Published online Oct 26, 2025. doi: 10.4330/wjc.v17.i10.109961
Revised: June 14, 2025
Accepted: September 17, 2025
Published online: October 26, 2025
Processing time: 151 Days and 2.8 Hours
Acute myocardial infarction (AMI) remains a leading global cause of morbidity and mortality, with high risk of recurrent adverse cardiovascular events. Conventional diagnostic markers often lack the sensitivity needed for early detection and prognostic stratification. Recent advances highlight the role of microRNAs (miRNAs) and their genetic polymorphisms in regulating inflammation, fibrosis, and endothelial function in atherosclerotic disease. This review summarizes evidence on circulating miRNA expression and miRNA-related single nucleotide polymorphisms as biomarkers in AMI. Literature from PubMed, Scopus, and Web of Science was evaluated, focusing on pathways involving NF-κB, interleukin-1 receptor/toll-like receptors, and JAK/STAT signaling. Circulating miRNAs such as miR-150, miR-208, miR-26a, and miR-483-5p demonstrate strong diagnostic accuracy, while polymorphisms, particularly rs2910164 in miR-146a, are con
Core Tip: Acute myocardial infarction (AMI) remains a leading cause of morbidity and mortality worldwide, and traditional biomarkers offer limited prognostic value. This review highlights the emerging significance of circulating microRNAs (miRNAs)-particularly miR-150, miR-208, miR-26a, and miR-483-5p and genetic polymorphisms such as rs2910164 in miR-146a in predicting AMI risk and major adverse cardiovascular events. These biomarkers regulate inflammation and endothelial dysfunction through key pathways, nuclear factor-kappaB, and nterleukin-1 receptor/toll-like receptors signaling. Integrating miRNA profiling with clinical assessment may enhance early diagnosis and enable personalized risk stratification in AMI patients.
- Citation: Ngo TH, Tran SK. Role of polymorphisms and microRNA levels in predicting cardiovascular events in patients with acute myocardial infarction. World J Cardiol 2025; 17(10): 109961
- URL: https://www.wjgnet.com/1949-8462/full/v17/i10/109961.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i10.109961
Coronary artery disease (CAD) is the leading cause of mortality among atherosclerotic cardiovascular diseases, accoun
In addition to its clinical impact, AMI imposes a significant economic burden. In South Korea, the total economic loss attributed to AMI in 2012 was estimated at $1.18 billion United States dollars, with 51.6% representing direct costs, such as medical expenses, transportation and caregiving, and the remainder due to productivity losses[4].
Despite advances in early intervention and coronary revascularization, patients remain at elevated risk for major adverse cardiovascular events (MACE), including death, recurrent MI, and coronary stent restenosis. A study conducted in Bangladesh reported an in-hospital MACE rate of 37.0%, increasing to 45.0% at 30 days post-intervention. In-hospital rates of all-cause mortality, post-procedural restenosis, and recurrent MI were 19.0%, 18.0%, and 0.0%, respectively. At 30 days, these rates were 15.0%, 26.0% and 4.0%[5]. Long-term follow-up data from Tini et al[6] showed that over a mean period of 3.06 ± 2.04 years, the MACE incidence was 11.2% in patients under 45 and 24.2% in those over 45. These findings underscore the urgent need for early and accurate risk stratification strategies to improve outcomes in AMI patients.
Recent advances in genetics and molecular biology have led to the discovery of small regulatory RNAs that modulate gene expression at the post-transcriptional level. Among these, microRNAs (miRNAs) are short, non-coding RNAs that bind to the 3’-untranslated region (UTR) of target mRNAs to suppress gene expression[7]. Growing evidence suggests that both circulating miRNA expression levels and miRNA-related genetic polymorphisms hold prognostic value in AMI. Notably, elevated circulating miRNA levels and the presence of the rs2910164 polymorphism have been associated with increased AMI risk and poorer clinical outcomes. Carriers of the CC + GC genotype at this locus demonstrated a 3.189-fold higher risk of cardiovascular events [odds ratio (OR) = 3.189, 95%CI: 1.450-7.015, P = 0.004]. Multivariate analysis further identified miRNA expression as an independent risk factor for MACE in AMI patients [hazard ratio (HR) = 2.712, 95%CI: 1.450-5.072, P = 0.002][8].
In addition, several miRNAs have been implicated in post-AMI cardiac remodeling and mortality prediction. For example, miRNA-150 suppresses myocardial hypertrophy and fibrosis by targeting serum response factor and c-Myb[9]. These findings underscore the significant potential of miRNAs and their polymorphisms as biomarkers for risk stratification in AMI. Given the high risk of mortality and complications both during hospitalization and after discharge, there is growing interest in the prognostic utility of these molecular markers. Consistent with this trend, we conducted a comprehensive review to evaluate the association between miRNA expression and genetic polymorphisms with AMI incidence and post AMI burden, particularly the risk of MACE.
This minireview was conducted through a comprehensive search of peer-reviewed articles published between January 2010 and April 2025. The databases of PubMed, Scopus, and Web of Science were queried using the following keywords and Boolean operators: ("microRNA" OR "miRNA") AND ("acute myocardial infarction" OR "AMI") AND ("polymor
RNA is a fundamental biological molecule involved in transcription, translation, gene regulation, and expression. Structurally, RNA consists of a single strand of nucleotide monomers composed of adenine (A), guanine (G), cytosine (C), and uracil (U). Three major types of RNA play critical roles in genetic processes, namely messenger RNA (mRNA), transfer RNA, and ribosomal RNA[10].
Advancements in genetics have led to the discovery of a novel class of small RNA molecules. In 1993, Ambros and Ruvkun first described a small RNA in Caenorhabditis elegans that regulates gene expression post-transcriptionally. Their studies identified a small RNA, lin-4, which downregulates lin-14 gene expression by binding to its 3’-UTR, thereby inhibiting its translation. The term "microRNA" was later introduced by Tuschl and Bartel to describe this class of small non-coding RNA molecules that act as post-transcriptional regulators[7]. Nearly a decade after their discovery, miRNAs were identified in higher organisms, including mammals, and have since been implicated in a wide range of human diseases[11,12].
Molecular structural analysis has shown that miRNAs are small, single-stranded non-coding RNA molecules ranging from 19 to 25 nucleotides in length. They play a critical role in post-transcriptional gene regulation[13]. In most cases, miRNAs inhibit gene expression by binding to the 3’-UTR of target mRNAs, resulting in either mRNA degradation or translational repression. However, recent studies have also identified miRNA interactions with the 5'-UTR. For example, Nitschke et al[14] demonstrated that miRNA-760 functions as a negative regulator by binding to the 5'-UTR of the ATXN-1 gene, which is implicated as a cause of spinocerebellar ataxia type 1 (commonly known as SCA1), leading to RNA degradation and translational inhibition. Furthermore, under specific conditions, miRNAs have been shown to activate gene expression, translocate between subcellular compartments to modulate translation rates, and even influence transcriptional activity[15].
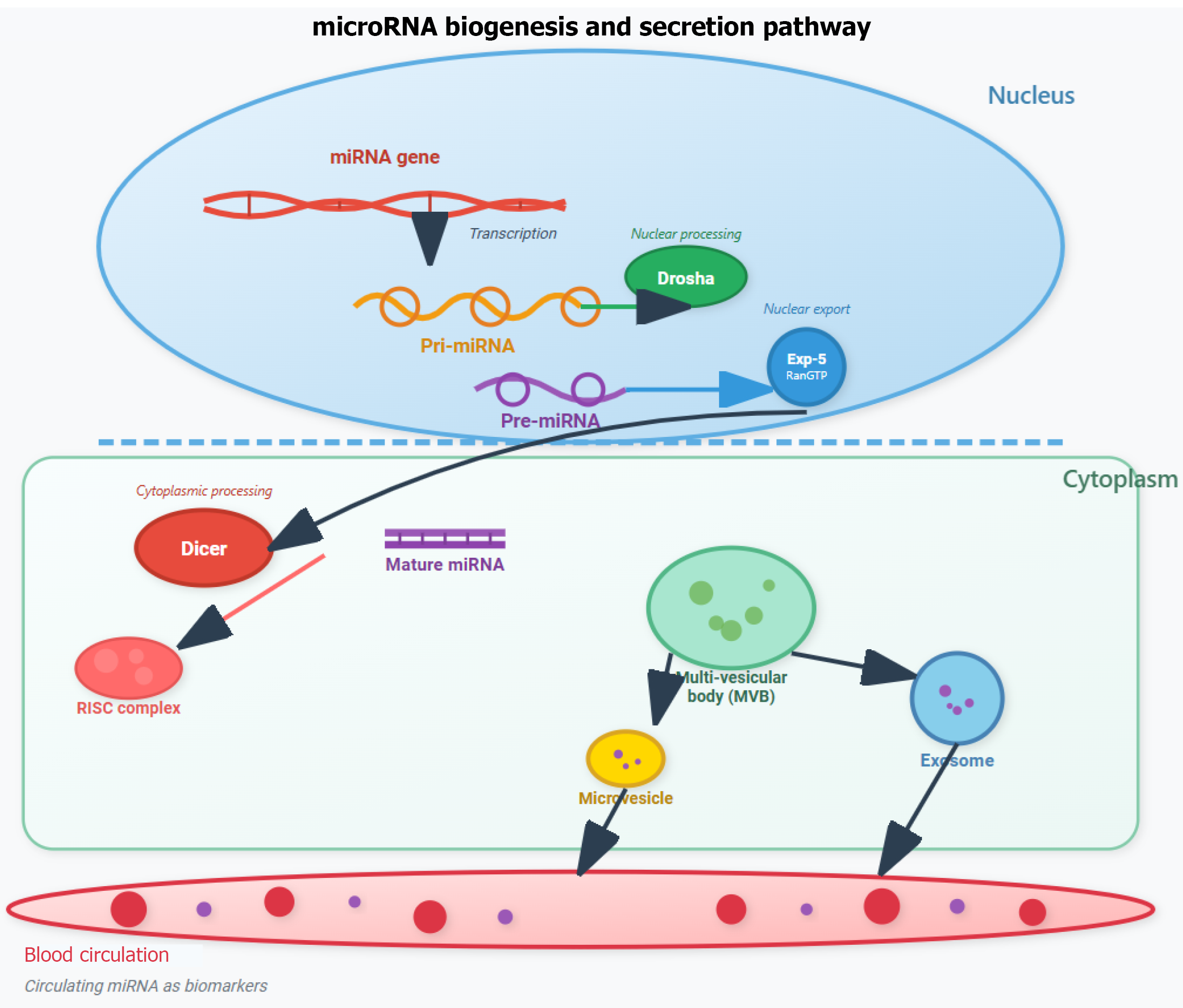
Circulating miRNAs are small, non-coding RNAs detectable in extracellular body fluids such as plasma, serum, synovial fluid, urine and saliva. Emerging evidence suggests that these miRNAs are transported through the circulatory system via small vesicles (e.g., exosomes), Argonaute protein complexes, or high-density lipoprotein cholesterol (HDL-c) particles[16]. Secretion mechanisms of miRNAs involve the ceramide-dependent pathway in COS7 and HEK293 cells. Ceramide, a bioactive sphingolipid whose biosynthesis is tightly controlled by neutral sphingomyelinase 2, triggers exosome secretion (Figure 1). Inhibition of this enzyme reduces miRNA secretion, while its overexpression increases miRNA secretion[17].
More recently, HDL-c has been identified as another carrier of endogenous miRNAs. With an average size of 8 nm to 12 nm-significantly smaller than exosomes–HDL-c contains lipids such as phosphatidylcholine, which can form stable ternary complexes with nucleic acids. Apolipoprotein A1, a major protein component of HDL-c, has also been shown to facilitate systemic delivery of miRNAs in animal models.
The physical and biochemical stability of circulating miRNAs makes them promising biomarkers for disease diagnosis and therapeutic monitoring. These molecules exhibit a long half-life (approximately 5 days in serum), resist RNase degra
Association between circulating miRNA levels and AMI diagnosis and prognosis of post-AMI burden (MACE): In recent years, several miRNAs have been implicated in the pathogenesis of atherosclerosis, largely through their re
Multiple miRNAs have been shown to predict post-AMI outcomes, such as mortality and left ventricular remodeling. For example, miRNA-150 has been found to play a cardioprotective role; its upregulation inhibits myocardial hyper
A growing number of studies have demonstrated that circulating miRNAs are closely associated with CAD and AMI. Chen et al[19] identified four specific miRNAs– miRNA-1291, miRNA-217, miRNA-455-3p, and miRNA-566–as potential biomarkers for the early diagnosis of AMI. In a cohort comprising 80 AMI patients and 80 controls, these miRNAs were significantly downregulated in AMI patients. Their individual diagnostic performance yielded area under the curve (AUC) values of 0.82, 0.79, 0.82, and 0.83, respectively. When combined, the four miRNAs produced a composite AUC of 0.87, with a sensitivity of 83% and a specificity of 87%. Notably, their peak expression occurred earlier than that of traditional biomarkers such as troponin I and creatine kinase-MB (CK-MB).
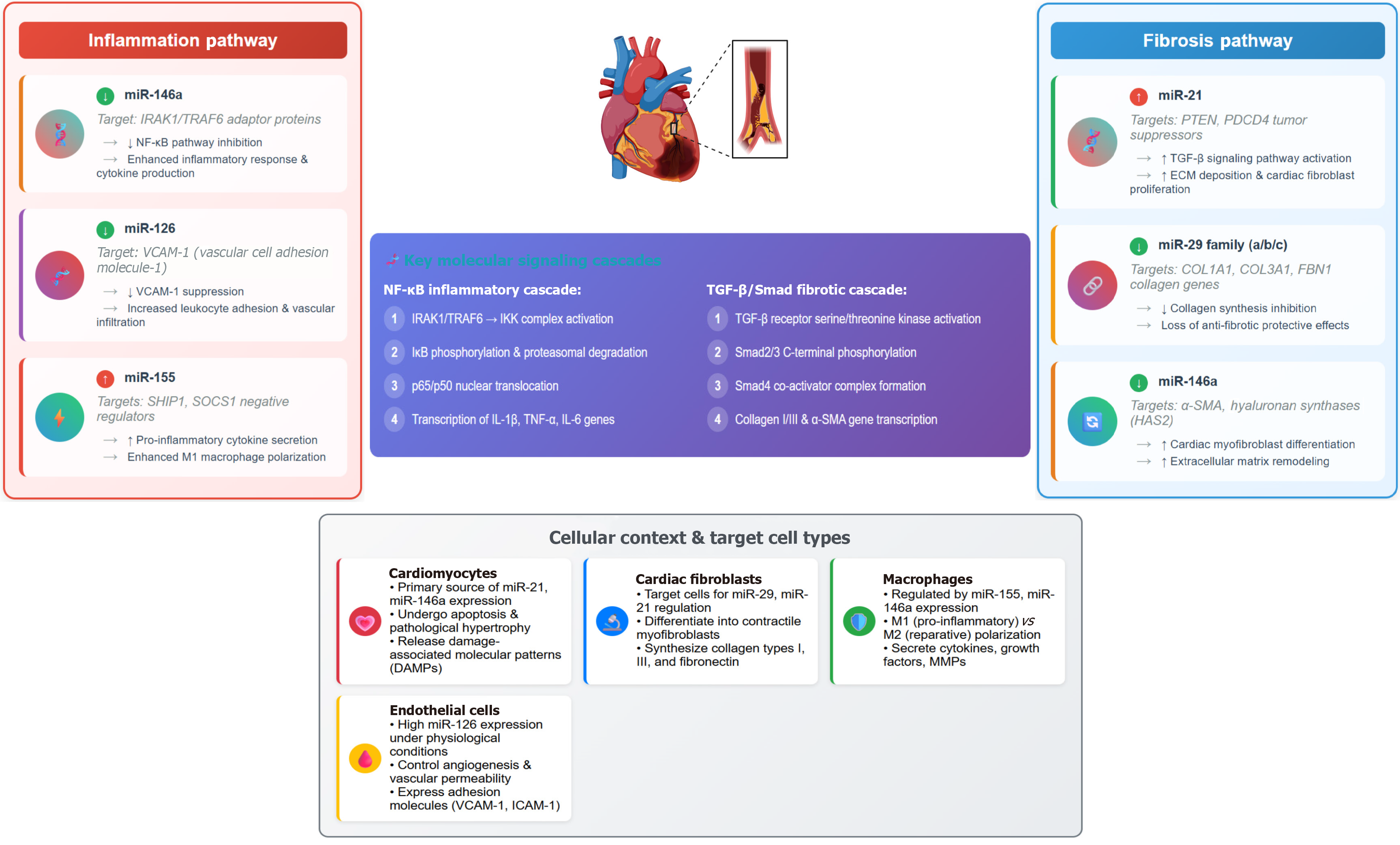
Similarly, Zhang et al[20] reported that miRNAs including miRNA-32–3p, miRNA-3149 and miRNA-26a-5p have strong diagnostic value for severe CAD requiring intervention. MiRNA-146 has been shown to suppress inflammatory cytokines via the IRAK-1 pathway and plays a dual role in regulating inflammatory responses and low-density lipo
Atherosclerotic CAD results from cholesterol deposition in the arterial intima, leading to luminal narrowing and ischemia. Inflammation and fibrosis are key features of this process, and miRNAs are central regulators. Raitoharju et al[24] and Takahashi et al[25] both found that circulating miRNA levels were elevated in patients with atherosclerosis and CAD compared to healthy controls, implicating the interleukin (IL)-1 receptor (IL-1R)/Toll-like receptors (TLRs)-nuclear factor kappa B (NF-κB) signaling pathway as a primary mediator of miRNA-induced inflammation. Additionally, circulating miRNA levels correlate with disease severity, with higher concentrations observed in patients with acute coronary syndrome (ACS) compared to those with stable angina[26].
Recent findings by Xiao et al[27] support the use of circulating miRNAs as prognostic biomarkers for MACE in STEMI patients. Other studies have reinforced the diagnostic performance of specific miRNAs in AMI (Table 1)[28-32]. Zhang et al[28] identified miRNA-486 and miRNA-150 with AUC values ranging from 0.678-0.771 (P < 0.001), while Peng et al[29] found miRNA-133 (AUC = 0.912, P < 0.001), miRNA-1291 (AUC = 0.695, P < 0.001) and miRNA-663b (AUC = 0.611, P < 0.01) to be promising candidates.
| Ref. | Patients (n) | miRNA | Standard | AUC | 95%CI | P value | ||
| Zhang et al[28], 2015 | 110 AMI | miRNA-486 | Quantitative real-time PCR | 0.731 | - | < 0.001 | ||
| miRNA-150 | 0.678 | - | < 0.001 | |||||
| miRNA-486 miRNA-150 | 0.771 | - | < 0.001 | |||||
| Peng et al[29], 2014 | 186 AMI | miRNA -133 | Quantitative reverse transcriptase-PCR | 0.912 | - | < 0.001 | ||
| miRNA-1291 | 0.695 | < 0.001 | ||||||
| miRNA-663b | 0.611 | < 0.01 | ||||||
| Wang et al[30], 2014 | 45 AMI | miRNA-361-5p | 0 hours | Quantitative real-time PCR | 0.881 | 0.777–0.985 | < 0.001 | |
| 4 hours | 0.883 | 0.777–0.989 | < 0.001 | |||||
| 24 hours | 0.838 | 0.716–0.961 | < 0.001 | |||||
| miRNA-21-5p | 0 hour | 0.949 | 0.872–1.000 | < 0.001 | ||||
| 4 hours | 0.947 | 0.000–1.000 | < 0.001 | |||||
| 24 hours | 0.791 | 0.655–0.927 | 0.001 | |||||
| miRNA-519e-5p | 0 hours | 0.798 | 0.663–0.934 | 0.001 | ||||
| 4 hours | 0.801 | 0.668–0.934 | 0.002 | |||||
| 24 hours | 0.908 | 0.818–0.997 | < 0.001 | |||||
| Combined score | 0 hour | 0.989 | 0.000–1.000 | < 0.001 | ||||
| 4 hours | 1.000 | 0.000–1.000 | < 0.001 | |||||
| 24 hours | 0.995 | 0.000–1.000 | < 0.001 | |||||
| Zhao et al[31], 2023 | 183 ACS | miRNA-483-5p | Quantitative reverse transcriptase-PCR | 0.919 | 0.881-0.957 | - | ||
| 78 AMI | 0.867 | 0.800-0.933 | - | |||||
| Xue et al[32], 2019 | 31 AMI | miRNA-26a-1 | Pre-PCI | Quantitative real-time PCR | 0.965 | - | < 0.001 | |
| Post-PCI | 0.939 | - | < 0.001 | |||||
| miRNA-146a | Pre-PCI | 0.911 | - | < 0.001 | ||||
| Post-PCI | 0.932 | - | < 0.001 | |||||
| miRNA-199a-1 | Pre-PCI | 0.855 | - | < 0.001 | ||||
| Post-PCI | 0.823 | - | < 0.001 | |||||
| Combined | Pre-PCI | 0.913 | - | < 0.001 | ||||
| Post-PCI | 0.890 | - | < 0.001 | |||||
Wang et al[30] further reported that miRNA-361-5p, miRNA-21-5p, and miRNA-519e-5p had AUCs > 0.7, all statistically significant (P < 0.01). Zhao et al[31] determined a diagnostic cutoff for circulating miRNA-483-5p in AMI; the values were 1.292 (AUC = 0.919, 95%CI: 0.881-0.957) for ACS and 1.536 (AUC = 0.867, 95%CI: 0.800-0.933) for AMI. Finally, Xue et al[32] reported that miRNA-26a-1, miRNA-146a, and miRNA-199a-1, individually and in combination, demonstrated high diagnosis accuracy in AMI patients undergoing percutaneous coronary intervention (PCI) (Table 1).
Several miRNAs have been identified as prognostic indicators of mortality and left ventricular remodeling following AMI. For example, miRNA-150 regulates myocardial hypertrophy and fibrosis by modulating serum response factor and c-Myb, while also inhibiting apoptotic gene expression. Additionally, miRNA-133a and miRNA-208 were the among the first miRNAs identified as prognostic markers, with levels at 6 months post-AMI correlating significantly with increased all-cause mortality[18].
MiRNAs have also emerged as independent predictors of ACS, regardless of traditional cardiovascular risk factors[26]. Xiao et al[27] demonstrated that circulating miRNA levels can predict MACE in patients with STEMI. Similarly, a multicenter study by Jakob et al[33] involving 1002 STEMI patients found that individuals who experienced MACE had significantly decreased levels of miRNA-26b-5p levels (P = 0.038) and increased levels of miRNA-320a (P = 0.047) and miRNA-660-5p (P = 0.01). These miRNAs were linked to distinct pathophysiological mechanisms: MiRNA-26b-5p was associated with decreased risk of adverse myocardial hypertrophy, miRNA-320a promoted cardiomyocyte death and apoptosis, and miRNA-660-5p correlated with increased platelet activity. In Cox regression models adjusted for age and sex, the AUCs were 0.707 for miRNA-26b-5p, 0.683 for miRNA-660-5p, and 0.672 for miRNA-320a. The combination of the three yielded an improved AUC of 0.718. Notably, incorporating these miRNAs into the GRACE risk score enhanced its AUC from 0.679 to 0.720, reinforcing their prognostic utility in ACS and AMI.
Yang et al[34] conducted a prospective case-control study with 932 STEMI patients undergoing primary PCI and found that patients who experienced MACE had significantly lower levels of miRNA-26a-5p, miRNA-21-5p, and miRNA-191-5p compared to those without MACE (P < 0.001). Multivariate logistic regression revealed that all three miRNAs were inversely associated with MACE risk (P < 0.01), supporting their role as prognostic biomarkers and potential therapeutic targets in AMI[34].
In another study, Alavi-Moghaddam et al[35] identified a cut-off value of 12.38 for miRNA-208b in screening for MACE at 6 months after discharge. Patients with levels above this threshold had an HR of 5.08 (95%CI: 1.13–22.82, P = 0.03) for experiencing MACE (Table 2). Consistently, Zhao et al[31] reported that elevated miRNA-483-5p levels were significantly associated with MACE in AMI patients, with an HR of 5.955 (95%CI: 1.928-18.389, P = 0.002) (Table 2).
| Ref. | Patients, | miRNA | Follow-up period in months | Standard | Cut off (%) | HR (95%CI) | P value |
| Xiao et al[27], 2021 | 192 AMI | miRNA-146a | 40 | Quantitative real-time PCR | - | 1.329 (1.060–1.664) | 0.01 |
| Alavi-Moghaddam et al[35], 2018 | 21 AMI | miRNA-208b | 6 | Quantitative real-time PCR | 12.38 | 5.08 (1.13–22.82) | 0.03 |
| Zhao et al[31], 2023 | 183 ACS | miRNA-483-5p | 6 | Quantitative reverse transcriptase-PCR | - | 5.955 (1.928-18.389) | 0.002 |
The above studies demonstrate that, beyond their role in post-transcriptional gene regulation, circulating miRNAs also have significant diagnostic and prognostic value in patients with AMI. These findings highlight the potential of miRNA-based molecular profiling to support individualized treatment strategies for AMI patients[36].
Association between miRNA polymorphisms, AMI diagnosis accuracy and prognosis of post-AMI MACE: Genetic variation plays a fundamental role in genomic diversity. While mutation refers to rare genetic sequence variations occurring in less than 1% of the population, more common variants are classified as polymorphisms. Among these, SNPs are the most prevalent, involving a single base-pair alteration in the genome. SNPs occur at an estimated frequency of 1 per 300 base pairs, resulting in approximately 10 million SNPs across the human genome[37].
MiRNAs are key regulators of inflammation and fibrosis, and structural variations within miRNA sequences can lead to functional impairments, contributing to various pathologies, particularly atherosclerosis-related cardiovascular diseases. Several SNPs in miRNAs have been linked to increased susceptibility to CAD. For instance, a meta-analysis conducted in China revealed that the rs2910164 SNP, particularly the GG and GG + GC genotypes and the G allele, was significantly associated with a heightened risk of CAD[38].
Another notable polymorphism, rs11614913T>C in miRNA-196a2 has shown potential in predicting CAD severity. In a study by Zhi et al[39], the CC and CC/CT genotypes were found to be associated with a 35% increased risk of severe CAD (HR = 1.34, 95%CI: 1.02–1.75 for CC; HR = 1.35, 95%CI: 1.03–1.75 for CC/CT) compared to the TT genotype. Cox regression analysis further identified age, smoking status, rs11614913T>C and diabetes as significant factors influencing severe CAD outcomes, suggesting that this SNP may serve as a prognostic marker in the Chinese population.
Qiao et al[40] also highlighted miRNA-146a and the rs2910164 SNP as modifiers of MACE risk in patients with coronary syndrome. Moreover, elevated plasma expression of miRNA-146a and its rs2910164 variant have been proposed as biomarkers for early STEMI diagnosis and shown to correlate with disease severity and prognosis[8]. Additional studies have demonstrated that miRNA-146a overexpression in patients with unstable angina correlated with CAD severity and predicted poorer clinical outcomes[41]. However, contradictory findings have been reported. For example, Huang et al[42] observed that the rs2910164C>G variant was associated with a reduced risk of ACS in a Chinese cohort, highlighting the need for larger-scale studies to validate these findings. A study in Vietnam identified SNPs rs2431697 and rs2910164 in the miRNA-146a gene as being associated with clinical severity in AMI patients. Specifically, the C allele of rs2431697 and the G allele of rs2910164 were found to be independent risk factors for severe complications following AMI[43].
Recent studies have increasingly focused on evaluating miRNA polymorphisms as prognostic markers for MACE following discharge in AMI patients (Table 3)[40,44]. Among these, miRNA-146a polymorphisms have been implicated in post-AMI prognosis. For example, Qiao et al[40] identified the rs2910164 (G/C) polymorphism as a risk factor for both AMI incidence and adverse outcomes in ACS patients. In a dominant model, the rs2910164 variant was associated with a significantly increased risk of post-PCI MACE (CG + GG vs CC, HR = 1.405, P = 0.038). Biochemical analyses further revealed that carriers of the G allele exhibited increased inflammatory markers and oxidative stress, leading to enhanced NF-κB activation and pro-inflammatory signaling in atherosclerotic plaques. In a broader analysis, Li et al[44] investigated several miRNA-related gene polymorphisms, including miRNA-149 rs71428439, miRNA-146a rs2910164, miRNA-499 rs3746444, miRNA-423 rs6505162, miRNA-4513 rs2168518 and FABP2 rs2168518. The primary endpoints included MI, stroke, heart failure, coronary revascularization or coronary artery bypass grafting, cardiovascular death and all-cause mortality. Among these, the rs2910164 polymorphism was initially associated with an increased risk of death; however, after multivariable adjustment, this association lost its statistical significance. Liu et al[8] conducted a study on the plasma distribution of miRNA146a and its rs2910164 polymorphism in 92 STEMI patients and 100 healthy controls, tracking MACE over a 28-month follow-up. They found a significantly higher prevalence of the CC + GC genotype among AMI patients, with carriers demonstrating a 3.189-fold increased risk of MACE (P = 0.004). Circulating miRNA-146a levels were markedly elevated in AMI patients (P = 0.0001), with an AUC of 0.742 for AMI diagnosis. Specifically, patients with higher miRNA gene expression levels had a significantly greater incidence of MACE compared to those with lower miRNA gene expression levels (P = 0.001), confirming miRNA gene expressions as an independent predictor of post-AMI MACE in STEMI patients (HR = 2.712, P = 0.002).
| Ref. | Patients | Genetic models | Analyzed model | Population-follow-up period | Standard | HR (95%CI) | P value |
| Qiao et al[40], 2023 | 612 ACS | miRNA-146a, rs2910164 | Dominant (CC vs CG + GG) | 42 months screening for MACE | ABI PRISM3730 DNA Sequencer | 1.405 (1.018-1.939) | 0.038 |
| Recessive (CC + CG vs GG) | 1.107 (0.821–1.492) | 0.506 | |||||
| Li et al[44], 2015 | 1004 CAD | miRNA-146a, rs2910164 | Dominant (CC vs CG + GG) | 5 years screening for MACE | PCR-based method | 1.119 (0.848-1.478) | 0.427 |
| Recessive (CC + CG vs GG) | 1.095 (0.787-1.524) | 0.591 | |||||
| Dominant (CC vs CG + GG) | 5 years screening for mortality | 0.762 (0.485-1.197) | 0.396 | ||||
| Recessive (CC + CG vs GG) | 0.757 (0.398-1.439) | 0.203 |
Before AMI onset, patients typically experience a prolonged period of atherosclerotic plaque development within the coronary arteries. This process is driven by multiple pathological mechanisms, endothelial dysfunction, infiltration and migration of inflammatory cells, comprised vascular cell integrity, and accumulation of vulnerable plaques[11]. The initial phase begins with the subendothelial deposition and gradual accumulation of LDL-c, which undergoes oxidative modi
MiRNA-31, miRNA-181b, miRNA-10a/b, miRNA-126, and miRNA-17-3p have been implicated in endothelial dys
Beyond its role in inflammation, miRNA regulates fibrosis. Recent evidence suggests that therapeutic interventions targeting miRNA significantly ameliorate fibrosis in multiple organ systems[51]. Excessive extracellular matrix (ECM) production is a hallmark of fibrotic disorders across various organs, predominantly driven by chronic inflammatory responses and epithelial-to-mesenchymal transition. Briefly, activated fibroblasts deposit ECM components, causing increased tissue stiffness, impaired oxygen and nutrient diffusion, and subsequent cellular injury[22]. In murine models of hepatic fibrosis, miRNAs are downregulated. In contrast, miRNA overexpression suppressed fibrogenesis by inhibiting the transforming growth factor-beta signaling pathway[52]. Additionally, miRNAs control fibrosis by regulating ECM production. Increased inflammation promotes fibroblast-mediated ECM deposition, leading to increased miRNA ex
Circulating miRNAs and miRNA polymorphisms hold clear potential in the early diagnosis and prognosis of AMI. Their early presence during atherosclerotic plaque formation, coupled with their demonstrated capacity to modulate fibrosis and inflammation through the IL-1R/TLRs/NF-κB and JAK-STAT pathways, underscores their crucial role. Notably, circulating miRNA levels for STEMI diagnosis significantly increase upon hospital admission, which is strongly corre
| miRNA/SNP | Biological function | AMI diagnostic value | MACE prognostic value | Associated pathway |
| miR-150 | Anti-apoptotic, anti-fibrotic | AUC 0.678–0.771[28] | Predictor of mortality, LV remodeling[18] | SRF, c-Myb, apoptosis |
| miR-208b | Cardiac-specific, involved in muscle gene regulation | AUC > 0.9 within 3–6 hours[19] | HR = 5.08 for MACE at 6 months[35] | Myocardial contractility genes |
| miR-483-5p | Inflammation-related | AUC = 0.867–0.919[31] | HR = 5.955 for MACE[31] | NF-κB, cytokines |
| miR-146a | Regulates IRAK1/TRAF6 | AUC = 0.91[32] | HR = 2.712 for MACE[8] | NF-κB, IL-1R/TLR |
| SNP rs2910164 (miR-146a) | Alters miRNA processing, G/C substitution | ↑Risk of AMI[38,40] | HR = 1.405 for MACE (dominant model)[57] | Inflammatory amplification |
| SNP rs11614913 (miR-196a2) | Influences mature miRNA levels | Associated with severe CAD[39] | HR = 2.44 (recessive model)[58] | Vascular remodeling, SIRT1 signaling |
| SNP rs3746444 (miR-499) | Affects cardiac muscle gene regulation | Predictor of MI susceptibility[58] | HR = 2.05 for MACE (recessive model)[58] | Myocardial apoptosis and necrosis |
Despite its promising potential, the clinical application of miRNA in AMI diagnosis and prognosis faces several challenges. According to Koshiol et al[54], one challenge is the inconsistency between detection methods, particularly between microarray and qRT-PCR, where only 44% of miRNAs (4/9) in a study of 49 Lung cancer samples showed correlated results between the two techniques. This discrepancy stems from differences in sensitivity, specificity, and technical execution. Additionally, miRNAs are short (approximately 22 nucleotides) with low copy numbers, leading to low detection sensitivity, especially when using non-amplification methods such as cloning or in situ hybridization, which require at least 5–25 µg RNA per sample and have lengthy processing times and poor sensitivity in detecting low-abundance miRNAs. Meanwhile, amplification-based methods such as qRT-PCR and microarray are susceptible to errors due to replication biases. There is currently no gold-standard assay for miRNA detection, which complicates standardization and reproducibility across studies. Different studies employ various validation methods without a unified protocol, reducing data reliability for cross-study comparisons. While capable of identifying novel miRNAs, next-generation sequencing technology still presents significant challenges. First, it requires large amounts of RNA as input (2–10 µg), is expensive, relies upon specialized equipment, and involves a lengthy processing time of 2–5 days per sample. Additionally, amplification biases may lead to false miRNA expression profiles, compromising result accuracy. Moreover, miRNA assays are highly susceptible to RNA contamination, laboratory handling errors, and amplification biases in qRT-PCR-based methods. Another challenge is data processing, as sequencing generates millions of reads, requiring complex bioinformatics algorithms to analyze and filter background noise. Notably, this technique may lead to underrepresentation of low-abundance miRNAs due to preferential amplification of highly expressed miRNAs[54]. Therefore, while miRNAs represent promising biomarkers, standardizing protocols and optimizing detection technologies are essential to enhance accuracy and clinical applicability.
To harness the full potential of miRNAs in cardiovascular disease management and treatment, future research should focus on their application in personalized medicine by improving diagnostic accuracy and enabling early therapeutic decision-making to enhance patient prognosis. Additionally, expanding genetic research on other miRNA polymor
Recent evidence suggests that miRNAs act within a complex network of conventional and non-conventional cardiova
It is increasingly clear that no single biomarker nor risk factor can fully predict adverse outcomes. Therefore, miRNAs should be interpreted in the context of both conventional and emerging risk factors, including age, diabetes, chronic kidney disease, inflammatory status, and genetic predisposition. Several studies utilized multivariable Cox regression models or integrated risk scoring systems (e.g., GRACE combined with miRNA panels) to assess the additional prognostic value of miRNAs. For example, Jakob et al[33] found that combining miR-26b-5p, miR-320a, and miR-660-5p expression with the GRACE score improved the AUC from 0.679 to 0.720, indicating an additive rather than substitutive value. Furthermore, individual miRNAs may reflect different pathophysiological mechanisms. MiR-146a is associated with inflammation through the IL-1R/TLR/NF-κB axis, miR-483-5p relates to endothelial dysfunction, and miR-208b corre
From a genetic perspective, evaluating the role of miRNA-related SNPs requires genotyping accuracy and appropriate model selection. Commonly used methods include PCR-restriction fragment length polymerase, real-time quantitative PCR, TaqMan SNP assays, and mass spectrometry-based genotyping platforms (e.g., matrix-assisted laser desorption/ionization time-of-flight mass spectrometry[55-57]. These techniques enable the detection of allelic variation and geno
| Ref. | Patients, n | miRNA | Analyzed model | Standard | OR (95%CI) | P value |
| Wang et al[55], 2017 | 353 CAD | microRNA-146a, rs2431697 | T allele carriers | MALDI-TOF MS, Sequenom MassARRAY system | 1.26 (1.04-1.53) | 0.018 |
| microRNA-146a, rs2910164 | G allele carriers | 0.73 (0.62-0.86) | < 0.001 | |||
| Tie et al[56], 2023 | 151 CAD | microRNA-146a, rs2910164 | Dominant (CG + GG vs CC) | Real-time PCR | 1.59 (0.76–2.81) | 0.014 |
| Recessive (CC vs TT + TC) | 0.91 (0.53–2.04) | 0.320 | ||||
| miRNA-146a, rs41291957 | Dominant (AA + GA vs GG) | 0.71 (0.53–1.35) | 0.680 | |||
| Recessive (AA vs GG + GA) | 0.66 (0.21–1.12) | 0.17 | ||||
| Agiannitopoulos et al[58], 2020 | 80 MI | miRNA-146a, rs2910164 | Dominant (CC + CG vs GG) | PCR-RFLP, HRM, Sanger Sequencing | 0.97 (0.57-1.63) | 1.000 |
| Recessive (CG + GG vs CC) | 1.37 (0.55-3.37) | 0.478 | ||||
| miRNA-149a, rs2292832 | Dominant (CT + TT vs CC) | 1.03 (0.61-1.74) | 1.000 | |||
| Recessive (CC + CT vs TT) | 1.18 (0.56-2.47) | 0.700 | ||||
| miRNA-196aC, rs11614913 | Dominant (CT + TT vs CC) | 1.73 (1.02-2.92) | 0.047 | |||
| Recessive (CC + CT vs TT) | 2.44 (1.13-5.27) | 0.031 | ||||
| miRNA-499, rs3746444 | Dominant (AG + GG vs AA) | 1.87 (1.08-3.24) | 0.031 | |||
| Recessive (AA + AG vs GG) | 2.05 (1.07-3.90) | 0.035 | ||||
| Huang et al[42], 2015 | 718 ACS | miRNA-146a, rs11614913 | Dominant (TC + CC vs TT) | Real time quantitative PCR system | 1.10 (0.84-1.44) | 0.488 |
| Recessive (CC vs TT + TC) | 1.00 | - | ||||
| 717 ACS | miRNA-146a, rs2910164 | Dominant (CG + GG vs CC) | 0.77 (0.60-0.99) | 0.044 | ||
| Recessive (CC + CG vs GG) | 1.07 (0.79-1.45) | 0.673 | ||||
| Zhao et al[59], 2019 | 5202 CHD | miRNA-146a, rs2910164 | Dominant (CC vs CG + GG) | Systematic review | 0.8 (0.73–0.87) | < 0.001 |
| Recessive (CC + CG vs GG) | 0.86 (0.76-0.98) | < 0.001 |
With advances in genetics, circulating miRNAs and their polymorphisms have significant potential in managing AMI and in the prognosis of post-AMI burden. MiRNAs herald a transformative era in CAD management, offering advancements in diagnosis, treatment, and prognosis, thereby enhancing both the quality of community life and healthcare. Despite its promising potential, the application of miRNAs as biomarkers in clinical practice faces numerous challenges, with a major barrier being limitations in miRNA testing. Further in-depth studies are necessary to expand the role and appli
The authors would like to thank the Can Tho University of Medicine and Pharmacy for creating favorable conditions for this study to be performed. We sincerely thank Dr. Tran Lam Thai Bao and Dr. Vo Anh Kiet for assisting us in the synthesis and revision of this manuscript.
| 1. | Ralapanawa U, Sivakanesan R. Epidemiology and the Magnitude of Coronary Artery Disease and Acute Coronary Syndrome: A Narrative Review. J Epidemiol Glob Health. 2021;11:169-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 387] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 2. | Salari N, Morddarvanjoghi F, Abdolmaleki A, Rasoulpoor S, Khaleghi AA, Hezarkhani LA, Shohaimi S, Mohammadi M. The global prevalence of myocardial infarction: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2023;23:206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 283] [Reference Citation Analysis (0)] |
| 3. | Yang J, Biery DW, Singh A, Divakaran S, DeFilippis EM, Wu WY, Klein J, Hainer J, Ramsis M, Natarajan P, Januzzi JL, Nasir K, Bhatt DL, Di Carli MF, Blankstein R. Risk Factors and Outcomes of Very Young Adults Who Experience Myocardial Infarction: The Partners YOUNG-MI Registry. Am J Med. 2020;133:605-612.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 4. | Seo H, Yoon SJ, Yoon J, Kim D, Gong Y, Kim AR, Oh IH, Kim EJ, Lee YH. Recent trends in economic burden of acute myocardial infarction in South Korea. PLoS One. 2015;10:e0117446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Akhtar Z, Aleem MA, Ghosh PK, Islam AKMM, Chowdhury F, MacIntyre CR, Fröbert O. In-hospital and 30-day major adverse cardiac events in patients referred for ST-segment elevation myocardial infarction in Dhaka, Bangladesh. BMC Cardiovasc Disord. 2021;21:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Tini G, Proietti G, Casenghi M, Colopi M, Bontempi K, Autore C, Volpe M, Musumeci B. Long-Term Outcome of Acute Coronary Syndromes in Young Patients. High Blood Press Cardiovasc Prev. 2017;24:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Leitão AL, Enguita FJ. A Structural View of miRNA Biogenesis and Function. Noncoding RNA. 2022;8:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 8. | Liu QZ, Xue TN, Xiao SJ, Wu Q, Pan DF, Zhu H. [Correlation of miRNA-146a SNP and its expression with the diagnosis and prognosis of patients with acute STEMI]. Linchuang Xonxueguan Zazhi. 2021;37:1002-1007. [DOI] [Full Text] |
| 9. | Scărlătescu AI, Micheu MM, Popa-Fotea NM, Dorobanțu M. MicroRNAs in Acute ST Elevation Myocardial Infarction-A New Tool for Diagnosis and Prognosis: Therapeutic Implications. Int J Mol Sci. 2021;22:4799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Minchin S, Lodge J. Understanding biochemistry: structure and function of nucleic acids. Essays Biochem. 2019;63:433-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 11. | Çakmak HA, Demir M. MicroRNA and Cardiovascular Diseases. Balkan Med J. 2020;37:60-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 12. | Yang Z, Liu Z. The Emerging Role of MicroRNAs in Breast Cancer. J Oncol. 2020;2020:9160905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol. 2018;141:1202-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 853] [Cited by in RCA: 1928] [Article Influence: 214.2] [Reference Citation Analysis (0)] |
| 14. | Nitschke L, Tewari A, Coffin SL, Xhako E, Pang K, Gennarino VA, Johnson JL, Blanco FA, Liu Z, Zoghbi HY. miR760 regulates ATXN1 levels via interaction with its 5' untranslated region. Genes Dev. 2020;34:1147-1160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | O'Brien J, Hayder H, Zayed Y, Peng C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front Endocrinol (Lausanne). 2018;9:402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2111] [Cited by in RCA: 3712] [Article Influence: 464.0] [Reference Citation Analysis (1)] |
| 16. | Phương HTB, Linh LTT, Thuý LHÁ. Microrna tuần hoàn – Dấu chứng sinh học tiềm năng cho chẩn đoán sớm bệnh thoái hóa khớp. HCMCOUJS. 2020;15:16-26. [DOI] [Full Text] |
| 17. | Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res. 2012;110:483-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 804] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 18. | Sun T, Dong YH, Du W, Shi CY, Wang K, Tariq MA, Wang JX, Li PF. The Role of MicroRNAs in Myocardial Infarction: From Molecular Mechanism to Clinical Application. Int J Mol Sci. 2017;18:745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 19. | Chen L, Bai J, Liu J, Lu H, Zheng K. A Four-MicroRNA Panel in Peripheral Blood Identified as an Early Biomarker to Diagnose Acute Myocardial Infarction. Front Physiol. 2021;12:669590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Zhang X, Cai H, Zhu M, Qian Y, Lin S, Li X. Circulating microRNAs as biomarkers for severe coronary artery disease. Medicine (Baltimore). 2020;99:e19971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Mahendra J, Mahendra L, Fageeh HN, Fageeh HI, Ibraheem W, Abdulkarim HH, Kanakamedala A, Prakash P, Srinivasan S, Balaji TM, Varadarajan S, Jagannathan R, Patil S. miRNA-146a and miRNA-126 as Potential Biomarkers in Patients with Coronary Artery Disease and Generalized Periodontitis. Materials (Basel). 2021;14:4692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Liao Z, Zheng R, Shao G. Mechanisms and application strategies of miRNA146a regulating inflammation and fibrosis at molecular and cellular levels (Review). Int J Mol Med. 2023;51:7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 23. | Paterson MR, Kriegel AJ. MiR-146a/b: a family with shared seeds and different roots. Physiol Genomics. 2017;49:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 24. | Raitoharju E, Lyytikäinen LP, Levula M, Oksala N, Mennander A, Tarkka M, Klopp N, Illig T, Kähönen M, Karhunen PJ, Laaksonen R, Lehtimäki T. miR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atherosclerosis. 2011;219:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 385] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 25. | Takahashi Y, Satoh M, Minami Y, Tabuchi T, Itoh T, Nakamura M. Expression of miR-146a/b is associated with the Toll-like receptor 4 signal in coronary artery disease: effect of renin-angiotensin system blockade and statins on miRNA-146a/b and Toll-like receptor 4 levels. Clin Sci (Lond). 2010;119:395-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 26. | Oerlemans MI, Mosterd A, Dekker MS, de Vrey EA, van Mil A, Pasterkamp G, Doevendans PA, Hoes AW, Sluijter JP. Early assessment of acute coronary syndromes in the emergency department: the potential diagnostic value of circulating microRNAs. EMBO Mol Med. 2012;4:1176-1185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 27. | Xiao S, Xue T, Pan Q, Hu Y, Wu Q, Liu Q, Wang X, Liu A, Liu J, Zhu H, Zhou Y, Pan D. MicroRNA-146a Serves as a Biomarker for Adverse Prognosis of ST-Segment Elevation Myocardial Infarction. Cardiovasc Ther. 2021;2021:2923441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Zhang R, Lan C, Pei H, Duan G, Huang L, Li L. Expression of circulating miR-486 and miR-150 in patients with acute myocardial infarction. BMC Cardiovasc Disord. 2015;15:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Peng L, Chun-guang Q, Bei-fang L, Xue-zhi D, Zi-hao W, Yun-fu L, Yan-ping D, Yang-gui L, Wei-guo L, Tian-yong H, Zhen-wen H. Clinical impact of circulating miR-133, miR-1291 and miR-663b in plasma of patients with acute myocardial infarction. Diagn Pathol. 2014;9:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 30. | Wang F, Long G, Zhao C, Li H, Chaugai S, Wang Y, Chen C, Wang DW. Atherosclerosis-related circulating miRNAs as novel and sensitive predictors for acute myocardial infarction. PLoS One. 2014;9:e105734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Zhao Y, Song X, Ma Y, Liu X, Peng Y. Circulating mir-483-5p as a novel diagnostic biomarker for acute coronary syndrome and its predictive value for the clinical outcome after PCI. BMC Cardiovasc Disord. 2023;23:360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 32. | Xue S, Zhu W, Liu D, Su Z, Zhang L, Chang Q, Li P. Circulating miR-26a-1, miR-146a and miR-199a-1 are potential candidate biomarkers for acute myocardial infarction. Mol Med. 2019;25:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 33. | Jakob P, Kacprowski T, Briand-Schumacher S, Heg D, Klingenberg R, Stähli BE, Jaguszewski M, Rodondi N, Nanchen D, Räber L, Vogt P, Mach F, Windecker S, Völker U, Matter CM, Lüscher TF, Landmesser U. Profiling and validation of circulating microRNAs for cardiovascular events in patients presenting with ST-segment elevation myocardial infarction. Eur Heart J. 2017;38:511-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Yang X, Du X, Ma K, Li G, Liu Z, Rong W, Miao H, Zhu F, Cui Q, Wu S, Li Y, Du J. Circulating miRNAs Related to Long-term Adverse Cardiovascular Events in STEMI Patients: A Nested Case-Control Study. Can J Cardiol. 2021;37:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Alavi-Moghaddam M, Chehrazi M, Alipoor SD, Mohammadi M, Baratloo A, Mahjoub MP, Movasaghi M, Garssen J, Adcock IM, Mortaz E. A Preliminary Study of microRNA-208b after Acute Myocardial Infarction: Impact on 6-Month Survival. Dis Markers. 2018;2018:2410451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Zhou SS, Jin JP, Wang JQ, Zhang ZG, Freedman JH, Zheng Y, Cai L. miRNAS in cardiovascular diseases: potential biomarkers, therapeutic targets and challenges. Acta Pharmacol Sin. 2018;39:1073-1084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 495] [Cited by in RCA: 508] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 37. | A.f. Al-koofee D, M.h. Mubarak S. Genetic Polymorphisms. Recent Top Genet Polymorphisms. 2020;. [DOI] [Full Text] |
| 38. | Bao MH, Xiao Y, Zhang QS, Luo HQ, Luo J, Zhao J, Li GY, Zeng J, Li JM. Meta-Analysis of miR-146a Polymorphisms Association with Coronary Artery Diseases and Ischemic Stroke. Int J Mol Sci. 2015;16:14305-14317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Zhi H, Wang L, Ma G, Ye X, Yu X, Zhu Y, Zhang Y, Zhang J, Wang B. Polymorphisms of miRNAs genes are associated with the risk and prognosis of coronary artery disease. Clin Res Cardiol. 2012;101:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Qiao XR, Zheng T, Xie Y, Yao X, Yuan Z, Wu Y, Zhou D, Chen T. MiR-146a rs2910164 (G/C) polymorphism is associated with the development and prognosis of acute coronary syndromes: an observational study including case control and validation cohort. J Transl Med. 2023;21:325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 41. | Shi B, Wang X, Xue T, Liu J, Wu W, Luo Y, Zhu H, Pan D. Expression level of miR-146a is associated with the coronary lesion severity and clinical prognosis in patients with unstable angina pectoris. Int J Cardiol Cardiovasc Risk Prev. 2025;24:200367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 42. | Huang S, Lv Z, Deng Q, Li L, Yang B, Feng J, Wu T, Zhang X, Cheng J. A Genetic Variant in Pre-miR-146a (rs2910164 C>G) Is Associated with the Decreased Risk of Acute Coronary Syndrome in a Chinese Population. Tohoku J Exp Med. 2015;237:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Toan NH, Son TK. study on characteristics of some microrna-146a gene polymorphisms and their relationship with the clinical severity of patients with acute myocardial infarction. TCNCYH. 2025;187:31-39. [DOI] [Full Text] |
| 44. | Li Q, Chen L, Chen D, Wu X, Chen M. Influence of microRNA-related polymorphisms on clinical outcomes in coronary artery disease. Am J Transl Res. 2015;7:393-400. [PubMed] |
| 45. | Chiong M, Wang ZV, Pedrozo Z, Cao DJ, Troncoso R, Ibacache M, Criollo A, Nemchenko A, Hill JA, Lavandero S. Cardiomyocyte death: mechanisms and translational implications. Cell Death Dis. 2011;2:e244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 369] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 46. | Lu Y, Thavarajah T, Gu W, Cai J, Xu Q. Impact of miRNA in Atherosclerosis. Arterioscler Thromb Vasc Biol. 2018;38:e159-e170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 47. | Tang Y, Luo X, Cui H, Ni X, Yuan M, Guo Y, Huang X, Zhou H, de Vries N, Tak PP, Chen S, Shen N. MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum. 2009;60:1065-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 570] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 48. | He X, Tang R, Sun Y, Wang YG, Zhen KY, Zhang DM, Pan WQ. MicroR-146 blocks the activation of M1 macrophage by targeting signal transducer and activator of transcription 1 in hepatic schistosomiasis. EBioMedicine. 2016;13:339-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 49. | Hartiala JA, Han Y, Jia Q, Hilser JR, Huang P, Gukasyan J, Schwartzman WS, Cai Z, Biswas S, Trégouët DA, Smith NL; INVENT Consortium; CHARGE Consortium Hemostasis Working Group; GENIUS-CHD Consortium, Seldin M, Pan C, Mehrabian M, Lusis AJ, Bazeley P, Sun YV, Liu C, Quyyumi AA, Scholz M, Thiery J, Delgado GE, Kleber ME, März W, Howe LJ, Asselbergs FW, van Vugt M, Vlachojannis GJ, Patel RS, Lyytikäinen LP, Kähönen M, Lehtimäki T, Nieminen TVM, Kuukasjärvi P, Laurikka JO, Chang X, Heng CK, Jiang R, Kraus WE, Hauser ER, Ferguson JF, Reilly MP, Ito K, Koyama S, Kamatani Y, Komuro I; Biobank Japan, Stolze LK, Romanoski CE, Khan MD, Turner AW, Miller CL, Aherrahrou R, Civelek M, Ma L, Björkegren JLM, Kumar SR, Tang WHW, Hazen SL, Allayee H. Genome-wide analysis identifies novel susceptibility loci for myocardial infarction. Eur Heart J. 2021;42:919-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 199] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 50. | Li X, Liao J, Su X, Li W, Bi Z, Wang J, Su Q, Huang H, Wei Y, Gao Y, Li J, Liu L, Wang C. Human urine-derived stem cells protect against renal ischemia/reperfusion injury in a rat model via exosomal miR-146a-5p which targets IRAK1. Theranostics. 2020;10:9561-9578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 51. | Morishita Y, Imai T, Yoshizawa H, Watanabe M, Ishibashi K, Muto S, Nagata D. Delivery of microRNA-146a with polyethylenimine nanoparticles inhibits renal fibrosis in vivo. Int J Nanomedicine. 2015;10:3475-3488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 52. | Zou Y, Cai Y, Lu D, Zhou Y, Yao Q, Zhang S. MicroRNA-146a-5p attenuates liver fibrosis by suppressing profibrogenic effects of TGFβ1 and lipopolysaccharide. Cell Signal. 2017;39:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 53. | Hoang Ngo T, Tran Khuong Nguyen N, Thi Ngoc Pham N, Tran BLT, Tuan Huynh A, Duy Nguyen K, Duy Nguyen K, Tran AV. The combination of CYP2C19 polymorphism and inflammatory cell ratios in prognosis cardiac adverse events after acute coronary syndrome. Int J Cardiol Cardiovasc Risk Prev. 2023;19:200222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 54. | Koshiol J, Wang E, Zhao Y, Marincola F, Landi MT. Strengths and limitations of laboratory procedures for microRNA detection. Cancer Epidemiol Biomarkers Prev. 2010;19:907-911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 55. | Wang Y, Wang X, Li Z, Chen L, Zhou L, Li C, Ouyang DS. Two Single Nucleotide Polymorphisms (rs2431697 and rs2910164) of miR-146a Are Associated with Risk of Coronary Artery Disease. Int J Environ Res Public Health. 2017;14:514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 56. | Tie J, Takanari H, Ota K, Okuda T. Role of miR-143 and miR-146 in Risk Evaluation of Coronary Artery Diseases in Autopsied Samples. Genes (Basel). 2023;14:471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 57. | Ramírez CM, Rotllan N, Vlassov AV, Dávalos A, Li M, Goedeke L, Aranda JF, Cirera-Salinas D, Araldi E, Salerno A, Wanschel A, Zavadil J, Castrillo A, Kim J, Suárez Y, Fernández-Hernando C. Control of cholesterol metabolism and plasma high-density lipoprotein levels by microRNA-144. Circ Res. 2013;112:1592-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 58. | Agiannitopoulos K, Samara P, Papadopoulou M, Efthymiadou A, Papadopoulou E, Tsaousis GN, Mertzanos G, Babalis D, Lamnissou K. miRNA polymorphisms and risk of premature coronary artery disease. Hellenic J Cardiol. 2021;62:278-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 59. | Zhao D, Li Y, Yu X, Zhu Y, Ma B. Associations between miR-146a rs2910164 polymorphisms and risk of ischemic cardio-cerebrovascular diseases. Medicine (Baltimore). 2019;98:e17106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/