial infarction, nonfatal stroke, cardiovascular death and hospitalization among the drug receiving patients[49]. Nevertheless, the increase of infection risk and the scarce cost-effectiveness of Canakinumab prevented this drug from becoming part of everyday clinical cardiology practice[50]. As for other anti-inflammatory drugs, lowdose methotrexate, tested in CIRT trial, failed to reduce IL1β, IL6, or CRP levels and did not decrease cardiovascular event rates compared to placebo in patients with stable atherosclerosis[51]. The anti-IL-6 receptor antibody tocilizumab was tested in the ASSAILMI trial to prove its efficacy in myocardial salvage in acute ST-segment elevation myocardial infarction (STEMI)[52]. This study randomized 199 STEMI patients (≤ 6 hours from symptom onset) to receive a single 280 mg tocilizumab infusion or placebo. Tocilizumab significantly increased the myocardial salvage index vs placebo and reduced microvascular obstruction but did not have any impact on the final infarct size. Because of these ambiguous results and its poor cost-effectiveness, tocilizumab never entered in routine cardiological clinical practice[52].
Other molecules such as Darapladib, Losmapimod and Varespladib, were all tested in the same context of inflammation in CAD and did not show any beneficial result[53-56].
On the other hand, colchicine has given some positive results. The capacity of colchicine in reducing hs-CRP levels in CAD patients has been demonstrated in an openlabel pilot study of 64 stable CAD patients with hsCRP > 2.0 mg/L despite aspirin and highdose atorvastatin. Twenty of those patients did not receive any additional therapy and 44 received colchicine 0.5 mg twice daily for 4 weeks. In the control group, hsCRP did not significantly change (4.28 ± 2.03 mg/L vs 3.70 ± 2.30 mg/L (mean change 11%, P value, not significant). In the colchicine group, hsCRP significantly decreased from 4.58 ± 2.05 mg/L to 1.78 ± 1.38 mg/L (P value < 0.001), with 64% of patients achieving > 50% reduction and 70% reducing to < 2.0 mg/L[5]. Beyond its mere effect on circulating hs-CRP levels, colchicine’s antiinflammatory action has been related with favorable remodeling of vulnerable coronary plaque[57] through its antiinflammatory effects, supporting its potential utility as an adjunctive secondary prevention therapy after acute coronary syndromes (ACS). In a recent prospective, nonrandomized study of 80 postACS patients (< 1 month), those receiving 0.5 mg/day colchicine plus optimal medical therapy (OMT) for 12.6 months exhibited a significantly greater reduction in lowattenuation plaque volume evaluated through computed tomography (CT) and hsCRP, compared with OMT alone patients[58]. Of note, calculated low-density lipoprotein cholesterol (cLDL) levels were similar between groups, showing that colchicine has a plaque-stabilizing effect independent of cLDL levels, corroborating the hypothesis that atherosclerosis cannot not be considered a mere “cholesterol excess” disease[58].
The most widespread use for colchicine in cardiovascular disease is in pericardial disease, in the context of both recurrent and acute pericarditis. ESC guidelines on pericardial disease recommend colchicine use as first-line therapy for acute pericarditis as an adjunct to aspirin/Non-Steroidal Anti-Inflammatory Drug (NSAID) therapy (Class of recommendation I, level of evidence A)[59]. The strongest evidence on the use of colchicine in this setting derives from two randomized controlled trials (RCTs) from Imazio et al[60,61]. The same guidelines recommend the use of colchicine in the setting of recurrent pericarditis. In this setting, this drug should be taken 0.5 mg twice daily or 0.5 mg daily for patients < 70 kg-or intolerant to higher doses-for 6 months, in addition to aspirin/NSAIDs (Class of recommendation I, level of evidence A)[59]. Its use for more than 6 months should be evaluated case by case (Class of recommendation IIA, level of evidence C)[59]. The indication on recurrent pericarditis derives from three pivotal clinical trials by Imazio et al[62-64].
While the use of colchicine in pericardial disease is broadly endorsed by major cardiology societies and clinical cardiologists-and has become a standard clinical practice-its application in the context of CAD remains marked by divergent viewpoints and centertocenter variability, owing in part to conflicting and often equivocal findings in the scientific literature.
As for CAD, the latest ESC guidelines on CCS recommend the use of colchicine for CAD patients with a low dose regimen (0.5 mg/day) to reduce myocardial infarction, stroke and need for revascularization (Class of recommendation IIA, level of evidence A)[7]. This recommendation derives from some pivotal RCTs, such as COLCOT[8] and LoDoCo2 trial[9] and from a metanalysis by Andreis et al[65] that included a total of 11 RCTs (12869 patients identified as eligible with a total of 6501 patients receiving colchicine and 6368 receiving placebo) and showed safety and efficacy in terms of secondary prevention from the use of colchicine in patients with CAD (Table 1).
| Study | Year | Study design | Comparison | Colchicine dose and duration | Primary endpoint | Sample size | Results |
| LoDoCo[72] | 2013 | Prospective, randomized, observer-blinded endpoint | Low-dose colchicine vs no colchicine | 0.5 mg/day; median 3 years follow-up | Composite incidence of acute coronary syndrome, out-of-hospital cardiac arrest, or noncardioembolic ischemic stroke | 532 patients | Primary endpoint: 5.3% (colchicine) vs 16.0% (no colchicine). Hazard ratio (HR) 0.33; 95%CI: 0.18 to 0.59; (P < 0.001). Significantly lower risk with colchicine |
| COLCOT[8] | 2019 | Randomized, double-blind, placebo-controlled, investigator initiated, event-driven trial | Low-dose colchicine vs Placebo | 0.5 mg once daily; median 22.6 months follow-up | Composite of death from cardiovascular causes, resuscitated cardiac arrest, myocardial infarction, stroke, or urgent hospitalization for angina leading to coronary revascularization | 4745 patients | Primary endpoint: 5.5% (colchicine) vs 7.1% (placebo). HR: 0.77; 95%CI: 0.61 to 0.96, (P = 0.02). Significantly lower risk with colchicine |
| COPS[71] | 2020 | Randomized, double-blind, placebo-controlled trial | Low-dose colchicine vs placebo | 0.5 mg twice daily for the first month, then 0.5 mg daily for 11 months; 12 months follow-up | Composite of all-cause mortality, ACS, ischemia-driven (unplanned) urgent revascularization and noncardioembolic ischemic stroke | 795 patients | Primary endpoint: 24 events (colchicine) vs 38 events (placebo). HR: 0.65; 95%CI: 0.38 to 1.09; (P = 0.10). The addition of colchicine to standard medical therapy did not significantly affect cardiovascular outcomes at 12 months in patients with ACS |
| LoDoCo2[9] | 2020 | Randomized, controlled, double-blind, investigator initiated, event-driven trial | Low-dose colchicine vs placebo | 0.5 mg once daily (after 1-month run-in phase); median 28.6 months follow-up | Composite of cardiovascular death, spontaneous (nonprocedural) myocardial infarction, ischemic stroke, or ischemia-driven coronary revascularization | 5522 patients | Primary endpoint: 6.8% (colchicine) vs 9.6% (placebo). HR: 0.69; 95%CI: 0.57 to 0.83; (P < 0.001). Significant risk reduction with colchicine |
| COVERT-MI[67] | 2021 | Investigator-initiated, randomized, double-blinded placebo-controlled, multicenter trial | Low-dose colchicine vs placebo | 2 mg loading dose followed by 0.5 mg twice a day for 5 days. Duration was from admission to day 5 | Infarct size in grams of left ventricular mass assessed by late gadolinium enhancement CMR at 5 days | 192 patients | At 5 days, gadolinium enhancement did not differ between colchicine and placebo groups (P = 0.87) |
| CONVINCE[13] | 2024 | Randomized, parallel-group, open-label, blinded endpoint assessed trial | Low-dose colchicine plus guideline-based usual care vs guideline-based usual care only | 0.5 mg orally per day; median 33.6 months follow-up | Composite of first recurrent non-fatal ischemic stroke, myocardial infarction, cardiac arrest, or hospitalization for unstable angina or vascular death | 3154 patients | Primary endpoint: 9.8% (colchicine plus usual care) vs 11.7% (usual care only). HR: 0.84; 95%CI: 0.68 to 1.05; (P = 0.12). The difference was not statistically significant in the intention-to-treat analysis |
| CHANCE-3[14] | 2024 | Multicenter, double blind, randomized, placebo controlled trial | Low-dose colchicine vs Placebo | 0.5 mg twice daily (days 1-3), then 0.5 mg daily (days 4-90). Duration was from admission to day 90 | Any new stroke (ischemic or hemorrhagic) within 90 days after randomization | 8343 patients | Primary endpoint (stroke): 6.3% (colchicine) vs 6.5% (placebo). HR: 0.98; 95%CI: 0.83 to 1.16; (P = 0.79). The trial did not provide sufficient evidence that low-dose colchicine could reduce the risk of subsequent stroke within 90 days |
| CLEAR[15] | 2024 | Multicenter trial with a 2-by-2 factorial design, randomized, placebo-controlled trial | Colchicine plus placebo vs colchicine plus spironolactone vs spironolactone plus placebo vs placebo only | Initially 0.5 mg twice daily (≥ 70 kg) or once daily (< 70 kg) for 90 days, then once daily for all. Modified to 0.5 mg once daily throughout from September 2020. Median 3 years follow-up | Composite of death from cardiovascular causes, recurrent myocardial infarction, stroke, or unplanned ischemia-driven coronary revascularization | 7062 | Primary endpoint: 9.1% (colchicine) vs 9.3% (placebo). HR: 0.99; 95%CI: 0.85 to 1.16; (P = 0.93). Treatment with colchicine, when started soon after myocardial infarction and continued for a median of 3 years, did not reduce the incidence of the composite primary outcome |
The COLCOT study (Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction; 2019) is the most important study that investigated the role of colchicine in the treatment of ACS[8]. A total of 4745 patients with a history of AMI within the last 30 days who had completed any planned percutaneous revascularization procedures and treated according to the guidelines of their country's cardiology societies, were randomized to receive colchicine at a dose of 0.5 mg/day and assessed according to a composite primary endpoint consisting of cardiovascular death, resuscitated cardiac arrest, AMI, stroke and urgent hospitalization for anginal pain leading to coronary revascularization. Colchicine reduced the incidence of primary endpoint events compared to placebo in a statistically significant manner (5.5% vs 7.1% P value = 0.02). More specifically, there was a reduction in the incidence of stroke and hospitalization for angina in the colchicine-treated population, while there were no statistically significant differences compared to placebo for the incidence of death from cardiovascular causes, AMI and resuscitated cardiac arrest. The incidence of adverse reactions was 16.0% in the colchicine-treated group and 15.8% in the placebo-treated group (P = 0.89): However, some gastrointestinal (nausea, diarrhea) or respiratory (pneumonia) adverse reactions were more frequent in the colchicine-treated population. Although outside the scope of this study, the effects of colchicine on certain biological markers were investigated in a specific subpopulation of patients. In a subgroup of 207 patients, CRP values were measured at the time of randomization and six months after randomization. In the experimental arm, there was a 70% reduction in CRP values at 6 months, compared to a 66% reduction in the control population. In contrast, the reduction in white blood cell count 1 year after randomization was not statistically significant between the two groups. Bouabdallaoui et al[66] investigated the role of time-to-treatment initiation in reducing the incidence of primary endpoint events in patients recruited in the COLCOT trial. They found that early colchicine administration (time to treatment initiation 0-3 days) showed statistically significant reductions in primary endpoint events in the experimental arm, whereas for later administrations (time to treatment initiation 4-7 days; time to treatment initiation 8-30 days), there was no statistically significant difference between the two groups.
The COVERT-MI study (Colchicine in STEMI Patients Study; 2021) set the ambitious goal of assessing the role of colchicine in reducing ischemic-reperfusion injury in patients with STEMI[67]. A total of 192 patients [first episode of STEMI, thrombolysis in myocardial infarction flow ≤ 1 and candidates for primary percutaneous coronary intervention (PCI) or rescue PCI] were randomized to receive either oral colchicine therapy (2 mg loading dose; 0.5 mg twice daily maintenance dose for 5 days) or a placebo. Colchicine therapy or placebo was administered whenever possible before the PCI procedure or, in residual cases, immediately afterwards. The primary endpoint was the reduction in the extent of the infarct area (expressed as percentage of left ventricular mass) as assessed by magnetic resonance imaging performed 5 ± 2 days after the acute event. Acute therapy with colchicine has not been shown to significantly reduce the extent of the infarcted area, except in women. Colchicine did not prove to be superior to placebo either with respect to secondary endpoints (reduction in left ventricular ejection fraction, reduction in left ventricular remodeling, incidence of microvascular obstruction) or in terms of reduction in inflammatory biomarkers (creatine kinase, white blood cells, neutrophil count, fibrinogen at entry, at 24 hours and at 48 hours, CRP); an increase in left ventricular thrombosis cases was found compared to the placebo-treated population. Bouleti et al[68] conducted a follow-up analysis of the COVERT-MI Study to assess clinical outcomes 1 year after the acute ischemic event. The primary endpoint of the study was assessed as the composite of the incidence of major cardiovascular events (all-cause death, ACSs, heart failure events, ischemic strokes, sustained ventricular arrhythmias and acute kidney injury at 1-year follow-up). At 1 year after the acute ischemic event, there were no statistically significant differences between the colchicine-treated and placebo-treated populations in terms of reduction in major adverse cardiovascular events (MACE). On the contrary, the colchicine-treated population had a lower incidence of heart failure events than the placebo-treated population, although the difference did not reach statistical significance.
The CLEAR study (Colchicine in AMI; 2024) investigated the potential of colchicine in reducing MACE in patients with AMI undergoing PCI[15]. A total of 7062 patients with a history of STEMI undergoing revascularization with PCI or with a history of non-STEMI undergoing revascularization with PCI and with at least one negative prognostic factor (ejection fraction ≤ 45%, diabetes mellitus, multivessel CAD, previous IMA, age ≥ 60 years) were randomized within a median time of 26.8 hours after the acute event to receive colchicine + spironolactone, colchicine + placebo, spironolactone + placebo or placebo alone. Colchicine was administered in the first 3 months after randomization at doses of 0.5 mg twice daily in patients > 70 kg and at doses of 0.5 mg daily in patients < 70 kg; in the following months, colchicine was administered at daily doses of 0.5 mg regardless of weight. The primary outcome was designed as the composite of death from cardiovascular causes, recurrent AMI, stroke or an unexpected ischemic event requiring coronary revascularization. The administration of colchicine has not been shown to reduce MACE in the investigated population. Furthermore, spironolactone did not change the effect of colchicine compared to placebo. The specific subpopulation treated with colchicine demonstrated a comparable incidence of events related to the primary endpoint, except for patients > 70 kg and treated with 0.5 mg colchicine twice daily for the first 3 months, who demonstrated a reduction in these events. In a heterogeneous patient population, CRP levels were measured at the time of randomization and 3 months after randomization. Administration of low-dose colchicine significantly reduced CRP values compared to placebo.
Although a recent meta-analysis demonstrated an increase in cardiovascular-related death in patients treated with colchicine[69], this study rather showed a reduction in the number of cardiovascular-related after treatment with low-dose colchicine, though the difference was not statistically significant. Of note, another recent meta-analysis of three randomized trials (12602 patients with acute or recent ACS) comparing low-dose colchicine vs placebo found no significant reduction in the composite of cardiovascular death, recurrent myocardial infarction (MI), stroke, or unplanned revascularization. Individual endpoints, including cardiovascular and all-cause mortality, recurrent MI, stroke, urgent revascularization, and diarrhea, were likewise unaffected. Leave-one-out analysis excluding the CLEAR trial yielded a significant benefit, underscoring its influence on overall findings[70].
The COPS trial (Colchicine in Patients With ACS; 2020)[71] was a multicenter, doubleblind, placebocontrolled trial carried out across 17 Australian acute cardiac centers, which enrolled 795 adults (mean age 59.8 ± 10.3 years; 21% female) with angiographically confirmed ACS. The patients were randomly assigned to colchicine (0.5 mg twice daily for 1 month, then 0.5 mg daily for 11 months) or placebo alongside standard secondary prevention and followed for ≥ 12 months. The primary composite endpoint (time to allcause mortality, ACS, urgent revascularization, or noncardioembolic stroke) occurred in 24 colchicinetreated vs 38 placebotreated patients. Colchicine was associated with higher total deaths (8 vs 1; P = 0.017), driven by noncardiovascular mortality (5 vs 0; P = 0.024), while adverse event (ACE) rates (predominantly gastrointestinal) were similar between groups (23.0% vs 24.3%). Post hoc analyses extending followup to 400 days and focusing on cardiovascular death alone revealed a statistically significant reduction in the primary composite outcome favoring colchicine, suggesting a timedependent benefit potentially mediated by colchicine antiinflammatory and plaquemodulating effects.
The COLCARDIO-ACS study (Colchicine Cardiovascular Outcomes in ACS Study-ACTRN12616000400460) is an ongoing randomized, doubleblind, placebocontrolled trial that aims to evaluate the efficacy of oral colchicine 0.5 mg once daily, on top of OMT, in reducing cardiovascular events in highrisk, postACS patients with inflammationmediated atherosclerosis (i.e., with hs-CRP ≥ 1.0 mg/L at time of registration, 4-52 weeks after discharge for an ACS event). The primary endpoint is the incidence of MACE, a composite of ACSs, urgent unscheduled revascularization, cardiovascular death, and nonfatal stroke, ascertained through scheduled followup visits and patientreported events or hospital admissions. The study is scheduled to conclude by the end of 2029. In a scientific landscape where the recent CLEAR-SINERGY trial has called into question colchicine’s efficacy in ACSs, this trial will serve as the decisive arbiter.
The LoDoCo trial (Low-Dose Colchicine for Secondary Prevention of Cardiovascular Disease; 2013) was one of the first studies to evaluate the role of colchicine in the treatment of CCS[72]. Five hundred thirty-two patients with stable CAD (angiographically proven coronary disease, age between 35-85 years, clinical stability for at least 6 months, absence of major comorbidities or contraindications to colchicine) and almost all of them being treated with Acetylsalicylic Acid/Clopidogrel and statin were randomized to receive colchicine 0.5 mg/day or not. The primary outcome was defined as the composite of the incidence of ACS, out-of-hospital cardiac arrests and non-cardioembolic ischemic stroke. The study results showed a significant reduction of primary outcome events in the experimental arm compared to the control arm (5.3% vs 16.0%, P value < 0.001), with an even clearer advantage when considering only treatment-compliant patients in the first 30 days after randomization.
In the wake of the promising results of the LoDoCo trial, LoDoCo2 (Colchicine in Patients with Chronic Coronary Disease; 2020) was born[9]. In LoDoCo2, a significantly larger population of patients with CCS was investigated than in the previous trial (5522) and some adjustments were made to make the study methodologically more robust (double-blind trial, placebo-control trial, larger number of participants, sample of patients from more than one country, different primary composite endpoint)[73]. Participants (age 35-82, evidence of CAD at coronarography or coronary CT scan, clinical stability for at least 6 months) were evaluated according to a composite primary endpoint consisting of cardiovascular death, spontaneous (nonprocedural) myocardial infarction, ischemic stroke and ischemia-driven coronary revascularization: Administration of colchicine at doses of 0.5 mg/day was shown to reduce the incidence of events associated with the primary endpoint compared to placebo (6.8% vs 9.6%, P value < 0.001). At the same time, the study demonstrated higher mortality from non-cardiovascular causes and higher overall mortality in the experimental arm compared to the control arm, although the differences in incidences were not statistically significant.
The CONVINCE study (Long-term colchicine for the prevention of vascular recurrent events in non-thromboembolic stroke: A RCT; 2024) focused on the role of colchicine in the prevention of recurrent cardiovascular events in patients with noncardioembolic stroke[13]. A population of 3154 hospitalized patients (clinically stable, age at least 40 years, history of non-severe ischemic stroke or high-risk transient ischemic attack caused by ipsilateral atherosclerosis of the carotid artery or vertebral artery or intracranial vessels or lacunar disease or cryptogenic embolism) with a history of non-severe ischemic stroke or high risk transient ischemic attack was randomized to receive colchicine at a dose of 0.5 mg/day or not, in combination with guideline-based therapy, over a period of 72 hours to 28 days after the acute event. The primary endpoint was evaluated as the composite of first recurrent non-fatal ischemic stroke, AMI, cardiac arrest and need for hospitalization for unstable angina or cardiovascular death. Colchicine administration did not significantly reduce the incidence of events associated with the primary endpoint (9.8% for colchicine + usual care vs 11.7% for usual care only, P-value 0.12). In patients with CAD, the benefits of colchicine administration were more pronounced than in patients without CAD, but statistical significance was not achieved. In specific subpopulations of patients, CRP levels were measured at baseline and 28 days, 1 year, 2 years, 3 years and 4 years after randomization. Basal CRP levels were similar between the two groups, whereas colchicine administration reduced CRP levels in all time intervals considered.
In contrast to LoDoCo2[9], mortality from non-cardiovascular causes and the incidence of neoplasms, infections and bleeding were similar in the two groups.
The CHANCE3 study (Colchicine in patients with acute ischemic stroke or transient ischemic attack: Multicenter, double blind, randomized, placebo controlled trial; 2024) evaluated the potential of colchicine in patients with minor or moderate stroke or transient ischemic attack[14]. A population of 8343 patients (at least 40 years of age, minor or moderate ischemic stroke with National Institutes of Health Stroke Scale of 5 or less or transient ischemic attack with ABCD scale greater than or equal to 4, baseline CRP values greater than or equal to 2 mg/dL) was randomized to receive colchicine (0.5 mg twice daily from day 1-3; 0.5 mg daily from day 4 onwards) or placebo within 24 hours after symptom onset. The primary outcome was stroke onset (ischemic or hemorrhagic) within 90 days after randomization. Colchicine was not superior to placebo in reducing the occurrence of stroke in this population (6.3% vs 6.5%, P value 0.79). It is relevant to note that colchicine showed greater efficacy in participants under 65 years of age, although systemic inflammation is known to increase with age[74]. This evidence is also consistent with a recent meta-analysis that found that colchicine reduced the incidence of cardiovascular events in CAD patients under 65 years of age[75].
As for colchicine’s role in stroke prevention, a recent meta-analysis[76] evaluated 16 RCTs with a total of 24967 patients. Colchicine reduced cardiovascular ACE by 33% (P < 0.001) and the incidence of myocardial infarction by 21% (P < 0.01). Overall, colchicine did not significantly reduce the incidence of stroke compared to placebo. However, the incidence of stroke was significantly lower in patients treated with colchicine and followed-up for 6 months or longer than in the control population.
Some research has focused on the role of colchicine in peripheral arterial obliterative disease (PAD)[10]. A retrospective study published in 2024 showed that colchicine was associated with a reduction in major adverse limb events in patients with PAD, especially in the case of concomitant therapy with hypouricemic agents[10].
The Peri-OPerative COlchicine to Reduce Negative Events (POPCORN trial-NCT05618353)[77] is a prospective, multicenter, doubleblind, placebocontrolled trial that aims to enroll 700 patients with prior coronary revascularization or high atherosclerotic burden undergoing intermediate or highrisk noncardiac surgery to receive a colchicine loading dose 1 day preoperatively followed by twicedaily dosing for 14 days postoperatively vs placebo. The primary objective is to compare the incidence of perioperative MACE. Secondary objectives include characterization of systemic inflammation and perioperative neutrophil profiles, as well as identification of clinical and genetic predictors of MACE and treatment response heterogeneity. The study is estimated to be completed by April 2029[77].
The Mono Antiplatelet and Colchicine Therapy (MACT trial-NCT0494951)[78] is a pilot trial that investigated whether monotherapy with a P2Y12 inhibitor (ticagrelor or prasugrel) combined with lowdose colchicine immediately after PCI was feasible and safe in ACS patients. The study enrolled 200 ACS patients treated with drugeluting stents who received colchicine 0.6 mg once daily plus maintenance ticagrelor or prasugrel from primary PCI. The primary endpoint was any stent thrombosis at 3 months. Key secondary endpoints included platelet reactivity prior to discharge and changes in hsCRP from 24 hours postPCI to 1 month. Only two patients (1.0%) experienced stent thrombosis (1 definite, 1 probable and median hsCRP decreased from 6.1 mg/L at 24 hours postPCI to 0.6 mg/L at 1 month), demonstrating that discontinuing aspirin and initiating lowdose colchicine with ticagrelor or prasugrel monotherapy after PCI in ACS patients is feasible, yields a low stent thrombosis and bleeding rate, maintains effective platelet inhibition, and rapidly attenuates postprocedural inflammation.
Low-dose colchicine (0.5 mg/day) is well tolerated in patients with normal renal function and normal liver function[79]. However, some adverse reaction on renal, liver, gastrointestinal function and on immune, neuromuscular and hematopoietic systems may still occur.
In patients with normal renal function, colchicine has been shown to be safe up to doses of 3 mg/day[80]. In patients with impaired renal function, administration of low-dose colchicine does not increase the risk of nephropathy progression[81,82]. However, colchicine administration should be avoided in patients with Chronic Kidney Disease IIIA or higher, as some serious adverse reactions to colchicine have occurred exclusively in this population (low dose for atherosclerosis link).
Like renal function, low-dose colchicine appears to be well tolerated in patients with normal liver function. Colchicine administration has been shown to be associated with a slight asymptomatic increase in alanine transaminase levels[83]. In contrast, a recent meta-analysis showed that continued treatment with colchicine was not associated with an increased risk of hepatotoxicity compared to the control population[84].
The administration of colchicine is associated with an increase in gastrointestinal adverse reactions (diarrhea, nausea, vomiting, abdominal pain, anorexia, gastrointestinal bleeding), particularly diarrhea[65]. More specifically, high doses of colchicine (> 0.5 mg/day) or a short duration of treatment (< 6 months) were associated with a statistically significant increase in gastrointestinal adverse reactions compared to placebo, whereas no significant differences were appreciated in patients treated with low doses of colchicine (< 0.5 mg/day) or for prolonged periods of time (> 6 months)[65].
Colchicine administration, even at low doses, is associated with an increased incidence of myalgias[9], although this has rarely led to discontinuation of therapy[85]. In contrast, the administration of colchicine was not associated with an increase in creatin kinase values compared to placebo, irrespective of the type and dose of statin with which it was associated[85]. Colchicine myoneuropathy is a rare event, which usually manifests with the symptoms of proximal myopathy and is associated with co-administration of CYP3A4 or glycoprotein P inhibitors or with renal or hepatic dysfunction[86].
Although some studies appear to show an increased risk of infection in patients treated with colchicine[8,87], two recent meta-analyses have shown that colchicine administration is not associated with an increased incidence of infection[84,86]. Low-dose colchicine administration has not been shown to be associated with an increased incidence of cancer[8,9]. On the contrary, the use of colchicine in certain rheumatological diseases, such as gout or familial Mediterranean fever, seems to reduce the incidence of neoplasias[88,89].
Continuous treatment with low-dose colchicine is not associated with a significantly higher risk of myelotoxicity than placebo[84].
Extinguishing the fire of inflammation in atherosclerotic disease is the next step that cardiovascular medicine aims to take. While the management of the epiphenomenon of coronary atherosclerosis, namely the atheromatous plaque and its potential disruption, is now well established in both acute and chronic settings, there remains limited therapeutic capability to address the underlying disease process of atherosclerosis itself. To date, colchicine represents the only antiinflammatory drug included in international cardiology societies guidelines on CAD and the only drug approved by the FDA in this context. Should it become definitively part of the “canonical” armamentarium for patients with CAD, this drug will have a dramatic impact on cardiovascular patients worldwide, owing to its widespread availability and ease of administration. However, despite demonstrated efficacy in reducing systemic inflammation and in reducing myocardial infarctions and cardiovascular death in patients at risk of or with established CAD, which lead to the subsequent inclusion in ESC and American Heart Association guidelines for chronic and ACSs, other findings have been contradictory. Further evidence from ongoing trials could shed light on the true efficacy of this old yet useful drug in reducing cardiovascular events. For now, in accordance with European and American cardiology society guidelines, colchicine should be considered an economical, well-tolerated and effective remedy in mitigating systemic inflammation, which is the cornerstone of atherosclerotic disease progression.
| 1. | Montone RA, Rinaldi R, Niccoli G, Andò G, Gragnano F, Piccolo R, Pelliccia F, Moscarella E, Zimarino M, Fabris E, de Rosa S, Calabrò P, Porto I, Burzotta F, Grigioni F, Barbato E, Chieffo A, Capodanno D, Al-Lamee R, Ford TJ, Brugaletta S, Indolfi C, Sinagra G, Perrone Filardi P, Crea F; Interventional Cardiology Working Group of the Italian Society of Cardiology. Optimizing Management of Stable Angina: A Patient-Centered Approach Integrating Revascularization, Medical Therapy, and Lifestyle Interventions. J Am Coll Cardiol. 2024;84:744-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 2. | Ralapanawa U, Sivakanesan R. Epidemiology and the Magnitude of Coronary Artery Disease and Acute Coronary Syndrome: A Narrative Review. J Epidemiol Glob Health. 2021;11:169-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 387] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 3. | Cappannoli L, Galli M, Borovac JA, Valeriani E, Animati FM, Fracassi F, Burzotta F. Editorial: Inflammation in ischemic heart disease: pathophysiology, biomarkers, and therapeutic implications. Front Cardiovasc Med. 2024;11:1469413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Liuzzo G, Montone RA, Gabriele M, Pedicino D, Giglio AF, Trotta F, Galiffa VA, Previtero M, Severino A, Biasucci LM, Crea F. Identification of unique adaptive immune system signature in acute coronary syndromes. Int J Cardiol. 2013;168:564-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Nidorf M, Thompson PL. Effect of colchicine (0.5 mg twice daily) on high-sensitivity C-reactive protein independent of aspirin and atorvastatin in patients with stable coronary artery disease. Am J Cardiol. 2007;99:805-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Del Buono MG, Bonaventura A, Vecchié A, Moroni F, Golino M, Bressi E, De Ponti R, Dentali F, Montone RA, Kron J, Lazzerini PE, Crea F, Abbate A. Pathogenic pathways and therapeutic targets of inflammation in heart diseases: A focus on Interleukin-1. Eur J Clin Invest. 2024;54:e14110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Vrints C, Andreotti F, Koskinas KC, Rossello X, Adamo M, Ainslie J, Banning AP, Budaj A, Buechel RR, Chiariello GA, Chieffo A, Christodorescu RM, Deaton C, Doenst T, Jones HW, Kunadian V, Mehilli J, Milojevic M, Piek JJ, Pugliese F, Rubboli A, Semb AG, Senior R, Ten Berg JM, Van Belle E, Van Craenenbroeck EM, Vidal-Perez R, Winther S; ESC Scientific Document Group. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur Heart J. 2024;45:3415-3537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1612] [Cited by in RCA: 1352] [Article Influence: 676.0] [Reference Citation Analysis (0)] |
| 8. | Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, Berry C, López-Sendón J, Ostadal P, Koenig W, Angoulvant D, Grégoire JC, Lavoie MA, Dubé MP, Rhainds D, Provencher M, Blondeau L, Orfanos A, L'Allier PL, Guertin MC, Roubille F. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Engl J Med. 2019;381:2497-2505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 2240] [Article Influence: 320.0] [Reference Citation Analysis (0)] |
| 9. | Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, The SHK, Xu XF, Ireland MA, Lenderink T, Latchem D, Hoogslag P, Jerzewski A, Nierop P, Whelan A, Hendriks R, Swart H, Schaap J, Kuijper AFM, van Hessen MWJ, Saklani P, Tan I, Thompson AG, Morton A, Judkins C, Bax WA, Dirksen M, Alings M, Hankey GJ, Budgeon CA, Tijssen JGP, Cornel JH, Thompson PL; LoDoCo2 Trial Investigators. Colchicine in Patients with Chronic Coronary Disease. N Engl J Med. 2020;383:1838-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 1565] [Article Influence: 260.8] [Reference Citation Analysis (0)] |
| 10. | Lin DS, Huang KC, Lin TT, Lee JK, Lin LY. Effects of Colchicine on Major Adverse Limb and Cardiovascular Events in Patients With Peripheral Artery Disease. Mayo Clin Proc. 2024;99:1374-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Kirov H, Caldonazo T, Runkel A, Medin D, Fischer J, Dallan LR, Mukharyamov M, Mejia OA, Jatene FB, Doenst T. Colchicine in Patients With Coronary Disease Who Underwent Coronary Artery Bypass Surgery: A Meta-Analysis of Randomized Controlled Trials. Am J Cardiol. 2024;231:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 12. | Rubin EJ, Leopold J, Morrissey S. NEJM at AHA - Routine Spironolactone in Acute Myocardial Infarction. N Engl J Med. 2025;392:e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Kelly P, Lemmens R, Weimar C, Walsh C, Purroy F, Barber M, Collins R, Cronin S, Czlonkowska A, Desfontaines P, De Pauw A, Evans NR, Fischer U, Fonseca C, Forbes J, Hill MD, Jatuzis D, Kõrv J, Kraft P, Kruuse C, Lynch C, McCabe D, Mikulik R, Murphy S, Nederkoorn P, O'Donnell M, Sandercock P, Schroeder B, Shim G, Tobin K, Williams DJ, Price C. Long-term colchicine for the prevention of vascular recurrent events in non-cardioembolic stroke (CONVINCE): a randomised controlled trial. Lancet. 2024;404:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 94] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 14. | Li J, Meng X, Shi FD, Jing J, Gu HQ, Jin A, Jiang Y, Li H, Johnston SC, Hankey GJ, Easton JD, Chang L, Shi P, Wang L, Zhuang X, Li H, Zang Y, Zhang J, Sun Z, Liu D, Li Y, Yang H, Zhao J, Yu W, Wang A, Pan Y, Lin J, Xie X, Jin WN, Li S, Niu S, Wang Y, Zhao X, Li Z, Liu L, Zheng H, Wang Y; CHANCE-3 Investigators. Colchicine in patients with acute ischaemic stroke or transient ischaemic attack (CHANCE-3): multicentre, double blind, randomised, placebo controlled trial. BMJ. 2024;385:e079061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 57] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 15. | Jolly SS, d'Entremont MA, Lee SF, Mian R, Tyrwhitt J, Kedev S, Montalescot G, Cornel JH, Stanković G, Moreno R, Storey RF, Henry TD, Mehta SR, Bossard M, Kala P, Layland J, Zafirovska B, Devereaux PJ, Eikelboom J, Cairns JA, Shah B, Sheth T, Sharma SK, Tarhuni W, Conen D, Tawadros S, Lavi S, Yusuf S; CLEAR Investigators. Colchicine in Acute Myocardial Infarction. N Engl J Med. 2025;392:633-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 163] [Article Influence: 163.0] [Reference Citation Analysis (0)] |
| 16. | D'Amario D, Cappetta D, Cappannoli L, Princi G, Migliaro S, Diana G, Chouchane K, Borovac JA, Restivo A, Arcudi A, De Angelis A, Vergallo R, Montone RA, Galli M, Liuzzo G, Crea F. Colchicine in ischemic heart disease: the good, the bad and the ugly. Clin Res Cardiol. 2021;110:1531-1542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Montecinos-Franjola F, Chaturvedi SK, Schuck P, Sackett DL. All tubulins are not alike: Heterodimer dissociation differs among different biological sources. J Biol Chem. 2019;294:10315-10324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Ouyang C, Mu J, Lu Q, Li J, Zhu H, Wang Q, Zou MH, Xie Z. Autophagic degradation of KAT2A/GCN5 promotes directional migration of vascular smooth muscle cells by reducing TUBA/α-tubulin acetylation. Autophagy. 2020;16:1753-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Zhang FS, He QZ, Qin CH, Little PJ, Weng JP, Xu SW. Therapeutic potential of colchicine in cardiovascular medicine: a pharmacological review. Acta Pharmacol Sin. 2022;43:2173-2190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 20. | Bhattacharyya B, Panda D, Gupta S, Banerjee M. Anti-mitotic activity of colchicine and the structural basis for its interaction with tubulin. Med Res Rev. 2008;28:155-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 366] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 21. | Brasier AR. The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res. 2010;86:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 460] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 22. | Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1825] [Cited by in RCA: 2177] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 23. | Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023-17023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2830] [Cited by in RCA: 6146] [Article Influence: 682.9] [Reference Citation Analysis (0)] |
| 24. | Mackenzie GG, Keen CL, Oteiza PI. Microtubules are required for NF-kappaB nuclear translocation in neuroblastoma IMR-32 cells: modulation by zinc. J Neurochem. 2006;99:402-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Jackman RW, Rhoads MG, Cornwell E, Kandarian SC. Microtubule-mediated NF-kappaB activation in the TNF-alpha signaling pathway. Exp Cell Res. 2009;315:3242-3249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Ben-David H, Livneh A, Lidar M, Feld O, Haj Yahia S, Grossman C, Ben-Zvi I. Toll-like receptor 2 is overexpressed in Familial Mediterranean fever patients and is inhibited by colchicine treatment. Best Pract Res Clin Rheumatol. 2018;32:651-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int J Mol Sci. 2019;20:3328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 925] [Cited by in RCA: 2634] [Article Influence: 376.3] [Reference Citation Analysis (0)] |
| 28. | Chen Y, Ye X, Escames G, Lei W, Zhang X, Li M, Jing T, Yao Y, Qiu Z, Wang Z, Acuña-Castroviejo D, Yang Y. The NLRP3 inflammasome: contributions to inflammation-related diseases. Cell Mol Biol Lett. 2023;28:51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 242] [Reference Citation Analysis (0)] |
| 29. | Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, Schröder GF, Fitzgerald KA, Wu H, Egelman EH. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156:1193-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 782] [Cited by in RCA: 1108] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 30. | Van Gorp H, Saavedra PH, de Vasconcelos NM, Van Opdenbosch N, Vande Walle L, Matusiak M, Prencipe G, Insalaco A, Van Hauwermeiren F, Demon D, Bogaert DJ, Dullaers M, De Baere E, Hochepied T, Dehoorne J, Vermaelen KY, Haerynck F, De Benedetti F, Lamkanfi M. Familial Mediterranean fever mutations lift the obligatory requirement for microtubules in Pyrin inflammasome activation. Proc Natl Acad Sci U S A. 2016;113:14384-14389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 150] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 31. | Park YH, Wood G, Kastner DL, Chae JJ. Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat Immunol. 2016;17:914-921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 358] [Cited by in RCA: 435] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 32. | Molad Y. Update on colchicine and its mechanism of action. Curr Rheumatol Rep. 2002;4:252-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 151] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | Ben-Chetrit E, Bergmann S, Sood R. Mechanism of the anti-inflammatory effect of colchicine in rheumatic diseases: a possible new outlook through microarray analysis. Rheumatology (Oxford). 2006;45:274-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | MALAWISTA SE, SEEGMILLER JE. THE EFFECT OF PRETREATMENT WITH COLCHICINE ON THE INFLAMMATORY RESPONSE TO MICROCRYSTALLINE URATE: A MODEL FOR GOUTY INFLAMMATION. Ann Intern Med. 1965;62:648-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Robertson S, Martínez GJ, Payet CA, Barraclough JY, Celermajer DS, Bursill C, Patel S. Colchicine therapy in acute coronary syndrome patients acts on caspase-1 to suppress NLRP3 inflammasome monocyte activation. Clin Sci (Lond). 2016;130:1237-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 36. | Silvis MJM, Fiolet ATL, Opstal TSJ, Dekker M, Suquilanda D, Zivkovic M, Duyvendak M, The SHK, Timmers L, Bax WA, Mosterd A, Cornel JH, de Kleijn DPV. Colchicine reduces extracellular vesicle NLRP3 inflammasome protein levels in chronic coronary disease: A LoDoCo2 biomarker substudy. Atherosclerosis. 2021;334:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 37. | Zhang J, Huang S, Zhu Z, Gatt A, Liu J. E-selectin in vascular pathophysiology. Front Immunol. 2024;15:1401399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 38. | Somers WS, Tang J, Shaw GD, Camphausen RT. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1. Cell. 2000;103:467-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 592] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 39. | Wagers AJ, Lowe JB, Kansas GS. An important role for the alpha 1,3 fucosyltransferase, FucT-VII, in leukocyte adhesion to E-selectin. Blood. 1996;88:2125-2132. [PubMed] |
| 40. | Meager A. Cytokine regulation of cellular adhesion molecule expression in inflammation. Cytokine Growth Factor Rev. 1999;10:27-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 167] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 41. | Cronstein BN, Molad Y, Reibman J, Balakhane E, Levin RI, Weissmann G. Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils. J Clin Invest. 1995;96:994-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 268] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 42. | Li Z, Davis GS, Mohr C, Nain M, Gemsa D. Inhibition of LPS-induced tumor necrosis factor-alpha production by colchicine and other microtubule disrupting drugs. Immunobiology. 1996;195:624-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Ding AH, Porteu F, Sanchez E, Nathan CF. Downregulation of tumor necrosis factor receptors on macrophages and endothelial cells by microtubule depolymerizing agents. J Exp Med. 1990;171:715-727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Buckley LF, Libby P. Colchicine's Role in Cardiovascular Disease Management. Arterioscler Thromb Vasc Biol. 2024;44:1031-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 45. | Zhang RS, Weber BN, Araiza-Garaygordobil D, Garshick MS. Colchicine for the Prevention of Cardiovascular Disease: Potential Global Implementation. Curr Cardiol Rep. 2024;26:423-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 46. | Sadiq NM, Robinson KJ, Terrell JM. Colchicine. 2025 Jan 19. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 47. | Camilli M, Iannaccone G, La Vecchia G, Cappannoli L, Scacciavillani R, Minotti G, Massetti M, Crea F, Aspromonte N. Platelets: the point of interconnection among cancer, inflammation and cardiovascular diseases. Expert Rev Hematol. 2021;14:537-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 48. | Rocco E, Grimaldi MC, Maino A, Cappannoli L, Pedicino D, Liuzzo G, Biasucci LM. Advances and Challenges in Biomarkers Use for Coronary Microvascular Dysfunction: From Bench to Clinical Practice. J Clin Med. 2022;11:2055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 49. | Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4997] [Cited by in RCA: 7155] [Article Influence: 795.0] [Reference Citation Analysis (0)] |
| 50. | Biasucci LM, Pedicino D, Liuzzo G. Promises and challenges of targeting inflammation to treat cardiovascular disease: the post-CANTOS era. Eur Heart J. 2020;41:2164-2167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 51. | Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, Gupta M, Tsigoulis M, Verma S, Clearfield M, Libby P, Goldhaber SZ, Seagle R, Ofori C, Saklayen M, Butman S, Singh N, Le May M, Bertrand O, Johnston J, Paynter NP, Glynn RJ; CIRT Investigators. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N Engl J Med. 2019;380:752-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 711] [Cited by in RCA: 1024] [Article Influence: 146.3] [Reference Citation Analysis (0)] |
| 52. | Broch K, Anstensrud AK, Woxholt S, Sharma K, Tøllefsen IM, Bendz B, Aakhus S, Ueland T, Amundsen BH, Damås JK, Berg ES, Bjørkelund E, Bendz C, Hopp E, Kleveland O, Stensæth KH, Opdahl A, Kløw NE, Seljeflot I, Andersen GØ, Wiseth R, Aukrust P, Gullestad L. Randomized Trial of Interleukin-6 Receptor Inhibition in Patients With Acute ST-Segment Elevation Myocardial Infarction. J Am Coll Cardiol. 2021;77:1845-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 314] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 53. | O'Donoghue ML, Braunwald E, White HD, Lukas MA, Tarka E, Steg PG, Hochman JS, Bode C, Maggioni AP, Im K, Shannon JB, Davies RY, Murphy SA, Crugnale SE, Wiviott SD, Bonaca MP, Watson DF, Weaver WD, Serruys PW, Cannon CP; SOLID-TIMI 52 Investigators, Steen DL. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. JAMA. 2014;312:1006-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 363] [Article Influence: 30.3] [Reference Citation Analysis (1)] |
| 54. | STABILITY Investigators; White HD, Held C, Stewart R, Tarka E, Brown R, Davies RY, Budaj A, Harrington RA, Steg PG, Ardissino D, Armstrong PW, Avezum A, Aylward PE, Bryce A, Chen H, Chen MF, Corbalan R, Dalby AJ, Danchin N, De Winter RJ, Denchev S, Diaz R, Elisaf M, Flather MD, Goudev AR, Granger CB, Grinfeld L, Hochman JS, Husted S, Kim HS, Koenig W, Linhart A, Lonn E, López-Sendón J, Manolis AJ, Mohler ER 3rd, Nicolau JC, Pais P, Parkhomenko A, Pedersen TR, Pella D, Ramos-Corrales MA, Ruda M, Sereg M, Siddique S, Sinnaeve P, Smith P, Sritara P, Swart HP, Sy RG, Teramoto T, Tse HF, Watson D, Weaver WD, Weiss R, Viigimaa M, Vinereanu D, Zhu J, Cannon CP, Wallentin L. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med. 2014;370:1702-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 441] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 55. | O'Donoghue ML, Glaser R, Cavender MA, Aylward PE, Bonaca MP, Budaj A, Davies RY, Dellborg M, Fox KA, Gutierrez JA, Hamm C, Kiss RG, Kovar F, Kuder JF, Im KA, Lepore JJ, Lopez-Sendon JL, Ophuis TO, Parkhomenko A, Shannon JB, Spinar J, Tanguay JF, Ruda M, Steg PG, Theroux P, Wiviott SD, Laws I, Sabatine MS, Morrow DA; LATITUDE-TIMI 60 Investigators. Effect of Losmapimod on Cardiovascular Outcomes in Patients Hospitalized With Acute Myocardial Infarction: A Randomized Clinical Trial. JAMA. 2016;315:1591-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 202] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 56. | Nicholls SJ, Kastelein JJ, Schwartz GG, Bash D, Rosenson RS, Cavender MA, Brennan DM, Koenig W, Jukema JW, Nambi V, Wright RS, Menon V, Lincoff AM, Nissen SE; VISTA-16 Investigators. Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. JAMA. 2014;311:252-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 262] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 57. | Gerhardt T, Seppelt C, Abdelwahed YS, Meteva D, Wolfram C, Stapmanns P, Erbay A, Zanders L, Nelles G, Musfeld J, Sieronski L, Stähli BE, Montone RA, Vergallo R, Haghikia A, Skurk C, Knebel F, Dreger H, Trippel TD, Rai H, Joner M, Klotsche J, Libby P, Crea F, Kränkel N, Landmesser U, Leistner DM; OPTICO-ACS study group. Culprit plaque morphology determines inflammatory risk and clinical outcomes in acute coronary syndrome. Eur Heart J. 2023;44:3911-3925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 58. | Vaidya K, Arnott C, Martínez GJ, Ng B, McCormack S, Sullivan DR, Celermajer DS, Patel S. Colchicine Therapy and Plaque Stabilization in Patients With Acute Coronary Syndrome: A CT Coronary Angiography Study. JACC Cardiovasc Imaging. 2018;11:305-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 230] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 59. | Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J, Brucato A, Gueret P, Klingel K, Lionis C, Maisch B, Mayosi B, Pavie A, Ristic AD, Sabaté Tenas M, Seferovic P, Swedberg K, Tomkowski W; ESC Scientific Document Group. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015;36:2921-2964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1457] [Cited by in RCA: 1705] [Article Influence: 155.0] [Reference Citation Analysis (7)] |
| 60. | Imazio M, Bobbio M, Cecchi E, Demarie D, Demichelis B, Pomari F, Moratti M, Gaschino G, Giammaria M, Ghisio A, Belli R, Trinchero R. Colchicine in addition to conventional therapy for acute pericarditis: results of the COlchicine for acute PEricarditis (COPE) trial. Circulation. 2005;112:2012-2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 399] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 61. | Imazio M, Cecchi E, Ierna S, Trinchero R; ICAP Investigators. Investigation on Colchicine for Acute Pericarditis: a multicenter randomized placebo-controlled trial evaluating the clinical benefits of colchicine as adjunct to conventional therapy in the treatment and prevention of pericarditis; study design amd rationale. J Cardiovasc Med (Hagerstown). 2007;8:613-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 62. | Imazio M, Bobbio M, Cecchi E, Demarie D, Pomari F, Moratti M, Ghisio A, Belli R, Trinchero R. Colchicine as first-choice therapy for recurrent pericarditis: results of the CORE (COlchicine for REcurrent pericarditis) trial. Arch Intern Med. 2005;165:1987-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 298] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 63. | Imazio M, Brucato A, Cemin R, Ferrua S, Belli R, Maestroni S, Trinchero R, Spodick DH, Adler Y; CORP (COlchicine for Recurrent Pericarditis) Investigators. Colchicine for recurrent pericarditis (CORP): a randomized trial. Ann Intern Med. 2011;155:409-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 279] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 64. | Imazio M, Belli R, Brucato A, Cemin R, Ferrua S, Beqaraj F, Demarie D, Ferro S, Forno D, Maestroni S, Cumetti D, Varbella F, Trinchero R, Spodick DH, Adler Y. Efficacy and safety of colchicine for treatment of multiple recurrences of pericarditis (CORP-2): a multicentre, double-blind, placebo-controlled, randomised trial. Lancet. 2014;383:2232-2237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 281] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 65. | Andreis A, Imazio M, Casula M, Avondo S, De Ferrari GM. Colchicine efficacy and safety for the treatment of cardiovascular diseases. Intern Emerg Med. 2021;16:1691-1700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 66. | Bouabdallaoui N, Tardif JC, Waters DD, Pinto FJ, Maggioni AP, Diaz R, Berry C, Koenig W, Lopez-Sendon J, Gamra H, Kiwan GS, Blondeau L, Orfanos A, Ibrahim R, Grégoire JC, Dubé MP, Samuel M, Morel O, Lim P, Bertrand OF, Kouz S, Guertin MC, L'Allier PL, Roubille F. Time-to-treatment initiation of colchicine and cardiovascular outcomes after myocardial infarction in the Colchicine Cardiovascular Outcomes Trial (COLCOT). Eur Heart J. 2020;41:4092-4099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 230] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 67. | Mewton N, Roubille F, Bresson D, Prieur C, Bouleti C, Bochaton T, Ivanes F, Dubreuil O, Biere L, Hayek A, Derimay F, Akodad M, Alos B, Haider L, El Jonhy N, Daw R, De Bourguignon C, Dhelens C, Finet G, Bonnefoy-Cudraz E, Bidaux G, Boutitie F, Maucort-Boulch D, Croisille P, Rioufol G, Prunier F, Angoulvant D. Effect of Colchicine on Myocardial Injury in Acute Myocardial Infarction. Circulation. 2021;144:859-869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 68. | Bouleti C, Viscogliosi S, Bresson D, Leboube S, Bochaton T, El-Jonhy N, Amaz C, Prunier F, Bidaux G, Roubille F, Angoulvant D, Mewton N. Colchicine in acute myocardial infarction: cardiovascular events at 1-year follow up. Open Heart. 2024;11:e002474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 69. | Fiolet ATL, Opstal TSJ, Mosterd A, Eikelboom JW, Jolly SS, Keech AC, Kelly P, Tong DC, Layland J, Nidorf SM, Thompson PL, Budgeon C, Tijssen JGP, Cornel JH. Efficacy and safety of low-dose colchicine in patients with coronary disease: a systematic review and meta-analysis of randomized trials. Eur Heart J. 2021;42:2765-2775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 183] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 70. | Cappannoli L, Fracassi F, Aurigemma C, Romagnoli E, Bianchini F, Lunardi M, Montone RA, Paraggio L, Trani C, Liuzzo G, Burzotta F. Colchicine In Acute Coronary Syndromes: A Meta-Analysis of 12.602 Patients. J Cardiovasc Pharmacol. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 71. | Tong DC, Quinn S, Nasis A, Hiew C, Roberts-Thomson P, Adams H, Sriamareswaran R, Htun NM, Wilson W, Stub D, van Gaal W, Howes L, Collins N, Yong A, Bhindi R, Whitbourn R, Lee A, Hengel C, Asrress K, Freeman M, Amerena J, Wilson A, Layland J. Colchicine in Patients With Acute Coronary Syndrome: The Australian COPS Randomized Clinical Trial. Circulation. 2020;142:1890-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 288] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 72. | Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61:404-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 808] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 73. | Banach M, Penson PE. Colchicine and Cardiovascular Outcomes: a Critical Appraisal of Recent Studies. Curr Atheroscler Rep. 2021;23:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 74. | Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1485] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 75. | Ma Z, Chen J, Jin K, Chen X. Colchicine and coronary heart disease risks: A meta-analysis of randomized controlled clinical trials. Front Cardiovasc Med. 2022;9:947959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 76. | Jaiswal V, Deb N, Hanif M, Wajid Z, Nasir YM, Naz S, Kalra K, Qaiser S, Shrestha AB, Bandyopadhyay D, Mattumpuram J. Efficacy of Colchicine for Prevention of Stroke and Adverse Cardiovascular Events: A Meta-analysis of 16 Randomized Controlled Trials. Am J Cardiovasc Drugs. 2025;25:83-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 77. | VA Office of Research and Development. The Peri-OPerative COlchicine to Reduce Negative Events Trial (POPCORN). In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/study/NCT05618353 ClinicalTrials.gov Identifier: NCT05618353. |
| 78. | Lee SY, Jeong YH, Yun KH, Cho JY, Gorog DA, Angiolillo DJ, Kim JW, Jang Y. P2Y(12) Inhibitor Monotherapy Combined With Colchicine Following PCI in ACS Patients: The MACT Pilot Study. JACC Cardiovasc Interv. 2023;16:1845-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 79. | Nidorf SM, Ben-Chetrit E, Ridker PM. Low-dose colchicine for atherosclerosis: long-term safety. Eur Heart J. 2024;45:1596-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 80. | Ozen S, Demirkaya E, Erer B, Livneh A, Ben-Chetrit E, Giancane G, Ozdogan H, Abu I, Gattorno M, Hawkins PN, Yuce S, Kallinich T, Bilginer Y, Kastner D, Carmona L. EULAR recommendations for the management of familial Mediterranean fever. Ann Rheum Dis. 2016;75:644-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 396] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 81. | Solak Y, Siriopol D, Yildiz A, Yilmaz MI, Ortiz A, Covic A, Kanbay M. Colchicine in Renal Medicine: New Virtues of an Ancient Friend. Blood Purif. 2017;43:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 82. | Nasri H. Colchicine and the concepts of nephroprotection; a new feature of an old drug. J Ren Endocrinol. 2022;8:e25072. [DOI] [Full Text] |
| 83. | van Broekhoven A, Mohammadnia N, Silvis MJM, Los J, Fiolet ATL, Opstal TSJ, Mosterd A, Eikelboom JW, Nidorf SM, Budgeon CA, Byrnes E, Bax WA, Tijssen JGP, de Kleijn DPV, Thompson PL, El Messaoudi S, Cornel JH. The Effect of Years-Long Exposure to Low-Dose Colchicine on Renal and Liver Function and Blood Creatine Kinase Levels: Safety Insights from the Low-Dose Colchicine 2 (LoDoCo2) Trial. Clin Drug Investig. 2022;42:977-985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 84. | Stewart S, Yang KCK, Atkins K, Dalbeth N, Robinson PC. Adverse events during oral colchicine use: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther. 2020;22:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 85. | van Broekhoven A, Eikelboom JW, Nidorf SM, Mosterd A, Cornel JH; LoDoCo2 investigators. Elevations in Creatine Kinase are Not Related to the Choice or Dose of Statins in Patients Taking Colchicine 0.5 mg Daily: Insights from the LoDoCo2 Trial. Clin Drug Investig. 2023;43:575-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 86. | McEwan T, Bhambra J, Liew DF, Robinson PC. Systematic review of colchicine neuromyopathy: Risk factors, duration and resolution. Semin Arthritis Rheum. 2023;58:152150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 87. | Zarpelon CS, Netto MC, Jorge JC, Fabris CC, Desengrini D, Jardim Mda S, Silva DG. Colchicine to Reduce Atrial Fibrillation in the Postoperative Period of Myocardial Revascularization. Arq Bras Cardiol. 2016;107:4-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 88. | Brenner R, Ben-Zvi I, Shinar Y, Liphshitz I, Silverman B, Peled N, Levy C, Ben-Chetrit E, Livneh A, Kivity S. Familial Mediterranean Fever and Incidence of Cancer: An Analysis of 8,534 Israeli Patients With 258,803 Person-Years. Arthritis Rheumatol. 2018;70:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 89. | Kuo MC, Chang SJ, Hsieh MC. Colchicine Significantly Reduces Incident Cancer in Gout Male Patients: A 12-Year Cohort Study. Medicine (Baltimore). 2015;94:e1570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/